Abstract
The Milan hypertensive strain (MHS) of rats is a model for hypertension in humans. Inherited defects in renal function have been well studied in MHS rats, but the mechanisms that underlie the elevated vascular resistance are unclear. Altered Ca2+ signaling plays a key role in the vascular dysfunction associated with arterial hypertension. Here we compared Ca2+ signaling in mesenteric artery smooth muscle cells from MHS rats and its normotensive counterpart (MNS). Systolic blood pressure was higher in MHS than in MNS rats (144 ± 2 vs. 113 ± 1 mmHg, P < 0.05). Resting cytosolic free Ca2+ concentration (measured with fura-2) and ATP-induced Ca2+ transients were augmented in freshly dissociated arterial myocytes from MHS rats. Ba2+ entry activated by the diacylglycerol analog 1-oleoyl-2-acetyl-sn-glycerol (a measure of receptor-operated channel activity) was much greater in MHS than MNS arterial myocytes. This correlated with a threefold upregulation of transient receptor potential canonical 6 (TRPC6) protein. TRPC3, the other component of receptor-operated channels, was marginally, but not significantly, upregulated. The expression of TRPC1/5, components of store-operated channels, was not altered in MHS mesenteric artery smooth muscle. Immunoblots also revealed that the Na+/Ca2+ exchanger-1 (NCX1) was greatly upregulated in MHS mesenteric artery (by ∼13-fold), whereas the expression of plasma membrane Ca2+-ATPase was not altered. Ca2+ entry via the reverse mode of NCX1 evoked by the removal of extracellular Na+ induced a rapid increase in cytosolic free Ca2+ concentration that was significantly larger in MHS arterial myocytes. The expression of α1/α2 Na+ pumps in MHS mesenteric arteries was not changed. Immunocytochemical observations showed that NCX1 and TRPC6 are clustered in plasma membrane microdomains adjacent to the underlying sarcoplasmic reticulum. In summary, MHS arteries exhibit upregulated TRPC6 and NCX1 and augmented Ca2+ signaling. We suggest that the increased Ca2+ signaling contributes to the enhanced vasoconstriction and elevated blood pressure in MHS rats.
Keywords: adducin, hypertension, Milan normotensive rats, C-type transient receptor potential channels, receptor-operated calcium entry
primary or essential hypertension is a multifactorial disorder resulting from the complex interplay between genetic predisposition (genetic heritability, ∼30%) and multiple environmental factors (1, 44, 71, 74). The major difficulty in identifying genes contributing to hypertension is the etiological heterogeneity of hypertension (1). Genetic, physiological, and biochemical studies reveal that an alteration in the genes encoding adducin, a ubiquitously expressed membrane-skeletal protein, is associated with a salt-sensitive form of hypertension and renal dysfunction in rats and humans (1, 10, 18).
Adducin is a tetramer comprised of either α/β- or α/γ-heterodimers (50). It is localized at spectrin-actin junctions affecting the assembly of the actin-based cytoskeleton and, thereby, the normal turnover and incorporation of plasma membrane (PM) ion transporters (29, 50) since the latter can be tethered to the membrane cytoskeleton (48, 56). The α-, β-, and γ-subunits of adducin are encoded by genes (ADD1, ADD2, and ADD3 or Add1, Add2, and Add3 in humans and rats, respectively) that map to different chromosomes (29, 50). The polymorphism of ADD1 (G460W/S586C) alone (or in combination with those of ADD2) and angiotensin-converting enzyme is associated with either essential hypertension (18, 45, 78) or with the related cardiovascular and renal complications (51, 54, 60, 70, 75).
The Milan hypertensive strain (MHS) of rats is a genetic model of hypertension in which cardiovascular phenotypes seem to be dependent, at least in part, on adducin gene polymorphisms (11, 62). In the MNS × MHS F2 hybrid population, the mutation of the Add1 gene accounts for the 50% of the blood pressure (BP) difference between MHS and its normotensive counterpart (MNS) (9, 11). Adducin polymorphisms are linked with higher Na+ pump activity and enhanced constitutive tubular Na+ reabsorption in the kidney in both rats and humans (22, 26, 27, 47, 66). Sodium retention should cause a plasma volume expansion and an increased cardiac output (17, 34). In the chronic phase, however, plasma volume and cardiac output are usually normal, and the elevated BP is the result of a blood flow-induced compensatory shift (whole body autoregulation) to increased vasoconstriction and total peripheral vascular resistance (17, 34). A relationship between the alteration in the adducin genes and renal dysfunction has been extensively studied (8, 18, 46, 64, 78), but the mechanisms involved in cardiovascular functional abnormalities in MHS rats are still unclear.
Chronic hypertension is typically associated with increased peripheral vascular resistance (65). This is due, in part, to enhanced arterial smooth muscle contractility, which is regulated by intracellular Ca2+ (39, 65, 81). Accumulating evidence indicates that Ca2+ influx through PM store- and receptor-operated Ca2+ channels (SOCs and ROCs, respectively), along with voltage-gated Ca2+ channels, may play a role in regulating myogenic tone and vasoconstriction (30, 53, 67, 73). Transient receptor potential canonical (TRPC)1, TRPC4, and/or TRPC5 proteins, mammalian homologs of the Drosophila transient receptor potential channel, form the endogenous SOCs that are activated by sarcoplasmic reticulum (SR) Ca2+ depletion (6, 57, 76, 77). In contrast, TRPC3 and TRPC6, which are components of ROCs, can be activated by diacylglycerols in a store depletion-independent manner (36). Several reports indicate that Ca2+ homeostasis in arterial smooth muscle cells (ASMCs) is influenced not only by direct Ca2+ entry through TRPC channels but also by Na+ entry through these nonselective cation channels. The entering Na+ apparently then also promotes Ca2+ entry through Na+/Ca2+ exchanger type 1 (NCX1) (4, 23, 58, 82).
TRPC channels (28, 43, 61) and NCX1 (39, 61, 82) have been implicated in the pathogenesis of various forms of hypertension. For example, TRPC1 and TRPC6 are involved in hypoxic pulmonary hypertension (42, 72). TRPC6 (79) and NCX1 (82) are both implicated in human primary pulmonary hypertension. We demonstrated that upregulated TRPC1, TRPC6, and NCX1 are involved in the augmented Ca2+ signaling in mesenteric artery myocytes from ouabain hypertensive rats (61).
Recent findings indicate that adducin polymorphisms are associated not only with renal dysfunction (18, 64, 78) but also with functional alterations in rat mesenteric small resistance arteries (62). Augmented Ca2+ signaling plays a key role in the vascular dysfunction associated with arterial hypertension. Here we explore the mechanisms responsible for altered Ca2+ signaling in MHS mesenteric artery myocytes. Our data demonstrate that upregulated NCX1 and TRPC6 contribute to the augmented Ca2+ signaling in ASMCs from MHS rats.
METHODS
Ethical approval.
All experiments were approved by the Institutional Animal Care and Use Committee of the University of Maryland School of Medicine.
Experimental animals.
MHS and MNS rats were obtained from Prassis Sigma Tau Research Institute (Milan, Italy) and bred in the Animal Care Facility of the University of Maryland, School of Medicine. Animals were maintained in a temperature- and humidity-controlled room with a 12-h:12-h light-dark cycle. Rats had free access to tap water and were fed standard rat chow ad libitum. Body weight was measured weekly. Genomic DNA was obtained from tail biopsies for genotyping by PCR. We studied male MHS and MNS rats at 10–12 wk of age. Rats were acclimated to the procedures of BP measurements for a week preceding actual data collection. After acclimation to the conditioning of BP measurements, baseline data were obtained over 1 to 2 wk. Systolic and mean BPs in MHS and age-matched MNS controls were recorded by tail-cuff plethysmography using a commercial photoelectric system (model 29 blood pressure meter/amplifier; IITC, Woodland Hill, CA) and a device providing constant rates of cuff inflation and deflation. The average values for BP in each rat were typically obtained from five sequential cuff inflation/deflation cycles.
Dissection of arteries for immunoblotting.
The superior mesenteric artery and aorta from a euthanized rat were rapidly removed and placed in ice-cold physiological salt solution 1 (PSS1) with the following composition: (in mM) 140 NaCl, 5.36 KCl, 0.34 Na2HPO4, 0.44 K2HPO4, 10 HEPES, 1.2 MgCl2, 1.8 CaCl2, and 10 d-glucose (pH 7.2). The arteries were cleaned of fat and connective tissue, deendothelialized, frozen in liquid nitrogen, and stored at −80°C before protein extraction, as previously described (7). Superior mesenteric arteries from 2 to 4 rats were pooled and homogenized in lysis buffer, whereas aortas were homogenized separately.
