Abstract
Cyclooxygenase (COX)-2 and inducible nitric oxide (NO) synthase (iNOS) are responsive to a wide array of inflammatory stimuli, have been localized to vascular smooth muscle cells (SMCs), and are intimately linked to the progression of vascular disease, including atherosclerotic lesion formation. We and others have shown that the production and subsequent impact of COX products appear to be correlative with the status of NO synthesis. This study examined the impact of inflammation-driven NO production on COX-2 expression in SMCs. Concurrent stimulation of quiescent rat aortic SMCs with lipopolysaccharide (LPS) and interferon (IFN)-γ increased COX-2, iNOS, and nitrite production. Pharmacological inhibition of NO synthase (NG-monomethyl-l-arginine) concentration- and time-dependently magnified LPS + IFN-γ-mediated COX-2 mRNA and protein induction in a cGMP-independent manner. COX-2 induction was associated with activation of the ERK, p38, and JNK mitogen-activated protein kinase (MAPK) pathways. Interestingly, NO synthase inhibition enhanced ERK, p38, and to a lesser extent JNK phosphorylation but suppressed MAPK phosphatase (MKP)-1 induction in response to LPS + IFN-γ. Similarly, the exposure of SMCs from iNOS−/− mice to LPS + IFN-γ produced an enhancement of COX-2 induction, p38, and JNK phosphorylation and an attenuated upregulation of MKP-1 versus their wild-type counterparts. Taken together, our data indicate that NO, in part derived from iNOS, negatively regulates the immediate early induction of COX-2 in response to inflammatory stimuli.
Keywords: smooth muscle cells, vascular inflammation, signaling, mitogen-activated protein kinase phosphatase
cyclooxygenase (COX)-1- and -2-derived prostanoids exert diverse and often opposing biological actions in the vasculature including the modulation of vascular tone, circulating cell adhesion, proliferation and migration, platelet aggregation, apoptosis, and the local inflammatory response (3, 5, 11). Clinical and experimental evidence has demonstrated that inflammation-driven vasculopathies such as atherosclerosis are often characterized by COX-2 induction, primarily in smooth muscle cells (SMCs) and macrophages in and around lesions and that a disruption of the delicate balance of prostanoid synthesis in the vessel wall is linked to disease progression (3, 5, 7, 8, 13, 42). Targeting this pathway in a global manner, such as the genetic deletion of COX-2 or chronic administration of selective COX-2 inhibitors (coxibs), reduces atherosclerosis in apolipoprotein E-null (ApoE−/−) and low-density lipoprotein receptor-null (LDLR−/−) mice (7, 8, 13). On the other hand, a predisposition to arterial thrombosis was observed in a recently designed targeted COX-2 knockdown model of coxib therapy, consistent with clinical trial data demonstrating an increase in cardiovascular events in patients receiving these drugs compared with traditional nonsteroidal anti-inflammatory agents (6, 43, 45). While a proinflammatory and atherogenic role for PGE2 derived from tightly coupled COX-2/microsomal prostaglandin E synthase-1 activities in macrophages has been established, both protective and detrimental roles for an array of prostanoids generated by vascular SMCs have been proposed (13, 38, 50, 53).
Analogous to COX-2, inducible nitric oxide (NO) synthase (iNOS) is responsive to inflammatory stimuli and is upregulated in atherosclerotic lesions (3, 51). iNOS is a high-output enzyme, capable of producing copious amounts of NO that directly influences SMC proliferation, platelet aggregation, and the adhesion and activation of leukocytes (34, 35). Similar to COX-derived prostanoids, a paradox exists regarding the role of iNOS-derived NO in the vessel wall. The genetic deletion of iNOS decreased neointimal thickening following arterial wall injury and reduced atherosclerosis in ApoE-null mice on a Western diet (10, 28, 34). On the other hand, iNOS is protective in transplant atherosclerosis and was reported to have no impact on early-stage atherosclerosis or in mice on normal chow (34, 44).
It has become apparent that in addition to its direct effects, NO indirectly modifies many of the major vasoregulatory pathways including the renin-angiotensin system, the production of heme oxygenase-derived carbon monoxide, and COX-mediated prostanoid synthesis (15). We and others have reported a critical role of NO derivatives in modulating prostanoid biosynthesis via direct modifications of COX-1 and -2 catalytic activities, e.g., via nitration and S-nitrosylation of critical residues (14, 15, 17, 18, 26, 47, 49). Despite the importance of posttranslational modifications that alter COX activity, the relatively short half-life, suicide inactivation, and N-glycosylation-mediated degradation of COX-2 support the notion that the capacity of de novo COX-2 synthesis to influence prostanoid production is significant (31). COX-2 is an immediate early gene whose expression is coupled to the rise and fall of stimuli in a chronic and cyclical inflammatory setting such as atherogenesis; thus the acute response to inflammatory stimuli may be highly relevant to atheroma progression.
In the present study, a pharmacological and genetic approach was implemented to identify the role of endogenous NO production on COX expression in vascular SMCs in an inflammatory setting. These studies were performed to further define the regulatory components of COX-2 that likely occur in both subclinical atherosclerosis, as well as mature lesion fibrous cap formation, when SMCs contribute significantly to prostanoid formation (20). Our results show that endogenously produced NO, derived largely from iNOS, negatively regulates COX-2 expression through an attenuation of autogenous signaling pathways.
EXPERIMENTAL PROCEDURES
Animals.
The animal protocol used in these studies was approved by the Institutional Animal Care and Use Committee at Weill Cornell Medical College of Cornell University. Adult Sprague-Dawley rats (250–300 g, Charles River, Wilmington, DE) and wild-type (WT) C57BL/6J and iNOS−/− (Jackson, Bar Harbor, ME) mice were maintained on standard chow and tap water ad libitum. Animals were anesthetized with pentobarbital sodium (50 mg/kg ip), a midline incision was made to expose the thoracic and abdominal cavities, and the aorta was excised from its origin at the left ventricle to the iliac bifurcation and prepared for aortic SMC isolation.
SMC isolation.
SMCs were isolated as previously described with minor modifications (18). Briefly, excised aortae were placed in a petri dish containing Hanks' balanced salt solution (HBSS; Gibco) and excess fat was removed under a dissecting microscope. Aortae were cut longitudinally and placed in digestion flasks with collagenase (type 1, 2 mg/ml) for 20 min at 37°C in a shaker bath. The adventitia was removed and aortae were cut into 1- to 2-mm segments, incubated with collagenase for 20 min at 37°C, followed by elastase (0.5–1 mg/aorta) in 1:1 HBSS/Dulbecco's modified Eagle's medium (DMEM; Gibco) [supplemented with 10% fetal bovine serum (FBS), 1% glutamine, 1% antibiotic-antimycotic; Invitrogen] mixture for 1 h at 37°C, maintaining pH 7.4 with sterile 1 M HCl-NaOH and mixing every 15 min. After 1 h, collagenase was added, and mixing continued every 15 min for 2 h until full dispersion into single-cell suspension was achieved. Cells were centrifuged at 900 rpm for 5 min, and the pellet was collected. Cells were resuspended in 7–10 ml DMEM (15% FBS and 1% insulin-transferrin-selenium) and transferred to Primaria dishes (BD Biosciences).
Cell culture and treatment protocols.
