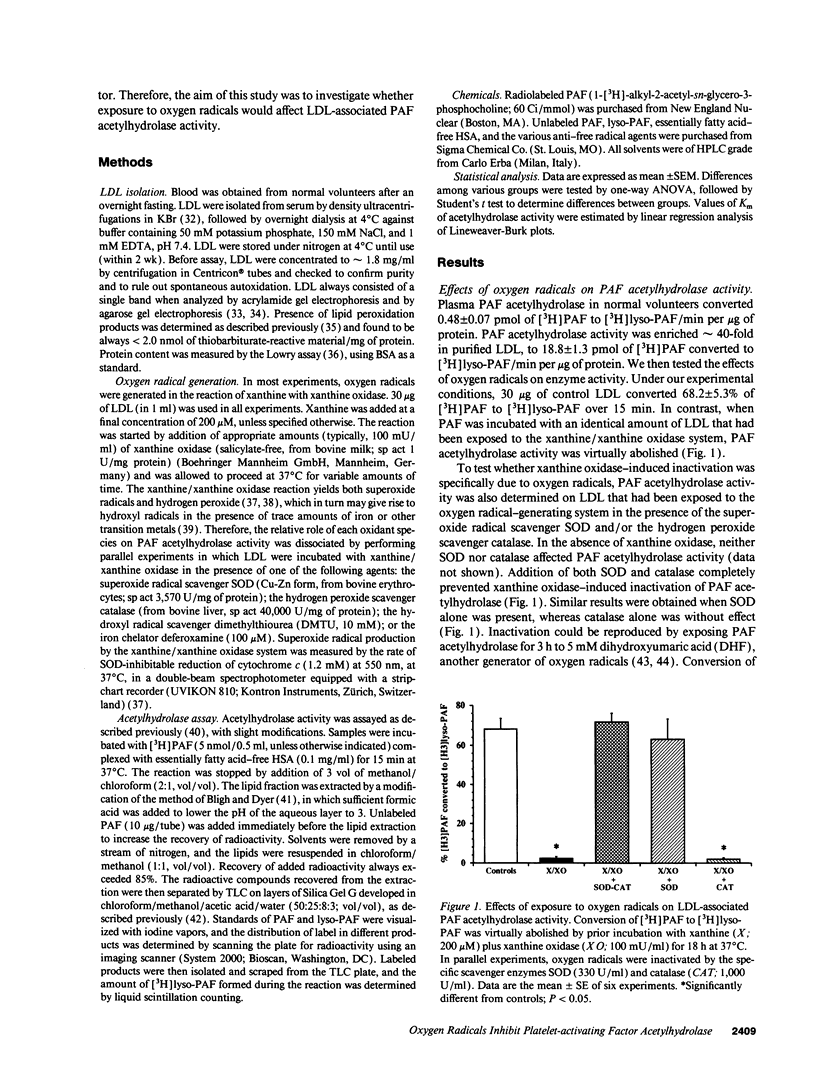
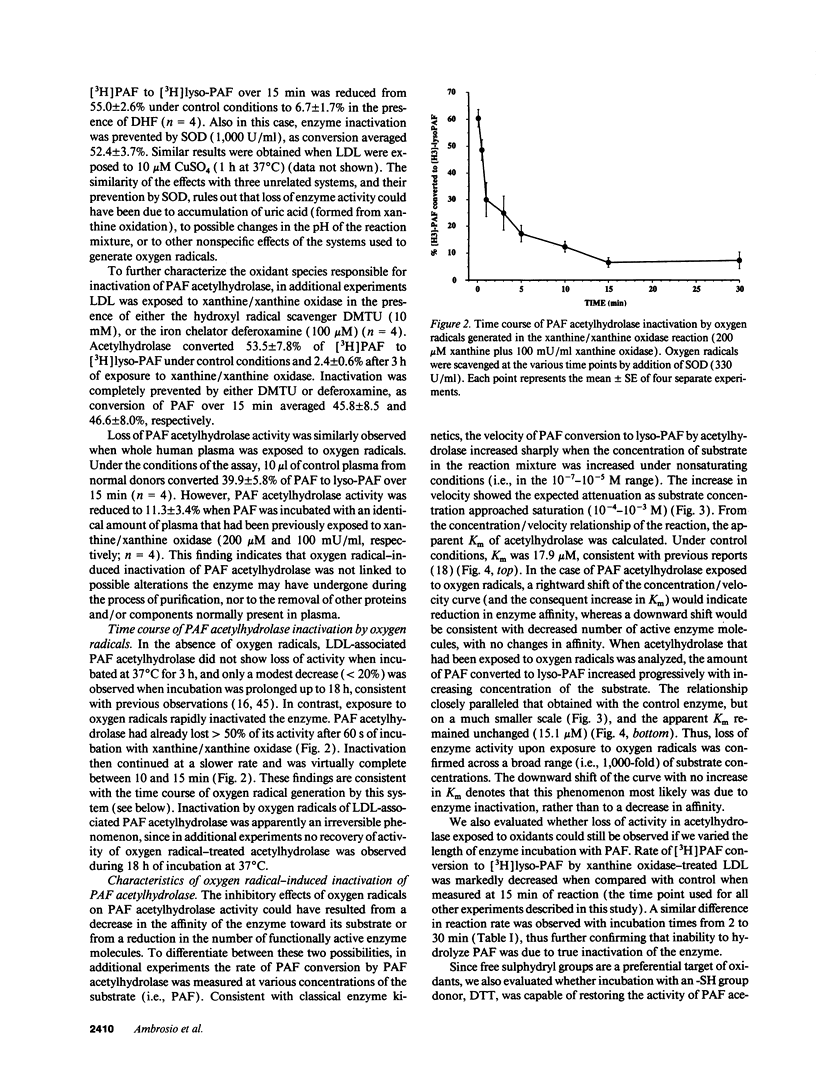
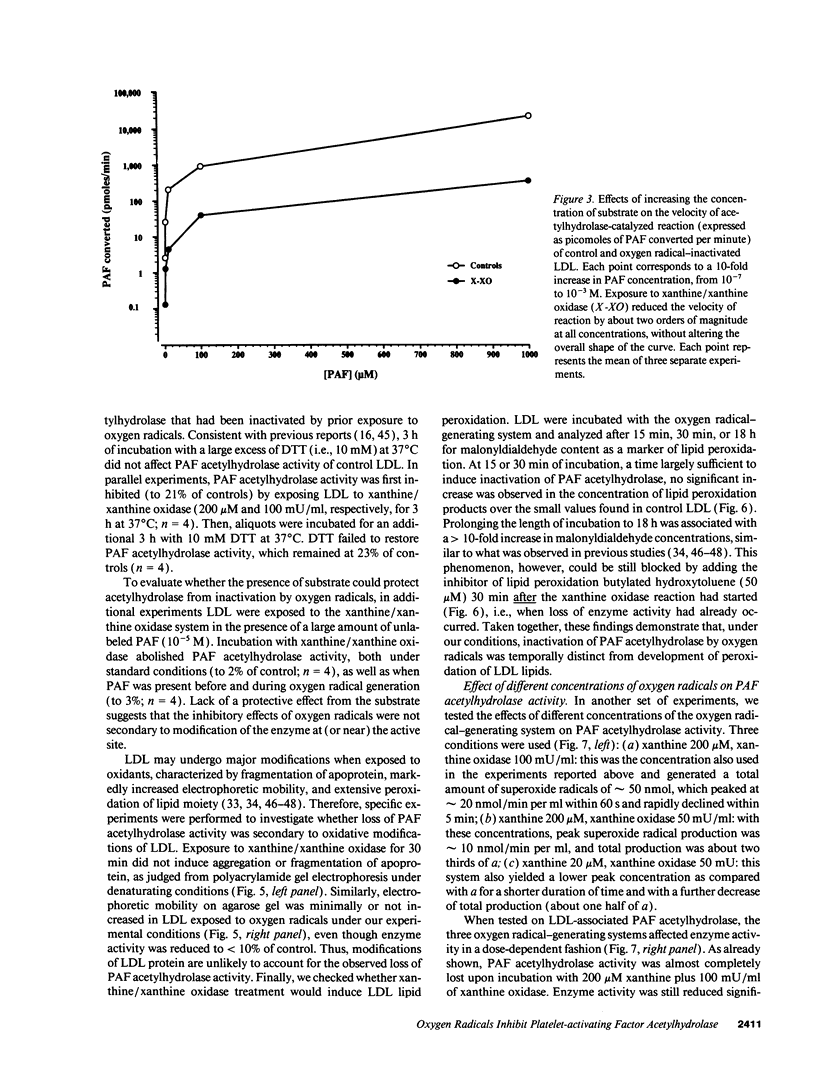
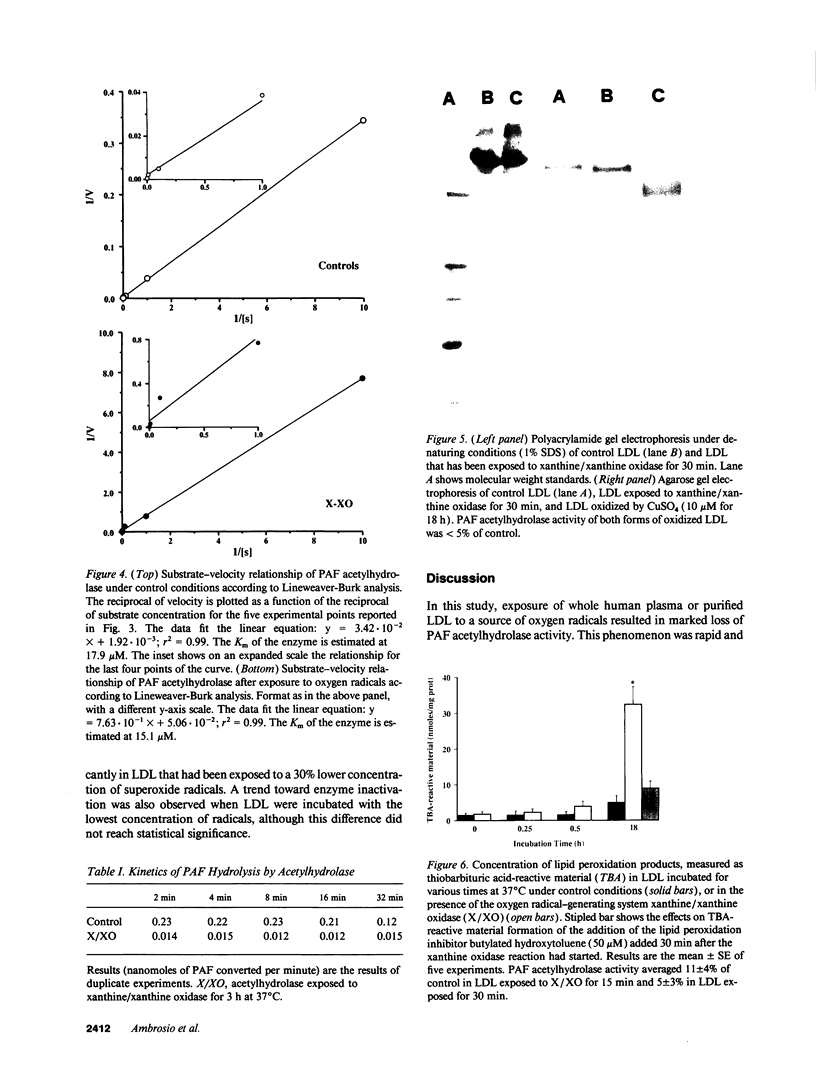
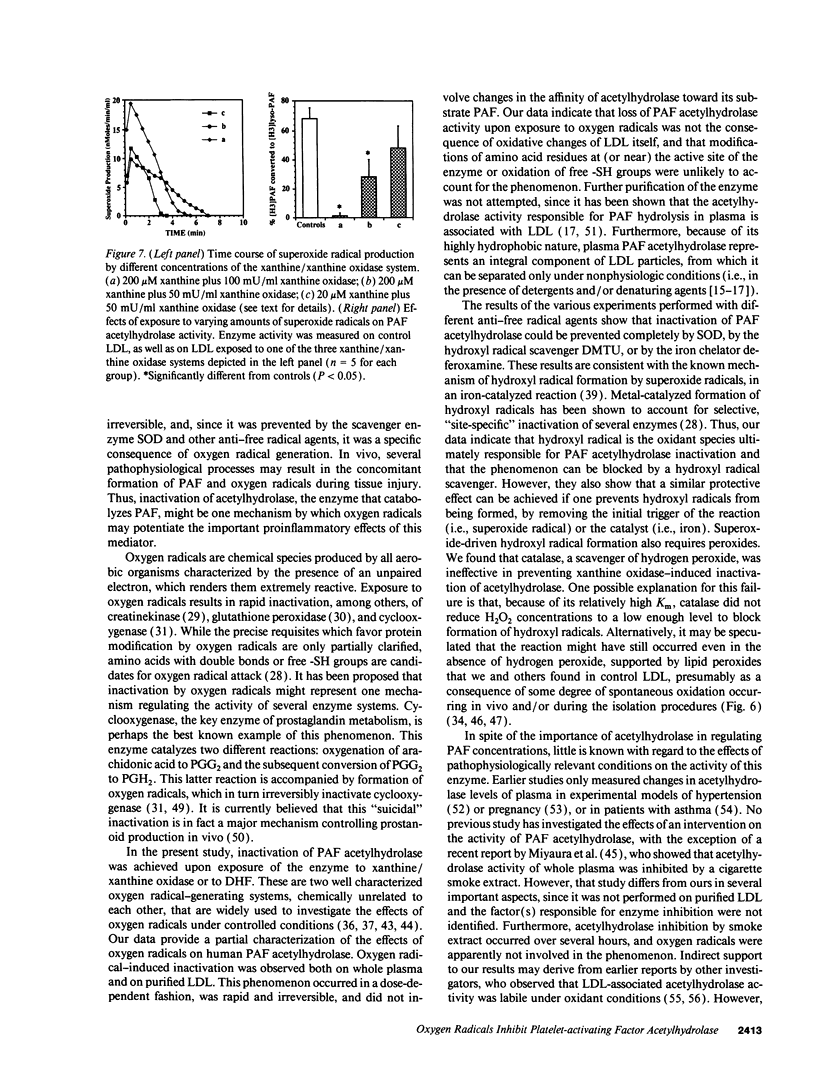
Abstract
Platelet-activating factor (PAF) can exert profound inflammatory effects at very low concentrations. In plasma, PAF is hydrolyzed to lyso-PAF by acetylhydrolase, an enzyme that circulates bound to LDL. Previous studies suggest that oxygen radicals may act synergistically with PAF to potentiate tissue injury. However, mechanisms underlying this interaction have not been elucidated. In this study we investigated whether oxygen radicals may inactivate PAF acetylhydrolase. PAF acetylhydrolase activity was measured in human plasma and purified LDL before and after exposure to radicals (10-20 nmol/min per ml) generated by xanthine/xanthine oxidase. Oxygen radicals induced > 50% loss of PAF acetylhydrolase activity within 60 s and almost complete inactivation by 10 min. This phenomenon was irreversible and independent of oxidative modification of LDL. Inactivation occurred without changes in the affinity constant of the enzyme (Km was 17.9 microM under control conditions and 15.1 microM after exposure to oxygen radicals). Inactivation was prevented by the scavengers superoxide dismutase or dimethylthiourea or by the iron chelator deferoxamine. Thus, superoxide-mediated, iron-catalyzed formation of hydroxyl radicals can rapidly and irreversibly inactivate PAF acetylhydrolase. Since concomitant production of PAF and oxygen radicals can occur in various forms of tissue injury, inactivation of acetylhydrolase might represent one mechanism by which oxygen radicals may potentiate and prolong the proinflammatory effects of PAF.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambrosio G., Flaherty J. T., Duilio C., Tritto I., Santoro G., Elia P. P., Condorelli M., Chiariello M. Oxygen radicals generated at reflow induce peroxidation of membrane lipids in reperfused hearts. J Clin Invest. 1991 Jun;87(6):2056–2066. doi: 10.1172/JCI115236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosio G., Zweier J. L., Duilio C., Kuppusamy P., Santoro G., Elia P. P., Tritto I., Cirillo P., Condorelli M., Chiariello M. Evidence that mitochondrial respiration is a source of potentially toxic oxygen free radicals in intact rabbit hearts subjected to ischemia and reflow. J Biol Chem. 1993 Sep 5;268(25):18532–18541. [PubMed] [Google Scholar]
