Abstract
The phosphodiesterase type-5 inhibitor sildenafil has powerful cardioprotective effects against ischemia-reperfusion injury. PKG-mediated signaling has been implicated in this protection, although the mechanism and the downstream targets of this kinase remain to be fully elucidated. In this study we assessed the role of phospholemman (PLM) phosphorylation, which activates the Na+/K+-ATPase, in cardioprotection afforded by sildenafil administered during reperfusion. Isolated perfused mouse hearts were optimally protected against infarction (indexed by tetrazolium staining) by 0.1 μM sildenafil treatment during the first 10 min of reperfusion. Extended sildenafil treatment (30, 60, or 120 min at reperfusion) did not alter the degree of protection provided. This protection was PKG dependent, since it was blocked by KT-5823. Western blot analysis using phosphospecific antibodies to PLM showed that sildenafil at reperfusion did not modulate PLM Ser63 or Ser68 phosphorylation but significantly increased Ser69 phosphorylation. The treatment of isolated rat ventricular myocytes with sildenafil or 8-bromo-cGMP (PKG agonist) enhanced PLM Ser69 phosphorylation, which was bisindolylmaleimide (PKC inhibitor) sensitive. Patch-clamp studies showed that sildenafil treatment also activated the Na+/K+-ATPase, which is anticipated in light of PLM Ser69 phosphorylation. Na+/K+-ATPase activation during reperfusion would attenuate Na+ overload at this time, providing a molecular explanation of how sildenafil guards against injury at this time. Indeed, using flame photometry and rubidium uptake into isolated mouse hearts, we found that sildenafil enhanced Na+/K+-ATPase activity during reperfusion. In this study we provide a molecular explanation of how sildenafil guards against myocardial injury during postischemic reperfusion.
Keywords: protein kinase G, sodium/potassium-adenosine 5′-triphosphatase, ischemia, heart
ischemic heart disease is a global burden on healthcare resources and is associated with severe morbidity and mortality. Novel treatment strategies are needed that can limit damage when, or if, myocardial blood flow is restored. Studies in multiple animal models have demonstrated that sildenafil is cardioprotective against injury during ischemia and reperfusion (7, 11, 34). Understanding the molecular basis of how this drug provides cardioprotection may lead to improved therapies.
Sildenafil, a selective inhibitor of phosphodiesterase enzyme type 5 (PDE5), prevents the hydrolysis of cGMP to elevate its cellular abundance. Although the cGMP-PKG signaling pathway has been implicated in sildenafil cardioprotection (7, 18), the precise molecular mechanism and downstream phosphorylation targets remain elusive and are the subject of this study.
There is a substantial body of evidence that perturbation of intracellular Na+ concentration ([Na+]i) may play a crucial role in the pathophysiology of myocardial ischemia-reperfusion injury (27). This rise in [Na+]i during ischemia has been attributed to a combination of an increased cellular influx of Na+ via the Na+ channel and the Na+/H+ exchanger, as well as a decreased Na+ extrusion by the Na+/K+-ATPase (23, 31). During reperfusion, Na+ entry is further potentiated, and as Na+/K+-ATPase activity remains depressed, this leads to elevated intracellular Ca2+ via the Na+/Ca2+ exchanger (Ca2+ overload). This failure of [Na+]i to recover completely during early reperfusion contributes to cardiac ischemia-reperfusion injury (41) and may be attributed in part to inadequate Na+/K+-ATPase activity at this time (38). Stimulating the Na+/K+-ATPase back to (or beyond) basal may be expected to limit injury resulting from high levels of [Na+]i. The activity of the Na+/K+-ATPase is regulated by FXYD-1, also known as phospholemman (PLM). PLM is highly expressed in the heart and has three established phosphorylation sites in its cytosolic COOH-terminal tail domain: Ser63, Ser68, and Thr/Ser69 (19). In the mouse PLM has a serine at position 69, while in rat (and human), this residue is a threonine. Both PKA and PKC phosphorylate Ser68, while PKC also phosphorylates Ser63 (30, 42). Recently, PKC has been found to also phosphorylate PLM at Thr/Ser69 (23). Unphosphorylated PLM inhibits the Na+/K+-ATPase (31), but this “brake” is relieved as a result of the COOH-terminal phophorylations described above.
Herein, we demonstrate that sildenafil-induced PKG-mediated phosphorylation of PLM during reperfusion may stimulate the Na+/K+-ATPase. This would limit Na+ and Ca2+ overload, providing a molecular explanation for how sildenafil reduces reperfusion injury. Indeed, we report that sildenafil at reperfusion stimulates PLM phosphorylation selectively at position 69, and the phosphorylation at this site in isolated myocytes is associated with the activation of the Na+/K+-ATPase. Stimulation of the Na+/K+-ATPase could contribute to infarct limitation by attenuating [Na+]i accumulation during ischemia and/or reperfusion. This protective phosphoactivation of the Na+/K+-ATPase involves both PKG- and PKC-dependent signaling.
EXPERIMENTAL PROCEDURES
Animals were maintained humanely in compliance with the “Principles of Laboratory Animal Care,” formulated by the National Society for Medical Research and the Guide for Care and Use of Laboratory Animals, prepared by the National Academy of Sciences and published by the National Institutes of Health (NIH Publication No. 85-23, Revised 1996). All animal protocols were approved both by the local King's College Ethical Review Process Committee and by the UK Government Home Office (Animals Scientific Procedures Group).
Perfusion of isolated mouse hearts.
As previously detailed (39), adult male C57/BL6 mice (25–30 g) were anesthetized by an intraperitoneal injection of pentobarbital sodium (300 mg/kg), mixed 50:50 with the anticoagulant heparin (150 units). The hearts were rapidly isolated, mounted onto a Langendorff apparatus, and retrogradely perfused at a constant pressure of 80 mmHg with Krebs-Henseleit buffer (KHB) containing (in mM) 118.5 NaCl, 25.0 NaHCO3, 4.75 KCl, 1.18 KH2PO4, 1.19 MgSO4, 11.0 d-glucose, and 1.4 CaCl2, equilibrated with 95% O2-5% CO2 at 37°C. A fluid-filled balloon inserted into the left ventricle cavity monitored contractile function. The balloon was gradually inflated until the left end-diastolic pressure was between 4 and 8 mmHg. Atrial pacing was performed at 600 beats/min, and coronary flow was measured by an electronic feedback circuit controlling the perfusion pump (STH Pump Controller, ADInstruments). Electrical pacing was stopped 2 min after contraction ceased during ischemia and was restarted 5 min into reperfusion. The hearts were allowed to stabilize for 40 min, followed by 30 min global ischemia and 120 min reperfusion.
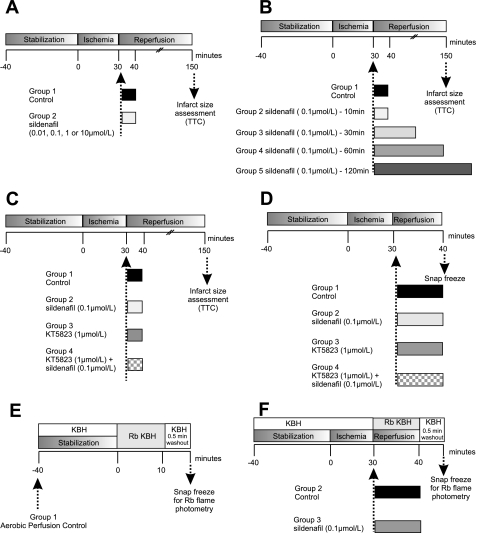
Specific isolated heart perfusion protocols were used to determine whether sildenafil is cardioprotective at reperfusion. Detailed protocol information is described below and in Fig. 1 (protocols A and B). Additional protocols were designed to investigate the potential role of the PKG activation (protocol C), natriuretic peptide receptor-A (NPR-A, protocol D), and Na+/K+-ATPase activity (protocol E) in sildenafil-induced protection (see Fig. 1).