Freshly dissociated ASMCs for Ca2+ imaging.
Myocytes were isolated from rat mesenteric arteries (7). The superior mesenteric artery was cleaned of fat and connective tissue and digested in low-Ca2+ (0.05 mM) PSS1 containing 2 mg/ml collagenase type XI, 0.16 mg/ml elastase type IV, and 2 mg/ml bovine serum album (fat free) for 35 min at 37°C. After digestion, the tissue was washed three times with low-Ca2+ PSS1 at 4°C. A suspension of single cells was obtained by gently triturating the tissue with a fire-polished Pasteur pipette in low-Ca2+ PSS1. Smooth muscle cells were differentiated by their characteristic elongated morphology. Dispersed cells were directly deposited on glass coverslips for fluorescence microscopy. ASMCs on coverslips were stored at 4°C and used within 4 h. Cells were allowed to settle on the coverslips for 20–30 min before loading with fura-2. Freshly dissociated cells that were markedly contracted under resting conditions (<5%) were excluded. At the conclusion of Ca2+ imaging experiments, the same cells were labeled for smooth muscle α-actin to identify the ASMCs (33). In these experiments, nuclei were also identified by labeling for 5 min with a 50 μM solution of 4′,6′-diamidino-2-phenylindole (DAPI) (31).
Primary cultured ASMCs for Western blot analysis and immunocytochemistry.
The methods used for isolation and culture of rat ASMCs are published (33). Briefly, the superior mesenteric artery is isolated in sterile conditions from euthanized male 10–12-wk-old MHS or MNS rats, as described in Freshly dissociated ASMCs for Ca2+ imaging. The artery was incubated for ∼45 min at 37°C in Ca2+- and Mg2+-free Hanks' balanced salt solution containing 1 mg/ml collagenase type 2. After the incubation, the adventitia was carefully stripped and the endothelium was removed (33). ASMCs in the remaining smooth muscle were dissociated by digestion for 35–40 min at 37°C in Hanks' balanced salt solution containing 1 mg/ml collagenase and 0.5 mg/ml elastase type IV. The dissociated cells were resuspended and plated on either 25-mm coverslips for use in fluorescent microscopy experiments or on 10-cm culture dishes for Western blot analysis. The plated cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum under a humidified atmosphere of 5% CO2-95% air at 37°C. The medium was changed on days 4 and 7. Experiments were performed on subconfluent cultures on days 8 to 9 in vitro if not indicated otherwise. The purity of ASMC cultures was verified by positive staining with smooth muscle-specific α-actin (31, 33). Most of the cells (>99.5%) were α-actin positive. The cells also did not cross-react with fibroblast (CD90/Thy-1)- and endothelial (factor VIII, von Willebrand)-specific antigens (33).
Ca2+ imaging.
Cytosolic free Ca2+ concentration ([Ca2+]cyt) was measured with fura-2 by using digital imaging as previously described (7). Freshly dissociated ASMCs were loaded with fura-2 by incubation for 35 min in PSS1 containing 3.3 μM fura-2 AM (20–22°C, 5% CO2-95% air). After dye loading, the coverslips were transferred to a tissue chamber mounted on a microscope stage where the cells were superfused for 15–20 min (35–36°C) with physiological salt solution 2 (PSS2) to wash away extracellular dye. The PSS2 contained (in mM) 140 NaCl, 5.0 KCl, 1.2 NaH2PO4, 5 NaHCO3, 1.4 MgCl2, 1.8 CaCl2, 11.5 glucose, and 10 HEPES (pH 7.4). In Ca2+-free PSS2, 50 μM EGTA was added to chelate residual Ca2+. In Na+-free PSS2, NaCl was replaced by equimolar N-methyl-d-glucamine (NMDG+). The cells were studied for 40–50 min during continuous superfusion with PSS2 (35°C).
The imaging system included a Zeiss Axiovert 100 microscope (Carl Zeiss, Thornwood, NY). The dye-loaded cells were illuminated with a diffraction grating-based system (Polychrome V, TILL Photonics). Fluorescent images were recorded with a CoolSnap HQ2 CCD camera (Photometrics, Tucson, AZ). Image acquisition and analysis were performed with a MetaFluor/MetaMorph Imaging System (Molecular Devices, Downingtown, PA). [Ca2+]cyt was calculated by determining the ratio of fura-2 fluorescent emission (510 nm) excited at 380 and 360 nm as previously described (7). Intracellular fura-2 was calibrated in situ in freshly dissociated ASMCs (7). Intracellular Ba2+ measurements are shown as fura-2 340/380 excitation ratios with fluorescent emission at 510 nm (7).
Immunobloting.
Membrane proteins were solubilized in sodium dodecyl sulfate buffer containing 5% 2-mercaptoethanol and were separated by polyacrylamide gel electrophoresis as previously described (7). The following antibodies were used: rabbit polyclonal anti-TRPC1, anti-TRPC3, anti-TRPC5, and anti-TRPC6 (dilution 1:200) (Alomone, Jerusalem, Israel); rabbit polyclonal anti-TRPC4 (dilution 1:300; Alomone); mouse monoclonal anti-NCX1 (dilution 1:500; R3F1; Swant, Bellinzona, Switzerland); rabbit polyclonal anti-Na+ pump α1-subunit isoform (dilution, 1:2,000; gift of Dr. Thomas Pressley); rabbit polyclonal anti-Na+ pump α2-subunit isoform (dilution 1:750; Millipore, Billerica, MA); mouse monoclonal anti-PMCA (dilution 1:1,000; Affinity Bioreagents, Golden, CO); and mouse monoclonal anti-sarco(endo)plasmic reticulum Ca2+-ATPase 2 (SERCA2) (dilution 1:1,500; Affinity Bioreagents). Gel loading was controlled with polyclonal or monoclonal anti-β-actin antibodies (dilution 1:10,000; Sigma-Aldrich, St. Louis, MO) or monoclonal anti-glyceraldehyde-3-phosphate dehydrogenase antibody (dilution 1:5,000; Abcam, Cambridge, MA). After being washed, the membranes were incubated with anti-rabbit horseradish peroxidase-conjugated IgG for 1 h at room temperature. The immune complexes on the membranes were detected by enhanced chemiluminescence plus (Amersham Biosciences, Piscattaway, NJ) and exposure to X-ray film (Eastman Kodak, Rochester, NY). Quantitative analysis of immunoblots was performed by using a Kodak DC120 digital camera and 1D Image Analysis Software (Eastman Kodak).
Immunocytochemistry.
ASMCs were immunolabeled, as previously described (32). Briefly, the cells were fixed in cyclohexylamine-formaldehyde fixative consisting of 0.45% (wt/vol) formaldehyde, 75 mM cyclohexylamine, 75 mM NaCl, 10 mM EGTA, 10 mM MgCl2, and 10 mM PIPES. After fixation, the cells were permeabilized in fixative containing 0.5% polyoxyethylene 20 cetyl ether (Brij 58) and were then incubated (4–17 h) in antibody buffer containing antibodies against TRPC6 (dilution 1:10; Alomone) and NCX1 (dilution 1:10; clone R3F1; Swant). FITC-labeled donkey anti-mouse IgG or Cy3-conjugated donkey anti-rabbit IgG (Jackson ImmunoResearch, West Grove, PA) were used to visualize the primary antibodies. The fluorescence from the secondary antibody in the absence of primary antibody (positive control) did not exceed 2 to 3% of the fluorescence in the presence of antiserum. To identify SR in arterial myocytes, the cells were treated (5 min) with 1 μM ER-Tracker (Invitrogen Detection Technologies, Eugene, OR).
Materials.
Fetal bovine serum was obtained from Atlanta Biologicals (Lawrenceville, GA). All other tissue culture reagents were obtained from GIBCO-BRL (Grand Island, NY). Fura-2 AM and DAPI were obtained from Molecular Probes (Invitrogen Detection Technologies). 1-Oleoyl-2-acetyl-sn-glycerol (OAG) was purchased from Calbiochem (San Diego, CA). Collagenase type 2 was obtained from Worthington Biochemical (Freehold, NJ). ATP, dimethylsulfoxide, β-actin, smooth muscle α-actin, collagenase type XI, elastase type IV, bovine serum album, nifedipine, ionomycin, penicillin G, and streptomycin were purchased from Sigma-Aldrich. All other reagents were analytic grade or the highest purity available.