- Ambrosio G., Zweier J. L., Flaherty J. T. The relationship between oxygen radical generation and impairment of myocardial energy metabolism following post-ischemic reperfusion. J Mol Cell Cardiol. 1991 Dec;23(12):1359–1374. doi: 10.1016/0022-2828(91)90183-m. [DOI] [PubMed] [Google Scholar]
- Ambrosio G., Zweier J. L., Jacobus W. E., Weisfeldt M. L., Flaherty J. T. Improvement of postischemic myocardial function and metabolism induced by administration of deferoxamine at the time of reflow: the role of iron in the pathogenesis of reperfusion injury. Circulation. 1987 Oct;76(4):906–915. doi: 10.1161/01.cir.76.4.906. [DOI] [PubMed] [Google Scholar]
- Aust S. D., Morehouse L. A., Thomas C. E. Role of metals in oxygen radical reactions. J Free Radic Biol Med. 1985;1(1):3–25. doi: 10.1016/0748-5514(85)90025-x. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Benveniste J., Henson P. M., Cochrane C. G. Leukocyte-dependent histamine release from rabbit platelets. The role of IgE, basophils, and a platelet-activating factor. J Exp Med. 1972 Dec 1;136(6):1356–1377. doi: 10.1084/jem.136.6.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank M. L., Hall M. N., Cress E. A., Snyder F. Inactivation of 1-alkyl-2-acetyl-sn-glycero-3-phosphocholine by a plasma acetylhydrolase: higher activities in hypertensive rats. Biochem Biophys Res Commun. 1983 Jun 15;113(2):666–671. doi: 10.1016/0006-291x(83)91778-3. [DOI] [PubMed] [Google Scholar]
- Blum J., Fridovich I. Inactivation of glutathione peroxidase by superoxide radical. Arch Biochem Biophys. 1985 Aug 1;240(2):500–508. doi: 10.1016/0003-9861(85)90056-6. [DOI] [PubMed] [Google Scholar]
- Bolli R., Patel B. S., Jeroudi M. O., Lai E. K., McCay P. B. Demonstration of free radical generation in "stunned" myocardium of intact dogs with the use of the spin trap alpha-phenyl N-tert-butyl nitrone. J Clin Invest. 1988 Aug;82(2):476–485. doi: 10.1172/JCI113621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braquet P., Touqui L., Shen T. Y., Vargaftig B. B. Perspectives in platelet-activating factor research. Pharmacol Rev. 1987 Jun;39(2):97–145. [PubMed] [Google Scholar]
- Cerbai E., Ambrosio G., Porciatti F., Chiariello M., Giotti A., Mugelli A. Cellular electrophysiological basis for oxygen radical-induced arrhythmias. A patch-clamp study in guinea pig ventricular myocytes. Circulation. 1991 Oct;84(4):1773–1782. doi: 10.1161/01.cir.84.4.1773. [DOI] [PubMed] [Google Scholar]