Fig. 1.
Experimental protocols implemented to assess infarction and signaling events that contribute to sildenafil-mediated protection via reduced Na+ loading during myocardial reperfusion. In all experiments, hearts were allowed to stabilize for 40 min and in relevant protocols were subjected to 30 min global ischemia and 120 min reperfusion. A: hearts were perfused with control vehicle or 0.01, 0.1, 1, or 10 μM sildenafil at the onset of reperfusion for the first 10 min, followed by perfusion with Krebs. B: hearts were perfused with control vehicle or 0.1 μM sildenafil for 10, 30, 60, 90, or 120 min. C: hearts were perfused with control vehicle or 0.1 μM sildenafil in the absence or presence of PKG inhibitor, KT-5823 (1 μM). D: hearts samples were collected following 30 min global ischemia and 10 min perfusion with control vehicle or 0.1 μM sildenafil in the absence or presence of 1 μM KT-5823 for protein analysis. E: protocols used to assess myocardial Na+/K+-ATPase activity. F: hearts were perfused with rubidium (Rb)-Krebs-Henseleit buffer (KHB) during aerobic perfusion or at reperfusion (with or without sildenafil) before a 0.5-min washout period with standard KHB to remove extracellular Rb. Each heart was then analyzed for its Rb content using flame photometry. TTC, triphenyltetrazolium chloride.
Langendorff protocol A.
In these experiments, the effects on infarct size of pharmacological inhibition of PDE5, sildenafil, at reperfusion were examined. As shown in Fig. 1A, C57/BL6 mice were divided into five groups: 1) standard ischemia reperfusion group (control) or perfusion with 2) 0.01 μM, 3) 0.1 μM, 4) 1 μM, or 5) 10 μM sildenafil for the first 10 min of reperfusion, followed by 110 min perfusion with Krebs. Sildenafil was dissolved in dimethyl sulfoxide (DMSO) and then diluted with perfusion buffer so that the final concentration of DMSO was 0.001%. The same volume of DMSO was added to the control buffer solution (vehicle control).
Langendorff protocol B.
These experiments set out to establish whether the cardioprotective effect of 0.1 μM sildenafil was enhanced by administering it for a longer period during reperfusion. After 30 min global ischemia, C57/BL6 hearts were subjected to control vehicle or perfusion with 0.1 μM sildenafil for 10, 30, 60, or 120 min (Fig. 1B).
Langendorff protocol C.
To determine whether PKG plays an important role in sildenafil-induced protection at reperfusion, KT-5823, a PKG inhibitor, was used (Fig. 1C). C57/BL6 mice were divided into four groups: 1) standard ischemia-reperfusion group (control) or perfusion with 2) 0.1 μM sildenafil or 3) 1 μM KT-5823 or 4) 1 μM KT-5823 and 0.1 μM sildenafil for the first 10 min of reperfusion followed by 110 min with Krebs.
Langendorff protocol D.
cGMP synthesis is controlled by two types of guanylate cyclases (GCs): 1) soluble GC (sGC), which is activated by nitric oxide (NO), and 2) particulate GC (pGC), which is activated by natriuretic peptides (atrial, brain, and C-type natriuretic peptides). The NPR-A is coupled to pGC, which generates cGMP upon natriuretic peptide binding/activation. Recently, Elrod and colleagues (11) found that sildenafil-mediated cardioprotection was NO-sGC-cGMP independent. To delineate whether the NPR-A-pGC-cGMP pathway played a role in sildenafil-mediated cardioprotection at reperfusion, we used NPR-A knockout (KO) mice. After 40 min stabilization and 30 min global ischemia, NPR-A wild-type (WT) or KO mice were subjected to either perfusion with buffer (control) or 0.1 μM sildenafil for the first 10 min of reperfusion, followed by 110 min Krebs buffer.
Infarction assessment in isolated murine hearts.
Infarct size was determined by triphenyltetrazolium chloride (TTC) staining. After 2 h of reperfusion, hearts were perfused for 1 min with 5 ml of 1% TTC in phosphate-buffered saline and then placed in an identical solution at 37°C for 10 min. The atria were removed, and the hearts were blotted dry, weighed, and stored at −20°C for up to 1 wk. The hearts were then thawed, placed in 2.5% glutaraldehyde for 1 min, and set in 5% agarose. The agarose heart blocks were sectioned from apex to base in 0.7-mm slices using a Vibratome (Agar Scientific). After being sectioned, the slices were placed overnight in 10% formaldehyde at room temperature before transferring to phosphate-buffered saline for an additional day at 4°C. The sections were then compressed between glass plates (0.7 mm apart) and scanned (Epson Model G850A). After magnification, planimetry was carried out using image analysis software (Adobe Photoshop 7.0), and the surface areas of the whole and TTC-negative myocardium were transformed to volume by multiplication with tissue thickness. Within each heart, after the summation of individual slices, the TTC-negative infarction volume was expressed as a percentage of heart volume. All analyses of infarct size were done by an investigator who was blinded with regard to the group assignments.
cGMP assay.
Isolated mouse hearts were stabilized for 40 min followed by 30 min global ischemia. At reperfusion, control vehicle or 0.1 μM sildenafil was administered for 10 min, after which the hearts were snap frozen for the assessment of cGMP. A 10% (wt/vol) suspension of tissue homogenates in homogenizing buffer [6% trichloroacetic acid] and was centrifuged and extracted with water-saturated ether. The aqueous layer was transferred and vacuum dried, and the pellet was resuspended in sodium acetate buffer for cGMP enzyme immunoassay (GE Healthcare).
In vitro kinase assay and dot blot analysis.
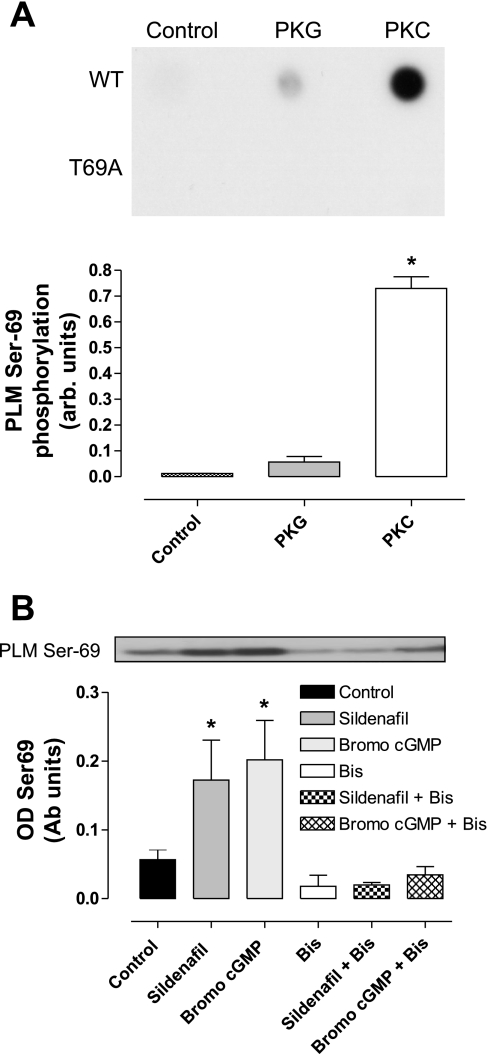
The ability of PKG1α or PKCε to phosphorylate PLM was determined using in vitro kinase activity assays. These used a purified recombinant glutathione S-transferase-tagged peptide sequence from the COOH-terminal intracellular domain of rat PLM, which has a threonine at position 69. A corresponding unphosphorylatable T69A mutant of this glutathione S-transferase-tagged peptide was also generated as a control. Each kinase activity assay contained 14 μM of PLM peptide and 15 mM MgCl2. Assays using PKG1α were also supplemented with 100 mM DTT and 2 mM of cGMP. All reactions were initiated by the addition of 100 μM of ATP and were quenched after 10 min with buffer containing 50 mM Tris·HCl, 2.5% 2-mercaptoethanol, 2% SDS, and 10% glycerol. One microliter of each sample was then dot blotted onto nitrocellulose, allowed to dry, and then immunostained using antibody CP69. In separate experiments (not shown), PKCε was shown to stoichiometrically phosphorylate the WT substrate as in previous studies (13).