Statistical analysis.
The numerical data presented in results are means ± SE from n single cells (1 value per cell). Immunoblots were repeated at least four to six times for each protein. The number of animals is presented where appropriate. Data from 6 to 18 rats were obtained for most protocols. Statistical significance was determined using Student's paired or unpaired t-test or two-way ANOVA, as appropriate. P < 0.05 was considered significant.
RESULTS
Abnormal Ca2+ homeostasis in freshly dissociated ASMCs from MHS rats.
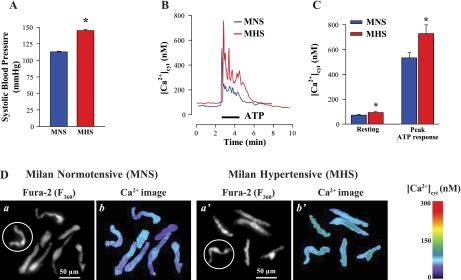
MNS rats (control group) had a mean systolic BP of 113 ± 1 mmHg (n = 16); the systolic BP of the MHS rats was significantly higher (144 ± 2 mmHg, n = 10; P < 0.05) (Fig. 1A). Freshly dissociated mesenteric artery myocytes from the MHS rats had significantly higher resting [Ca2+]cyt than did MNS myocytes (97 ± 2 vs. 78 ± 3 nM, P < 0.001; Fig. 1, B–D). Furthermore, activation by 5 μM ATP in physiological media induced augmented Ca2+ signals in the MHS ASMCs. Both the peak initial response, believed to be the result of inositol 1,4,5-trisphosphate-mediated SR Ca2+ release (15), and the later plateau, perhaps mediated by Ca2+ entry through ROCs and/or SOCs, were greater in MHS than in MNS rat ASMCs (Fig. 1, B and C). The plateau was frequently accompanied by low-amplitude [Ca2+]cyt oscillations or fluctuations in both MHS and MNS arterial myocytes (Fig. 1B). Superimposed records of the ATP-induced Ca2+ response show that the integral of the rise of [Ca2+]cyt (area under the [Ca2+]cyt curve) in MHS arterial myocytes was increased 184 ± 8% of the area in MNS ASMCs (n = 28 MNS cells; 24 MHS cells, P < 0.05). To eliminate the contribution of extracellular Ca2+, the experiments were repeated in Ca2+-free medium. Under these circumstances, the peak amplitudes of ATP-induced Ca2+ transients were also greater in MHS than in MNS rat ASMCs (643 vs. 438 nM, P < 0.05, n = 56 cells). In subsequent experiments, we investigated the role of ROCs and SOCs that can contribute to the altered Ca2+ homeostasis and augmented signaling in ASMCs from MHS rats.
Fig. 1.
Ca2+ homeostasis in freshly dissociated mesenteric artery myocytes from Milan hypertensive rats (MHS) and their control normotensive strain (MNS). A: systolic blood pressures in a representative group of MHS (n = 10 rats) and age-matched MNS controls (n = 16 rats). MHS = *P < 0.05 vs. MNS rats (ANOVA). B: ATP-induced Ca2+ transients in arterial smooth muscle cells (ASMCs) from MNS and MHS rats. Representative records show the time course of cytosolic free Ca2+ concentration ([Ca2+]cyt) changes induced by 5 μM ATP. C: summarized resting [Ca2+]cyt (181 MNS cells, and 198 MHS cells) and peak ATP-induced Ca2+ transient (28 MNS cells, and 24 MHS cells) in ASMCs from 7 MHS and 8 MNS rats. *P < 0.05 vs. ASMCs from normotensive rats. D: representative fura-2 fluorescent images (a and a′) and pseudocolor Ca2+ images show resting [Ca2+]cyt (b and b′) in freshly dissociated ASMCs from MNS (a and b) and MHS (a′ and b′) rats. Data in B were recorded from the circled cells in a and a′. F360, fluorescent emission excited at 360 nm.
Increased ROC-mediated Ca2+ entry and augmented expression of TRPC6 in MHS rat artery smooth muscle.
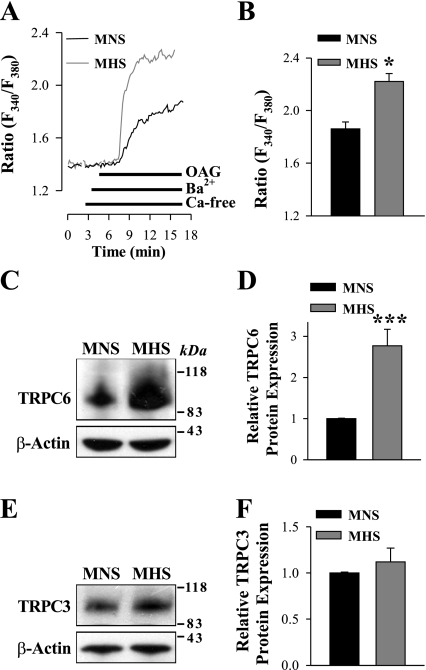
To determine whether ROC-mediated Ca2+ entry (ROCE) is indeed increased in mesenteric ASMCs from MHS rats, freshly dissociated myocytes were stimulated with OAG, the cell-permeable diacylglycerol analog. OAG opens TRPC3 and TRPC6 channels in a protein kinase C-independent manner (36). SOCs have high-Ca2+ selectivity and, unlike ROCs, are virtually impermeable to other alkaline-earth cations, such as Ba2+ (69). Therefore, to distinguish ROCs from SOCs, we measured Ba2+ entry. Ba2+ is not transported by SERCA or PM Ca2+ pumps (41). In the presence of extracellular Ba2+, 100 μM OAG induced significantly larger elevations of cytosolic Ba2+ (fura-2 340/380nm ratio; see methods) in myocytes from MHS rats than in those from normotensive rats (Fig. 2, A and B). To eliminate the contribution of voltage-gated Ca2+ channels to OAG-induced Ba2+ entry, all solutions in the experiments contained 10 μM nifedipine. At this concentration, nifedipine blocks not only L-type but also T-type voltage-gated Ca2+ channels (2). The implication is that ROC activity is augmented in MHS arterial myocytes. Whether this was simply due to an increased entry through an unchanged number of channels or to an increase in the number of channels available was tested by immunoblot analysis. The results reveal that the increased ROCE correlates with the approximately threefold augmentation of TRPC6 expression in deendothelialized mesenteric artery (Fig. 2, C and D) and aorta (not shown). In contrast, the expression of TRPC3, which also belongs to the TRPC3/6/7 subfamily of diacylglycerol-activated ROCs, was not significantly different in the MHS arteries (Fig. 2, E and F); messenger RNA for TRPC7 was not detected in rat mesenteric artery smooth muscle (35) and, therefore, was not studied. Notably, the expression of TRPC1 and TRPC5, which are believed to form subunits of SOCs (6), was not altered in ASMCs from MHS rats (Fig. 3, A–D). TRPC4, also a protein component of SOCs in other cell types, is not expressed in rat mesenteric arteries (7). Augmented ATP-induced Ca2+ response in MHS ASMCs (Fig. 1, B and C) can result not only from increased ROCE (Fig. 2, A and B) but also from increased Ca2+ release from augmented SR Ca2+ stores. Indeed, upregulated SERCA2 expression (Fig. 3, E and F) implies that SR Ca2+ concentration is higher in MHS arterial myocytes than in MNS ASMCs.
Fig. 2.
Augmented 1-oleoyl-2-acetyl-sn-glycerol (OAG)-induced [receptor-operated channel (ROC) mediated] Ba2+ entry and transient receptor potential canonical 6 (TRPC6) protein expression in freshly dissociated ASMCs from MHS rats. A: representative records show the time course of the fura-2 fluorescence ratio (F340/F380) signals induced by 100 μM OAG in freshly dissociated ASMCs from MNS and MHS rats. Extracellular Ca2+ was replaced by 1 mM Ba2+ during the period indicated on the graph. Nifedipine (10 μM) was applied 10 min before the trace shown and was maintained throughout the experiment. B: summarized data show the OAG-induced, ROC-mediated Ba2+ entry in 31 MNS and 42 MHS rat mesenteric ASMCs. Each bar corresponds to data from 8 rats. C–F: Western blot analysis of TRPC6 and TRPC3 expression in ASMCs from MNS and MHS rats. C and E: representative immunoblots (30 μg protein/lane). Summarized data from 4 (D) and 5 (F) immunoblots (total of 18 rats) are normalized to β-actin. *P < 0.05 and ***P < 0.001 vs. MNS ASMCs.