- Cronstein B. N., Kramer S. B., Weissmann G., Hirschhorn R. Adenosine: a physiological modulator of superoxide anion generation by human neutrophils. J Exp Med. 1983 Oct 1;158(4):1160–1177. doi: 10.1084/jem.158.4.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cueva J. P., Hsueh W. Role of oxygen derived free radicals in platelet activating factor induced bowel necrosis. Gut. 1988 Sep;29(9):1207–1212. doi: 10.1136/gut.29.9.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demopoulos C. A., Pinckard R. N., Hanahan D. J. Platelet-activating factor. Evidence for 1-O-alkyl-2-acetyl-sn-glyceryl-3-phosphorylcholine as the active component (a new class of lipid chemical mediators). J Biol Chem. 1979 Oct 10;254(19):9355–9358. [PubMed] [Google Scholar]
- Doebber T. W., Wu M. S., Robbins J. C., Choy B. M., Chang M. N., Shen T. Y. Platelet activating factor (PAF) involvement in endotoxin-induced hypotension in rats. Studies with PAF-receptor antagonist kadsurenone. Biochem Biophys Res Commun. 1985 Mar 29;127(3):799–808. doi: 10.1016/s0006-291x(85)80014-0. [DOI] [PubMed] [Google Scholar]
- Engler R. L., Dahlgren M. D., Peterson M. A., Dobbs A., Schmid-Schönbein G. W. Accumulation of polymorphonuclear leukocytes during 3-h experimental myocardial ischemia. Am J Physiol. 1986 Jul;251(1 Pt 2):H93–100. doi: 10.1152/ajpheart.1986.251.1.H93. [DOI] [PubMed] [Google Scholar]
- Esterbauer H., Puhl H., Dieber-Rotheneder M., Waeg G., Rabl H. Effect of antioxidants on oxidative modification of LDL. Ann Med. 1991;23(5):573–581. doi: 10.3109/07853899109150520. [DOI] [PubMed] [Google Scholar]
- Fantone J. C., Ward P. A. Role of oxygen-derived free radicals and metabolites in leukocyte-dependent inflammatory reactions. Am J Pathol. 1982 Jun;107(3):395–418. [PMC free article] [PubMed] [Google Scholar]
- Farr R. S., Cox C. P., Wardlow M. L., Jorgensen R. Preliminary studies of an acid-labile factor (ALF) in human sera that inactivates platelet-activating factor (PAF). Clin Immunol Immunopathol. 1980 Mar;15(3):318–330. doi: 10.1016/0090-1229(80)90044-6. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Hoult J. R., Blake D. R. Oxidants, inflammation, and anti-inflammatory drugs. FASEB J. 1988 Oct;2(13):2867–2873. doi: 10.1096/fasebj.2.13.2844616. [DOI] [PubMed] [Google Scholar]
- Hanahan D. J. Platelet activating factor: a biologically active phosphoglyceride. Annu Rev Biochem. 1986;55:483–509. doi: 10.1146/annurev.bi.55.070186.002411. [DOI] [PubMed] [Google Scholar]
- Hatch F. T. Practical methods for plasma lipoprotein analysis. Adv Lipid Res. 1968;6:1–68. [PubMed] [Google Scholar]
