Abstract
Healthy cardiovascular function relies on a balanced and responsive integration of noradrenergic and cholinergic innervation of the heart. High-affinity choline uptake by cholinergic terminals is pivotal for efficient ACh production and release. To date, the cardiovascular impact of diminished choline transporter (CHT) expression has not been directly examined, largely due to the transporter's inaccessibility in vivo. Here, we describe findings from cardiovascular experiments using transgenic mice that bear a CHT genetic deficiency. Whereas CHT knockout (CHT−/−) mice exhibit early postnatal lethality, CHT heterozygous (CHT+/−) mice survive, grow, and reproduce normally and exhibit normal spontaneous behaviors. However, the CHT+/− mouse heart displays significantly reduced levels of high-affinity choline uptake accompanied by significantly reduced levels of ACh. Telemeterized recordings of cardiovascular function in these mice revealed tachycardia and hypertension at rest. After treadmill exercise, CHT+/− mice exhibited slower heart rate recovery, consistent with a diminished cholinergic reserve, a contention validated through direct vagal nerve stimulation. Echocardiographic and histological experiments revealed an age-dependent decrease in fractional shortening, increased left ventricular dimensions, and increased ventricular fibrosis, consistent with ventricular dysfunction. These cardiovascular phenotypes of CHT+/− mice encourage an evaluation of humans bearing reduced CHT expression for their resiliency in maintaining proper heart function as well as risk for cardiovascular disease.
Keywords: acetylcholine, autonomic nervous system, heart, echocardiography, polymorphism
the sympathetic and parasympathetic branches of the autonomic nervous system provide tight control of cardiovascular function, as evident in their contribution to heart rate (HR) and consequent cardiac output (CO) and blood pressure (BP) modulation (40). An imbalance in these two systems leads to increased HR and BP (64) and, if sustained, may confer an increased risk for cardiovascular disorders, including arrhythmias, hypertension, cardiac hypertrophy, and heart failure (14, 23, 25, 31, 56, 66). Indeed, chronic elevation of HR is an established risk factor for cardiac-related morbidity and mortality (25, 31, 53). Clinical perturbations in the autonomic control of the heart can be assessed by measurements of HR variability as well as sensitivity of the baroreceptor reflex (BRS) to pharmacological and physiological challenges (37, 44, 54). Reductions in parasympathetic components of these measures are significant risk factors for mortality associated with cardiovascular disease (5, 59, 60). The effectiveness of β-adrenergic receptor blockers in improving overall morbidity and mortality for patients with hypertension and heart failure demonstrate the clinical significance of attenuating cardiac sympathetic tone (25, 39).
Parasympathetic effects on the heart are mediated by the right and left vagus nerves, which predominantly innervate the sinoatrial and atrioventricular nodes, respectively, and thereby decrease the sinoatrial node discharge rate and atrioventricular node conduction velocity (67). Vagal inhibition of cardiac nodal discharge is mediated by the neurotransmitter ACh, which signals through the M2 muscarinic receptor (M2AChR) to hyperpolarize pacemaker cells, in opposition to the positive chronotropic effects of sympathetic noradrenergic innervation (40, 51). Although modest reductions in cholinergic tone likely recruit adaptations elsewhere to sustain normal cardiovascular function, a capacity for sustained reductions in vagal drive are likely needed to diminish life-threatening alterations in heart function. Patients with myocardial infarction demonstrate reductions in HRV and BRS, consistent with a diminished capacity for regulatory adaptations in cholinergic transmission (38, 45, 64). Disruptions in cholinergic transmission that lead to diminished cholinergic regulation of the heart can arise from many potential sources, including the structural integrity of parasympathetic innervation, to the expression of various molecular components that support ACh synthesis, release, and responses. One such component yet to be explored for its support of cardiovascular function is the presynaptic choline transporter (CHT; SLC5A7) (1). As the Km of choline acetyltransferase for choline is thought to be above that of tissue choline levels, the uptake of choline, rather than its enzymatic conversion to ACh, is likely rate limiting for neurotransmitter production (21). Consistent with this idea, pharmacological blockade of high-affinity choline uptake (HACU) by hemicholinium-3 (HC-3) impairs both the synthesis and release of ACh (21, 35, 47). The toxicity of HC-3 in vivo and the absence of other pharmacological probes of the transporter, as well as CHT-directed imaging agents, has to date precluded a direct analysis of this protein's contributions to cardiovascular structure and function.
We (20) developed CHT knockout (CHT−/−) mice and demonstrated that these animals die shortly after birth due to asphyxiation, presumably arising from a loss of cholinergic signaling to respiratory muscles. In contrast, CHT heterozygous (CHT+/−) mice survive and grow normally but display signs of a diminished cholinergic capacity, including reduced brain levels of ACh, altered brain ACh receptor densities, and impairments in sustained motor activity in the face of physiological or pharmacological challenges (7). Here, we explore the biochemical, structural, and in vivo physiological impact of the genetically imposed reduction in CHT expression present in CHT+/− mice. Our study established an important role for CHT in normal heart function, most evident in the recovery of HR to physiological or pharmacological perturbations. Additionally, we uncovered age-dependent phenotypes linking diminished CHT expression to impairments in cardiac structure and function. Despite its clear contribution to cholinergic neurotransmission, no studies exist that reveal the contribution of genetic or functional alterations in CHT for normal cardiovascular function.
MATERIALS AND METHODS
Drugs.
(±)-Metoprolol (+)-tartrate (M-5391) and methscopolamine bromide (S-8502) were obtained from Sigma Aldrich (St. Louis, MO) and dissolved in sterile saline (0.9% NaCl). Both drugs were injected intraperitoneally at a dose of 2 mg/kg in a 100-μl volume. Isoflurane, USP (Terrell), was obtained from RxElite (Meridian, ID) and used as a general anesthetic mixed at 1–3% with 100% O2.
Mice.
All animal procedures were approved by the Vanderbilt University Institutional Animal Care and Use Committee (protocol no. M/04/075). Male mice [4–6 mo old (young) and 11–12 mo old (aged)] were housed at up to 5 mice/cage on a 12:12-h light-dark cycle (lights on at 0600 hours). Telemetry and cardiovascular experiments were performed during the light part of the cycle. Food (Purina Rodent Chow no. 5001) and water were provided ad libitum. All mice were back crossed at least seven generations to the C57BL/6 background. In all cases, CHT+/+ littermates were used as controls. All cardiovascular experiments were performed in the laboratory of D. Robertson of the Autonomic Dysfunction Center of Vanderbilt University.
Animal preparation and surgery.
For long-term ambulatory ECG or BP monitoring in conscious mice, telemetry devices [model TA10ETA-F20 (ECG) or TA10-C20 (BP), DataSciences, St. Paul, MN] were implanted using sterile techniques. Mice were anesthetized with isoflurane (1%) in 100% O2 at 1.5 l/min, and body temperature was maintained at 36–37°C with an isothermal pad (Braintree Scientific, Braintree, MA). Following antiseptic preparation, a midline incision was made subcutaneously along the back. For ECG determination, an implantable, radiofrequency transmitter (TA10ETA-F20, 3.9 g) was inserted into the subcutaneous pocket with the leads directed caudally. Using a trochar, the cathodal lead was placed over the scapula and anchored in place with permanent suture. Another incision was made subcutaneously over the apex of the heart, through which, using the trochar, the anodal lead was tunneled underneath the left front paw and sutured in place over the heart apex. For BP determination, an implantable transmitter (TA10-C20, 3.2 g) was inserted into a subcutaneous pocket with the lead placed into the left carotid artery and advanced toward the bifurcation. Skin was sutured and secured with veterinary adhesive (Nexaband, Veterinary Products Laboratories, Phoenix, AZ). Mice were allowed to recover for 5 days before use in the experimental protocols.
ECG (HR) and BP experiments.
After a 5-day postoperative recovery period, 24-h continuous ECG recordings of HR and BP recordings were performed in conscious, telemeterized mice (n = 8 CHT+/+ mice and 8 CHT+/− mice) for a period of 5 days. Average HR and BP values were determined in individual CHT+/+ and CHT+/− mice during resting (light cycle) and awake (dark cycle) time periods. ECG and BP signals were recorded in 1-s intervals using flatbed radiofrequency receivers (DSI PhysioTel Receiver RPC-1, DataSciences) and a digital acquisition system (Dataquest A.R.T., DataSciences). Pharmacological experiments were performed in conscious, telemeterized mice (n = 6 CHT+/+ mice and 6 CHT+/− mice) by giving metoprolol (2 mg/kg) and methscopolamine (2 mg/kg) by an intraperitoneal injection. “Tachycardia,” for the purposes of this study, was defined as a statistically significantly elevation in resting HR above that seen under the equivalent condition in wild-type animals. Similarly, “hypertension” was defined as a statistically significantly elevation in resting BP above that seen under the equivalent condition in wild-type animals.
Echocardiography.