Rat or murine aortic SMCs were grown in appropriately sized culture plates in DMEM (10% FBS) at 37°C in 5% CO2-95% room air. At ∼85% confluency, SMCs (passages 4–8) were quiesced in serum-free DMEM containing 1% insulin-transferrin-selenium liquid media supplement (Sigma-Aldrich) for 48 h before the experimental protocols were run. In an effort to induce both COX-2 and iNOS, SMCs were exposed to the combination of proinflammatory stimuli lipopolysaccharide (LPS) and interferon (IFN)-γ for 0–240 min. Because of assay sensitivity constraints and characteristic lag phase associated with iNOS induction and nitrite production, the cells for these measurements were exposed to stimuli for 4 h. Where noted, the cells were pretreated for 30 min with pharmacological inhibitors of NO synthase [NG-monomethyl-l-arginine (l-NMMA); 0.01–5 mmol/l], ERK (U-0126; 10 μmol/l), p38 (SB-202190; 30 μmol/l), JNK (SP-600125; 10 μmol/l; Calbiochem), or guanylate cyclase {1H-[1,2,4] oxadiazolo[4,3-a]quinoxalin-1-one (ODQ); 10 μmol/l}. All other working concentrations of stimuli and drugs are listed in figure legends and the main text. In all cases, the vehicle for each stimulus or drug was used as a control. Following the treatment protocols, the cell culture media was removed for nitrite production measurements and the phosphate-buffered saline-washed cells were prepared for subsequent analysis.
Real-time quantitative PCR RT-PCR.
Total RNA was isolated using the RNAeasy Mini Kit (Qiagen) as per the manufacturer's protocol. Reverse transcription of total RNA (50 ng) was performed using the high-capacity cDNA reverse transcription kit (ABI). Primers were designed using IDT software, and SYBR Green master mixes were prepared for PCR. Each RT-PCR reaction was performed in triplicate with primers for COX-2 and actin. Quantification was performed by the AB1 7500 system (Applied Biosystems). The results are presented as the fold induction of target mRNA relative to basal expression and normalized to the level of actin mRNA. The PCR primer set specificity was verified with a dissociation curve analysis of the reactions compared with no-template and no-RT controls.
Western blot analysis.
Cells were lysed in lysis buffer containing 50 mmol/l Tris·HCl (pH 7.5), 150 mmol/l NaCl, 1% Triton X-100, 1 mmol/l sodium fluoride, 1 mmol/l sodium orthovanadate, and complete protease inhibitor cocktail; sonicated; and centrifuged at 13,000 rpm for 10 min at 4°C. The cell lysates supernatant was collected, and the protein concentration was determined using the Lowry method (30). Cell lysates (10–20 μg of protein) were separated using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS/PAGE) and transferred to Immobilon-FL polyvinylidene difluoride membranes (Millipore). The membranes were blocked overnight in 5% milk, incubated with primary antibody targeting the protein of interest for 1 h (1% milk), washed, and incubated with a species-appropriate secondary antibody for 1 h (1% milk). Following an additional wash, ECL-plus (GE Healthcare) was used to visualize immunocomplexed bands on BioMax film (Kodak) with subsequent quantification by densitometric analysis (ImageJ; expressed as protein-to-actin ratio).
Measurement of nitrite production.
Nitrite production was colorimetrically quantified using the Griess Assay as an index of NO production by SMCs following exposure to inflammatory stimuli. Detection of nitrite accumulation required exposure of cells to LPS + IFN-γ for a period of 4 h to ensure reproducibility of this assay. Nitrite standards (0–80 μM) were prepared using sodium nitrite solution in distilled H2O and working Griess reagent was prepared using equal parts of stock Griess A (1% sulfanilamide in 5% ortho-phosphoric acid) and Griess B [1% N-(1-napthyl)-ethylenediamine dihydrochloride] solutions. Nitrite standards or conditioned media was added to working Griess reagent into a 96-well plate, and absorbance was measured at 540–550 nm.
Chemicals.
All chemicals and reagents were purchased from Sigma-Aldrich unless otherwise noted. The following antibodies were used: COX-1 (sc-70877), COX-2 (sc-1745), iNOS (sc-650), MKP-1 (sc-10796) actin (sc-1615), and anti-goat (sc-2922) from Santa Cruz; ERK p44/42 (No. 9102) and phospho-ERK (No. 9101S) from Biolabs; and JNK (No. 9252), phospho-SAPK/JNK (No. 4671), p38 MAPK (No. 9212), phospho-P38 MAPK (No. 9215), and anti-rabbit (No.9215S) from Cell Signaling.
Statistics.
Results are presented as means ± SE with n = number of replicates listed in figure legends. Analysis by ANOVA followed by the Newman-Keuls post hoc test was conducted, and the null hypothesis was rejected at P < 0.05.
RESULTS
Combination of LPS and IFN-γ increases iNOS, nitrite production, and COX-2 expression in rat aortic SMCs.
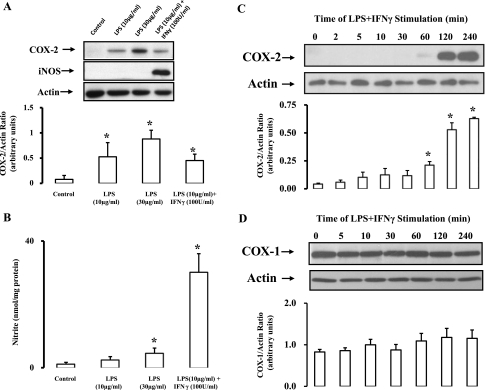
In the course of inflammation-driven pathophysiological settings such as atherogenesis, vascular components are susceptible to a plethora of COX-2- and iNOS-inducing agents including cytokines, growth factors, physical stress, lipoproteins, and a variety of pathogens (1, 3, 42). It is the collective sum of these stimuli, acting via diverse signaling pathways, which ultimately determines the expression profile of COX-2 and iNOS. In the present study, the combination of LPS and IFN-γ were used to mimic a global activation of these pathways. While LPS (10 μg/ml) alone upregulated COX-2 in rat aortic SMCs, a concurrent induction of iNOS (Fig. 1A) and an associated increase in NO synthesis (measured by its oxidized product NO2−; Fig. 1B) required adjunct stimulation with IFN-γ (100 U/ml). COX-2 is an immediate early gene product; thus the time course of its induction following exposure to stimuli was examined. COX-2 protein was upregulated in a time-dependent manner by LPS + IFN-γ, reaching near maximal induction 2–4 h poststimulus (Fig. 1C). COX-1 expression in SMCs was not significantly altered by LPS + IFN-γ (Fig. 1D).
Fig. 1.
Combination of lipopolysaccharide (LPS) and interferon (IFN)-γ upregulates cyclooxygenase (COX)-2, inducible nitric oxide (NO) synthase (iNOS), and nitrite production. A: rat aortic smooth muscle cells (SMCs) were exposed to LPS ± IFN-γ for 4 h, lysed, and probed for COX-2, iNOS, or actin by Western blot analysis. B: media collected from SMCs was analyzed for nitrite accumulation as measured by the Griess assay and normalized to protein. C and D: time course (0–240 min) of COX-1 and COX-2 expression following exposure of SMCs to LPS + IFN-γ. Shown are representative blots [n = 4 (A and B) and n = 3 (C and D)], and the ratio of protein of interest to actin control was expressed following densitometric analysis. Data are means ± SE. *P ≤ 0.05 vs. control or zero time.
Inhibition of NO synthase enhances COX-2 induction in rat aortic SMCs.