Fig. 3.
Expression of TRPC1, TRPC5, and sarco(endo)plasmic reticulum Ca2+-ATPase 2 (SERCA2) in deendothelialized mesenteric arteries of MNS and MHS rats. A–F: Western blot analysis of TRPC1 (A and B), TRPC5 (C and D), and SERCA2 (E and F) protein expression (30 μg/lane) in smooth muscle cell membranes from mesenteric arteries of MNS and MHS rats. A, C, and E: representative immunoblots. Summarized data from 4 (B), 4 (D), and 4 (F) Western blots (total of 16 rats) are normalized to the amount of β-actin. ***P < 0.001 vs. MNS ASMCs.
Na+ pumps in MHS rat artery smooth muscle.
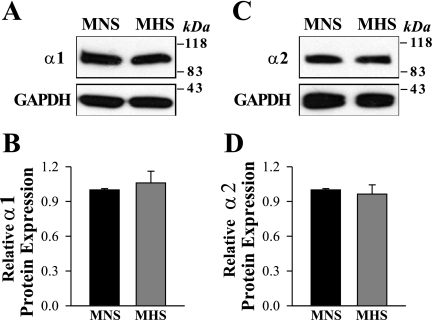
Recently, we demonstrated that the expression of the highly ouabain-sensitive α2 Na+ pump isoform is ∼2.5-fold higher in mesenteric arteries of ouabain hypertensive rats versus their normotensive controls, whereas the expression of α1 Na+ pumps, which have very low affinity for ouabain (55), was not altered (61). The Na+ pump α2-subunits control myogenic tone and BP in ouabain-induced hypertension (21, 81). It is noteworthy that adducin polymorphisms have been associated with elevated levels of circulating ouabain-like factor (24, 25). Furthermore, the genetic alteration in the adducin genes in rats and humans is associated with the upregulation of renal Na+-K+-ATPase; this might be implicated in abnormal Na+ reabsorption and high BP (22, 26, 27, 66). The aforementioned findings suggest that the expression of α2 and possibly α1 Na+ pumps might be altered in deendothelialized mesenteric arteries of MHS rats. Figure 4 shows that neither the α2 nor α1 Na pump subunits are upregulated in MHS arterial myocytes.
Fig. 4.
Expression of Na+ pump α1- and α2-subunit isoforms in deendothelialized mesenteric arteries of MNS and MHS rats. Western blot analysis of α1 (A and B) and α2 Na+ pumps (C and D) protein expression in smooth muscle cell membranes from mesenteric arteries of MNS and MHS rats. A and C: representative blots. All lanes were loaded with 30 μg of membrane protein. Summary data (B and D) are normalized to the amount of β-actin and are expressed as means ± SE from 4 (B) and 4 (D) immunoblots (total of 12 rats).
Upregulated NCX in MHS rat artery smooth muscle.
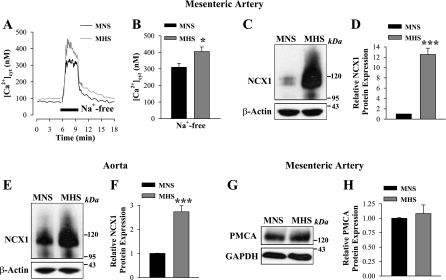
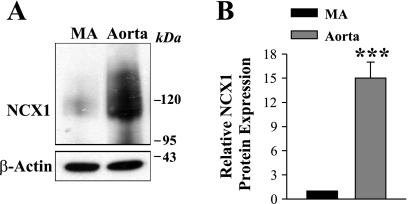
The expression of TRPC6 and NCX1 in rat ASMCs is interrelated (61). If TRPC6 is upregulated in MHS mesenteric arteries, it is logical to ask whether NCX1 is also upregulated. Figure 5, A and B, shows that the removal of extracellular Na+ [conditions that favor Na+ extrusion and Ca2+ entry via NCX1 (12)] induced a rapid increase in [Ca2+]cyt. The increase was significantly larger in MHS mesenteric artery myocytes (404 ± 28 nM, n = 26) compared with that in MNS rats (309 ± 25 nM, n = 33). This was associated with a robust upregulation of NCX1 expression in MHS mesenteric artery (Fig. 5, C and D). Notably, the upregulation of NCX1 was dramatically greater in MHS mesenteric artery (∼13-fold) than in aorta (∼3-fold) (Fig. 5, C–F). The expression of PM Ca2+-ATPase (PMCA) was not altered in mesenteric arteries (Fig. 5, G and H) or aortas from MHS rats (not shown). Interestingly, the expression of NCX1 substantially varies in different vascular beds. As shown in Fig. 6, in the MNS control rats, NCX1 expression was ∼15-fold higher in the aorta than in the mesenteric artery smooth muscle, whereas there was no difference in PMCA and TRPC6 expression in these arteries (not shown).
Fig. 5.
Enhanced Ca2+ entry via the reverse mode of Na+/Ca2+ exchanger-1 (NCX1) and augmented NCX1 expression in freshly dissociated myocytes from MHS rats. A and B: activation of the reverse mode of NCX1 in ASMCs from MNS (black) and MHS (gray) rats. A: representative time course records showing changes in [Ca2+]cyt in single ASMCs; time of treatment with Na+-free solution is indicated. Nifedipine (10 μM) was added 10 min before the records shown and was maintained throughout the experiment. B: summarized data show the NCX1-mediated Ca2+ entry in 33 MNS and 23 MHS mesenteric ASMCs. *P < 0.05 vs. MNS arterial myocytes. C–H: Western blot analysis of NCX1 (C–F) and plasma membrane Ca2+-ATPase (PMCA; G and H) protein expression (30 μg/lane) in smooth muscle cell membranes from mesenteric arteries (C, D, G, and H) and aortas (E and F) of MNS and MHS rats. C, E, and G: representative blots. Summary data (D, F, and H) are normalized to the amount of β-actin and are expressed as means ± SE from 9 (D), 18 (F), and 5 (H) immunoblots (total of 22 rats). ***P < 0.001 vs. MNS ASMCs.
Fig. 6.
NCX1 expression in deendothelialized mesenteric arteries (MAs) and aortas of control normotensive rats. A: representative immunoblot (35 μg protein/lane). B: summarized data from 5 immunoblots (total of 8 rats) are normalized to β-actin. Aorta = ***P < 0.001 vs. mesenteric artery.
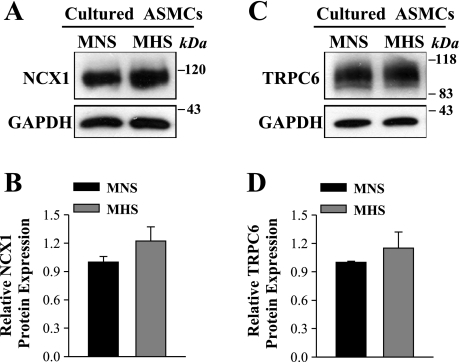
Expression of NCX1 and TRPC6 in primary cultured MHS mesenteric artery myocytes.
The aforementioned changes in NCX1 and ROCs (TRPC6) in MHS mesenteric artery smooth muscle in vivo might be the result of adducin polymorphisms or, alternatively perhaps, the consequence of the elevated BP or some other in vivo factor(s), such as the level of endogenous ouabain that is elevated in MHS rats (24, 25). To explore this issue, we tested the expression of NCX1 and TRPC6 in primary cultured MHS mesenteric artery myocytes. Figure 7 shows that NCX1 and TRPC6 were marginally, but not significantly, upregulated in cultured MHS arterial myocytes when compared with ASMCs from MNS rats.
Fig. 7.
Expression of NCX1 and TRPC6 in primary cultured mesenteric artery myocytes from MNS and MHS rats. A and C: representative immunoblots (30 μg protein/lane). B and D: summarized data from 5 (B) and 4 (D) immunoblots are normalized to β-actin.