Transthoracic echocardiography was performed in conscious mice using a Sonos 5500 (Agilent, Andover, MA) 15-MHz high-frequency linear transducer at a frame rate of 100 frames/s. All images were acquired at a depth setting of 20 mm. Optimal parasternal long- and short-axis views were obtained by visualization of the endocardial and epicardial walls. Two-dimensional guided M-mode echocardiographic images were obtained to determine left ventricular (LV) internal dimensions at systole (LVIDs) and diastole (LVIDd) as previously described (58, 63). These parameters allowed the determination of LV fractional shortening (FS; in %) using the following equation: FS (in %) = [(LVIDd − LVIDs)/LVIDd] × 100%. Additional echocardiographic parameters (i.e., CO, LV mass, etc.) were calculated using M-mode data as previously described (16, 63).
Treadmill testing.
Mice (n = 9 CHT+/+ mice and 8 CHT+/− mice) chronically telemeterized with BP transducers (as described above) were run on a two-lane motorized treadmill (Columbus Instruments, Columbus, OH) equipped with an adjustable-speed belt (0–90 m/min) and an electric shock grid at one end. On day 1 (training 1), mice were exposed to the treadmill for 10 min without shock. Mice were then exposed to two timed runs (5-min duration) at 5 and 10 m/min with 10-min recovery periods between runs. On day 2 (training 2), mice were then exposed to the treadmill in the presence of shock (2 mA, 4 min-1 frequency) activated by physical contact with the grid. Mice were then run on the treadmill starting at 5 m/min and gradually increased 2 m/min every 2 min until exhaustion. Exhaustion was defined as resting on the electric grid >15 s/min or falling back onto the grid >15 times/min (7). On day 3 (fixed speed/time), mice were run on the treadmill for 13 m/min for 5 min. HR and BP were collected in the home cage 30 min before the exercise challenge and during the 60-min recovery period after the 5-min treadmill run.
BRS experiments.
To determine the baroreceptor-mediated cardioinhibitory response, mice (n = 8 CHT+/+ mice and 8 CHT+/− mice) underwent a challenge with phenylephrine (PE; 5–30 μg/kg) given intravenously using a syringe pump (CMA 400 pump, CMA Microdialysis, Stockholm, Sweden) in a dose-response manner in anesthetized mice. Baseline ECG and BP were recorded for 1.0 min before the administration of drug, and the postdrug response was recorded for 3.0 min after administration. The ratio of the maximal change in HR over the change of mean arterial BP (MABP) was calculated and averaged at each dose in each animal. BRS was determined by the averaged ratio of HR change over MAPB change (ΔHR/ΔMABP) (42).
Vagal nerve stimulation.
Mice (n = 8 CHT+/+ mice and 7 CHT+/− mice) were anesthetized as described above, a cervical midline incision was performed, and the right vagus nerve was isolated from surrounding tissues. The right vagus nerve was ligated using vicryl suture, and the distal segment was then placed on a pair of platinum hook electrodes [PT101 (25 mm), World Precision Instruments, Sarasota, FL] and covered with silicone gel for insulation and immobilization (65). To determine cardiac sensitivity to vagal nerve stimulation (VNS), two protocols were used. In protocol 1 (frequency response), the vagus nerve was stimulated with rectangular wave pulses of 1-ms duration in randomized frequencies of 1–50 Hz at 20-s duration. Baseline HR was recorded for 1 min before the stimulus and 5 min after the stimulus. Mice were given 10-min recovery periods between stimulations. In protocol 2 (saturation), the vagus nerve was stimulated at 40 Hz for 5 min continuously to determine the duration of bradycardia with constant vagal stimulation.
Assessment of ACh and norepinephrine, precursors, and metabolites.
ACh levels in heart tissue were quantified by HPLC using electrochemical detection (n = 11 CHT+/+ mice and 10 CHT+/− mice, Vanderbilt Neurochemistry Core Resource) as previously described (7, 17). Briefly, animals were decapitated and microwaved for 5 s to inactivate acetylcholinesterase degradation of ACh (8). Hearts were then quickly removed and placed onto dry ice. Heart samples were then homogenized in acetonitrile, and lipids were removed using heptane and vacuum drying. Catecholamines were measured in urine using spot collection from conscious adult male mice. Resting mice were immediately removed from their home cages, and urine was collected in microtubes. Urine was preserved in 6 N HCl to prevent catecholamine breakdown and frozen at −80°C until analysis. Plasma catecholamine levels were measured from isoflurane-anesthetized mice. Blood was removed via cardiac puncture and preserved with EGTA and reduced glutathione. Catecholamines were measured after alumina extraction by HPLC with electrochemical detection (34).
Immunoblot analysis of CHT and M2AChR expression.
Freshly dissected heart atrial tissue (n = 4 mice/genotype) was first homogenized in 0.32 M sucrose + HEPES buffer and then vortexed at 3,650 g at 4°C for 20 min. The pellet was solubilized in 200 μl for 24 h at 4°C in lysis buffer [1.0% Triton, 0.1% SDS, 50 mM Tris (pH 7.4), 100 mM NaCl, and protease inhibitors]. Insoluble material was removed by centrifugation at 15,000 g. The protein concentration was measured and normalized using the Bradford method, and samples were loaded into SDS-PAGE 1× Laemmali buffer [1% SDS, 31.25 mM Tris (pH 6.8), 5% glycerol, and 200 mM 2-mercaptoethanol]. Samples were then removed and centrifuged at 13,000 rpm at 4°C for 20 min to remove cellular debris. Samples were then normalized for protein concentration using the Bradford method, resolved by standard SDS-PAGE, and transferred electrophoretically to polyvinylidene diflouride (PVDF) membranes (Amersham Biosciences, Arlington Heights, IL) following standard procedures (22) and subjected to immunoblot analysis using mouse monoclonal CHT and mouse polyclonal M2AChR antibodies (41). Analysis of CHT, GAPDH, and M2AChR proteins from a single PVDF membrane was performed after blots were stripped between incubations with 2% SDS, 62.5 mM Tris·HCl (pH 8), and 100 mM 2-mercaptoethanol at 55°C for 20 min. After being washing with PBS-Tween 20, blots were then blocked in 5% milk with PBS-Tween 20 before analysis with the next antibody.
HACU assays.
Crude atrial tissue extracts from hearts of adult male mice were prepared as previously described (22, 43). Assays of choline transport activity in heart atrial tissue were performed in triplicate for 5 min at 37°C in Krebs-Ringer-HEPES buffer (130 mM NaCl, 3 mM KCl, 2.2 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, 10 mM glucose, and 10 mM HEPES; pH 7.4) with a final choline concentration of 100 nM (specific activity: 82 Ci/mmol, Amersham Pharmacia; 1 Ci = 37 GBq). HC-3 at 10 μM was used to define CHT-mediated choline uptake. Uptake assays were terminated by aspiration and washing onto polyethyleneimine-coated glass fiber filters with a Brandel (Gaithersburg, MD) cell harvester. The low yield of tissue from the heart precluded the analysis of saturation kinetics in these samples.
Histology.
Mice (n = 6 CHT+/+ mice and 6 CHT+/− mice) were weighed, and hearts dissected at 2 and 12 mo of age. Dissected mouse hearts were rinsed and weighed in PBS. Hearts were cut in cross section just below the papillary muscle, and the top half was fixed in formalin and embedded in paraffin. Sections (5 μm) were prepared at 200-μm intervals and fixed with hematoxylin and eosin for gross examination, Masson's trichrome (MT) for the quantification of fibrosis, and periodic acid-Schiff (PAS) counterstained with hematoxylin to determine cardiomyocyte size (4). Histological images were taken using an inverted wide-field microscope at ×40 (MT images) or ×20 (PAS images) (Leica DM-IRB, Bannockburn, IL) and captured with a Nikon DXM 1200C camera (Nikon Instruments, Melville, NY). Morphometric analysis and quantification were performed using Metamorph imaging software (Molecular Devices, Sunnyvale, CA) and ImageJ for MT and PAS images, respectively. Values from each heart were calculated using measurements from three representative areas from each heart. Results are expressed as means ± SE from each group.
Statistical analyses.
Data are expressed as means ± SE. Statistical comparisons were with a one-tailed unpaired Student's t-test with 95% confidence limits comparing transgenic values with controls or two-way ANOVA followed by the Bonferroni procedure for multiple-group comparisons, as indicated in the figures. Results were considered statistically significant if P < 0.05.
RESULTS
HACU and ACh levels are significantly diminished in the CHT+/− heart.