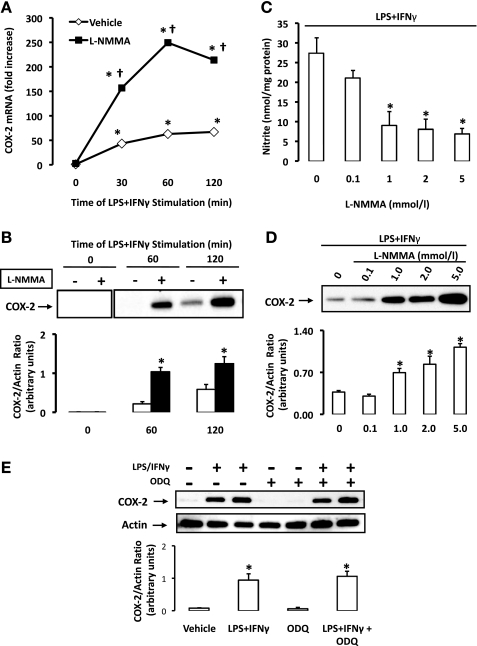
A pharmacological approach was first implemented to evaluate the impact of endogenous NO production on COX-2 induction in rat aortic SMCs. Cells were pretreated with the nonselective inhibitor of NO synthase (NOS), l-NMMA (1 mmol/l), for 30 min before stimulation with LPS + IFN-γ. When compared with vehicle-treated cells, real-time PCR analysis revealed an increase in COX-2 mRNA in cells pretreated with l-NMMA, as early as 30 min following stimulation with LPS + IFN-γ (Fig. 2A). This suprainduction of COX-2 mRNA peaked at 60 min poststimulation with LPS + IFN-γ, at which point COX-2 mRNA was increased more than 250-fold versus time zero in cells undergoing NOS inhibition compared with a ∼50-fold increase in cells receiving vehicle. Accordingly, the induction of COX-2 protein was significantly enhanced in cells pretreated with l-NMMA at 60 and 120 min (Fig. 2B). The effectiveness of NOS inhibition was confirmed as endogenous NO production was concentration-dependently suppressed by l-NMMA (Fig. 2C). The capacity of NOS inhibition to enhance COX-2 induction was also found to occur in a concentration-dependent manner (2 h poststimulus; Fig. 1D). l-NMMA did not significantly alter COX-2 protein levels when either LPS or IFN-γ was used as a solitary stimulus (data not shown). The incubation of SMCs with ODQ, an inhibitor of guanylate cyclase, had no effect on LPS + IFN-γ-induced COX-2 expression (Fig. 2E). These findings suggest that the target of NO may be independent of cGMP-mediated signaling, a traditional secondary messenger pathway for NO in SMCs.
Fig. 2.
NO synthase (NOS) inhibition enhances COX-2 induction in rat aortic SMCs. A: SMCs were pretreated with vehicle (n = 3) or NG-monomethyl-l-arginine (l-NMMA, 1 mmol/l; n = 3), an inhibitor of NOS, for 30 min before stimulation with LPS + IFN-γ, and COX-2 mRNA was detected by real-time PCR (expressed as fold increase over baseline). *P ≤ 0.05 vs. time zero; †P ≤ 0.05 vs. vehicle. B: SMCs were pretreated with l-NMMA (n = 4) or vehicle (n = 4) before stimulation with LPS + IFN-γ, and COX-2 expression was measured by Western blot analysis. C: SMCs were pretreated with various concentrations of l-NMMA (0.1–5.0 mmol/l; n = 5) before stimulation, and nitrite accumulation was determined by the Griess assay. D: SMCs were again treated with l-NMMA (0.1–5.0 mmol/l; n = 5) before stimulation and COX-2 expression was assessed. E: SMCs were pretreated with 1H-[1,2,4] oxadiazolo[4,3-a]quinoxalin-1-one (ODQ, 10 μmol/l; n = 4) or vehicle (n = 4) before stimulation, and COX-2 expression was measured by Western blot analysis. The ratio of protein of interest to actin control was expressed following densitometric analysis. Data are means ± SE. *P ≤ 0.05 vs. vehicle.
iNOS−/− mice display enhanced COX-2 induction in response to LPS + IFN-γ.
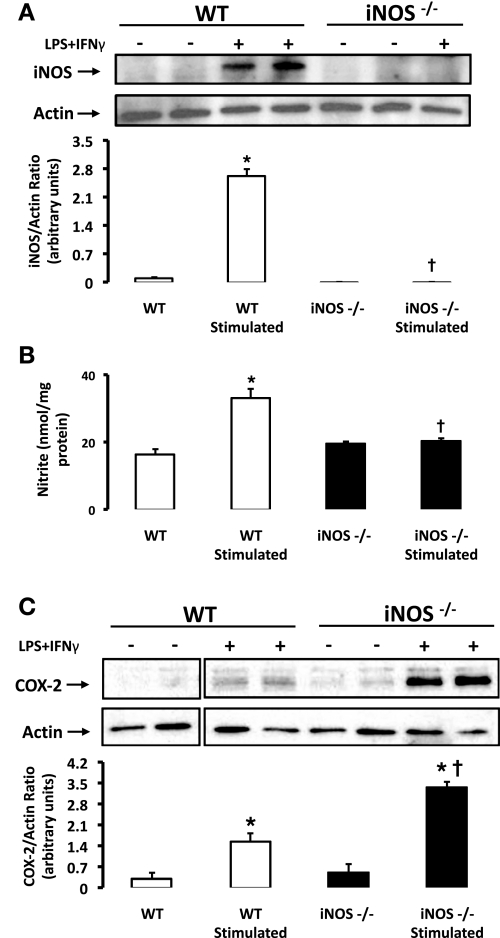
iNOS is upregulated by a variety of stimuli in SMCs and significantly contributes to NO production in inflammatory settings (34). Nonstimulated SMCs isolated from WT and iNOS−/− mice were found to express extremely low or undetectable levels of iNOS protein, respectively (Fig. 3A). Under basal conditions, NO production was detected and comparable in both strains of mice (Fig. 3B). Similar to rat aortic SMCs, exposure of WT SMCs to LPS + IFN-γ led to the induction of iNOS protein and an increase in NO production (Fig. 3, A and B, respectively). As anticipated, SMCs isolated from iNOS−/− mice were unable to upregulate iNOS protein (Fig. 3A) or augment NO synthesis (Fig. 3B) in response to LPS + IFN-γ.
Fig. 3.
COX-2 induction in response to LPS and IFN-γ is enhanced in SMCs from iNOS−/− mice. A: wild-type (WT; n = 6) and iNOS−/− (n = 5) SMCs were exposed to LPS + IFN-γ or vehicle for 4 h, lysed, and probed for iNOS by Western blot analysis. B: media collected from SMCs was analyzed for nitrite accumulation as measured by the Griess assay and normalized to protein. C: WT and iNOS−/− SMCs were exposed to LPS + IFN-γ for 2 h, lysed, and probed for COX-2 or loading control actin by Western blot analysis. Shown are representative blots, and the ratio of protein of interest to actin control was expressed following densitometric analysis. Data are means ± SE. *P ≤ 0.05 vs. nonstimulated cells; †P ≤ 0.05 vs. WT.
We then investigated whether the lack of iNOS and an associated reduction in the ability to upregulate NO synthesis would modify COX-2 induction in SMCs. Interestingly, COX-2 expression was greatly enhanced in response to LPS + IFN-γ in SMCs from iNOS−/− mice versus their WT counterparts (Fig. 3C). COX-1 expression was similar in WT and iNOS-deficient mice (data not shown). Consistent with pharmacological evidence in rat aortic SMCs, these findings implicate an inhibitory influence of NO or a NO derivative during the immediate early induction of COX-2 in SMCs that is generated, at least in part, by the inducible isoform of NOS.