- Heinecke J. W., Baker L., Rosen H., Chait A. Superoxide-mediated modification of low density lipoprotein by arterial smooth muscle cells. J Clin Invest. 1986 Mar;77(3):757–761. doi: 10.1172/JCI112371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson W. R., Kaliner M. Immunologic and nonimmunologic generation of superoxide from mast cells and basophils. J Clin Invest. 1978 Jan;61(1):187–196. doi: 10.1172/JCI108917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson P. M. Release of vasoactive amines from rabbit platelets induced by sensitized mononuclear leukocytes and antigen. J Exp Med. 1970 Feb;131(2):287–306. doi: 10.1084/jem.131.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuta N., Sugiyama S., Takagi K., Satake T., Ozawa T. Implication of oxygen radicals on airway hyperresponsiveness after ovalbumin challenge in guinea pigs. Am Rev Respir Dis. 1992 Mar;145(3):561–565. doi: 10.1164/ajrccm/145.3.561. [DOI] [PubMed] [Google Scholar]
- Jackson C. V., Schumacher W. A., Kunkel S. L., Driscoll E. M., Lucchesi B. R. Platelet-activating factor and the release of a platelet-derived coronary artery vasodilator substance in the canine. Circ Res. 1986 Feb;58(2):218–229. doi: 10.1161/01.res.58.2.218. [DOI] [PubMed] [Google Scholar]
- Jessup W., Rankin S. M., De Whalley C. V., Hoult J. R., Scott J., Leake D. S. Alpha-tocopherol consumption during low-density-lipoprotein oxidation. Biochem J. 1990 Jan 15;265(2):399–405. doi: 10.1042/bj2650399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa H., Kurihara N., Hirata K., Takeda T. The role of free radicals in airway obstruction in asthmatic patients. Chest. 1991 Nov;100(5):1319–1322. doi: 10.1378/chest.100.5.1319. [DOI] [PubMed] [Google Scholar]
- Kenzora J. L., Pérez J. E., Bergmann S. R., Lange L. G. Effects of acetyl glyceryl ether of phosphorylcholine (platelet activating factor) on ventricular preload, afterload, and contractility in dogs. J Clin Invest. 1984 Oct;74(4):1193–1203. doi: 10.1172/JCI111528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubes P., Arfors K. E., Granger D. N. Platelet-activating factor-induced mucosal dysfunction: role of oxidants and granulocytes. Am J Physiol. 1991 Jun;260(6 Pt 1):G965–G971. doi: 10.1152/ajpgi.1991.260.6.G965. [DOI] [PubMed] [Google Scholar]
- Kubes P., Ibbotson G., Russell J., Wallace J. L., Granger D. N. Role of platelet-activating factor in ischemia/reperfusion-induced leukocyte adherence. Am J Physiol. 1990 Aug;259(2 Pt 1):G300–G305. doi: 10.1152/ajpgi.1990.259.2.G300. [DOI] [PubMed] [Google Scholar]