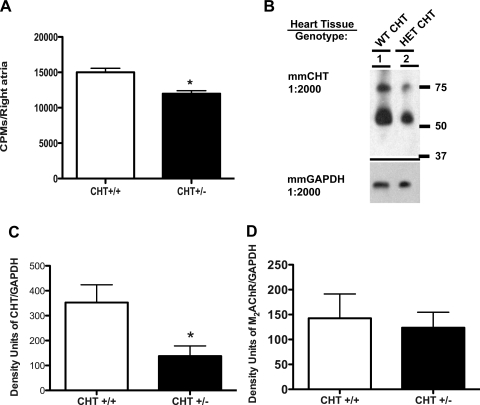
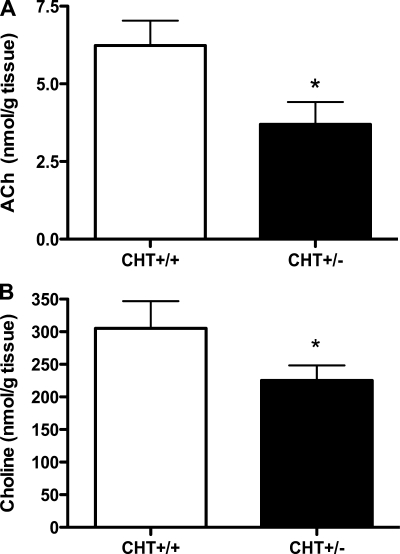
Our previous studies demonstrated that CHT+/− mice exhibited an ∼50% loss of CHT protein (22) and significant reductions in brain ACh levels (7). As shown in Fig. 1A, isolated atria from CHT+/− mice displayed reduced HC-3-sensitive [3H]choline transport rates compared with CHT+/+ atria. These reductions in HACU were supported by a reduction in atrial CHT protein expression in CHT+/− mice compared with CHT+/+ mice (P < 0.05; Fig. 1, B and C). Quantitation of postsynaptic M2AChRs in the same samples did not reveal significant differences in receptor expression between genotypes (Fig. 1D). Moreover, in keeping with a critical role of CHT in maintaining synaptic ACh levels, CHT+/− mice displayed an ∼50% loss of whole heart ACh levels (P < 0.05; Fig. 2A). We also found heart choline levels to be significantly diminished (P < 0.05; Fig. 2B).
Fig. 1.
Reduction of high-affinity choline uptake (HACU) and choline transporter (CHT) expression in CHT heterozygous (Het; CHT+/−) atrial preparations. A: in cardiac atrial preparations, CHT+/− tissue showed diminished hemicholinium-3 (HC-3)-sensitive [3H]choline uptake compared with wild-type (WT; CHT+/+) atria. CPM, counts/min. B: CHT expression was significantly reduced in CHT+/− atria compared with CHT+/+ atria. C and D: quantitation of CHT and M2 muscarinic ACh receptor (M2AChR) expression in CHT+/+ and CHT+/− mice. The mouse monoclonal CHT antibody recognizes the same mature glycosylated and immature protein. Values are means ± SE; n = 4 mice/genotype. Significance was determined using a one-tailed, unpaired Student's t-test (*P < 0.05).
Fig. 2.
Cardiac tissue levels of ACh levels are lower in CHT+/− mice. A: tissue ACh levels measured by HPLC were significantly lower in whole hearts of CHT+/− mice. B: tissue levels of choline were lower in CHT+/− versus CHT+/+ mice. Values are means ± SE; n = 9 mice/genotype. Significance was determined using a one-tailed, unpaired Student's t-test (*P < 0.05).
CHT+/− mice exhibit resting tachycardia.
The deficits in HACU and ACh noted above in the hearts of CHT+/− mice could prove insignificant in the context of functional compensation, as, for example, with structural or physiological adjustments in the sympathetic branch of autonomic innervation. To explore this issue, we monitored HR and BP by telemetry in unanesthetized animals during rest (day) and active (night) periods. We observed that CHT+/− mice exhibited significantly higher mean resting HRs than CHT+/+ mice (P = 0.017; Table 1). In contrast, there were no significant differences between genotypes in HR during active periods. However, during the active period, when HR was monitored after an injection of metoprolol (2 mg/kg ip) to eliminate adrenergic drive, CHT+/− mice displayed a significantly reduced ability to lower their HR (P < 0.022; Table 1). To examine whether CHT+/− mice possess elevated intrinsic HRs, we injected mice with both methscopolamine (2 mg/kg ip) and metoprolol (2 mg/kg ip) to achieve full autonomic blockade. HRs under these conditions did not differ between genotypes (data not shown). Finally, we found that CHT+/− mice exhibited a small but significantly higher mean resting BP (P < 0.05; Table 1). The increase in mean resting BP was driven by a significant increase in systolic BP since diastolic BP did not differ between the two genotypes (Table 1). As with HR, activity-dependent BP did not differ between genotypes.
Table 1.
Baseline cardiovascular characteristics and neurochemistry profiles of CHT+/+ and CHT+/− mice
| Parameter | No. of Mice/Group | CHT+/+ Mice | CHT+/− Mice | P Value |
|---|---|---|---|---|
| HR (day, resting), beats/min | 8 | 538.8 ± 8.16 | 567.0 ± 8.12* | 0.0172 |
| HR (night, activity), beats/min | 8 | 682.6 ± 6.50 | 696.6 ± 8.50 | 0.1951 |
| Mean BP (day), mmHg | 7 | 98.20 ± 1.18 | 104.9 ± 2.06* | 0.035 |
| Systolic BP (resting), mmHg | 7 | 111.0 ± 1.48 | 118.4 ± 1.90* | 0.035 |
| Diastolic BP (resting), mmHg | 7 | 84.90 ± 1.63 | 91.54 ± 3.23 | 0.181 |
| Systolic BP (activity), mmHg | 7 | 117.5 ± 3.79 | 124.1 ± 3.93 | 0.291 |
| Diastolic BP (activity), mmHg | 7 | 108.4 ± 0.051 | 103.8 ± 0.77 | 0.180 |
| ΔHR with metoprolol (2 mg/kg ip), beats/min | 8 | −166.0 ± 36.57 | −66.83 ± 22.23* | 0.022 |
| ACh, nmol/g tissue | 6.2 ± 0.8 | 3.7 ± 0.7 | 0.05 | |
| Choline, nmol/g tissue | 305.2 ± 41.6 | 225.3 ± 23.0 | 0.05 | |
| Urinary norepinephrine, pg/μl | 125 ± 10.3 | 195.8 ± 34.5 | 0.05 | |
| Urinary epinephrine, pg/μl | 3.9 ± 0.3 | 6.8 ± 1.3 | 0.05 | |
| Plasma norepinephrine, pmol/ml | 17.40 ± 5.86 | 31.93 ± 8.96 | 0.123 | |
| Plasma 3,4-dihydroxyphenylethylene glycol, pmol/ml | 13.68 ± 1.94 | 19.23 ± 2.14 | 0.05 |
Values are reported as means ± SE. CHT, choline transporter; CHT+/+ mice, wild-type mice; CHT+/− mice, CHT heterozygous mice; HR, heart rate; BP, blood pressuer. Significance was determined using a one-tailed, unpaired Student's t-test.
P < 0.05, CHT+/− compared with CHT+/+ mice.
Evidence of elevated sympathetic tone in CHT+/− mice.
Reduced parasympathetic tone has been shown to contribute to autonomic instability, leading to increased sympathetic tone (53). To gain a measure of such alterations in CHT+/− mice, we collected urine from conscious mice during their resting period and measured catecholamine levels by HPLC. We observed that CHT+/− mice displayed elevated urinary levels of both norepinephrine (P = 0.05) and epinephrine (P = 0.05). We also measured plasma catecholamines in a separate cohort of CHT+/− and wild-type mice. Although a small increase in the plasma levels of norepinephrine was observed in CHT+/− mice, the difference did not reach statistical significance (Table 1). However, CHT+/− mice did show significantly higher levels of the norepinephrine metabolite 3,4-dihydroxyphenylethylene glycol (DHPG) compared with wild-type mice (P < 0.05; Table 1).
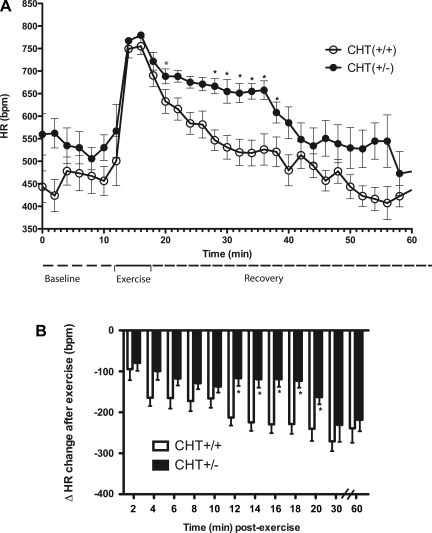
CHT+/− mice exhibit diminished postexercise HR recovery.