NOS inhibition enhances MAPK activation.
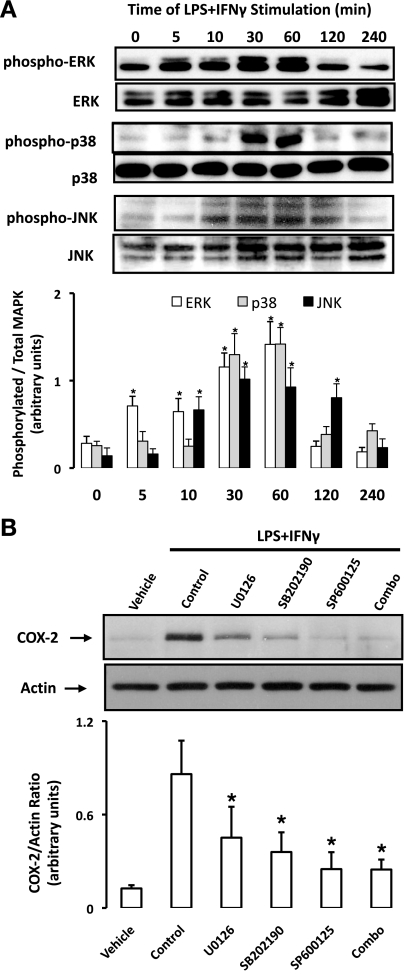
The enhanced induction of COX-2 mRNA (30 min) and protein expression (∼1–2 h) in rat aortic SMCs undergoing pharmacological NOS inhibition occurred in the immediate early phase of induction; thus we examined the well-characterized MAPK pathways that propagate signals linking extracellular ligands to intracellular and nuclear targets such as those regulating COX-2 induction (40). Consistent with global activation of these cells, the stimulation of rat aortic SMCs with LPS + IFN-γ led to an increase in the phosphorylated forms of ERK, p38, and JNK (Fig. 4A). The activation of MAPKs was observed as early as 5–10 min poststimulus, tended to peak by 30–60 min, and returned to baseline levels between 1 and 2 h (Fig. 4A). As reported previously (48), the induction of COX-2 by LPS + IFN-γ was found to be dependent on all three MAPK pathways, as specific inhibitors of ERK (U-0126) and p38 (SB-202190) partially reduced COX-2 expression, whereas an inhibitor of JNK (SP-600125) or the combination of all three inhibitors completely abolished COX-2 induction in response to LPS + IFN-γ (2 h, Fig. 4B).
Fig. 4.
ERK, p38, and JNK mediate COX-2 induction in response to LPS + IFN-γ. A: rat aortic SMCs were exposed to LPS + IFN-γ for 0–240 min, lysed, and probed for the basal and phosphorylated isoforms of ERK, p38, and JNK by Western blot analysis. Mitogen-activated protein kinase (MAPK) phosphorylation was quantified by densitometric analysis and expressed as phosphorylated-to-total MAPK ratio. Data are means ± SE. *P ≤ 0.05 vs. time zero. B: SMCs were pretreated with inhibitors of ERK (U-0126), p38 (SB-202190), JNK (SP-600125), or in combination for 30 min before stimulation with LPS + IFN-γ (2 h) and were lysed, and COX-2 expression was determined by Western blot analysis. Shown are representative blots. Data are means ± SE. *P ≤ 0.05 vs. control.
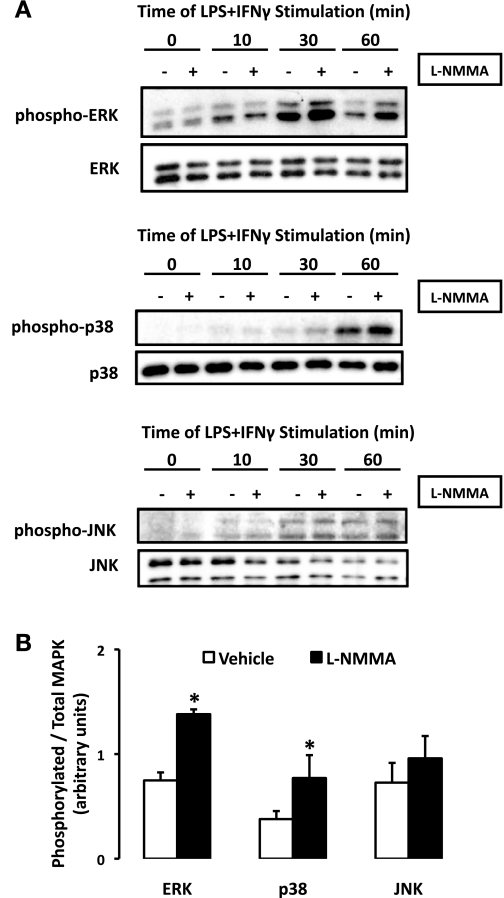
To determine whether these MAPK pathways were influenced by endogenous NO production, LPS + IFN-γ-induced activation of ERK, p38, and JNK was examined in rat aortic SMCs pretreated with l-NMMA or vehicle (Fig. 5A). l-NMMA (0.1–10 mmol/l) had no effect on ERK, p38, or JNK phosphorylation in the absence of stimuli (data not shown). On the other hand, the activation of ERK and p38 by LPS + IFN-γ was enhanced in the presence of l-NMMA (1 mmol/l; Fig. 5A). MAPK phosphorylation was quantified using densitometric analysis and revealed that ERK and p38 activation was about twofold greater (P < 0.05) 30 min poststimulus in cells pretreated with l-NMMA (Fig. 5B). While JNK phosphorylation tended to be elevated in cells undergoing NOS inhibition, at no time point did this reach statistical significance (Fig. 5B).
Fig. 5.
MAPK phosphorylation profile is modified by NOS inhibition. A: rat aortic SMCs were pretreated with l-NMMA (1 mmol/l) exposed to LPS + IFN-γ for 0–60 min, lysed, and probed for the basal and phosphorylated isoforms of ERK, p38, and JNK, respectively, by Western blot analysis. B: MAPK phosphorylation (30 min) was quantified by densitometric analysis and expressed as phosphorylated-to-total MAPK ratio (means ± SE; *P ≤ 0.05 vs. vehicle).
Deletion of iNOS enhances MAPK activation in SMCs.