Isolation and culture of adult rat ventricular myocytes.
Adult rat ventricular myocytes (ARVMs) were isolated, as described previously (35), and washed with modified medium 199 (M199) with added penicillin (100 IU/ml), streptomycin (100 IU/ml), l-carnitine (2 mM), creatine (5 mM), and taurine (5 mM). The cell suspension was allowed to settle 1 h to pellet the myocytes, which were then resuspended in M199. To each well of a laminated six-well culture plate, 2 ml of cell suspension were added, and the plates were maintained in a humidified 95% O2-5% CO2 incubator at 37°C. After 2 h of preplating, the medium was aspirated, leaving only adherent cells, and 2 ml of fresh, prewarmed M199 were added. ARVM were bathed in standard culture medium 18–24 h after plating.
ARVM were exposed to sildenafil (0.1 μM), 8-bromo-cGMP (0.1 μM, PKG agonist), or control vehicle (DMSO, 0.001% vol) for 10 min. The PKC inhibitor bisindolylmaleimide (1 μM) was added to the cells 30 min before sildenafil or 8-bromo-cGMP, and the final concentrations of these control vehicles (0.1%) were included in the appropriate solutions in the untreated groups.
Western blot analysis.
Samples were prepared from perfused hearts that had been freeze clamped at the end of defined protocols. The samples were prepared for Western blot analysis as previously described (13). Briefly, a 10% (wt/vol) solution of tissues homogenate in homogenization buffer containing 100 mM Tris·HCL (pH 7.4), protease complete C tablet per 50 ml, 2 mM sodium orthovanadate, and 5 mM sodium fluoride. The homogenate sample was resuspended in 2× sample buffer containing 250 mM Tris·HCl, 4% SDS, 10% glycerol, and 2% β-mercaptoethanol (pH 6.8). After being blocked with 5% nonfat milk, the nitrocellulose membranes were exposed to the following phosphospecific primary antibodies—CP69 (1:100 with 1 μg/ml unphosphorylated blocking peptide), CP68, CP63 (1:10,000), or total PLM antibody (N1) (1:100)—and then an appropriate second antibody (21). It is important to note that the phosphospecific CP69 antibody used in these studies does not distinguish between these amino acids but simply recognizes the phosphorylated form irrespective of the underlying residue. Studies in this article referring to rat and mouse may therefore describe Thr69 or Ser69, respectively, but as far as we are aware, it is the phosphorylation of this residue that may be important in its function and not the identity of the underlying amino acid. Enhanced chemiluminescence detection (GE Healthcare) was used. Images of the Western blots were scanned and then quantified using Gel-Pro analyzer software.
Na+ pump activity measurements with voltage clamping.
To measure the effects of sildenafil acutely on the Na+/K+-ATPase, isolated rat adult ventricular myocytes were voltage-clamped in the whole cell-perforated patch configuration, as described previously (31). Briefly, myocytes were placed on a microscope slide and allowed to settle before being perfused with Tyrode solution containing (in mM) 137 NaCl, 5.4 KCl, 0.5 MgCl2, 1.0 CaCl2, 10 glucose, and 10 HEPES. Patch electrodes were made from thin-walled (1.65 mm outer diameter, and 1.20 mm inner diameter) Schott capillary glass (A-M systems) and pulled to a resistance of 1 to 2 MΩ (P-97 Brown-Flaming-electrode puller, Sutter Instruments) when filled with 30 mM Na+ pipette internal solution containing (in mM) 15 NaCl, 15 NaCH3O3S, 1 MgCl2, 8 CsCl, 95 CsCH3O3S, 10 HEPES (pH 7.2) at 35°C and were lowered onto myocytes and a gigaohm seal was formed. Myocytes were then perfused with K-Test solution and membrane permeabilization occurred within 10 min with series resistance typically falling to between 10–15 MΩ. Membrane capacitance was recorded after permeabilization by imposing a 25-ms square step from −80 to −75 mV and integrating the area under the capacitance transient.
The Na+/K+-ATPase pump current (Ip) was continuously recorded at 0 mV, sampled at 100 Hz and filtered at 10 Hz while being perfused with K-Test solution. When a stable recording was observed, a control Ip measurement was taken. In all experiments, Ip was defined as the current sensitive to the removal of extracellular K+ and was calculated as steady-state K-Test minus the K+-free current. When the K-Test recording was stable again, myocytes were perfused with K-Test solution containing 0.1 μM sildenafil. Following 20 min perfusion with sildenafil, the perfusion was switched to K+-free solution to define the “treated” Ip current. Once the trace returned to baseline, the recording was terminated. Ip was measured in both groups (vehicle control and sildenafil treatment) from every cell isolation, therefore reducing isolation bias.
Na+/K+-ATPase activity in isolated hearts measured by rubidium uptake using flame photometry.
To determine the Na+/K+-ATPase activity in the intact mouse myocardium, we measured tissue rubidium (Rb) accumulation. The perfusion protocols used in this study are shown in Fig. 1E. These studies used the same KHB formula as described in the perfusion of isolated hearts protocol, but the KCl and NaH2PO4 were replaced with 4.7 mM RbCl as before (Rb-KHB) (22). After periods of perfusion with Rb-KHB, perfusion was switched back to Rb-free KBH for 0.5 min to remove extracellular Rb. The hearts were then immediately snap frozen in liquid nitrogen until further analysis. To determine myocardial Rb content, the hearts were dried for 12 h at 80°C, weighed, and then finely chopped in 50% trifluoroacetic acid before being shaken (Eppendorf Thermomixer Compact) at 55°C for 1 h. The mixture was then centrifuged at 20,000 g for 5 min, and the supernatant was collected and diluted in water. The Rb content was determined using a Sherwood Model 410 Classic Flame Photometer (with a Rb filter set) using RbCl standards to produce a linear calibration line.
Data analysis and statistics.
All data are presented as means ± SE. Comparisons between multiple groups were performed by one-way ANOVA with subsequent Student-Newman-Keuls post hoc test. A two-tailed t-test was used to test for differences in Na+/K+-ATPase between two groups. P < 0.05 was considered significant.
RESULTS
Sildenafil concentration-response and duration of treatment study.