- Kubes P., Suzuki M., Granger D. N. Modulation of PAF-induced leukocyte adherence and increased microvascular permeability. Am J Physiol. 1990 Nov;259(5 Pt 1):G859–G864. doi: 10.1152/ajpgi.1990.259.5.G859. [DOI] [PubMed] [Google Scholar]
- Kuehl F. A., Jr, Humes J. L., Egan R. W., Ham E. A., Beveridge G. C., Van Arman C. G. Role of prostaglandin endoperoxide PGG2 in inflammatory processes. Nature. 1977 Jan 13;265(5590):170–173. doi: 10.1038/265170a0. [DOI] [PubMed] [Google Scholar]
- Kukreja R. C., Kontos H. A., Hess M. L., Ellis E. F. PGH synthase and lipoxygenase generate superoxide in the presence of NADH or NADPH. Circ Res. 1986 Dec;59(6):612–619. doi: 10.1161/01.res.59.6.612. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lambeir A. M., Markey C. M., Dunford H. B., Marnett L. J. Spectral properties of the higher oxidation states of prostaglandin H synthase. J Biol Chem. 1985 Dec 5;260(28):14894–14896. [PubMed] [Google Scholar]
- Laurindo F. R., Goldstein R. E., Davenport N. J., Ezra D., Feuerstein G. Z. Mechanisms of hypotension produced by platelet-activating factor. J Appl Physiol (1985) 1989 Jun;66(6):2681–2690. doi: 10.1152/jappl.1989.66.6.2681. [DOI] [PubMed] [Google Scholar]
- Mak I. T., Misra H. P., Weglicki W. B. Temporal relationship of free radical-induced lipid peroxidation and loss of latent enzyme activity in highly enriched hepatic lysosomes. J Biol Chem. 1983 Nov 25;258(22):13733–13737. [PubMed] [Google Scholar]
- Maki N., Hoffman D. R., Johnston J. M. Platelet-activating factor acetylhydrolase activity in maternal, fetal, and newborn rabbit plasma during pregnancy and lactation. Proc Natl Acad Sci U S A. 1988 Feb;85(3):728–732. doi: 10.1073/pnas.85.3.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall P. J., Kulmacz R. J., Lands W. E. Constraints on prostaglandin biosynthesis in tissues. J Biol Chem. 1987 Mar 15;262(8):3510–3517. [PubMed] [Google Scholar]
- Mayo L. A., Curnutte J. T. Kinetic microplate assay for superoxide production by neutrophils and other phagocytic cells. Methods Enzymol. 1990;186:567–575. doi: 10.1016/0076-6879(90)86151-k. [DOI] [PubMed] [Google Scholar]
- McCord J. M. Free radicals and inflammation: protection of synovial fluid by superoxide dismutase. Science. 1974 Aug 9;185(4150):529–531. doi: 10.1126/science.185.4150.529. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. The reduction of cytochrome c by milk xanthine oxidase. J Biol Chem. 1968 Nov 10;243(21):5753–5760. [PubMed] [Google Scholar]