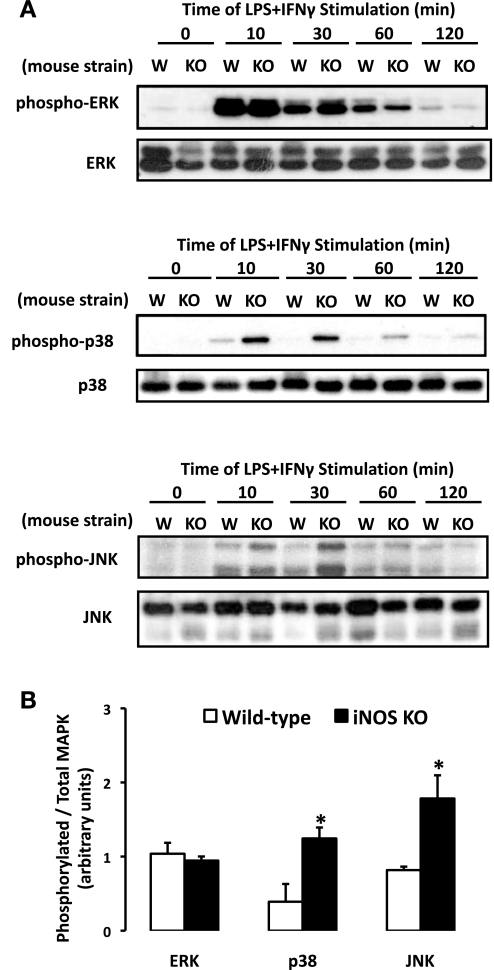
To investigate the relationship between iNOS-derived NO production and signaling pathways associated with COX-2 induction, MAPK activation was observed following the stimulation with LPS + IFN-γ in WT and iNOS−/−-derived SMCs. Under basal conditions, the phosphorylation of ERK, p38, and JNK in WT and iNOS−/− SMCs was absent (Fig. 6A). The exposure of WT SMCs to LPS + IFN-γ resulted in a rapid activation of ERK, p38, and JNK that was near maximal at 10 min poststimulus followed by a gradual return to baseline by 60–120 min. SMCs isolated from iNOS−/− mice displayed a modified activation profile compared with their WT counterparts (Fig. 6A). ERK activation did not significantly differ in SMCs from either strain of mice (Fig. 6B). In contrast, the phosphorylation of p38 and JNK was enhanced (P < 0.05) in SMCs from iNOS−/− mice at 30 min (Fig. 6B). The activation of p38 and JNK also appeared to be prolonged, since the phosphorylation of these signaling molecules was sustained for a longer duration in iNOS−/− SMCs versus their WT counterparts. Collectively, these findings implicate a role of endogenous NO in the modulation of MAPK activation.
Fig. 6.
MAPK phosphorylation profile elicited by LPS + IFN-γ is modified in SMCs isolated from iNOS−/− vs. WT mice. A: SMCs were exposed to LPS + IFN-γ for 0–120 min, lysed, and probed for the basal and phosphorylated isoforms of ERK, p38, and JNK by Western blot analysis. W, WT; KO, knockout. B: MAPK phosphorylation (30 min) was quantified by densitometric analysis and expressed as phosphorylated-to-total MAPK ratio (means ± SE; *P ≤ 0.05 vs. WT).
NOS inhibition or deletion of iNOS suppressed MAPK phosphatase-1 induction in SMCs.
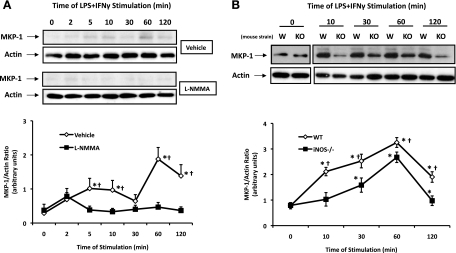
MAPKs are regulated by dual-specificity protein phosphatases or MKPs, which remove a phosphate group from threonine and tyrosine residues, resulting in MAPK deactivation. The prototype and most prolifically expressed isoform of this group, MKP-1, has been previously shown to be expressed and amenable to regulation in SMCs (33). Thus we investigated whether MKP-1 activity, which is primarily regulated at the level of its expression, was altered by a reduction in endogenous NO synthesis (29). Basal rat aortic SMC expression of MKP-1 was found to be low, with very little protein expression detected by Western blot analysis (Fig. 7A). MKPs are known to follow a similar induction profile as their targets, MAPKs, and indeed we observed a rapid induction of MKP-1 in response to LPS + IFN-γ in vehicle-treated rat aortic SMCs (Fig. 7A). The inhibition of NO synthesis by l-NMMA in rat aortic SMCs was associated with a reduced capacity to upregulate MKP-1 following stimulation with LPS + IFN-γ (Fig. 7A). Shown in Fig. 7B, SMCs from WT and iNOS−/− express similar basal levels of MKP-1. However, the capacity of SMCs from iNOS−/− mice to enhance MKP-1 expression following stimulation with LPS + IFN was greatly impaired versus their WT counterparts (Fig. 7B). We believe that these findings implicate NO as an endogenous modulator of MKP-1 expression following exposure to inflammatory stimuli.
Fig. 7.
MAPK phosphatase (MKP)-1 induction is attenuated in settings where NO production is suppressed. A: rat aortic SMCs were pretreated with l-NMMA (1 mmol/l; n = 4) or vehicle (n = 3) and exposed to LPS + IFN-γ for 0–120 min, lysed, and probed for MKP-1. B: SMCs from WT (n = 4) and iNOS−/− mice (n = 4) were exposed to LPS + IFN-γ for 0–120 min, lysed, and probed for MKP-1. Data represent the MKP-1-to-actin ratio as determined by densitometric analysis and expressed as means ± SE. *P ≤ 0.05 vs. time zero; †P ≤ 0.05 vs. vehicle treated or WT.
DISCUSSION
In inflammation-driven atherogenesis, SMCs undergo phenotypic changes, proliferate, migrate into the nearby intima, and secrete extracellular matrix, factors that are particularly relevant during the progression from early to intermediate lesions and in advanced fibrous plaques in which they are the most predominant cell type (20, 39). Additionally, SMCs produce functionally diverse COX-derived lipid mediators such as PGI2, PGE2, thromboxane A2, PGD2, PGJ2, endocannabinoids, and the novel aspirin-triggered lipoxins, which in turn impact vascular inflammation and plaque evolution. We and others have reported the ability of NO-derived species to alter COX catalytic activity and prostanoid synthesis through nitration and S-nitrosylation of specific amino acid residues (14, 15, 17, 18, 26, 47, 49). Herein, we report that in an inflammatory setting 1) SMC-derived NO is a negative regulator of COX-2 expression in the immediate early phase of induction, 2) the inducible form of NOS is a contributing source of NO in mediating this effect, and, finally, 3) we present evidence for the MKP-1/MAPK pathway as a molecular target of endogenous NO production.
Our findings indicate that a reduced capacity to upregulate NO synthesis sensitizes SMCs to COX-2 induction by inflammatory stimuli. COX-2 levels were enhanced as early as ∼2 h poststimulus following treatment with an inhibitor of NOS or in iNOS−/−-derived SMCs, implicating a molecular target that is involved in the immediate early phase of COX-2 induction. We observed an increase in ERK, p38, and JNK activation as indicated by an increase in their phosphorylation in rat and murine vascular SMCs stimulated with LPS and IFN-γ. While these data are consistent with previous reports in smooth muscle and other cell types, they also serve to highlight the redundancy of MAPK signaling (37, 46, 48). Indeed, MAPK cascades are not independent of one another and have been shown to interact via a variety of mechanisms such as the competition for scaffolding protein binding sites and the differential modulation by phosphatases (29). As COX-2 products govern both pro- and anti-inflammatory processes, overlapping MAPK signaling pathways also allow for a fine-tuned control of COX product generation (12, 23). In the present study, JNK appeared to be critical to COX-2 induction and our data implicate JNK as a modulatory component to ERK and p38 signaling. Importantly, the phosphorylation of p38 and JNK was enhanced in stimulated SMCs from mice lacking iNOS, whereas the nonselective inhibitor of NOS isoforms enhanced the activation of p38 and ERK and, to a lesser extent, JNK. These findings suggest an inhibitory influence of NO on MAPK signaling that, when ablated, allows for a more robust response to inflammatory stimuli. Consistent with our observations, NO was recently shown to negatively regulate angiotensin II-elicited MAPK phosphorylation in vascular SMCs and in vivo, and exogenous peroxynitrite decreased phosphorylation of ERK1/2 and p38 in vascular SMCs (4, 54, 55). On the other hand, previous studies have also shown a stimulatory or biphasic role of NO-cGMP signaling on MAPK signaling (2, 27, 49). Although our data did not uncover a role for cGMP in modulating COX-2 induction in response to LPS + IFN-γ, these reports raise the possibility that NO may initiate parallel pathways with opposing biological actions (37).