Our findings of resting tachycardia and hypertension accompanied by deficits in cardiac HACU and ACh in CHT+/− mice suggests a limited capacity of vagal efferents to offset sympathetic drive to the heart. We examined this hypothesis by monitoring HR before, during, and after treadmill exercise. As expected, baseline HR in CHT+/− mice assessed before treadmill activity was significantly higher compared with CHT+/+ mice (P < 0.001; Fig. 3). After a 5-min treadmill exercise period, CHT+/− mice produced a similar onset and maximal HR level as wild-type mice (Fig. 3A). Whereas, with cessation of exercise, both CHT+/+ and CHT+/− mice achieved a resting HR equivalent to their preexercise levels, CHT+/− mice required a significantly longer time to achieve full HR recovery (CHT+/+ mice: 24 ± 3.4 min and CHT+/− mice: 33 ± 2.4 min, P < 0.05). When changes in HR from peak values were calculated during the recovery period (Fig. 3B), CHT+/− mice also displayed significant overall deficits in HR recovery (P < 0.05, n = 8 mice/genotype; Fig. 3B). In these analyses, three periods of differential HR adjustment were evident. Although CHT+/− animals generated consistently smaller reductions in HR during the first ∼10 min of recovery, these differences were not statistically significant. In contrast, over the next 10 min, the change in HR from peak values was significantly blunted for CHT+/− animals. Finally, over the final 40 min of recording, genotype differences in HR recovery were lost.
Fig. 3.
Altered heart rate (HR) recovery from moderate exercise in CHT+/− mice. A: average HR traces between genotypes at baseline and during exercise and recovery periods. bpm, Beats/min. B: quantitative differences of HR between genotypes at baseline and during exercise and recovery periods. Values are means ± SE; n = 8 mice/genotype. *P < 0.05 for differences between HR by two-way ANOVA (time vs. genotype interaction).
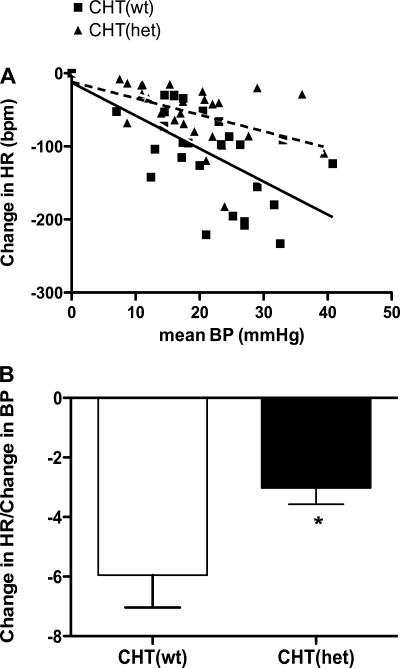
CHT+/− mice display impaired baroreceptor sensitivity.
The increase in resting HR and the inability to return from an activity-dependent HR after exercise suggested a decrease in vagal regulation of chronotropic control of the heart. To further elucidate the vagal contribution to the HR response in CHT+/− mice, we measured baroreceptor-mediated changes in the HR response after an intravenous administration of PE in anesthetized CHT+/+ and CHT+/− mice. CHT+/+ and CHT+/− mice exhibited similar baseline MABP, whereas CHT+/− mice displayed higher baseline HR compared with CHT+/+ mice. Both genotypes exhibited similar increases in BP to PE challenge, but CHT+/− mice demonstrated significantly reduced HR changes to PE-induced BP changes (Fig. 4A). The ratio of the HR change to BP change during exposure to pressor agents has been used as an index of BRS (42). CHT+/− mice also demonstrated a significant reduction in the ratio of the mean change in HR to the maximal mean BP change in response to 20 μg/kg PE challenge (P = 0.03; Fig. 4B). These results indicate that CHT+/− mice exhibited an impaired vagal regulation of the HR response to acute changes in BP and reduced BRS.
Fig. 4.
CHT+/− mice exhibit blunted baroreceptor sensitivity. A: averaged HR response (in beats/min) in CHT+/+ (solid line) and CHT+/− (dotted line) mice to phenylephrine-induced changes in blood pressure (BP) in anesthetized CHT+/+ and CHT+/− mice (P < 0.001, F = 12.76). B: estimation of the baroreflex sensitivity index (ΔHR/Δmean BP) to phenylephrine (20 μg/kg ip). Values are means ± SE; n = 8 mice/genotype. Significance was determined using a one-tailed, unpaired Student's t-test (*P = 0.03).
CHT+/− mice show altered responses to acute and chronic VNS.
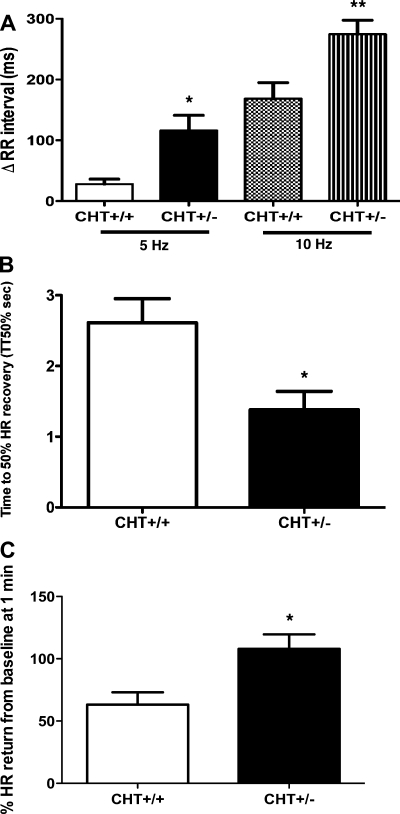
To directly determine the impact of CHT heterzygosity on the vagal regulation of HR, the effect of stimulation of right vagal efferents on HR was determined using two separate VNS protocols. In protocol 1, we examined the frequency-dependent, acute change in the RR interval in anesthetized mice. Similar to HR recordings in conscious mice, anesthetized CHT+/− mice exhibited significantly elevated baseline HRs. During protocol 1, acute stimulation of the right vagus nerve significantly increased the RR interval in a frequency-dependent manner in CHT+/− mice compared with CHT+/+ mice (P = 0.004; Fig. 5A), consistent with an intact capacity for acute cholinergic transmission and possibly a hypersensitivity of postsynaptic M2AChRs. Given the importance of CHT in sustaining cholinergic tone, as well as deficits observed in total atrial ACh, we hypothesized that CHT+/− mice should be unable to maintain the bradycardic response to chronic, continuous VNS. Indeed, during chronic VNS (5 min) at 40 Hz, CHT+/− mice exhibited a more pronounced acute decrease in HR, similar to that seen in protocol 1; however, the average rate of return of HR in response to chronic VNS (measured as the time to 50% HR recovery; P = 0.002, Fig. 5B) was significantly faster in CHT+/− mice compared with CHT+/+ mice. CHT+/− mice also exhibited a greater percent loss of the bradycardic response compared with CHT+/+ mice (P = 0.02; Fig. 5C) indicating an inability of CHT+/− mice to sustain prolonged vagal stimulation of HR.
Fig. 5.
CHT+/− mice display differential HR responses to acute and chronic vagal nerve stimulation (VNS). A: change in RR interval (in ms) during acute VNS at 5 and 10 Hz. CHT+/− mice exhibited increased bradycardic responses during acute VNS compared with CHT+/+ mice. Values are means ± SE. Significance was determined using a one-tailed, unpaired Student's t-test (*P = 0.004, CHT+/+ vs. CHT+/− mice at 5 Hz; **P = 0.007, CHT+/+ vs. CHT+/− mice at 10 Hz). B: time to 50% of HR recovery during chronic VNS at 40 Hz for 5 min (*P = 0.002). C: percent bradycardic response lost at 1.0 min (*P = 0.02). Values are means ± SE. Significance was determined using a one-tailed, unpaired Student's t-test.
CHT+/− mice exhibit functional and structural signs of cardiac hypertrophy.