- Miwa M., Miyake T., Yamanaka T., Sugatani J., Suzuki Y., Sakata S., Araki Y., Matsumoto M. Characterization of serum platelet-activating factor (PAF) acetylhydrolase. Correlation between deficiency of serum PAF acetylhydrolase and respiratory symptoms in asthmatic children. J Clin Invest. 1988 Dec;82(6):1983–1991. doi: 10.1172/JCI113818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyaura S., Eguchi H., Johnston J. M. Effect of a cigarette smoke extract on the metabolism of the proinflammatory autacoid, platelet-activating factor. Circ Res. 1992 Feb;70(2):341–347. doi: 10.1161/01.res.70.2.341. [DOI] [PubMed] [Google Scholar]
- Montrucchio G., Alloatti G., Tetta C., De Luca R., Saunders R. N., Emanuelli G., Camussi G. Release of platelet-activating factor from ischemic-reperfused rabbit heart. Am J Physiol. 1989 Apr;256(4 Pt 2):H1236–H1246. doi: 10.1152/ajpheart.1989.256.4.H1236. [DOI] [PubMed] [Google Scholar]
- Nathan C. F. Secretory products of macrophages. J Clin Invest. 1987 Feb;79(2):319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy S., Barnett J. Phospholipase A2 activity of low density lipoprotein: evidence for an intrinsic phospholipase A2 activity of apoprotein B-100. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9741–9745. doi: 10.1073/pnas.87.24.9741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porras A. G., Olson J. S., Palmer G. The reaction of reduced xanthine oxidase with oxygen. Kinetics of peroxide and superoxide formation. J Biol Chem. 1981 Sep 10;256(17):9006–9103. [PubMed] [Google Scholar]
- Postma D. S., Renkema T. E., Noordhoek J. A., Faber H., Sluiter H. J., Kauffman H. Association between nonspecific bronchial hyperreactivity and superoxide anion production by polymorphonuclear leukocytes in chronic air-flow obstruction. Am Rev Respir Dis. 1988 Jan;137(1):57–61. doi: 10.1164/ajrccm/137.1.57. [DOI] [PubMed] [Google Scholar]
- Prasad K., Kalra J., Bharadwaj B., Chaudhary A. K. Increased oxygen free radical activity in patients on cardiopulmonary bypass undergoing aortocoronary bypass surgery. Am Heart J. 1992 Jan;123(1):37–45. doi: 10.1016/0002-8703(92)90744-g. [DOI] [PubMed] [Google Scholar]
- Robertson D. A., Genovese A., Levi R. Negative inotropic effect of platelet-activating factor on human myocardium: a pharmacological study. J Pharmacol Exp Ther. 1987 Dec;243(3):834–839. [PubMed] [Google Scholar]
- Rouis M., Nigon F., Chapman M. J. Platelet activating factor is a potent stimulant of the production of active oxygen species by human monocyte-derived macrophages. Biochem Biophys Res Commun. 1988 Nov 15;156(3):1293–1301. doi: 10.1016/s0006-291x(88)80773-3. [DOI] [PubMed] [Google Scholar]