The capacity of NO to influence more than one MAPK pathway suggests that NO may indirectly target these signaling cascades via intermediary molecules. MKPs function to deactivate MAPK signaling in an energy-efficient manner and have been recently heralded as critical regulators of the immune response (29). We observed low levels of basal MKP-1 expression in both rat and murine SMCs. Interestingly, we report an impairment in MKP-1 induction in response to LPS + IFN-γ under conditions in which NO production is suppressed by pharmacological or genetic means, a notion that is supported by recent findings. Ridnour et al. (41) observed a biphasic response of MKP-1 expression to an NO donor, which at elevated concentrations enhanced the expression of this phosphatase in human endothelial cells (41). Similarly, the inhibition of vascular SMC migration by insulin was found to occur in part via a NO-mediated induction of MKP-1 with subsequent inactivation of MAPK signaling (25).
One would anticipate that the impact of NO on the phenotype of MAPK phosphorylation would reflect the inherent selectivity of the targeted MKP. MKP-1 negatively regulates the immune response through the dephosphorylation of p38 and JNK to a much greater affinity than ERK (9, 29). This would be consistent with the observation that SMCs from iNOS−/− mice exhibit elevated levels of phosphorylated p38 and JNK, but not ERK. Although the phosphorylation of p38 was similarly enhanced in SMCs undergoing NOS inhibition, the phosphorylation of ERK and JNK did not fully adhere to the paradigm established by iNOS−/− mice. To this end, we must consider that incomplete inhibition of iNOS by l-NMMA or the combined inhibition of other NOS isoforms differentially impact MAPK pathways. Interestingly, MKPs have been shown to be amenable to regulation via phosphorylation events that modify protein stability and/or activity (29). Whether NO or NO derivatives can analogously modify residues on MKPs as demonstrated for other proteins (16) has not been reported, but MKP-1 is a clear candidate for future studies.
A significant reduction in nitrite accumulation and an enhancement in stimuli-induced COX-2 expression were observed at concentrations of l-NMMA beginning at 1 mmol/l. l-NMMA is an l-arginine analog that must overcome endogenous concentrations of this amino acid found in mammalian cultured cells (0.2–3.5 mmol/l). Taken together, these data imply that a threshold of NOS inhibition must be reached to achieve a functional response in terms of COX-2 expression (52). The finding in a previous report that the inhibition of NOS with aminoguanidine did not alter TNF-induced COX-2 expression at 24 h in rat SMCs suggests that the stimulus and resulting signaling mechanisms may be critical determinants of NO-mediated influences (22). Nevertheless, one would anticipate the atherogenic milieu of the vessel wall to be subjected to a transient, overlapping, and potentially synergistic array of stimuli. Interestingly, it has been reported that in the absence of inflammatory stimuli, COX-2 upregulation was magnified in mesenteric arteries from rats chronically receiving the nonselective NOS inhibitor l-NAME and that this de novo COX-2 functioned in a compensatory manner to maintain flow-induced dilation (24).
Our findings identify iNOS as a critical source of NO with the capacity to modify SMC COX expression in response to inflammatory stimuli. Although we were unable to detect basal expression of iNOS, SMCs associated with human atheromas have been documented to express this protein at various stages of lesion progression, and low levels of constitutive iNOS expression have been reported in rat carotid artery, as well as other cell types (51, 56). The progression of SMCs from a contractile to synthetic phenotype may further facilitate the susceptibility of these cells to cytokines and other iNOS-inducing stimuli that are abundant in and around sites of lesion formation (36, 39).
COX-2 is a so-called immediate early gene, which indicates that its expression pattern tends to follow a 2–4-h induction window, followed by varied rates of decline in expression. During atherogenesis, the blood vessel wall is likely exposed to cycles of proinflammatory mediators that dictate COX expression and thus the critical equilibrium of prostanoid synthesis. Once present, the products of COX-2 are determined by the availability of substrate, coupling to downstream isomerases and the inherent life span of the COX-2 enzyme. We and others have shown that the production and subsequent impact of these products appear to be correlative with the status of NO synthesis and bioavailability, since NO can positively or negatively regulate COX catalytic activity, alter substrate bioavailability, and modify downstream components of prostanoid synthesis such as PGI2 synthase (14, 15, 17, 18, 26, 47, 49, 57). Although they were not observed in the present study, it should be noted that fluctuations in COX-1 expression in vascular disease have been reported and demonstrated to contribute to lesion formation (5, 32). As the catalytic activity of COX-1 is amenable to NO-mediated regulation, a global assessment of COX products in pathophysiological settings must be considered.
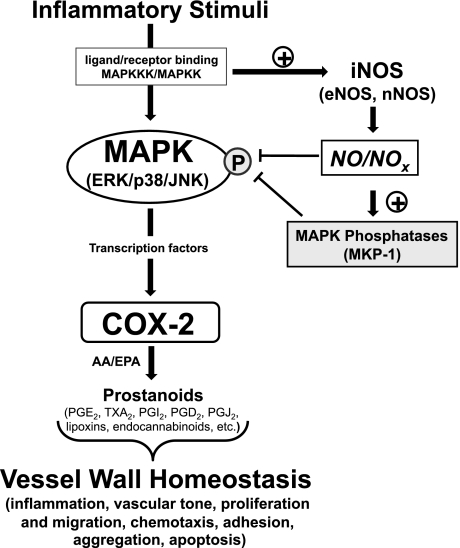
Herein, we provide new evidence that NO production negatively regulates COX-2 expression in response to inflammatory stimuli in vascular SMCs via an inhibitory influence on MAPK signaling (Fig. 8). A coupling of these findings with previously identified effects of NO on the COX pathway should aid in the elucidation of the “net” effect of NO and NO-derived species in the vasculature, particularly with regard to whether these pathways ameliorate or accentuate the inflammatory response. Such findings will be paramount in the appropriate therapeutic targeting of these systems for cardiovascular disease, including the use of nonsteroidal anti-inflammatory drugs and COX-2-selective inhibitors and in the development of novel hybrid compounds such as NO-donating aspirin (19, 21).
Fig. 8.
The blood vessel wall is susceptible to a plethora of inflammatory stimuli that have been linked to the progression of vascular disease such as atherosclerosis. COX-2 is an immediate early gene that is tightly coupled to the rise and fall of these stimuli. Induction of iNOS appears to support a negative feedback mechanism involving MKP-1 to suppress MAPK-mediated signaling events responsible for COX-2 induction. As COX products have been shown to regulate both pro- and anti-inflammatory pathways, vessel wall homeostasis is likely to be linked to the precise control of the acute and cyclical induction of COX-2. eNOS, endothelial NOS; nNOS, neuronal NOS; NOX, NO-derived species; AA, arachidonic acid; EPA, eicosapentaenoic acid; TXA2, thromboxane A2.
GRANTS
This research was funded by National Heart, Lung, and Blood Institute Grants HL-46403, HL-072942, HL-091101, and HL-07423; the Abercrombie Foundation and the Julia and Seymour Gross Foundation (to D. P. Hajjar); The Hartwell Foundation (to B. D. Lamon); and the Alice Bohmfalk Charitable Trust award (to R. K. Upmacis).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Barbara Summers for technical assistance. Portions of this study were presented at the Experimental Biology 2009 Meeting in New Orleans, Louisiana (FASEB J 23: 357.4, 2009) and at the 2009 International Winter Eicosanoid Conference in Baltimore, Maryland (SFS4).