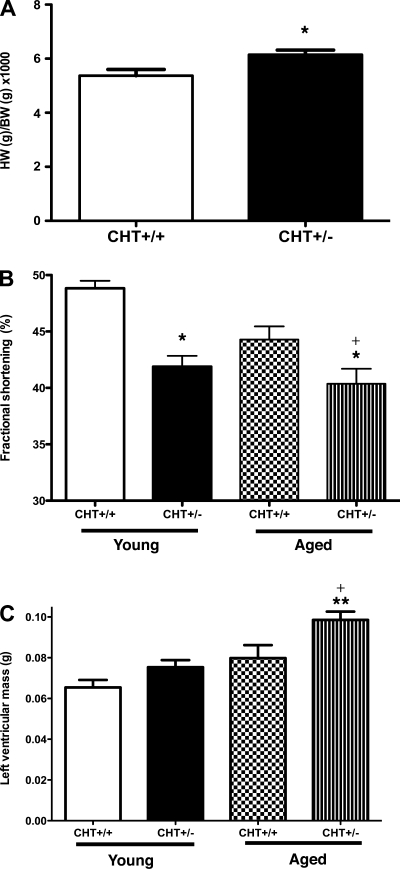
To examine the impact of chronically elevated HR on cardiac structure and function, histological and echocardiographic experiments were performed in both young and aged mice. Whereas the body weights of CHT+/− and CHT+/+ mice did not differ, CHT+/− mice exhibited significantly increased heart-to-body weight ratios (P < 0.05; Fig. 6A). To explore this observation further, we used two-dimensional guided, M-mode echocardiography to assess the structural dynamics supporting cardiac function in both young (4–6 mo old) and aged (10–12 mo old) CHT+/− mice and their littermate wild-type controls. Both young and aged CHT+/− mice (Table 2) showed significantly diminished cardiac contractility, as shown by a reduction in LV function [FS (in %), P < 0.05; Fig. 6B]. Although there were no significant differences in CO in between genotypes in young mice, aged CHT+/− mice exhibited significant reductions in CO compared with CHT+/+ mice (Table 2). Changes in CO may reflect differences in HR; however, both genotypes displayed similar HRs within their respective age group. Indeed, HRs monitored during this procedure were not different between genotypes and were elevated compared with HRs monitored by telemetry, likely due to handling stress. LV mass was increased in both young and old CHT+/− mice relative to age-matched CHT+/+ mice, although only in aged CHT+/− mice did increased LV mass reach statistical significance (P < 0.05; Table 2). A comparison of changes in FS between young and aged CHT+/− mice showed aged-dependent deficits in cardiac function (P < 0.05; Fig. 6C and Table 2). Our findings thus provide evidence of resting and dynamic changes in heart structure associated with CHT heterozygosity.
Fig. 6.
Histological and echocardiographic detection of enlarged hearts and diminished cardiac contractility in CHT+/− mice. A: ratio of heart weight (HW; in g) to body weight (BW; in g) × 1,000. Values are means ± SE; n = 7 CHT+/+ mice and 9 CHT+/− mice. Significance was determined using a one-tailed, unpaired Student's t-test (*P < 0.05 compared with the CHT+/+ group). B: fractional shortening (in %) calculated by images at both maximal systole and diastole. C: measure of left ventricular mass (in g) in young (2–3 mo old) and aged (10–12 mo old) CHT+/+ and CHT+/− mice. Values are means ± SE; n = 8 mice/group (young CHT+/+, young CHT+/−, aged CHT+/+, and CHT+/− groups) Significance was determined using a one-tailed, unpaired Student's t-test (genotype × same age group) (*P < 0.04, **P < 0.02, and +P < 0.05, young vs. aged CHT+/− mice).
Table 2.
Echocardiographic measurements in young and aged CHT+/+ and CHT+/− mice
| Young Mice |
Aged Mice |
|||
|---|---|---|---|---|
| Parameter | CHT+/+ | CHT+/− | CHT+/+ | CHT+/− |
| No. of mice/group | 8 | 9 | 8 | 8 |
| LV internal dimension at diastole, mm | 0.292 ± 0.005 | 0.330 ± 0.006* | 0.342 ± 0.008 | 0.333 ± 0.009 |
| LV internal dimension at systole, mm | 0.149 ± 0.004 | 0.191 ± 0.006* | 0.186 ± 0.008 | 0.198 ± 0.002 |
| Cardiac output, ml/min | 15.36 ± 0.7310 | 19.59 ± 1.221 | 23.86 ± 1.08 | 20.66 ± 1.38† |
| Stroke volume, ml | 0.021 ± 0.001 | 0.029 ± 0.001* | 0.031 ± 0.002 | 0.029 ± 0.002 |
| HR (beats/min) | 704.5 ± 5.61 | 675.8 ± 16.59 | 692.2 ± 7.4 | 705 ± 7.55 |
| Fractional shortening, % | 48.83 ± 0.667 | 41.89 ± 0.9484*‡ | 44.29 ± 1.16 | 39.26 ± 0.853†‡ |
| LV mass, g | 0.065 ± 0.004 | 0.075 ± 0.004 | 0.079 ± 0.006 | 0.098 ± 0.004† |
Values are means ± SE. LV, left ventricular. Significance was determined using a one-tailed, unpaired Student's t-test.
P < 0.05 in young CHT+/− vs. CHT+/+ mice;
P < 0.05 in aged CHT+/− vs. CHT+/+ mice;
P < 0.05 in young vs. aged CHT+/− mice.
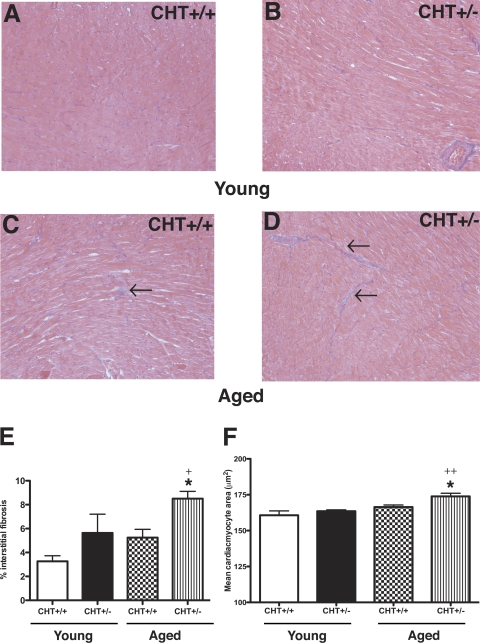
Cardiomyocyte hypertrophy and interstitial fibrosis are evident in association with elevated sympathetic tone (18, 26, 69). Quantitative analysis of MT-stained sections revealed increased interstitial fibrosis in aged but not young CHT+/− mice compared with CHT+/+ littermates (P < 0.01; Fig. 7, C and D). An examination of cardiomyocyte area using PAS staining with hematoxylin counterstaining also revealed a significant age-dependent increase in interstitial fibrosis (P = 0.04) and mean cardiomyocyte area (P = 0.002; Fig. 7, E and F). Together, these results indicate that the diminished cholinergic drive to the hearts of CHT+/− mice is sufficient to produce, over time, overt cellular changes, which can derive from or support cardiac hypertrophy and its functional consequences.
Fig. 7.
Cardiac interstitial fibrosis and mean myocyte alterations in CHT+/+ and CHT+/− mice. A–D: Masson's trichrome-stained 5-μm sections of the paraffin-embedded left ventricular myocardium from young (2–3 mo; A and B) and aged (10–12 mo; C and D) CHT+/+ mice (A and C) and CHT+/− mice (B and D). E: morphometrical analysis of myocyte fibrosis showed that aged CHT+/− mice exhibited significantly higher left ventricular fibrosis compared with aged CHT+/+ mice. *P < 0.01 and +P = 0.04, young vs. aged CHT+/− mice. F: morphometrical analysis of mean cardiomyocyte area from the periodic acid-Schiff-stained left ventricular myocardium showed similar cardiomyocyte area in young CHT+/+ and CHT+/− mice. However, aged CHT+/− mice displayed significantly increased cardiomyocyte area versus aged CHT+/+ mice. *P = 0.01 and ++P = 0.002, young vs. aged CHT+/− mice. Arrows reflect areas of cardiac interstitial fibrosis. Values are means ± SE; n = 5 mice/genotype. Significance was determined using a one-tailed, unpaired Student's t-test (genotype × same age group).
DISCUSSION
In this study, we demonstrated that a partial genetic loss of CHT function results in attenuated vagal regulation of cardiac structure and function. The physiological importance of CHT in maintaining cholinergic tone in other systems has been demonstrated by studies where selective pharmacological blockade or genetic ablation of CHT reduced ACh synthesis and release, thus impairing cholinergic transmission (6, 20, 46). Loss of cholinergic transmission at the neuromuscular junction due to genetic ablation of CHT leads to respiratory paralysis and hypoxic death in CHT−/− mice (20). Loss of cholinergic tone has been demonstrated to increase the risk of cardiovascular disease (5, 9, 52), and a study (24) conducted in M2AChR knockout mice showed a loss of vagally mediated bradycardia. Although M2AChR knockout mice displayed similar resting HRs compared with wild-type mice, chronic stimulation with isoproterenol resulted in significantly impaired ventricular function, indicating that chronically elevated sympathetic tone in the absence of a parasympathetic counterbalance results in altered cardiac function (38). These studies, however, focused primarily on the postsynaptic cholinergic receptors mediating the effects of vagal tone in the heart. We hypothesized that, with the role of CHT in maintaining presynaptic ACh levels, a genetically imposed reduction in CHT function could also impact heart function. Thus, a genetic loss of CHT function may serve as a risk allele in promoting worsening of cardiovascular function.
Activation of parasympathetic inputs via vagal efferent projections to the heart results in bradycardia and contributes to resting HR (25, 40). Our telemetric experiments demonstrated that CHT+/− mice exhibited a basal resting tachycardia and increased BP compared with CHT+/+ mice, whereas activity-dependent increases in HR and BP were not different between genotypes (Table 1). The overall increase in resting mean BP was driven primarily by the increase in systolic rather than diastolic BP, possibly reflecting the increased chronotropic effects seen in CHT+/− mice. Although the differences in resting HR and BP were statistically significant, the actual differences between both genotypes were not large. These results could be explained by the limited localization of cholinergic neurons within the intrinsic cardiac ganglia in mice (28) and thus reflect the predominance of sympathetic tone in driving HR in mice (24). Although we observed that CHT+/− mice exhibited similar M2AChR expression, additional studies are needed to assess M2AChR/G protein coupling, which has been reported to be altered in sinoaortic-denervated rats (62).