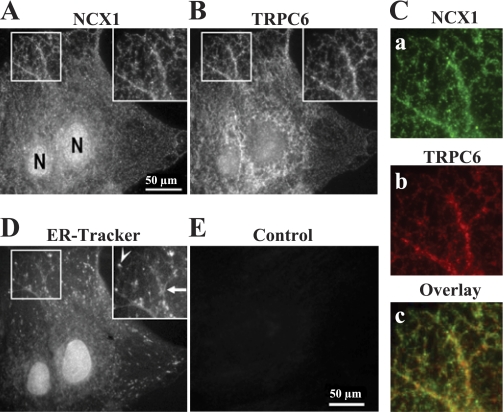
NCX1 and TRPC6 are localized in the PM in close proximity.
As already noted, NCX1 and TRPC6 in rat ASMCs are functionally associated (61). This raises the possibility of their close proximity in the PM. Immunocytochemistry was used to elucidate the relationship between the specific location of NCX1 and TRPC6 proteins in the PM of cultured ASMCs from MNS and MHS rats. High-power images of a portion of an MNS ASMC show that the NCX1 labeling pattern (Fig. 8A, inset) is remarkably similar to the pattern observed with antibodies directed against the TRPC6 (Fig. 8B, inset). Indeed, when Fig. 8C,a (green) is overlaid on Fig. 8C,b (red), an extensive overlap of the labels is observed (Fig. 8C,c), as indicated by the large amount of yellow in the image. Both NCX1 and TRPC6 labels are distributed in a distinct reticular pattern that parallels the organization of the underlying ER-Tracker-stained SR (Fig. 8D, inset). This reticular pattern indicates that clusters of NCX1 and TRPC6 in the PM are organized around the underlying SR. Notably, reactivity was not detected in the PM in the absence of the primary anti-NCX1 (Fig. 8E) or anti-TRPC6 (not shown) antibodies. A similar distribution of NCX1 and TRPC6 was observed in arterial myocytes from MHS rats (not shown).
Fig. 8.
Immunofluorescent localization of NCX1 and TRPC6 in primary cultured mesenteric artery myocytes from normotensive rat. A–D: images of cell triple labeled with anti-NCX1 antibody (A), anti-TRPC6 antibody (B), and ER-Tracker (D). Insets in A, B, and D (enlargements of boxed areas): NCX1, TRPC6, and SR labels show similar distribution. C,a and b: pseudocolor images (green, anti-NCX1; red, anti-TRPC6) of enlarged boxes from A and B, respectively. C,c: colocalization of NCX1 (a) and TRPC6 (b) staining; yellow/orange, and yellow/green areas in overlay indicate regions of overlap between the 2 epitopes. E: fluorescence detected from the secondary antibody (Cy3) in the absence of primary anti-NCX1 antibody (control). Scale bar = 50 μm (A and E). N, nucleus. Similar results were obtained in 12 ASMCs.
DISCUSSION
Arterial hypertension in MHS rats has been associated with functional alterations of small resistance arteries including increased contractility (62). As intracellular Ca2+ plays a fundamental role in the genesis and maintenance of arterial tone (39, 65, 81), alterations of vascular Ca2+ signaling might be pivotal events for the development and maintenance of adducin-dependent hypertension. The present report describes the first study of some of the key mechanisms involved in the arterial myocyte Na+ and Ca2+ metabolism in the MHS rat model of hypertension. The results show there is augmented expression of NCX1 and TRPC6 and remodeling of Ca2+ signaling in Milan rats carrying the hypertensive adducin phenotype. The identity of the specific transport proteins involved and their direct role in cellular Ca2+ metabolism imply a primary contribution to increased myogenic tone and peripheral vascular resistance in MHS rats.
Ca2+ homeostasis is altered in MHS rat arterial myocytes.
Arterial contractility is augmented in many forms of hypertension (13, 21, 81), including adducin-dependent hypertension (62). Some evidence points to dynamic increases in contraction that may be associated directly with Ca2+ dysregulation, such as enhanced responses to vasoconstrictors (13, 62, 80).
Based on the evidence for altered arterial function in MHS rats, we studied aspects of Ca2+ homeostasis in ASMCs from the MHS and MNS rats. Indeed, freshly dissociated myocytes from MHS exhibit Ca2+ dysregulation: elevated resting [Ca2+]cyt and augmented ATP-induced Ca2+ signals (Fig. 1, B–D). The MHS myocytes also exhibit increased ROCE (Fig. 2, A and B). Furthermore, immunoblots indicated that the latter effect is a consequence of upregulated TRPC6 protein expression (Fig. 2, C and D). The expression of other TRPCs such as TRPC3 (Fig. 2, E and F), as well as TRPC1 and TRPC5 (Fig. 3, A–D), was not significantly different in the MHS rats. The upregulation of TRPC6 is thus specific and, when taken with the data showing increased ROCE, is consistent with the augmented norepinephrine-evoked vasoconstriction of intact mesenteric small resistance arteries from MHS rats, especially at high norepinephrine concentrations (62). The increased basal [Ca2+]cyt, as well as the increased Ca2+ influx through TRPC6-encoded ROCs (20, 37, 73), is likely to contribute to the augmented myogenic reactivity of MHS rat arteries. Indeed, ouabain-induced hypertension (61), mineralocorticoid-salt hypertension (5), and human idiopathic pulmonary hypertension (79) are all associated with a marked upregulation of TRPC6. Thus the biochemistry and physiology appear to be directly linked, and the upregulation of TRPC6 may provide a molecular explanation for the observed augmentation of vascular contractile responses in hypertension (13, 62, 80). Vascular structural remodeling and artery narrowing (49, 52) and increased arterial stiffness (14) have also been linked with sustained increases in peripheral vascular resistance. Recent findings, however, revealed that the structure of MHS mesenteric small resistance arteries is not altered when compared with arteries from MNS rats (62).
It is noteworthy that adducin-dependent hypertension in rats has been associated with elevated plasma levels of an endogenous ouabain-like factor (24, 25). Indeed, the plasma concentration of endogenous ouabain is twofold greater in MHS (0.565 ± 0.06 nM, n = 17) than in MNS rats (0.284 ± 0.05 nM, n = 15, P < 0.01) (24). This begs the question, Is the expression of α2 Na+ pump and NCX1, both of which are upregulated in ouabain hypertensive rats (61), also augmented in the arteries of MHS rats? Here we demonstrate that the expression of α2 and α1 Na+ pump subunits is not changed (Fig. 4). The absence of upregulated Na+ pump abundance implies that either the elevated circulating ouabain-like factors, previously reported in MHS (25), were mitigated or that some other factor is involved. In addition, our results do not exclude the possibility that the activity of the Na+ pumps in MHS arteries might be increased. NCX1 is greatly upregulated in both deendothelialized mesenteric artery (Fig. 5, C and D) and aorta (Fig. 5, E and F) from MHS rats. Interestingly, the upregulation of NCX1 is more pronounced in MHS mesenteric artery than in aorta. This may be important because resistance arteries develop pressure-induced tone and thus play a major role in the maintenance of arterial BP (19), whereas aorta is primarily considered to be a conduit blood vessel.
The mechanism(s) by which the upregulation of TRPC6 and NCX1 occurs is (are) not yet understood. PM ion transporters and channels can be tethered to the membrane cytoskeleton (29, 50). For example, a direct interaction between human TRPC4 and the spectrin cytoskeleton is involved in the regulation of human TRPC4 surface expression and activation (56). Moreover, NCX activity is modulated by changes in the actin cytoskeleton (16). Similarly, a modulation of actin-spectrin assembly by adducin polymorphisms can affect turnover and the incorporation of PM ion transporters and channels, including NCX1, TRPC6, and Na+ pumps. In fact, mutant adducin variants lead to a lowering of endocytosis of Na+ pump and thereby an increase in the number of basolateral Na+ pumps in kidney epithelial cells (22, 68). It is not clear whether TRPC6 and NCX1 in arteries of MHS rats are simultaneously affected or whether an upregulation of one of them affects the expression of the other protein. As the expression of NCX1 and TRPC6 in rat ASMCs is highly interrelated (61), a mutual interaction between the regulation of Ca2+ homeostasis by NCX1 and the regulation of Ca2+ (and Na+) entry through ROCs, which are nonselective cation channels, is clearly implied (3, 20, 38). Regardless of the mechanism(s) involved in their upregulation, both transporters may play an important role in the development and/or maintenance of hypertension (5, 39, 43, 79). This concept is further underscored in ouabain-induced hypertension (61) and in human primary pulmonary hypertension, where NCX1 (82) and TRPC6 (79) are both upregulated.