- Royston D., Fleming J. S., Desai J. B., Westaby S., Taylor K. M. Increased production of peroxidation products associated with cardiac operations. Evidence for free radical generation. J Thorac Cardiovasc Surg. 1986 May;91(5):759–766. [PubMed] [Google Scholar]
- Schuh J., Fairclough G. F., Jr, Haschemeyer R. H. Oxygen-mediated heterogeneity of apo-low-density lipoprotein. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3173–3177. doi: 10.1073/pnas.75.7.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shappell S. B., Taylor A. A., Hughes H., Mitchell J. R., Anderson D. C., Smith C. W. Comparison of antioxidant and nonantioxidant lipoxygenase inhibitors on neutrophil function. Implications for pathogenesis of myocardial reperfusion injury. J Pharmacol Exp Ther. 1990 Feb;252(2):531–538. [PubMed] [Google Scholar]
- Shaw J. O., Pinckard R. N., Ferrigni K. S., McManus L. M., Hanahan D. J. Activation of human neutrophils with 1-O-hexadecyl/octadecyl-2-acetyl-sn-glycerol-3-phosphorylcholine (platelet activating factor). J Immunol. 1981 Sep;127(3):1250–1255. [PubMed] [Google Scholar]
- Sparrow C. P., Parthasarathy S., Steinberg D. Enzymatic modification of low density lipoprotein by purified lipoxygenase plus phospholipase A2 mimics cell-mediated oxidative modification. J Lipid Res. 1988 Jun;29(6):745–753. [PubMed] [Google Scholar]
- Stadtman E. R. Protein oxidation and aging. Science. 1992 Aug 28;257(5074):1220–1224. doi: 10.1126/science.1355616. [DOI] [PubMed] [Google Scholar]
- Stafforini D. M., Carter M. E., Zimmerman G. A., McIntyre T. M., Prescott S. M. Lipoproteins alter the catalytic behavior of the platelet-activating factor acetylhydrolase in human plasma. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2393–2397. doi: 10.1073/pnas.86.7.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafforini D. M., McIntyre T. M., Carter M. E., Prescott S. M. Human plasma platelet-activating factor acetylhydrolase. Association with lipoprotein particles and role in the degradation of platelet-activating factor. J Biol Chem. 1987 Mar 25;262(9):4215–4222. [PubMed] [Google Scholar]
- Stafforini D. M., Prescott S. M., McIntyre T. M. Human plasma platelet-activating factor acetylhydrolase. Purification and properties. J Biol Chem. 1987 Mar 25;262(9):4223–4230. [PubMed] [Google Scholar]
- Stahl G. L., Terashita Z., Lefer A. M. Role of platelet activating factor in propagation of cardiac damage during myocardial ischemia. J Pharmacol Exp Ther. 1988 Mar;244(3):898–904. [PubMed] [Google Scholar]