A previous study (20) has shown that CHT+/− mice exhibit similar levels of CHT-mediated HACU within the central nervous system (CNS), even though they express 50% of wild-type levels of CHT protein. The latter findings suggest a degree of functional compensation not evident with our heart study. Indeed, we showed that CHT+/− mice exhibited a significant reduction in total atrial CHT protein and that CHT+/− mice demonstrated a significant reduction in CHT-mediated choline uptake (Fig. 1, B and C). The reduction in cardiac CHT-mediated HACU may also reflect differences in the mobilization or reduction of various vesicular pools of CHT between the CNS and the heart (22, 57). Additionally, differences in CHT-mediated HACU may reflect the availability of the ACh precursor choline. In cardiac tissue, CHT+/− mice displayed reduced choline levels (Fig. 2B), whereas within the CNS, CHT+/− mice demonstrated increases in tissue choline levels. These differences may reflect the presence of additional choline uptake mechanisms within the CNS neurons or glia that are absent in cardiac muscles or its innervation (7).
Since changes in HR recovery and BRS have been shown to be an independent predictor of mortality (15, 36), we asked 1) if deficits in cholinergic tone in CHT+/− mice would be more pronounced during increased phasic demands on vagal tone during HR recovery after exercise or challenge to PE and 2) whether structural changes in the heart could be detected. In our treadmill exercise model, CHT+/− mice exhibited a biphasic HR recovery (Fig. 3). The HR recovery plateau seen in CHT+/− mice may reflect a lack of sustainable ACh due to an inability to mobilize sufficient intracellular CHT pools to drive HR back to resting levels, although feedback modulation of sympathetic tone cannot be discounted. Although our data demonstrating reduced cardiac ACh levels in the presence of similar postsynaptic M2AChR expression point to a presynaptic mechanism responsible for mediating these different HR recovery effects, our lack of pharmacological antagonists during the HR recovery period present a limitation in interpreting which specific branch of the autonomic nervous system is responsible for the temporal changes in HR. Indeed, significant controversy exists as to the temporal contributions of the sympathetic and parasympathetic regulation of HR recovery (15). Although the majority of the data in human and canine models supports parasympathetic activation as dictating the early phase of HR recovery after exercise (33, 55, 61), these data are lacking in rodent models. One study (13) conducted in rats demonstrated that parasympathetic contributions to HR recovery in hypertensive rats occurred within 20 min after exercise, a finding similar to our observations in CHT+/− mice.
Consistent with reduced vagal tone, CHT+/− mice exhibited blunted BRS, as reflected by an inability to elicit HR reductions in response to increases in BP induced by PE (Fig. 4). Reductions in BRS have been found to correlate with the reduction of parasympathetic activity and increase in sympathetic activity that occurs during the development and progression of heart failure (36) and to serve as an independent predictor of mortality in patients with heart failure and myocardial infarction (36, 45). Whereas the blunting of the baroreceptor reflex in CHT+/− mice may reflect deficits in the availability of presynaptic ACh, alterations in both postsynaptic muscarinic ACh receptors and central regulatory mechanisms may be involved (45). Failure of ACh to produce acute variability of HR in response to pressure changes may involve nitric oxide, which has been shown to facilitate ACh release (68), and postsynaptic muscarinic ACh regulated-regulated G protein-activated inwardly rectifying K+ (GIRK4) channels (ACh-sensitive K+ current), as GIRK4 knockout mice display reduced BRS reflexes (70). Additionally, altered sensitivity of mechanostretch receptors located in the aortic arch and common carotid artery may disrupt afferent signals into the cardioinhibitory centers of the medulla (45).
Our findings of similar HR recovery in both CHT+/+ and CHT+/− mice during the immediate postexercise recovery period indicated that despite having significant reductions in CHT expression and tissue ACh levels, CHT+/− mice may exhibit similar bradycardic responses during acute increases in vagal demand. To explore this, we used a standard frequency-response protocol. During acute VNS, CHT+/− mice exhibited a significant increase in RR interval (reduced HR) compared with CHT+/+ mice, indicative of hypersensitivity of postsynaptic M2AChRs (Fig. 5A). Our Western blot images of M2AChRs (Fig. 1D) did not show significant differences between genotypes; however, the enhanced bradycardic response seen in our CHT+/− mice may reflect increased coupling of the receptor to its respective G protein. Indeed, changes in M2AChR expression and/or coupling have been seen in animal models and patients with heart failure (10, 38, 62). Previous pharmacological (22) and physiological (7) studies in CHT+/− mice have revealed deficits in their ability to maintain sufficient ACh levels during chronic increases in cholinergic demand. As demonstrated during chronic VNS, although CHT+/− mice exhibited an acute bradycardic response, this effect was unsustainable, as CHT+/− mice showed a significant loss of bradycardia and faster return to baseline compared with CHT+/+ mice (Fig. 5, B and C). The difference in bradycardic responses to acute and chronic VNS may correspond to changes seen during the initial phases of HR recovery in our treadmill exercises, where CHT+/− mice showed normal, acute changes in HR recovery but were unable to sustain this effect, as evidenced by a HR recovery plateau.
Long-term elevations in HR and BP have been shown in both humans and animal models to produce heart failure (10, 12). Overexpression of the β1-adrenergic receptor resulted in significant reductions in FS (a measure of cardiac function) and increased myocyte area due to chronically elevated sympathetic tone (18). To determine the impact of genetic loss of CHT on cardiac structure and function, we performed echocardiography and histopathological experiments. CHT+/− mice exhibited reduced FS, which could be identified in young mice and was further reduced in aged mice (Fig. 6). Histopathological examination of myocyte area and fibrosis produced similar findings in that aged CHT+/− mice exhibited significantly increased myocyte area and fibrotic areas (Fig. 7) compared with age-matched controls and that CHT+/− mice displayed age-dependent structural changes in cardiac myocytes. This reduction in cardiac function also resulted in an age-dependent increase in LV mass (Fig. 6). Finally, CHT+/− mice exhibited increased heart weight-to-body weight ratios, suggestive of cardiac hypertrophy. The age-dependent structural changes seen in CHT+/− mice may reflect loss of cholinergic inhibition of matrix metalloproteinase mediated by M2AChRs (38). Elevated NE levels may promote cardiac maladaptive hypertrophy secondary to increased ROS produced by monoamine oxidase-A (MAO-A) activity (32). In fact, the elevated levels of DHPG observed in CHT+/− mice may reflect an increase in MAO-A activity, induced by elevated NE, resulting in enhanced ROS generation contributing to the hypertrophic remodeling (32, 68). Interestingly, VNS has been shown to modulate the myocardial redox state and suppress adrenergic drive, leading to reduced free radical production in mouse models of heart failure (3, 65). The possibility of elevated myocardial MAO-A and production of ROS contributing to the myocardial alterations seen in our CHT+/− mice warrant further studies, especially in light of our VNS experiments.
Our study focused on the impact of a genetic loss of function in the presynaptic CHT on parasympathetically mediated regulation of cardiovascular function and demonstrated the importance of presynaptic mechanisms regulating ACh biosynthesis and its contributions to maintaining cholinergic tone in the heart. Whereas CHT hemizygosity did not produce overt heart failure in our CHT+/− mice, these mice clearly exhibited significant impairments in vagally mediated cardiac regulation such as BRS and HR recovery after exercise, findings similar to risk factors associated with increased mortality and morbidity and similar to findings found in humans with myocardial infaction and hypertension (5, 15, 60, 61). Since loss of vagal-derived parasympathetic tone is associated with heart failure and myocardial infarction, further studies in CHT+/− mice with hypotension or surgically induced myocardial infarction are needed to determine the impact of CHT hemizygosity in mediating the loss of these vagally regulated protective reflexes (67).
Rodent and human CHT genes were among the last of the presynpatic transporters connected to neuromuscular signaling to be cloned (1, 2, 49). Thus, to date, only a few studies have addressed the physiological contribution of CHT genetic variation. The major allele of a polymorphic site within the 3′-flanking sequence of the human CHT gene (rs333229) has been reported to be associated with reduced HR variability (48), although neither the molecular impact of this variant nor its association with functional variation elsewhere in the gene are understood. Importantly, Okuda and coworkers (50) identified a common single-nucleotide polymorphism within human CHT (rs1013940) that produces an isolucine to valine substitution at amino acid position 89 (I89V). The I89V CHT protein supports normal total and surface CHT expression in transfected cells but results in a 40–56% reduction in choline transport Vmax (50). Okuda and collegues (50) estimated the frequency of the I89V variant in Ashkenazi Jewish samples at 6%, findings we have corroborated in a separate study of Caucasian subjects. In two recent studies, we found the frequency of the 89Val variant to be doubled in subjects with either major depressive disorder (27) or attention-deficit hyperactivity disorder (19), consistent with a role of cholinergic signaling in CNS circuits supporting mood and cognitive function. Interestingly, depression and attention-deficit hyperactivity disorder are comorbid with alterations in cardiovascular function (11, 29, 30), suggesting that subjects with reduced function CHT gene variants may exhibit both brain and cardiovascular phenotypes.