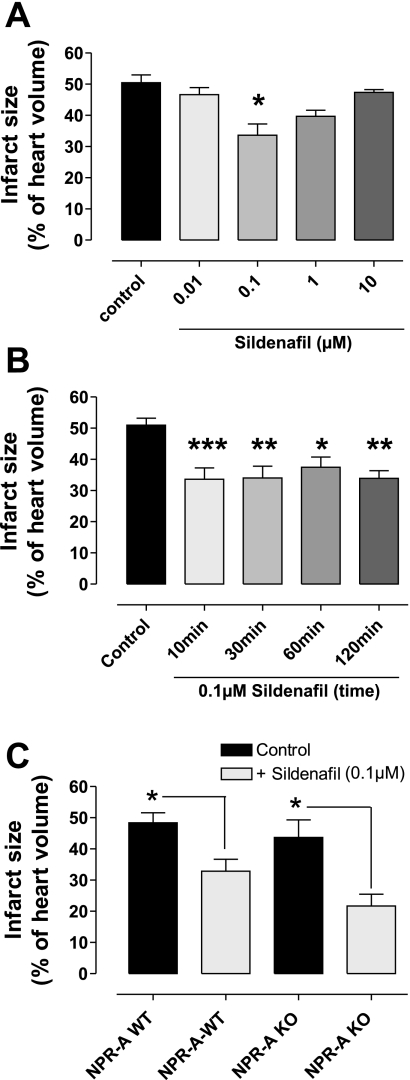
Figure 2A shows that infarct size (expressed as the percentage of area at risk) was 50.5 ± 2.5% under control conditions. Treatment with 0.1 μM sildenafil during the first 10 min of reperfusion significantly reduced infarct size (33.65 ± 3.61%; P < 0.001), but lower (0.01 μM) or higher concentrations (1 or 10 μM) during the first 10 min of reperfusion did not protect the heart (n = 4–9).
Fig. 2.
A: the effect of different concentrations of sildenafil (0.01–10 μM) at reperfusion on infarct size. Mouse hearts were subjected to 30 min global ischemia and 120 min of reperfusion. Bars represent means ± SE; n = 4–9 animals. *P < 0.001 vs. control (1-way ANOVA). B: the time dependence of protection by 0.1 μM sildenafil at reperfusion, assessing 10, 30, 60, or 120 min. Bars represent means ± SE; n = 5–11 animals. ***P < 0.001 vs. control; **P < 0.01 vs. control; *P < 0.05 vs. control (1-way ANOVA). Sildenafil administration beyond 10 min did not further attenuate infarction. C: the effect of 0.1 μM sildenafil administered at reperfusion on myocardial infarction in wild-type (WT) or natriuretic peptide receptor-A (NPR-A) null tissue following ischemia and reperfusion. Sildenafil (0.1 μM) was given at the onset of reperfusion for the first 10 min and protected both WT and NPR-A null hearts. Bars represent means ± SE; n = 5–13 animals. *P < 0.05 vs. control (1-way ANOVA). Infarction has been measured as infarction volume as a percentage of total myocardial volume. KO, knockout.
To determine whether 10 min was a sufficient duration of treatment for optimal protection, extended periods (30, 60, or 120 min) of sildenafil (0.1 μM) at reperfusion were investigated. Figure 2B shows that none of these prolonged treatments altered infarct size compared with the protective 10-min sildenafil treatment. However, they were all protective compared with vehicle control (P < 0.01–0.05; n = 5–11). Therefore, in the rest of these investigations, 0.1 μM sildenafil for 10 min was used.
The protective mechanism of sildenafil is independent of NPR-A-pGC pathway.
To test whether the sildenafil-induced protection was NPR-A-pGC dependent, NPR-A KO and NPR-A WT mice were subjected to 40 min stabilization, 30 min global ischemia, and 120 min reperfusion. Mice received either 0.1 μM sildenafil or vehicle control for the first 10 min of reperfusion. Sildenafil significantly reduced infarct size in both NPR-A WT (32.86 ± 3.82) and KO (21.68 ± 3.77) mice compared with control-treated hearts in WT (48.30 ± 3.21) and KO (43.67 ± 5.63) hearts, respectively (n = 5–13; P < 0.05; Fig. 2C).
Sildenafil did not alter total myocardial cGMP levels at reperfusion.
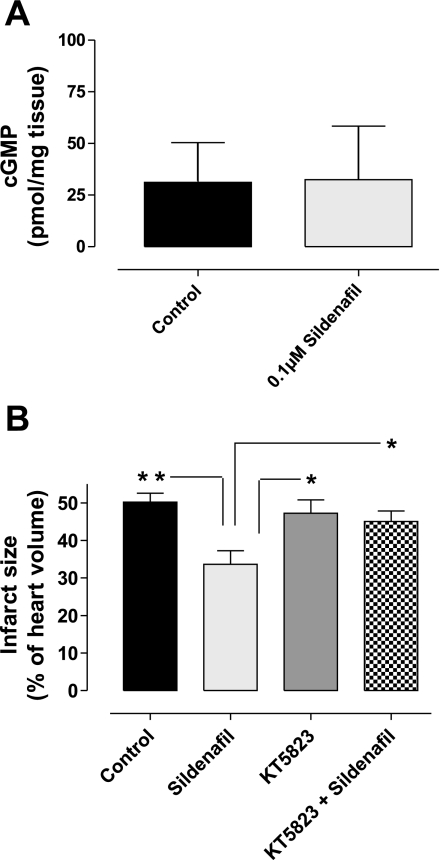
Hearts treated with control vehicle or sildenafil (0.1 μM) for 10 min at the onset of reperfusion had been snap frozen for the assessment of myocardial cGMP. Figure 3A shows that sildenafil treatment did not alter myocardial cGMP levels. The hearts receiving vehicle contained 31.17 pmol/mg tissue cGMP, whereas those treated with sildenafil contained 32.44 pmol/mg tissue (n = 6; P = not significant).
Fig. 3.
A: whole heart cGMP levels. Isolated mouse hearts were subjected to 30 min global ischemia followed by 10 min reperfusion with control vehicle or the cardioprotective 0.1 μM sildenafil treatment. cGMP analysis showed that the cyclic nucleotide levels were similar in both groups. Bars represent means ± SE; n = 6 animals; not significantly different when compared with controls. B: the effect of 0.1 μM sildenafil at reperfusion in the absence or presence of the PKG inhibitor KT-5823 on infarct size. Infarction has been measured as a percentage of total myocardial volume. PKG inhibition blocked the sildenafil-dependent protection from infarct. Bars represent means ± SE; n = 3–5 animals. *P < 0.05 vs. control (1-way ANOVA); **P < 0.01.
PKG activity is important for sildenafil-mediated protection.
To investigate whether PKG activity is involved in sildenafil-induced cardioprotection, isolated mouse hearts were perfused with the kinase inhibitor KT-5823 (1 mM) in the absence or presence of 0.1 μM sildenafil at the onset of reperfusion for 10 min (see protocol in Fig. 1C). As shown in Fig. 3B, the infarct-limiting affect of sildenafil alone (33.65 ± 3.61%) was significantly abolished when KT-5823 was coadministered with this PKG inhibitor (45.04 ± 2.83%, P < 0.05). In contrast, KT-5823 treatment alone had no affect on infarct size compared with control hearts (47.25 ± 3.52% and 50.17 ± 2.43%, respectively; n = 7–13; P = not significant).
Sildenafil increased Na+/K+ ATPase activity.
Figure 4A shows that in isolated rat ventricular myocytes, 0.1 μM sildenafil (1.72 ± 0.08 pA/pF) significantly increased the Na+/K+-ATPase pump activity compared with controls (1.51 ± 0.09 pA/pF, P < 0.05, n = 8 preparations).
Fig. 4.