NCX1 and TRPC6 in primary cultured MHS mesenteric artery myocytes.
Another key observation made in this study is that NCX1 and TRPC6 expression is not significantly augmented in primary cell cultures of MHS arterial myocytes when compared with ASMCs from MNS rats. The results imply that the upregulation of NCX1 and TRPC6 in MHS rats in vivo is not directly triggered by adducin polymorphisms per se, because cultured cells from each of the strains continue to express their respective adducins. Thus we suggest that the differences in the in vivo expression of these transporters may be triggered and maintained by the elevated level of endogenous ouabain in MHS rats or other neurohumoral factors that are absent in the cell culture environment. Consistent with this idea, we have shown that prolonged (72 h) treatment of cultured myocytes from control normotensive rats with nanomolar ouabain significantly upregulates NCX1 and TRPC6 (61). While we cannot entirely exclude the possibility that increases in BP trigger the changes in NCX1 and TRPC6 expression, the aforementioned observations suggest that endogenous ouabain likely accounts for most of the increase in NCX1, TRPC6, and BP in the MHS rat.
The functional interaction of NCX1 and TRPC6 in ASMCs (61) raises the possibility of their clustering in the PM-junctional SR regions. Notably, NCX1 (40) and TRPC channels (32) are confined to the PM microdomains that overlie the closely apposed junctional SR. Moreover, coimmunoprecipitation experiments provide evidence for the association of NCX1 with TRPC3 in protein complexes in HEK293 cells (63). In the present study, immunocytochemistry with anti-TRPC6 and anti-NCX1 antibodies revealed the close proximity of TRPC6 and NCX1 proteins in the PM-junctional SR regions of the mesenteric ASMC (Fig. 8). These findings show that PM microdomains which include TRPC6-containing channels and NCX1 function as integrated units help to regulate Ca2+ signals in vascular smooth muscle cells. The buffering of NCX1-mediated Ca2+ entry in the PM-junctional SR regions by the SR and mitochondria limits its diffusion into the cytosol (59). As a result, the estimated transient increase in the sub-PM Ca2+ concentration upon the substitution of extracellular Na+ by NMDG in rat ASMCs is 13-fold greater than the observed increase in [Ca2+]cyt (59). In this regard also, it is noteworthy that the expression of SERCA2 is ∼2.5-fold higher in arterial myocytes from MHS rats than in MNS ASMCs (Fig. 3, E and F). The large effect of multiorganelle buffering therefore can explain the relatively modest difference in changes of bulk [Ca2+]cyt in response to Na+-free solution in ASMCs from MHS and MNS rats despite a robust upregulation of NCX1 in MHS arteries (Fig. 5, A–D).
In summary, this report provides the first evidence for the upregulation of specific molecular mechanisms and augmented Ca2+ signaling in ASMCs from MHS rats. The alterations in Ca2+ entry via the reverse mode of NCX1 and augmented ROC-mediated Ba2+ entry in MHS arterial myocytes are highly consistent with the upregulation of NCX1 and TRPC6 expression, respectively. Similar alterations in Ca2+ signaling and an upregulation of NCX1 and TRPC6 have been observed in ouabain-induced hypertension (61) and in human primary pulmonary hypertension (79, 82). Thus the aforementioned findings indicate that the upregulation of these two transporters is intrinsic to many forms of hypertension. Therefore, the results of this study are likely to be relevant not only to those patients with essential hypertension harboring specific adducin polymorphisms but also to a broader population of patients with hypertension.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant HL-PO1-078870 Project 2 (to V. A. Golovina) and by funds from the University of Maryland School of Medicine.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank M. P. Blaustein for comments on the manuscript.
Present address of A. Zulian: Dept. of Biological Science, Univ. of Padova, Padova, Italy.
REFERENCES
- 1.Agarwal A, Williams GH, Fisher ND. Genetics of human hypertension. Trends Endocrinol Metab 16: 127–133, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Akaike N, Kostyuk PG, Osipchuk YV. Dihydropyridine-sensitive low-threshold calcium channels in isolated rat hypothalamic neurones. J Physiol 412: 181–195, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albert AP, Large WA. Signal transduction pathways and gating mechanisms of native TRP-like cation channels in vascular myocytes. J Physiol 570: 45–51, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnon A, Hamlyn JM, Blaustein MP. Na+ entry via store-operated channels modulates Ca2+ signaling in arterial myocytes. Am J Physiol Cell Physiol 278: C163–C173, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Bae YM, Kim A, Lee YJ, Lim W, Noh YH, Kim EJ, Kim J, Kim TK, Park SW, Kim B, Cho SI, Kim DK, Ho WK. Enhancement of receptor-operated cation current and TRPC6 expression in arterial smooth muscle cells of deoxycorticosterone acetate-salt hypertensive rats. J Hypertens 25: 809–817, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Beech DJ, Muraki K, Flemming R. Non-selective cationic channels of smooth muscle and the mammalian homologues of Drosophila TRP. J Physiol 559: 685–706, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berra-Romani R, Mazzocco-Spezzia A, Pulina MV, Golovina VA. Ca2+ handling is altered when arterial myocytes progress from a contractile to a proliferative phenotype in culture. Am J Physiol Cell Physiol 295: C779–C790, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bianchi G, Cusi D. Association and linkage analysis of alpha-adducin polymorphism: is the glass half full or half empty? Am J Hypertens 13: 739–743, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Bianchi G, Ferrari P, Staessen JA. Adducin polymorphism: detection and impact on hypertension and related disorders. Hypertension 45: 331–340, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Bianchi G, Tripodi G. Genetics of hypertension: the adducin paradigm. Ann NY Acad Sci 986: 660–668, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Bianchi G, Tripodi G, Casari G, Salardi S, Barber BR, Garcia R, Leoni P, Torielli L, Cusi D, Ferrandi M, Pinna LA, Baralle FE, Ferrari P. Two point mutations within the adducin genes are involved in blood pressure variation. Proc Natl Acad Sci USA 91: 3999–4003, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blaustein MP, Lederer WJ. Sodium/calcium exchange: its physiological implications. Physiol Rev 79: 763–854, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Bohr DF, Dominiczak AF, Webb RC. Pathophysiology of the vasculature in hypertension. Hypertension 18: III69–III75, 1991 [DOI] [PubMed] [Google Scholar]
- 14.Briones AM, Xavier FE, Arribas SM, Gonzalez MC, Rossoni LV, Alonso MJ, Salaices M. Alterations in structure and mechanics of resistance arteries from ouabain-induced hypertensive rats. Am J Physiol Heart Circ Physiol 291: H193–H201, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Chin TY, Chueh SH. Distinct Ca2+ signalling mechanisms induced by ATP and sphingosylphosphorylcholine in porcine aortic smooth muscle cells. Br J Pharmacol 129: 1365–1374, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Condrescu M, Gardner JP, Chernaya G, Aceto JF, Kroupis C, Reeves JP. ATP-dependent regulation of sodium-calcium exchange in Chinese hamster ovary cells transfected with the bovine cardiac sodium-calcium exchanger. J Biol Chem 270: 9137–9146, 1995 [DOI] [PubMed] [Google Scholar]
- 17.Cowley AW., Jr Long-term control of arterial blood pressure. Physiol Rev 72: 231–300, 1992 [DOI] [PubMed] [Google Scholar]