In summary, our study provides evidence for a functional impact of genetically imposed deficits in CHT expression on autonomic regulation of cardiovascular function. Our experiments with CHT+/− mice warrant further efforts to define the role of human CHT in modifying cardiovascular health outcomes. Efforts to enhance CHT expression or activity may provide protective influences over disorders supported by enhanced sympathetic tone. Moreover, indirect targeting of CHT by medications could represent an unsuspected determinant of cardiovascular dysfunction and should be considered in future medication development.
GRANTS
This work was support by an American Heart Association Predoctoral Fellowship Award (to B. A. English) and by National Institutes of Health Grants HL-56693 (to D. Robertson and R. D. Blakely) and MH-073159 (to R. D. Blakely).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Apparsundaram S, Ferguson SM, Blakely RD. Molecular cloning and characterization of a murine hemicholinium-3-sensitive choline transporter. Biochem Soc Trans 29: 711–716, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Apparsundaram S, Ferguson SM, George AL, Jr, Blakely RD. Molecular cloning of a human, hemicholinium-3-sensitive choline transporter. Biochem Biophys Res Commun 276: 862–867, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Azevedo ER, Parker JD. Parasympathetic control of cardiac sympathetic activity: normal ventricular function versus congestive heart failure. Circulation 100: 274–279, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Barrick CJ, Rojas M, Schoonhoven R, Smyth SS, Threadgill DW. Cardiac response to pressure overload in 129S1/SvImJ and C57BL/6J mice: temporal- and background-dependent development of concentric left ventricular hypertrophy. Am J Physiol Heart Circ Physiol 292: H2119–H2130, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Barron HV, Lesh MD. Autonomic nervous system and sudden cardiac death. J Am Coll Cardiol 27: 1053–1060, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Bazalakova MH, Blakely RD. The high-affinity choline transporter: a critical protein for sustaining cholinergic signaling as revealed in studies of genetically altered mice. Handb Exp Pharmacol: 525–544, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Bazalakova MH, Wright J, Schneble EJ, McDonald MP, Heilman CJ, Levey AI, Blakely RD. Deficits in acetylcholine homeostasis, receptors and behaviors in choline transporter heterozygous mice. Genes Brain Behav 6: 411–424, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Bertrand N, Beley P, Beley A. Brain fixation for acetylcholine measurements. J Neurosci Methods 53: 81–85, 1994 [DOI] [PubMed] [Google Scholar]
- 9.Binkley P. Parasympathetic withdrawal is an integral component of autonomic imbalance in congestive heart failure: demonstration in human subjects and verification in a paced canine model of ventricular failure. J Am Coll Cardiol 18: 464–472, 1991 [DOI] [PubMed] [Google Scholar]
- 10.Brodde OE, Leineweber K. Autonomic receptor systems in the failing and aging human heart: similarities and differences. Eur J Pharmacol 500: 167–176, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Brown AD, Barton DA, Lambert GW. Cardiovascular abnormalities in patients with major depressive disorder: autonomic mechanisms and implications for treatment. CNS Drugs 23: 583–602, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Brum PC, Kosek J, Patterson A, Bernstein D, Kobilka B. Abnormal cardiac function associated with sympathetic nervous system hyperactivity in mice. Am J Physiol Heart Circ Physiol 283: H1838–H1845, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Chen Y, Chandler MP, DiCarlo SE. Acute exercise attenuates cardiac autonomic regulation in hypertensive rats. Hypertension 26: 676–683, 1995 [DOI] [PubMed] [Google Scholar]
- 14.Cirillo M, Laurenzi M, Trevisan M, Stamler Hematocrit J. blood pressure, hypertension. The Gubbio Population Study. Hypertension 20: 319–326, 1992 [DOI] [PubMed] [Google Scholar]
- 15.Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med 341: 1351–1357, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Collins KA, Korcarz CE, Lang RM. Use of echocardiography for the phenotypic assessment of genetically altered mice. Physiol Genomics 13: 227–239, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Damsma G, Westerink BH, Horn AS. A simple, sensitive, and economic assay for choline and acetylcholine using HPLC, an enzyme reactor, and an electrochemical detector. J Neurochem 45: 1649–1652, 1985 [DOI] [PubMed] [Google Scholar]
- 18.Engelhardt S, Hein L, Wiesmann F, Lohse MJ. Progressive hypertrophy and heart failure in β1-adrenergic receptor transgenic mice. Proc Natl Acad Sci USA 96: 7059–7064, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.English BA, Hahn MK, Gizer IR, Mazei-Robinson M, Steele A, Kurnik DM, Stein MA, Waldman ID, Blakely RD. Choline transporter gene variation is associated with attention-deficit hyperactivity disorder. J Neurodev Disorder. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferguson SM, Bazalakova M, Savchenko V, Tapia JC, Wright J, Blakely RD. Lethal impairment of cholinergic neurotransmission in hemicholinium-3-sensitive choline transporter knockout mice. Proc Natl Acad Sci USA 101: 8762–8767, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferguson SM, Blakely RD. The choline transporter resurfaces: new roles for synaptic fesicles? Mol Interv 4: 22–37, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Ferguson SM, Savchenko V, Apparsundaram S, Zwick M, Wright J, Heilman CJ, Yi H, Levey AI, Blakely RD. Vesicular localization and activity-dependent trafficking of presynaptic choline transporters. J Neurosci 23: 9697–9709, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Filipovsky J, Ducimetiere P, Safar ME. Prognostic significance of exercise blood pressure and heart rate in middle-aged men. Hypertension 20: 333–339, 1992 [DOI] [PubMed] [Google Scholar]
- 24.Fisher JT, Vincent SG, Gomeza J, Yamada M, Wess J. Loss of vagally mediated bradycardia and bronchoconstriction in mice lacking M2 or M3 muscarinic acetylcholine receptors. FASEB J 18: 711–713, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Fox K, Borer JS, Camm AJ, Danchin N, Ferrari R, Lopez Sendon JL, Steg PG, Tardif JC, Tavazzi L, Tendera M. Resting heart rate in cardiovascular disease. J Am Coll Cardiol 50: 823–830, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Gava AL, Peotta VA, Cabral AM, Meyrelles SS, Vasquez EC. Decreased baroreflex sensitivity in isoproterenol-treated mice with cardiac hypertrophy. Auton Neurosci 114: 47–54, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Hahn MK, Blackford JU, Haman K, Mazei-Robison M, English BA, Prasad HC, Steele A, Hazelwood L, Fentress HM, Myers R, Blakely RD, Sanders-Bush E, Shelton R. Multivariate permutation analysis associates multiple polymorphisms with subphenotypes of major depression. Genes Brain Behav 7: 487–495, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoard JL, Hoover DB, Mabe AM, Blakely RD, Feng N, Paolocci N. Cholinergic neurons of mouse intrinsic cardiac ganglia contain noradrenergic enzymes, norepinephrine transporters, and the neurotrophin receptors tropomyosin-related kinase A and p75. Neuroscience 156: 129–142, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang KL, Su TP, Chen TJ, Chou YH, Bai YM. Comorbidity of cardiovascular diseases with mood and anxiety disorder: a population based 4-year study. Psychiatry Clin Neurosci 63: 401–409, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Hughes JW, York KM, Li Q, Freedland KE, Carney RM, Sheps DS. Depressive symptoms predict heart rate recovery after exercise treadmill testing in patients with coronary artery disease: results from the Psychophysiological Investigation of Myocardial Ischemia study. Psychosom Med 70: 456–460, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Iyer VR. Ventricular dysfunction: tachycardia induced cardiomyopathy. Indian Pacing Electrophysiol J 8: S122–S129, 2008 [PMC free article] [PubMed] [Google Scholar]