A: the effect of sildenafil on Na+/K+-ATPase pump current (Ip). Isolated rat ventricular myocytes were superfused with solutions containing control vehicle or 0.1 μM sildenafil. The sildenafil treatment robustly activated the Na+/K+-ATPase. Bars represent means ± SE; n = 8 animals. *P < 0.05 vs. control (2-tailed t-test). The effect of sildenafil on phospholemman (PLM) phosphorylation at Ser63 (B), Ser68 (C), and Ser69 (D). Isolated mouse hearts were subjected to 30 min global ischemia and 10 min perfusion with control vehicle or 0.1 μM sildenafil at the onset of reperfusion. Heart samples were collected at the end of 10-min treatment for immunoblotting analysis. Representative blots are shown with quantitative analysis of repeat experiments. The dotted line on the immunoblots indicates that they were edited using computer software to present the control and sildenafil treatment samples directly adjacent to each other. The band intensities are, however, directly comparable as the sample pairs are from the same immunoblots and the data are normalized to the control levels. Sildenafil treatment selectively induced the phosphorylation of Ser69, whereas the two other sites remain unchanged. Bars represent means ± SE; n = 3–5 animals. *P < 0.05 vs. control (1-way ANOVA).
PLM Ser69 phosphorylation coincides with sildenafil-induced cardioprotection.
We compared the phosphorylation of PLM after 10 min perfusion with or without sildenafil. This was measured by Western blot analysis with Ser63, Ser68, and Ser69 PLM phosphospecific antibodies. Basal PLM Ser63 or Ser68 was not altered by sildenafil treatment (Fig. 4, B and C, respectively). However, sildenafil did enhance phosphorylation at Ser69 (Fig. 4D), which increased significantly (P < 0.05) 1.9-fold over control.
Inhibition of PKG decreases PLM phosphorylation at Ser69 site.
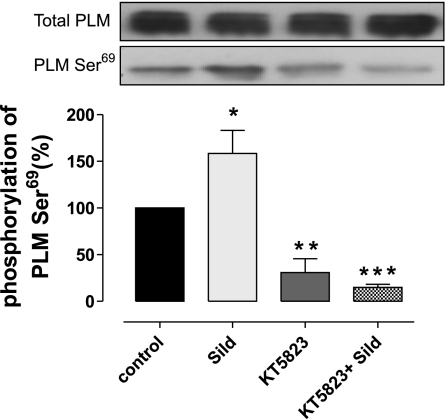
To determine whether increased phosphorylation at the Ser69 site following sildenafil treatment was PKG dependent, we tested whether the PKG inhibitor KT-5823 reduced phosphorylation at this site (see protocol in Fig. 1D). Figure 5 reaffirms that sildenafil significantly increases PLM Ser69 phosphorylation. PKG inhibition with KT-5823 significantly reduced basal, as well as sildenafil-induced, PLM Ser69 phosphorylation. Thus KT5823 treatment at reperfusion blocks both sildenafil-induced PLM Ser69 phosphorylation and sildenafil-mediated cardioprotection.
Fig. 5.
The effect of sildenafil (Sild) at reperfusion in the absence or presence of KT-5823 on PLM Ser69 phosphorylation and total PLM. The PKG inhibitor KT-5823 reduced basal Ser69 phosphorylation and also blocked the sildenafil-dependant increase. *P < 0.05; **P < 0.01; ***P < 0.001.
Kinases involved in sildenafil-induced PLM Thr/Ser69 phosphorylation.
To investigate the signaling pathways involved in sildenafil-dependent cardioprotection, we assessed the ability of PKG and PKCε to directly phosphorylate PLM Thr69 using an in vitro kinase assay. Figure 6A shows that PKCε efficiently phosphorylated WT (but not mutant T69A) PLM at Thr69, as detected in dot blots probed with an anti-phospho-Thr/Ser69 antibody. Indeed, in separate experiments (not shown) described previously (13), this level of phosphorylation represents a complete stoichiometric modification of PLM. This allows an absolute quantitative comparison of the ability of PKG to phosphorylate PLM compared with PKC. Indeed, while PKG does not phosphorylate the T69A mutant peptide at all, it similarly does not phosphorylate the WT protein efficiently (n = 3). This suggests that PKG, despite the evidence presented from the Langendorff and ARVM studies for an upstream role in cardioprotection induced by sildenafil, is unlikely to be the kinase that directly modifies PLM during sildenafil or other stimuli that culminate in Thr/Ser69 phosphorylation.
Fig. 6.
A: the ability of PKG or PKC to phosphorylate a recombinant PLM peptide in vitro was assessed using WT or a T69A (which cannot be phosphorylated at that site) recombinant peptide. Dot blots were probed with an anti-PLM CP69 antibody to index phosphorylation. The T69A serves as a control that shows the phosphospecific antibody only recognizes modification of Ser/Thr69, and does not cross-react with other sites. Whilst PKC efficiently phosphorylated the PLM peptide, PKG did not. From previous HPLC studies (not shown), we know that the PKC-dependent signal represents a stoichiometric phosphorylation of the PLM peptide. Arb units, arbitrary units. B: the effect of PKC inhibition on PLM Thr69 phosphorylation in cultured adult rat ventricular myocytes. Sildenafil or 8-bromo-cGMP each increased PLM Thr69 phosphorylation, and this was robustly blocked by the PKC inhibitor bisindolylmaleimide (Bis). OD, optical density; Ab units, arbitrary units. Representative blots are shown with quantitative analysis of repeat experiments. Bars represent means ± SE; n = 3–5 experiments. *P < 0.05 vs. control (1-way ANOVA).
To further elucidate the possible links between PKG, PKC, and PLM Thr/Ser69, we investigated the phosphorylation of this site in ARVMs. Treatment of ARVM with sildenafil or 8-bromo-cGMP, a potent PKG activator, strongly induced PLM Thr69 phosphorylation (Fig. 6B). The induction of Thr69 phosphorylation by either of these agents was robustly inhibited by the pretreatment of the cells with bisindolylmaleimide. It is notable that there is a basal level of PLM Thr69 phosphorylation, which can also be attenuated by treatment with bisindolylmaleimide.
Sildenafil reduces Na+ overload at reperfusion.
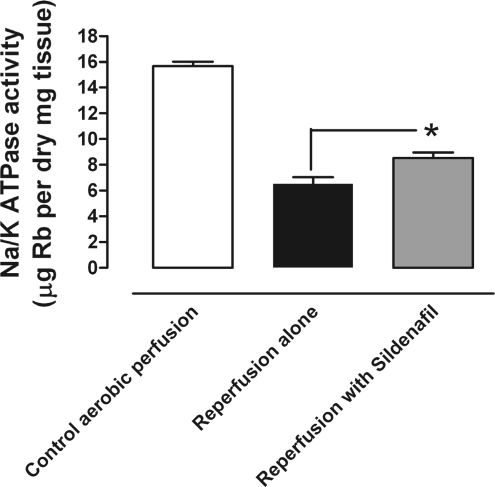
To test whether sildenafil reduced Na+ overload at reperfusion, isolated mouse hearts were perfused with Rb-KBH in the absence and presence of sildenafil (0.1 μM) at reperfusion. Rb accumulation provides an index of Na+/K+-ATPase activity and showed that sildenafil significantly improved pump activity at reperfusion compared with control reperfused hearts (n = 6; 8.53 ± 0.42 and 6.50 ± 0.53 μg/Rb per mg dry tissue, respectively; Fig. 7). Na+/K+-ATPase activity was also measured in aerobically perfused hearts (15.68 ± 0.34 μg/Rb per mg dry tissue; n = 5; Fig. 7) and was markedly higher than that observed during early postischemic reperfusion.