- 18.Cusi D, Barlassina C, Azzani T, Casari G, Citterio L, Devoto M, Glorioso N, Lanzani C, Manunta P, Righetti M, Rivera R, Stella P, Troffa C, Zagato L, Bianchi G. Polymorphisms of alpha-adducin and salt sensitivity in patients with essential hypertension. Lancet 349: 1353–1357, 1997 [DOI] [PubMed] [Google Scholar]
- 19.Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev 79: 387–423, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Dietrich A, Chubanov V, Kalwa H, Rost BR, Gudermann T. Cation channels of the transient receptor potential superfamily: their role in physiological and pathophysiological processes of smooth muscle cells. Pharmacol Ther 112: 744–760, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Dostanic I, Paul RJ, Lorenz JN, Theriault S, Van Huysse JW, Lingrel JB. The α2-isoform of Na-K-ATPase mediates ouabain-induced hypertension in mice and increased vascular contractility in vitro. Am J Physiol Heart Circ Physiol 288: H477–H485, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Efendiev R, Krmar RT, Ogimoto G, Zwiller J, Tripodi G, Katz AI, Bianchi G, Pedemonte CH, Bertorello AM. Hypertension-linked mutation in the adducin alpha-subunit leads to higher AP2-mu2 phosphorylation and impaired Na+, K+-ATPase trafficking in response to GPCR signals and intracellular sodium. Circ Res 95: 1100–1108, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Fellner SK, Arendshorst WJ. Angiotensin II-stimulated Ca2+ entry mechanisms in afferent arterioles: role of transient receptor potential canonical channels and reverse Na+/Ca2+ exchange. Am J Physiol Renal Physiol 294: F212–F219, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Ferrandi M, Manunta P, Balzan S, Hamlyn JM, Bianchi G, Ferrari P. Ouabain-like factor quantification in mammalian tissues and plasma: comparison of two independent assays. Hypertension 30: 886–896, 1997 [DOI] [PubMed] [Google Scholar]
- 25.Ferrandi M, Minotti E, Salardi S, Florio M, Bianchi G, Ferrari P. Ouabainlike factor in Milan hypertensive rats. Am J Physiol Renal Fluid Electrolyte Physiol 263: F739–F748, 1992 [DOI] [PubMed] [Google Scholar]
- 26.Ferrandi M, Salardi S, Tripodi G, Barassi P, Rivera R, Manunta P, Goldshleger R, Ferrari P, Bianchi G, Karlish SJ. Evidence for an interaction between adducin and Na+-K+-ATPase: relation to genetic hypertension. Am J Physiol Heart Circ Physiol 277: H1338–H1349, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Ferrandi M, Tripodi G, Salardi S, Florio M, Modica R, Barassi P, Parenti P, Shainskaya A, Karlish S, Bianchi G, Ferrari P. Renal Na,K-ATPase in genetic hypertension. Hypertension 28: 1018–1025, 1996 [DOI] [PubMed] [Google Scholar]
- 28.Firth AL, Remillard CV, Yuan JX. TRP channels in hypertension. Biochim Biophys Acta 1772: 895–906, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gardner K, Bennett V. Modulation of spectrin-actin assembly by erythrocyte adducin. Nature 328: 359–362, 1987 [DOI] [PubMed] [Google Scholar]
- 30.Gibson A, McFadzean I, Wallace P, Wayman CP. Capacitative Ca2+ entry and the regulation of smooth muscle tone. Trends Pharmacol Sci 19: 266–269, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Golovina VA. Cell proliferation is associated with enhanced capacitative Ca2+ entry in human arterial myocytes. Am J Physiol Cell Physiol 277: C343–C349, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Golovina VA. Visualization of localized store-operated calcium entry in mouse astrocytes. Close proximity to the endoplasmic reticulum. J Physiol 564: 737–749, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Golovina VA, Blaustein MP. Preparation of primary cultured mesenteric artery smooth muscle cells for fluorescent imaging and physiological studies. Nat Protoc 1: 2681–2687, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Guyton AC, Granger HJ, Coleman TG. Autoregulation of the total systemic circulation and its relation to control of cardiac output and arterial pressure. Circ Res 28, Suppl 1: 93–97, 1971 [PubMed] [Google Scholar]
- 35.Hill AJ, Hinton JM, Cheng H, Gao Z, Bates DO, Hancox JC, Langton PD, James AF. A TRPC-like non-selective cation current activated by alpha 1-adrenoceptors in rat mesenteric artery smooth muscle cells. Cell Calcium 40: 29–40, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature 397: 259–263, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Inoue R, Jensen LJ, Shi J, Morita H, Nishida M, Honda A, Ito Y. Transient receptor potential channels in cardiovascular function and disease. Circ Res 99: 119–131, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Inoue R, Okada T, Onoue H, Hara Y, Shimizu S, Naitoh S, Ito Y, Mori Y. The transient receptor potential protein homologue TRP6 is the essential component of vascular alpha(1)-adrenoceptor-activated Ca2+-permeable cation channel. Circ Res 88: 325–332, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Iwamoto T, Kita S, Zhang J, Blaustein MP, Arai Y, Yoshida S, Wakimoto K, Komuro I, Katsuragi T. Salt-sensitive hypertension is triggered by Ca2+ entry via Na+/Ca2+ exchanger type-1 in vascular smooth muscle. Nat Med 10: 1193–1199, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Juhaszova M, Shimizu H, Borin ML, Yip RK, Santiago EM, Lindenmayer GE, Blaustein MP. Localization of the Na+-Ca2+ exchanger in vascular smooth muscle, and in neurons and astrocytes. Ann NY Acad Sci 779: 318–335, 1996 [DOI] [PubMed] [Google Scholar]
- 41.Kwan CY, Putney JW., Jr Uptake and intracellular sequestration of divalent cations in resting and methacholine-stimulated mouse lacrimal acinar cells. Dissociation by Sr2+ and Ba2+ of agonist-stimulated divalent cation entry from the refilling of the agonist-sensitive intracellular pool. J Biol Chem 265: 678–684, 1990 [PubMed] [Google Scholar]
- 42.Lin MJ, Leung GP, Zhang WM, Yang XR, Yip KP, Tse CM, Sham JS. Chronic hypoxia-induced upregulation of store-operated and receptor-operated Ca2+ channels in pulmonary arterial smooth muscle cells: a novel mechanism of hypoxic pulmonary hypertension. Circ Res 95: 496–505, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Liu D, Yang D, He H, Chen X, Cao T, Feng X, Ma L, Luo Z, Wang L, Yan Z, Zhu Z, Tepel M. Increased transient receptor potential canonical type 3 channels in vasculature from hypertensive rats. Hypertension 53: 70–76, 2009 [DOI] [PubMed] [Google Scholar]
- 44.Luft FC. Molecular genetics of salt-sensitivity and hypertension. Drug Metab Dispos 29: 500–504, 2001 [PubMed] [Google Scholar]
- 45.Manunta P, Barlassina C, Bianchi G. Adducin in essential hypertension. FEBS Lett 430: 41–44, 1998 [DOI] [PubMed] [Google Scholar]
- 46.Manunta P, Bianchi G. Pharmacogenomics and pharmacogenetics of hypertension: update and perspectives–the adducin paradigm. J Am Soc Nephrol 17: S30–S35, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Manunta P, Maillard M, Tantardini C, Simonini M, Lanzani C, Citterio L, Stella P, Casamassima N, Burnier M, Hamlyn JM, Bianchi G. Relationships among endogenous ouabain, alpha-adducin polymorphisms and renal sodium handling in primary hypertension. J Hypertens 26: 914–920, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marrs JA, Napolitano EW, Murphy-Erdosh C, Mays RW, Reichardt LF, Nelson WJ. Distinguishing roles of the membrane-cytoskeleton and cadherin mediated cell-cell adhesion in generating different Na+, K+-ATPase distributions in polarized epithelia. J Cell Biol 123: 149–164, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martinez-Lemus LA, Hill MA, Meininger GA. The plastic nature of the vascular wall: a continuum of remodeling events contributing to control of arteriolar diameter and structure. Physiology (Bethesda) 24: 45–57, 2009 [DOI] [PubMed] [Google Scholar]