REFERENCES
- 1.Ali K, Lund-Katz S, Lawson J, Phillips MC, Rader DJ. Structure-function properties of the apoE-dependent COX-2 pathway in vascular smooth muscle cells. Atherosclerosis 196: 201–209, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arejian M, Li Y, Anand-Srivastava MB. Nitric oxide attenuates the expression of natriuretic peptide receptor C and associated adenylyl cyclase signaling in aortic vascular smooth muscle cells: role of MAPK. Am J Physiol Heart Circ Physiol 296: H1859–H1867, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Baker CS, Hall RJ, Evans TJ, Pomerance A, Maclouf J, Creminon C, Yacoub MH, Polak JM. Cyclooxygenase-2 is widely expressed in atherosclerotic lesions affecting native and transplanted human coronary arteries and colocalizes with inducible nitric oxide synthase and nitrotyrosine particularly in macrophages. Arterioscler Thromb Vasc Biol 19: 646–655, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Bassil M, Li Y, Anand-Srivastava MB. Peroxynitrite inhibits the expression of Giα protein and adenylyl cyclase signaling in vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 294: H775–H784, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Belton O, Byrne D, Kearney D, Leahy A, Fitzgerald DJ. Cyclooxygenase-1 and -2-dependent prostacyclin formation in patients with atherosclerosis. Circulation 102: 840–845, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Bresalier RS, Sandler RS, Quan H, Bolognese JA, Oxenius B, Horgan K, Lines C, Riddell R, Morton D, Lanas A, Konstam MA, Baron JA. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med 352: 1092–1102, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Burleigh ME, Babaev VR, Oates JA, Harris RC, Gautam S, Riendeau D, Marnett LJ, Morrow JD, Fazio S, Linton MF. Cyclooxygenase-2 promotes early atherosclerotic lesion formation in LDL receptor-deficient mice. Circulation 105: 1816–1823, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Burleigh ME, Babaev VR, Yancey PG, Major AS, McCaleb JL, Oates JA, Morrow JD, Fazio S, Linton MF. Cyclooxygenase-2 promotes early atherosclerotic lesion formation in ApoE-deficient and C57BL/6 mice. J Mol Cell Cardiol 39: 443–452, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Chi H, Barry SP, Roth RJ, Wu JJ, Jones EA, Bennett AM, Flavell RA. Dynamic regulation of pro- and anti-inflammatory cytokines by MAPK phosphatase 1 (MKP-1) in innate immune responses. Proc Natl Acad Sci USA 103: 2274–2279, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chyu KY, Dimayuga P, Zhu J, Nilsson J, Kaul S, Shah PK, Cercek B. Decreased neointimal thickening after arterial wall injury in inducible nitric oxide synthase knockout mice. Circ Res 85: 1192–1198, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Cipollone F, Cicolini G, Bucci M. Cyclooxygenase and prostaglandin synthases in atherosclerosis: recent insights and future perspectives. Pharmacol Ther 118: 161–180, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Cipollone F, Fazia M, Iezzi A, Ciabattoni G, Pini B, Cuccurullo C, Ucchino S, Spigonardo F, De Luca M, Prontera C, Chiarelli F, Cuccurullo F, Mezzetti A. Balance between PGD synthase and PGE synthase is a major determinant of atherosclerotic plaque instability in humans. Arterioscler Thromb Vasc Biol 24: 1259–1265, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Cipollone F, Prontera C, Pini B, Marini M, Fazia M, De Cesare D, Iezzi A, Ucchino S, Boccoli G, Saba V, Chiarelli F, Cuccurullo F, Mezzetti A. Overexpression of functionally coupled cyclooxygenase-2 and prostaglandin E synthase in symptomatic atherosclerotic plaques as a basis of prostaglandin E2-dependent plaque instability. Circulation 104: 921–927, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Deeb RS, Hao G, Gross SS, Laine M, Qiu JH, Resnick B, Barbar EJ, Hajjar DP, Upmacis RK. Heme catalyzes tyrosine 385 nitration and inactivation of prostaglandin H2 synthase-1 by peroxynitrite. J Lipid Res 47: 898–911, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Deeb RS, Lamon BD, Hajjar DP. Silent partner in blood vessel homeostasis? Pervasive role of nitric oxide in vascular disease. Curr Hypertens Rev 5: 273–282, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deeb RS, Nuriel T, Gross SS. Untargeted discovery of nitric-oxide-modified proteins. In: Nitric Oxide: Biology and Pathobiology, edited by Ignarro LJ. San Diego: Elsevier, 2010, p. 327–390 [Google Scholar]
- 17.Deeb RS, Resnick MJ, Mittar D, McCaffrey T, Hajjar DP, Upmacis RK. Tyrosine nitration in prostaglandin H2 synthase. J Lipid Res 43: 1718–1726, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Deeb RS, Shen H, Gamss C, Gavrilova T, Summers BD, Kraemer R, Hao G, Gross SS, Laine M, Maeda N, Hajjar DP, Upmacis RK. Inducible nitric oxide synthase mediates prostaglandin H2 synthase nitration and suppresses eicosanoid production. Am J Pathol 168: 349–362, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deeb RS, Upmacis RK, Lamon BD, Gross SS, Hajjar DP. Maintaining equilibrium by selective targeting of cyclooxygenase pathways: promising offensives against vascular injury. Hypertension 51: 1–7, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Doran AC, Meller N, McNamara CA. Role of smooth muscle cells in the initiation and early progression of atherosclerosis. Arterioscler Thromb Vasc Biol 28: 812–819, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gresele P, Momi S. Pharmacologic profile and therapeutic potential of NCX 4016, a nitric oxide-releasing aspirin, for cardiovascular disorders. Cardiovasc Drug Rev 24: 148–168, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Haider A, Lee I, Grabarek J, Darzynkiewicz Z, Ferreri NR. Dual functionality of cyclooxygenase-2 as a regulator of tumor necrosis factor-mediated G1 shortening and nitric oxide-mediated inhibition of vascular smooth muscle cell proliferation. Circulation 108: 1015–1021, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Haworth O, Buckley CD. Resolving the problem of persistence in the switch from acute to chronic inflammation. Proc Natl Acad Sci USA 104: 20647–20648, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henrion D, Dechaux E, Dowell FJ, Maclour J, Samuel JL, Levy BI, Michel JB. Alteration of flow-induced dilatation in mesenteric resistance arteries of l-NAME treated rats and its partial association with induction of cyclo-oxygenase-2. Br J Pharmacol 121: 83–90, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacob A, Molkentin JD, Smolenski A, Lohmann SM, Begum N. Insulin inhibits PDGF-directed VSMC migration via NO/cGMP increase of MKP-1 and its inactivation of MAPKs. Am J Physiol Cell Physiol 283: C704–C713, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Kim SF, Huri DA, Snyder SH. Inducible nitric oxide synthase binds, S-nitrosylates, and activates cyclooxygenase-2. Science 310: 1966–1970, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Komalavilas P, Shah PK, Jo H, Lincoln TM. Activation of mitogen-activated protein kinase pathways by cyclic GMP and cyclic GMP-dependent protein kinase in contractile vascular smooth muscle cells. J Biol Chem 274: 34301–34309, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Kuhlencordt PJ, Chen J, Han F, Astern J, Huang PL. Genetic deficiency of inducible nitric oxide synthase reduces atherosclerosis and lowers plasma lipid peroxides in apolipoprotein E-knockout mice. Circulation 103: 3099–3104, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Liu Y, Shepherd EG, Nelin LD. MAPK phosphatases—regulating the immune response. Nat Rev Immunol 7: 202–212, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275, 1951 [PubMed] [Google Scholar]