- 32.Kaludercic N, Takimoto E, Nagayama T, Feng N, Lai EW, Bedja D, Chen K, Gabrielson KL, Blakely RD, Shih JC, Pacak K, Kass DA, Di Lisa F, Paolocci N. Monoamine oxidase A-mediated enhanced catabolism of norepinephrine contributes to adverse remodeling and pump failure in hearts with pressure overload. Circ Res 106: 193–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kannankeril PJ, Goldberger JJ. Parasympathetic effects on cardiac electrophysiology during exercise and recovery. Am J Physiol Heart Circ Physiol 282: H2091–H2098, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Keller NR, Diedrich A, Appalsamy M, Miller LC, Caron MG, McDonald MP, Shelton RC, Blakely RD, Robertson D. Norepinephrine transporter-deficient mice respond to anxiety producing and fearful environments with bradycardia and hypotension. Neuroscience 139: 931–946, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Kuhar MJ, Murrin LC. Sodium-dependent, high affinity choline uptake. J Neurochem 30: 15–21, 1978 [DOI] [PubMed] [Google Scholar]
- 36.La Rovere M. Baroreceptor sensitivity and heart rate variability in the identification of patients at risk for life threatening arrhythmias. Implications for clinical trials. Circulation 94: 2027–2077, 2001 [DOI] [PubMed] [Google Scholar]
- 37.La Rovere MT, Pinna GD, Raczak G. Baroreflex sensitivity: measurement and clinical implications. Ann Noninvasive Electrocardiol 13: 191–207, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.LaCroix C, Freeling J, Giles A, Wess J, Li YF. Deficiency of M2 muscarinic acetylcholine receptors increases susceptibility of ventricular function to chronic adrenergic stress. Am J Physiol Heart Circ Physiol 294: H810–H820, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Lanza GA, Fox K, Crea F. Heart rate: a risk factor for cardiac diseases and outcomes? Pathophysiology of cardiac diseases and the potential role of heart rate slowing. Adv Cardiol 43: 1–16, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Lefkowitz RR, Koch WJ. Neurotransmission: the autonomic and somatic motor nervous systems. In: Goodman & Gilman's The Pharmacologic Basis of Therapeutics, edited by Hardman JG, Limbird LE, Gilman AG. New York: McGraw-Hill, 1996, p. 105–139. [Google Scholar]
- 41.Li B, Duysen EG, Volpicelli-Daley LA, Levey AI, Lockridge O. Regulation of muscarinic acetylcholine receptor function in acetylcholinesterase knockout mice. Pharmacol Biochem Behav 74: 977–986, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Lin M, Liu R, Gozal D, Wead WB, Chapleau MW, Wurster R, Cheng ZJ. Chronic intermittent hypoxia impairs baroreflex control of heart rate but enhances heart rate responses to vagal efferent stimulation in anesthetized mice. Am J Physiol Heart Circ Physiol 293: H997–H1006, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Lindmar R, Loffelholz K, Weide W, Witzke J. Neuronal uptake of choline following release of acetylcholine in the perfused heart. J Pharmacol Exp Ther 215: 710–715, 1980 [PubMed] [Google Scholar]
- 44.Lombardi F. Heart rate variability in the early hours of an acute myocardial infarction. Am J Cardiol 77: 1037–1044, 1996 [DOI] [PubMed] [Google Scholar]
- 45.Ma X, Abboud FM, Chapleau MW. Analysis of afferent, central, and efferent components of the baroreceptor reflex in mice. Am J Physiol Regul Integr Comp Physiol 283: R1033–R1040, 2002 [DOI] [PubMed] [Google Scholar]
- 46.Maire JC, Wurtman RJ. Effects of electrical stimulation and choline availability on the release and contents of acetylcholine and choline in superfused slices from rat striatum. J Physiol 80: 189–195, 1985 [PubMed] [Google Scholar]
- 47.Murrin LC, DeHaven RN, Kuhar MJ. On the relationship between (3H)choline uptake activation and (3H)acetylcholine release. J Neurochem 29: 681–687, 1977 [DOI] [PubMed] [Google Scholar]
- 48.Neumann SA, Lawrence EC, Jennings JR, Ferrell RE, Manuck SB. Heart rate variability is associated with polymorphic variation in the choline transporter gene. Psychosom Med 67: 168–171, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Okuda T, Haga T, Kanai Y, Endou H, Ishihara T, Katsura I. Identification and characterization of the high-affinity choline transporter. Nat Neurosci 3: 120–125, 2000 [DOI] [PubMed] [Google Scholar]
- 50.Okuda T, Okamura M, Kaitsuka C, Haga T, Gurwitz D. Single nucleotide polymorphism of the human high affinity choline transporter alters transport rate. J Biol Chem 277: 45315–45322, 2002 [DOI] [PubMed] [Google Scholar]
- 51.Olshansky B, Sabbah HN, Hauptman PJ, Colucci WS. Parasympathetic nervous system and heart failure: pathophysiology and potential implications for therapy. Circulation 118: 863–871, 2008 [DOI] [PubMed] [Google Scholar]
- 52.Palatini P. Elevated heart rate as a predictor of increased cardiovascular morbidity. J Hypertens Suppl 17: S3–S10, 1999 [PubMed] [Google Scholar]
- 53.Palatini P, Julius S. Elevated heart rate: a major risk factor for cardiovascular disease. Clin Exp Hypertens 26: 637–644, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Pieper SJ. Heart rate variability: technique and investigational applications in cardiovascular medicine. Mayo Clin Proc 70: 955–964, 1995 [DOI] [PubMed] [Google Scholar]
- 55.Pierpont GL, Stolpman DR, Gornick CC. Heart rate recovery post-exercise as an index of parasympathetic activity. J Auton Nerv Syst 80: 169–174, 2000 [DOI] [PubMed] [Google Scholar]
- 56.Reed D, McGee D, Yano K. Biological and social correlates of blood pressure among Japanese men in Hawaii. Hypertension 4: 406–414, 1982 [DOI] [PubMed] [Google Scholar]
- 57.Ribeiro FM, Pinthong M, Black SA, Gordon AC, Prado VF, Prado MA, Rylett RJ, Ferguson SS. Regulated recycling and plasma membrane recruitment of the high-affinity choline transporter. Eur J Neurosci 26: 3437–3448, 2007 [DOI] [PubMed] [Google Scholar]
- 58.Rottman JN, Ni G, Khoo M, Wang Z, Zhang W, Anderson ME, Madu EC. Temporal changes in ventricular function assessed echocardiographically in conscious and anesthetized mice. J Am Soc Echocardiogr 16: 1150–1157, 2003 [DOI] [PubMed] [Google Scholar]
- 59.Routledge HC. Heart rate variability–a therapeutic target? J Clin Pharm Ther 27: 85–92, 2002 [DOI] [PubMed] [Google Scholar]
- 60.Schwartz PJ, Zaza A, Pala M, Locati E, Beria G, Zanchetti A. Baroreflex sensitivity and its evolution during the first year after myocardial infarction. J Am Coll Cardiol 12: 629–636, 1988 [DOI] [PubMed] [Google Scholar]
- 61.Smith LL, Kukielka M, Billman GE. Heart rate recovery after exercise: a predictor of ventricular fibrillation susceptibility after myocardial infarction. Am J Physiol Heart Circ Physiol 288: H1763–H1769, 2005 [DOI] [PubMed] [Google Scholar]
- 62.Soares PP, Porto CS, Abdalla FM, De La Fuente RN, Moreira ED, Krieger EM, Irigoyen MC. Effects of rat sinoaortic denervation on the vagal responsiveness and expression of muscarinic acetylcholine receptors. J Cardiovasc Pharmacol 47: 331–336, 2006 [DOI] [PubMed] [Google Scholar]
- 63.Syed F, Diwan A, Hahn HS. Murine echocardiography: a practical approach for phenotyping genetically manipulated and surgically modeled mice. J Am Soc Echocardiogr 18: 982–990, 2005 [DOI] [PubMed] [Google Scholar]
- 64.Thayer JFL, Richard D. The role of vagal function in the risk for cardiovascular disease and mortality. Biol Psychol 74: 224–242, 2007 [DOI] [PubMed] [Google Scholar]
- 65.Tsutsumi T, Ide T, Yamato M, Kudou W, Andou M, Hirooka Y, Utsumi H, Tsutsui H, Sunagawa K. Modulation of the myocardial redox state by vagal nerve stimulation after experimental myocardial infarction. Cardiovasc Res 77: 713–721, 2008 [DOI] [PubMed] [Google Scholar]
- 66.Umana E, Solares CA, Alpert MA. Tachycardia-induced cardiomyopathy. Am J Med 114: 51–55, 2003 [DOI] [PubMed] [Google Scholar]
- 67.Vaseghi M, Shivkumar K. The role of the autonomic nervous system in sudden cardiac death. Prog Cardiovasc Dis 50: 404–419, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vecoli C, Paolocci N. When the heart sleeps … is the vagus resetting the myocardial “redox clock”? Cardiovasc Res 77: 609–611, 2008 [DOI] [PubMed] [Google Scholar]
- 69.Weber KT, Brilla CG, Janicki JS. Myocardial remodeling and pathologic hypertrophy. Hosp Pract (Off Ed) 26: 73–80, 1991 [DOI] [PubMed] [Google Scholar]
- 70.Wickman K, Nemec J, Gendler SJ, Clapham DE. Abnormal heart rate regulation in GIRK4 knockout mice. Neuron 20: 103–114, 1998 [DOI] [PubMed] [Google Scholar]