- 50.Matsuoka Y, Li X, Bennett V. Adducin: structure, function and regulation. Cell Mol Life Sci 57: 884–895, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morrison AC, Bray MS, Folsom AR, Boerwinkle E. ADD1 460W allele associated with cardiovascular disease in hypertensive individuals. Hypertension 39: 1053–1057, 2002 [DOI] [PubMed] [Google Scholar]
- 52.Mulvany MJ. Small artery remodeling in hypertension. Curr Hypertens Rep 4: 49–55, 2002 [DOI] [PubMed] [Google Scholar]
- 53.Ng LC, Gurney AM. Store-operated channels mediate Ca2+ influx and contraction in rat pulmonary artery. Circ Res 89: 923–929, 2001 [DOI] [PubMed] [Google Scholar]
- 54.Nicod J, Frey BM, Frey FJ, Ferrari P. Role of the alpha-adducin genotype on renal disease progression. Kidney Int 61: 1270–1275, 2002 [DOI] [PubMed] [Google Scholar]
- 55.O'Brien WJ, Lingrel JB, Wallick ET. Ouabain binding kinetics of the rat alpha two and alpha three isoforms of the sodium-potassium adenosine triphosphate. Arch Biochem Biophys 310: 32–39, 1994 [DOI] [PubMed] [Google Scholar]
- 56.Odell AF, Van Helden DF, Scott JL. The spectrin cytoskeleton influences the surface expression and activation of human transient receptor potential channel 4 channels. J Biol Chem 283: 4395–4407, 2008 [DOI] [PubMed] [Google Scholar]
- 57.Parekh AB, Putney JW., Jr Store-operated calcium channels. Physiol Rev 85: 757–810, 2005 [DOI] [PubMed] [Google Scholar]
- 58.Poburko D, Liao CH, Lemos VS, Lin E, Maruyama Y, Cole WC, van Breemen C. Transient receptor potential channel 6-mediated, localized cytosolic [Na+] transients drive Na+/Ca2+ exchanger-mediated Ca2+ entry in purinergically stimulated aorta smooth muscle cells. Circ Res 101: 1030–1038, 2007 [DOI] [PubMed] [Google Scholar]
- 59.Poburko D, Potter K, van Breemen E, Fameli N, Liao CH, Basset O, Ruegg UT, van Breemen C. Mitochondria buffer NCX-mediated Ca2+-entry and limit its diffusion into vascular smooth muscle cells. Cell Calcium 40: 359–371, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Psaty BM, Smith NL, Heckbert SR, Vos HL, Lemaitre RN, Reiner AP, Siscovick DS, Bis J, Lumley T, Longstreth WT, Jr, Rosendaal FR. Diuretic therapy, the alpha-adducin gene variant, and the risk of myocardial infarction or stroke in persons with treated hypertension. JAMA 287: 1680–1689, 2002 [DOI] [PubMed] [Google Scholar]
- 61.Pulina MV, Zulian A, Berra-Romani R, Beskina O, Mazzocco-Spezzia A, Baryshnikov SG, Papparella I, Hamlyn JM, Blaustein MP, Golovina VA. Upregulation of Na+ and Ca2+ transporters in arterial smooth muscle from ouabain hypertensive rats. Am J Physiol Heart Circ Physiol 298: H263–H274, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rizzoni D, Castellano M, Porteri E, Giacche M, Ferrari P, Cusi D, De Ciuceis C, Boari GE, Rosei EA. Functional alterations of mesenteric small resistance arteries in Milan hypertensive and normotensive rats. Hypertens Res 32: 581–585, 2009 [DOI] [PubMed] [Google Scholar]
- 63.Rosker C, Graziani A, Lukas M, Eder P, Zhu MX, Romanin C, Groschner K. Ca2+ signaling by TRPC3 involves Na+ entry and local coupling to the Na+/Ca2+ exchanger. J Biol Chem 279: 13696–13704, 2004 [DOI] [PubMed] [Google Scholar]
- 64.Staessen JA, Bianchi G. Adducin and hypertension. Pharmacogenomics 6: 665–669, 2005 [DOI] [PubMed] [Google Scholar]
- 65.Thompson LP, Bruner CA, Lamb FS, King CM, Webb RC. Calcium influx and vascular reactivity in systemic hypertension. Am J Cardiol 59: 29A–34A, 1987 [DOI] [PubMed] [Google Scholar]
- 66.Torielli L, Tivodar S, Montella RC, Iacone R, Padoani G, Tarsini P, Russo O, Sarnataro D, Strazzullo P, Ferrari P, Bianchi G, Zurzolo C. α-Adducin mutations increase Na+/K+ pump activity in renal cells by affecting constitutive endocytosis: implications for tubular Na+ reabsorption. Am J Physiol Renal Physiol 295: F478–F487, 2008 [DOI] [PubMed] [Google Scholar]
- 67.Tosun M, Paul RJ, Rapoport RM. Coupling of store-operated Ca2+ entry to contraction in rat aorta. J Pharmacol Exp Ther 285: 759–766, 1998 [PubMed] [Google Scholar]
- 68.Tripodi G, Valtorta F, Torielli L, Chieregatti E, Salardi S, Trusolino L, Menegon A, Ferrari P, Marchisio PC, Bianchi G. Hypertension-associated point mutations in the adducin alpha and beta subunits affect actin cytoskeleton and ion transport. J Clin Invest 97: 2815–2822, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Venkatachalam K, Ma HT, Ford DL, Gill DL. Expression of functional receptor-coupled TRPC3 channels in DT40 triple receptor InsP3 knockout cells. J Biol Chem 276: 33980–33985, 2001 [DOI] [PubMed] [Google Scholar]
- 70.Wang JG, Staessen JA, Tizzoni L, Brand E, Birkenhager WH, Fagard R, Herrmann SM, Bianchi G. Renal function in relation to three candidate genes. Am J Kidney Dis 38: 1158–1168, 2001 [DOI] [PubMed] [Google Scholar]
- 71.Ward R. Familiar aggregation and genetic epidemiology of blood pressure. In: Hypertension (2nd ed.), edited by Laragh JH, Brenner BM. New York: Raven Press, 1995, p. 67–88 [Google Scholar]
- 72.Weigand L, Foxson J, Wang J, Shimoda LA, Sylvester JT. Inhibition of hypoxic pulmonary vasoconstriction by antagonists of store-operated Ca2+ and nonselective cation channels. Am J Physiol Lung Cell Mol Physiol 289: L5–L13, 2005 [DOI] [PubMed] [Google Scholar]
- 73.Welsh DG, Morielli AD, Nelson MT, Brayden JE. Transient receptor potential channels regulate myogenic tone of resistance arteries. Circ Res 90: 248–250, 2002 [DOI] [PubMed] [Google Scholar]
- 74.Williams GH. Genetic factors associated with volume-sensitive hypertension. Mol Cell Endocrinol 217: 41–44, 2004 [DOI] [PubMed] [Google Scholar]
- 75.Winnicki M, Somers VK, Accurso V, Hoffmann M, Pawlowski R, Frigo G, Visentin P, Palatini P. alpha-Adducin Gly460Trp polymorphism, left ventricular mass and plasma renin activity. J Hypertens 20: 1771–1777, 2002 [DOI] [PubMed] [Google Scholar]
- 76.Xu SZ, Beech DJ. TrpC1 is a membrane-spanning subunit of store-operated Ca2+ channels in native vascular smooth muscle cells. Circ Res 88: 84–87, 2001 [DOI] [PubMed] [Google Scholar]
- 77.Xu SZ, Boulay G, Flemming R, Beech DJ. E3-targeted anti-TRPC5 antibody inhibits store-operated calcium entry in freshly isolated pial arterioles. Am J Physiol Heart Circ Physiol 291: H2653–H2659, 2006 [DOI] [PubMed] [Google Scholar]
- 78.Yamagishi K, Iso H, Tanigawa T, Cui R, Kudo M, Shimamoto T. Alpha-adducin G460W polymorphism, urinary sodium excretion, and blood pressure in community-based samples. Am J Hypertens 17: 385–390, 2004 [DOI] [PubMed] [Google Scholar]
- 79.Yu Y, Fantozzi I, Remillard CV, Landsberg JW, Kunichika N, Platoshyn O, Tigno DD, Thistlethwaite PA, Rubin LJ, Yuan JX. Enhanced expression of transient receptor potential channels in idiopathic pulmonary arterial hypertension. Proc Natl Acad Sci USA 101: 13861–13866, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang J, Hamlyn JM, Karashima E, Raina H, Mauban JR, Izuka M, Berra-Romani R, Zulian A, Wier WG, Blaustein MP. Low-dose ouabain constricts small arteries from ouabain-hypertensive rats: implications for sustained elevation of vascular resistance. Am J Physiol Heart Circ Physiol 297: H1140–H1150, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang J, Lee MY, Cavalli M, Chen L, Berra-Romani R, Balke CW, Bianchi G, Ferrari P, Hamlyn JM, Iwamoto T, Lingrel JB, Matteson DR, Wier WG, Blaustein MP. Sodium pump alpha2 subunits control myogenic tone and blood pressure in mice. J Physiol 569: 243–256, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang S, Dong H, Rubin LJ, Yuan JX. Upregulation of Na+/Ca2+ exchanger contributes to the enhanced Ca2+ entry in pulmonary artery smooth muscle cells from patients with idiopathic pulmonary arterial hypertension. Am J Physiol Cell Physiol 292: C2297–C2305, 2007 [DOI] [PubMed] [Google Scholar]