- 31.Mbonye UR, Yuan C, Harris CE, Sidhu RS, Song I, Arakawa T, Smith WL. Two distinct pathways for cyclooxygenase-2 protein degradation. J Biol Chem 283: 8611–8623, 2008 [DOI] [PubMed] [Google Scholar]
- 32.McClelland S, Gawaz M, Kennerknecht E, Konrad CS, Sauer S, Schuerzinger K, Massberg S, Fitzgerald DJ, Belton O. Contribution of cyclooxygenase-1 to thromboxane formation, platelet-vessel wall interactions and atherosclerosis in the ApoE null mouse. Atherosclerosis 202: 84–91, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Metzler B, Li C, Hu Y, Sturm G, Ghaffari-Tabrizi N, Xu Q. LDL stimulates mitogen-activated protein kinase phosphatase-1 expression, independent of LDL receptors, in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 19: 1862–1871, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Miyoshi T, Li Y, Shih DM, Wang X, Laubach VE, Matsumoto AH, Helm GA, Lusis AJ, Shi W. Deficiency of inducible NO synthase reduces advanced but not early atherosclerosis in apolipoprotein E-deficient mice. Life Sci 79: 525–531, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Nathan C. Inducible nitric oxide synthase: what difference does it make? J Clin Invest 100: 2417–2423, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peng HB, Libby P, Liao JK. Induction and stabilization of I kappa B alpha by nitric oxide mediates inhibition of NF-kappa B. J Biol Chem 270: 14214–14219, 1995 [DOI] [PubMed] [Google Scholar]
- 37.Perez-Sala D, Lamas S. Regulation of cyclooxygenase-2 expression by nitric oxide in cells. Antioxid Redox Signal 3: 231–248, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Pritchard KA, Jr, O'Banion MK, Miano JM, Vlasic N, Bhatia UG, Young DA, Stemerman MB. Induction of cyclooxygenase-2 in rat vascular smooth muscle cells in vitro and in vivo. J Biol Chem 269: 8504–8509, 1994 [PubMed] [Google Scholar]
- 39.Raines EW, Ross R. Smooth muscle cells and the pathogenesis of the lesions of atherosclerosis. Br Heart J 69: S30–S37, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raman M, Chen W, Cobb MH. Differential regulation and properties of MAPKs. Oncogene 26: 3100–3112, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Ridnour LA, Isenberg JS, Espey MG, Thomas DD, Roberts DD, Wink DA. Nitric oxide regulates angiogenesis through a functional switch involving thrombospondin-1. Proc Natl Acad Sci USA 102: 13147–13152, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schonbeck U, Sukhova GK, Graber P, Coulter S, Libby P. Augmented expression of cyclooxygenase-2 in human atherosclerotic lesions. Am J Pathol 155: 1281–1291, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seta F, Chung AD, Turner PV, Mewburn JD, Yu Y, Funk CD. Renal and cardiovascular characterization of COX-2 knockdown mice. Am J Physiol Regul Integr Comp Physiol 296: R1751–R1760, 2009 [DOI] [PubMed] [Google Scholar]
- 44.Shears LL, Kawaharada N, Tzeng E, Billiar TR, Watkins SC, Kovesdi I, Lizonova A, Pham SM. Inducible nitric oxide synthase suppresses the development of allograft arteriosclerosis. J Clin Invest 100: 2035–2042, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Solomon SD, Wittes J, Finn PV, Fowler R, Viner J, Bertagnolli MM, Arber N, Levin B, Meinert CL, Martin B, Pater JL, Goss PE, Lance P, Obara S, Chew EY, Kim J, Arndt G, Hawk E. Cardiovascular risk of celecoxib in 6 randomized placebo-controlled trials: the cross trial safety analysis. Circulation 117: 2104–2113, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sprague AH, Khalil RA. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem Pharmacol 78: 539–552, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trostchansky A, O'Donnell VB, Goodwin DC, Landino LM, Marnett LJ, Radi R, Rubbo H. Interactions between nitric oxide and peroxynitrite during prostaglandin endoperoxide H synthase-1 catalysis: a free radical mechanism of inactivation. Free Radic Biol Med 42: 1029–1038, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Tsatsanis C, Androulidaki A, Venihaki M, Margioris AN. Signalling networks regulating cyclooxygenase-2. Int J Biochem Cell Biol 38: 1654–1661, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Upmacis RK, Deeb RS, Resnick MJ, Lindenbaum R, Gamss C, Mittar D, Hajjar DP. Involvement of the mitogen-activated protein kinase cascade in peroxynitrite-mediated arachidonic acid release in vascular smooth muscle cells. Am J Physiol Cell Physiol 286: C1271–C1280, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Vinals M, Martinez-Gonzalez J, Badimon L. Regulatory effects of HDL on smooth muscle cell prostacyclin release. Arterioscler Thromb Vasc Biol 19: 2405–2411, 1999 [DOI] [PubMed] [Google Scholar]
- 51.Wilcox JN, Subramanian RR, Sundell CL, Tracey WR, Pollock JS, Harrison DG, Marsden PA. Expression of multiple isoforms of nitric oxide synthase in normal and atherosclerotic vessels. Arterioscler Thromb Vasc Biol 17: 2479–2488, 1997 [DOI] [PubMed] [Google Scholar]
- 52.Xie L, Hattori Y, Tume N, Gross SS. The preferred source of arginine for high-output nitric oxide synthesis in blood vessels. Semin Perinatol 24: 42–45, 2000 [DOI] [PubMed] [Google Scholar]
- 53.Young W, Mahboubi K, Haider A, Li I, Ferreri NR. Cyclooxygenase-2 is required for tumor necrosis factor-alpha- and angiotensin II-mediated proliferation of vascular smooth muscle cells. Circ Res 86: 906–914, 2000 [DOI] [PubMed] [Google Scholar]
- 54.Zhang GX, Kimura S, Murao K, Shimizu J, Matsuyoshi H, Takaki M. Role of neuronal NO synthase in regulating vascular superoxide levels and mitogen-activated protein kinase phosphorylation. Cardiovasc Res 81: 389–399, 2009 [DOI] [PubMed] [Google Scholar]
- 55.Zhang GX, Nagai Y, Nakagawa T, Miyanaka H, Fujisawa Y, Nishiyama A, Izuishi K, Ohmori K, Kimura S. Involvement of endogenous nitric oxide in angiotensin II-induced activation of vascular mitogen-activated protein kinases. Am J Physiol Heart Circ Physiol 293: H2403–H2408, 2007 [DOI] [PubMed] [Google Scholar]
- 56.Zhang R, Bai YG, Lin LJ, Bao JX, Zhang YY, Tang H, Cheng JH, Jia GL, Ren XL, Ma J. Blockade of AT1 receptor partially restores vasoreactivity, NOS expression, and superoxide levels in cerebral and carotid arteries of hindlimb unweighting rats. J Appl Physiol 106: 251–258, 2009 [DOI] [PubMed] [Google Scholar]
- 57.Zou M, Martin C, Ullrich V. Tyrosine nitration as a mechanism of selective inactivation of prostacyclin synthase by peroxynitrite. Biol Chem 378: 707–713, 1997 [DOI] [PubMed] [Google Scholar]