Fig. 7.
The effect of sildenafil on Na+/K+-ATPase activity. Isolated mouse hearts were stabilized and aerobically perfused with KBH for 40 min. Mouse hearts were then subjected to aerobic perfusion with Rb-KBH for 10 min, or 30 min global ischemia followed by 10 min reperfusion with Rb-KBH in the absence or presence of 0.1 μM sildenafil. Clearly, ischemia and reperfusion lead to Na+/K+-ATPase inhibition, and this is partially alleviated by sildenafil treatment. Bars represent means ± SE; n = 5 to 6 animals. *P < 0.05 vs. control ischemia-reperfusion (1-way ANOVA).
DISCUSSION
Our observation that sildenafil administered during the first 10 min of reperfusion efficiently reduced infarct size is consistent with a number of studies also showing that it limits injury in several models of myocardial ischemia and reperfusion injury. Sildenafil administered before ischemia has been found to reduce infarct size in an in vivo rabbit model (3). Similar results have been observed in isolated rabbit (28), rat (8), and mouse hearts (33). Sildenafil also protects against other forms of cardiac dysfunction, such as progression of hypertrophy (26).
Despite these observations, the precise mechanism by which sildenafil is cardioprotective remains to be fully elucidated. Also, most studies investigating sildenafil-mediated protection have used healthy animals before the onset of ischemia (28) or treatment 5 min before reperfusion, which may equate to partial early reperfusion (11, 34). Such interventions are not as clinically relevant as sildenafil treatment at reperfusion, which theoretically could be used during nonelective revascularization procedures. Consequently, we focused on sildenafil at reperfusion, trying to define the underlying cellular basis of this cardioprotective intervention. Sildenafil given for 10 min at reperfusion was protective at 0.1 μM but surprisingly was not protective at lower (0.01 μM) or higher (1 or 10 μM) concentrations. We cannot explain the loss of protection by sildenafil at higher concentrations, leading to a bell-shaped dose-response curve. Nevertheless, this finding is consistent with others (10, 25) who also found that sildenafil only reduced myocardial infarct size in a relatively narrow therapeutic concentration window. However, the loss of protection at higher concentrations of sildenafil may involve the nonspecific activation of additional molecular targets (other than PDE5) that mediate deleterious effects. We then tested whether longer reperfusion times (of 30, 60, or 120 min) with sildenafil improved protection, which they did not. This observation is consistent with the early minutes of reperfusion being a crucial period for establishing tissue survival and viability (49), especially in the context of ion handling (9, 47).
We compared myocardial cGMP levels in hearts subjected to ischemia and reperfusion with or without sildenafil. We had anticipated that sildenafil would elevate cGMP, as it inhibits PDE5 to prevent the breakdown of this second messenger (2). Perhaps surprisingly, sildenafil did not increase cGMP above controls, although it is clearly “bioactive” since it reduces infarction and, as outlined below, triggers phosphorylation of PLM. Our observations are consistent with those of Wilson and colleagues (44) who also found sildenafil did not elevate global cGMP. The likely explanation of this is that sildenafil increases cGMP in defined compartments of cardiomyocytes, which has been observed by others (4). In addition, Elrod and colleagues (11) recently found that sildenafil protected through endothelial and inducible NO synthases but, as in our studies, this did not alter myocardial cGMP. These findings contradict those of Das and colleagues (6) who found endothelial and inducible NO synthases were required for sildenafil-mediated protection. There is a recognized basis for the elevation of disparate, compartmentalized cGMP pools, as opposed to global cell-wide increases, namely pGC. sGC is a cytosolic enzyme that is activated by NO to produce cGMP (16), whereas pGC enzymes are membrane-bound receptors that produce cGMP after natriuretic peptide (atrial, brain, and C-type) binding (24). pGC can trigger discrete signal transduction events compared with the soluble enzyme (37), elevating cGMP locally instead of globally throughout the cell (4). Sildenafil can synergize with particulate-derived cGMP, for example potentiating the protective effects of brain natriuretic peptide against heart failure (12). In addition, NPR-A KO mice, which cannot produce cGMP following atrial natriuretic peptide treatment, have a deficit in their response to sildenafil compared with WT mice. These KO mice have enhanced hypoxia-induced pulmonary hypertension, right ventricular hypertrophy, and vascular remodeling (48). These observations highlight the prospect that sildenafil-mediated cardioprotection could involve stabilization of cGMP produced by receptor cyclases in the particulate fraction, as opposed to that derived from sGC. This would be consistent with the failure of sildenafil to globally elevate cGMP. To address this issue, we assessed the protective effect of sildenafil at reperfusion in NPR-A KO mice, comparing it with littermate WT controls. Sildenafil significantly protected both groups of mice, suggesting that cGMP derived from the particulate NPR-A receptor was not required for protection. Therefore, it remains possible that total myocardial cGMP does not necessarily reflect localized cGMP signaling, which may exist within compartmentalized subdomains within the cell.
While this study has focused on elevating cGMP using sildenafil-induced PDE5 inhibition, the scheme outlined in Fig. 8 is consistent with other mechanisms of elevating cGMP being cardioprotective. One way in which this might be achieved would be to use GC activators (36). Elevating cGMP by alternate mechanisms such as GC activators may provide protection from reperfusion injury without the potential limitations of using sildenafil, such as coronary “steal.”
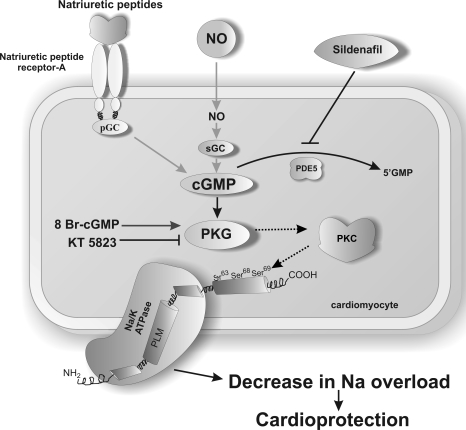
Fig. 8.
Schematic diagram to illustrate the potential signaling cardioprotective mechanism for sildenafil at reperfusion. NO, nitric oxide; sGC, soluble guanylate cyclase; PDE5, phosphodiesterase enzyme type 5; 8-Br-cGMP, 8-bromo-cGMP.
Having established that sildenafil protects against reperfusion injury, we performed further experiments to test whether this was PKG mediated. A role for PKG might be expected, since this kinase is activated by cGMP and sildenafil prevents the hydrolysis of this cyclic nucleotide (2). The PKG inhibitor, KT-5823, attenuated sildenafil-induced protection from reperfusion injury, confirming a role for this kinase as hypothesized. This is consistent with the general perception of PKG as a “good” kinase, integral to several forms of cardioprotection, including that by NO (32) and ischemic preconditioning (15).
In this study we used KT-5823, choosing this antagonist for a combination of reasons. This inhibitor is the most commonly used in isolated heart studies investigating PKG-dependent cardioprotection (1, 20, 25), perhaps primarily because it is affordable in perfusion studies. cGMP-derivative inhibitors are generally too expensive for use in heart perfusion work and indeed can have their own issues due to actually activating PKG in some scenarios (40). Other options for pharmacological inhibition are several DT peptides, some of which are cell permeable as well as being PKG isoform selective. While some studies have shown these to be useful in experiments investigating vasotone in cerebral arteries, they were ineffective in isolated heart preparations, being trapped in the endothelium and failing to reach the myocytes (21). Routine genetic manipulation of PKG signaling is not possible in the isolated heart, and PKG null mice are not available to us and also have a complex phenotype and have health issues that shorten their life (17). Following these considerations, we used KT-5823 for this study.