- Steinbrecher U. P., Pritchard P. H. Hydrolysis of phosphatidylcholine during LDL oxidation is mediated by platelet-activating factor acetylhydrolase. J Lipid Res. 1989 Mar;30(3):305–315. [PubMed] [Google Scholar]
- Stephens C. J., Graham R. M., Yadava O. P., Leong L. L., Sturm M. J., Taylor R. R. Plasma platelet activating factor degradation and serum lipids after coronary bypass surgery. Cardiovasc Res. 1992 Jan;26(1):25–31. doi: 10.1093/cvr/26.1.25. [DOI] [PubMed] [Google Scholar]
- Triggiani M., Chilton F. H. Influence of immunologic activation and cellular fatty acid levels on the catabolism of platelet-activating factor within the murine mast cell (PT-18). Biochim Biophys Acta. 1989 Nov 6;1006(1):41–51. doi: 10.1016/0005-2760(89)90321-4. [DOI] [PubMed] [Google Scholar]
- Triggiani M., Chilton F. H. Metabolism of platelet-activating factor in the guinea-pig heart. J Mol Cell Cardiol. 1992 Oct;24(10):1101–1111. doi: 10.1016/0022-2828(92)93175-j. [DOI] [PubMed] [Google Scholar]
- Triggiani M., D'Souza D. M., Chilton F. H. Metabolism of 1-acyl-2-acetyl-sn-glycero-3-phosphocholine in the human neutrophil. J Biol Chem. 1991 Apr 15;266(11):6928–6935. [PubMed] [Google Scholar]
- Wallace J. L., Whittle B. J. Picomole doses of platelet-activating factor predispose the gastric mucosa to damage by topical irritants. Prostaglandins. 1986 May;31(5):989–998. doi: 10.1016/0090-6980(86)90028-6. [DOI] [PubMed] [Google Scholar]
- Wardlow M. L., Cox C. P., Meng K. E., Greene D. E., Farr R. S. Substrate specificity and partial characterization of the PAF-acylhydrolase in human serum that rapidly inactivates platelet-activating factor. J Immunol. 1986 May 1;136(9):3441–3446. [PubMed] [Google Scholar]
- White G. J. Inhibition of oxidative enzymes by anti-allergy drugs. Agents Actions. 1981 Nov;11(5):503–509. doi: 10.1007/BF02004713. [DOI] [PubMed] [Google Scholar]
- Zweier J. L., Flaherty J. T., Weisfeldt M. L. Direct measurement of free radical generation following reperfusion of ischemic myocardium. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1404–1407. doi: 10.1073/pnas.84.5.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweier J. L., Kuppusamy P., Williams R., Rayburn B. K., Smith D., Weisfeldt M. L., Flaherty J. T. Measurement and characterization of postischemic free radical generation in the isolated perfused heart. J Biol Chem. 1989 Nov 15;264(32):18890–18895. [PubMed] [Google Scholar]