The downstream targets of PKG in the context of cardioprotection are less well established, although pathways involving PKCε and phosphoactivation of the mitochondrial ATP-sensitive K+ channel, inhibition of the mitochondrial transition pore, are routinely cited (5, 29). However, the precise order of these events is less clear, such as the molecular connections between PKG and the putative end-effector kinase PKCε. We therefore attempted to define whether there was a possible link between PKC, PKG, and PLM Ser/Thr69. Either sildenafil or the PKG activator 8-bromo-cGMP stimulated efficient PLM Thr69 phosphorylation, and in both cases this was blocked by PKC inhibition using bisindolylmaleimide. It is tempting to speculate that PKCε is the end-effector kinase that phosphorylates PLM, consistent with the known protective role of this protein. However, this idea is difficult to reconcile with the fact that Ser68, an established target for PKC, was not phosphorylated concomitantly with Ser69. One possibility is that the detection of Ser/Thr69 phosphorylation by antibody binding was compromised when the adjacent Ser68 site was modified. However, the precise relationship between PKG, PKC, and PLM phosphorylation remains to be defined. The involvement of PKG and PKC in sildenafil-induced cardioprotection is consistent with other studies that also implicate these kinases in cardioprotection during ischemic pre- or postconditioning. However, as in these other studies on protection, it remains unclear exactly how the PKG pathway and PKC pathways integrate with each other. Substrates of PKG, other than PLM, such as vasodilator-stimulated phosphoprotein or the L-type Ca2+ channel may also be phosphorylated in response to sildenafil (46) and could contribute to the cardioprotection observed. cGMP can also directly modulate the activity of ion translocating membrane proteins and so could recruit their activity and signaling to protection.
The loss of ionic homeostasis is a key event in reperfusion injury (9), and it is logical that protective interventions such as sildenafil treatment should somehow address this major mode of dysfunction, especially Na+ and Ca2+ overload. NO donors stimulate the Na+/K+-ATPase in isolated ventricular myocytes (43), with additional evidence that this enzyme has reduced function during ischemia and early reperfusion (14). This NO-mediated activation highlights a potential role for PKG in stimulating the Na+/K+-ATPase. The Na+/K+-ATPase is regulated by PLM, the phosphorylation of which activates ion pump activity. Although there are no studies reporting that PKG phosphorylates PLM, it is a target for PKA that can target the same substrates as PKG (45). We undertook several experiments that indicate that stimulating the PKG pathway culminates in PLM phosphorylation. This phosphorylation was not at the established Ser63 or Ser68 sites but at a newly confirmed phosphorylation site (13), namely, Ser/Thr69. In isolated myocytes treated with 8-bromo-cGMP, a highly selective activator of PKG, we observed PLM Thr69 phosphorylation. Thr69 phosphorylation was also induced by sildenafil treatment of isolated myocytes, an event that stimulated the Na+/K+-ATPase. The prospect that sildenafil activates the Na+/K+-ATPase in isolated hearts when given at reperfusion was supported by our observation that PLM Ser/Thr69 was phosphorylated during this intervention in isolated hearts. Furthermore, sildenafil also induced PLM Ser/Thr69 phosphorylation in isolated ventricular myocytes, which patch-clamp analysis confirmed activated the Na+/K+-ATPase. Consequently, we examined whether sildenafil enhanced Na+/K+-ATPase activity during early reperfusion, using Rb uptake into the intact myocardium to index this. Consistent with the other supporting data, we found that sildenafil did indeed enhance Na+/K+-ATPase activity in the isolated perfused heart, which would serve to limit Na+ accumulation and attenuate injury. In addition, these Rb uptake experiments also confirmed a central tenet of these studies, namely, that the Na+/K+-ATPase activity is attenuated during ischemia and reperfusion. Na+/K+-ATPase inhibition during ischemia and reperfusion contributes to injury, and interventions that limits this deficit (here sildenafil) provide protection.
Although our studies are consistent with the phosphorylation of PLM being integral to the protection afforded by sildenafil against injury during postischemic reperfusion, this is only a correlative association. A role for PLM phosphorylation is supported by the fact that PLM is phosphorylated during this intervention, and that the Na+ loading at reperfusion is attenuated, this is only correlative. We cannot exclude the possibility that sildenafil triggers other cardioprotective mechanisms, and indeed there may be multiple, synergistic pathways that are recruited by PDE5 inhibition. Establishing the cause and effect in cardioprotection is notoriously difficult, but perhaps this could only be most definitive if a PLM T69A knock-in mouse was generated and this was not protected by sildenafil.
Our observations are consistent with a recent study that identified improved Ca2+ handling as a crucial mechanism in sildenafil-mediated cardioprotection from hypertrophy (26). Sildenafil enhanced the expression of sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA2a) sarcoplasmic reticulum Ca2+-pump expression, as well as the phosphorylation of its phosphoregulatory protein phospholamban (PLB). In our acute protection experiments involving 10 min of sildenafil treatment at reperfusion, an alteration of ion-translocating protein expression such as the Na+/K+-ATPase or SERCA2a is unlikely. However, their observations of PLB activation and enhanced Ca2+ handling have strong parallels with our studies of enhanced Na+ handling as a result of PLM Ser/Thr69 phosphorylation. Indeed, both PLM and PLB serve as a brake on the activities of their respective ATPase enzyme, which is relieved by the phosphorylation of these small accessory proteins.
In summary, we have shown that sildenafil protects against myocardial reperfusion injury and clarified in part the mechanism involved. Figure 8 outlines the cardioprotective pathway that our data suggest underlies sildenafil-mediated protection. Sildenafil at reperfusion activates PKG, leading to the phosphorylation of PLM Ser/Thr69, but not Ser63 or Ser68, in a PKC-dependent manner. The phosphorylation of PLM at position 69 leads to the activation of the Na+/K+-ATPase, which provides a mechanism for sildenafil-mediated cardioprotection against reperfusion injury, namely, by attenuating an otherwise damaging Na+ overload that occurs at this time.
GRANTS
We thank The British Heart Foundation and The Wellcome Trust for their support.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Angelone T, Filice E, Quintieri AM, Imbrogno S, Recchia A, Pulera E, Mannarino C, Pellegrino D, Cerra MC. Beta3-adrenoceptors modulate left ventricular relaxation in the rat heart via the NO-cGMP-PKG pathway. Acta Physiol (Oxf) 193: 229–239, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev 58: 488–520, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Bremer YA, Salloum F, Ockaili R, Chou E, Moskowitz WB, Kukreja RC. Sildenafil citrate (viagra) induces cardioprotective effects after ischemia/reperfusion injury in infant rabbits. Pediatr Res 57: 22–27, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Castro LR, Verde I, Cooper DM, Fischmeister R. Cyclic guanosine monophosphate compartmentation in rat cardiac myocytes. Circulation 113: 2221–2228, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costa AD, Garlid KD, West IC, Lincoln TM, Downey JM, Cohen MV, Critz SD. Protein kinase G transmits the cardioprotective signal from cytosol to mitochondria. Circ Res 97: 329–336, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Das A, Xi L, Kukreja RC. Phosphodiesterase-5 inhibitor sildenafil preconditions adult cardiac myocytes against necrosis and apoptosis. Essential role of nitric oxide signaling. J Biol Chem 280: 12944–12955, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Das A, Xi L, Kukreja RC. Protein kinase G-dependent cardioprotective mechanism of phosphodiesterase-5 inhibition involves phosphorylation of ERK and GSK3beta. J Biol Chem 283: 29572–29585, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Das S, Maulik N, Das DK, Kadowitz PJ, Bivalacqua TJ. Cardioprotection with sildenafil, a selective inhibitor of cyclic 3′,5′-monophosphate-specific phosphodiesterase 5. Drugs Exp Clin Res 28: 213–219, 2002 [PubMed] [Google Scholar]
- 9.Dirksen MT, Laarman GJ, Simoons ML, Duncker DJ. Reperfusion injury in humans: a review of clinical trials on reperfusion injury inhibitory strategies. Cardiovasc Res 74: 343–355, 2007 [DOI] [PubMed] [Google Scholar]
- 10.du Toit EF, Rossouw E, Salie R, Opie LH, Lochner A. Effect of sildenafil on reperfusion function, infarct size, and cyclic nucleotide levels in the isolated rat heart model. Cardiovasc Drugs Ther 19: 23–31, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Elrod JW, Greer JJ, Lefer DJ. Sildenafil-mediated acute cardioprotection is independent of the NO/cGMP pathway. Am J Physiol Heart Circ Physiol 292: H342–H347, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Forfia PR, Lee M, Tunin RS, Mahmud M, Champion HC, Kass DA. Acute phosphodiesterase 5 inhibition mimics hemodynamic effects of B-type natriuretic peptide and potentiates B-type natriuretic peptide effects in failing but not normal canine heart. J Am Coll Cardiol 49: 1079–1088, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Fuller W, Howie J, McLatchie LM, Weber RJ, Hastie CJ, Burness K, Pavlovic D, Shattock MJ. FXYD1 phosphorylation in vitro and in adult rat cardiac myocytes: threonine 69 is a novel substrate for protein kinase C. Am J Physiol Cell Physiol 296: C1346–C1455, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuller W, Parmar V, Eaton P, Bell JR, Shattock MJ. Cardiac ischemia causes inhibition of the Na/K ATPase by a labile cytosolic compound whose production is linked to oxidant stress. Cardiovasc Res 57: 1044–1051, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Hausenloy DJ, Yellon DM. Preconditioning and postconditioning: united at reperfusion. Pharmacol Ther 116: 173–191, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Hobbs AJ. Soluble guanylate cyclase: an old therapeutic target re-visited. Br J Pharmacol 136: 637–640, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hofmann F, Feil R, Kleppisch T, Schlossmann J. Function of cGMP-dependent protein kinases as revealed by gene deletion. Physiol Rev 86: 1–23, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Kass DA, Champion HC, Beavo JA. Phosphodiesterase type 5: expanding roles in cardiovascular regulation. Circ Res 101: 1084–1095, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Kemp BE, Pearson RB. Protein kinase recognition sequence motifs. Trends Biochem Sci 15: 342–346, 1990 [DOI] [PubMed] [Google Scholar]
- 20.Krieg T, Liu Y, Rutz T, Methner C, Yang XM, Dost T, Felix SB, Stasch JP, Cohen MV, Downey JM. BAY 58-2667, a nitric oxide-independent guanylyl cyclase activator, pharmacologically post-conditions rabbit and rat hearts. Eur Heart J 30: 1607–1613, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Krieg T, Philipp S, Cui L, Dostmann WR, Downey JM, Cohen MV. Peptide blockers of PKG inhibit ROS generation by acetylcholine and bradykinin in cardiomyocytes but fail to block protection in the whole heart. Am J Physiol Heart Circ Physiol 288: H1976–H1981, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Kupriyanov VV, Xiang B, Sun J, Jilkina O, Kuzio B. Imaging of ischemia and infarction in blood-perfused pig hearts using 87Rb MRI. Magn Reson Med 49: 99–107, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Lansbery KL, Burcea LC, Mendenhall ML, Mercer RW. Cytoplasmic targeting signals mediate delivery of phospholemman to the plasma membrane. Am J Physiol Cell Physiol 290: C1275–C1286, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Maack T. Role of atrial natriuretic factor in volume control. Kidney Int 49: 1732–1737, 1996 [DOI] [PubMed] [Google Scholar]
- 25.Maas O, Donat U, Frenzel M, Rutz T, Kroemer HK, Felix SB, Krieg T. Vardenafil protects isolated rat hearts at reperfusion dependent on GC and PKG. Br J Pharmacol 154: 25–31, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagayama T, Hsu S, Zhang M, Koitabashi N, Bedja D, Gabrielson KL, Takimoto E, Kass DA. Sildenafil stops progressive chamber, cellular, and molecular remodeling and improves calcium handling and function in hearts with pre-existing advanced hypertrophy caused by pressure overload. J Am Coll Cardiol 53: 207–215, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neubauer S, Newell JB, Ingwall JS. Metabolic consequences and predictability of ventricular fibrillation in hypoxia. A 31P- and 23Na-nuclear magnetic resonance study of the isolated rat heart. Circulation 86: 302–310, 1992 [DOI] [PubMed] [Google Scholar]
- 28.Ockaili R, Salloum F, Hawkins J, Kukreja RC. Sildenafil (Viagra) induces powerful cardioprotective effect via opening of mitochondrial KATP channels in rabbits. Am J Physiol Heart Circ Physiol 283: H1263–H1269, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Oldenburg O, Qin Q, Krieg T, Yang XM, Philipp S, Critz SD, Cohen MV, Downey JM. Bradykinin induces mitochondrial ROS generation via NO, cGMP, PKG, and mitoKATP channel opening and leads to cardioprotection. Am J Physiol Heart Circ Physiol 286: H468–H476, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Palmer CJ, Scott BT, Jones LR. Purification and complete sequence determination of the major plasma membrane substrate for cAMP-dependent protein kinase and protein kinase C in myocardium. J Biol Chem 266: 11126–11130, 1991 [PubMed] [Google Scholar]
- 31.Pavlovic D, Fuller W, Shattock MJ. The intracellular region of FXYD1 is sufficient to regulate cardiac Na/K ATPase. FASEB J 21: 1539–1546, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Rakhit RD, Marber MS. Nitric oxide: an emerging role in cardioprotection? Heart 86: 368–372, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salloum F, Yin C, Xi L, Kukreja RC. Sildenafil induces delayed preconditioning through inducible nitric oxide synthase-dependent pathway in mouse heart. Circ Res 92: 595–597, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Salloum FN, Takenoshita Y, Ockaili RA, Daoud VP, Chou E, Yoshida K, Kukreja RC. Sildenafil and vardenafil but not nitroglycerin limit myocardial infarction through opening of mitochondrial KATP channels when administered at reperfusion following ischemia in rabbits. J Mol Cell Cardiol 42: 453–458, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Snabaitis AK, D'Mello R, Dashnyam S, Avkiran M. A novel role for protein phosphatase 2A in receptor-mediated regulation of the cardiac sarcolemmal Na+/H+ exchanger NHE1. J Biol Chem 281: 20252–20262, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Stasch JP, Hobbs AJ. NO-independent, haem-dependent soluble guanylate cyclase stimulators. Handb Exp Pharmacol 191: 277–308, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Su J, Scholz PM, Weiss HR. Differential effects of cGMP produced by soluble and particulate guanylyl cyclase on mouse ventricular myocytes. Exp Biol Med (Maywood) 230: 242–250, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Tani M, Neely JR. Role of intracellular Na+ in Ca2+ overload and depressed recovery of ventricular function of reperfused ischemic rat hearts. Possible involvement of H+-Na+ and Na+-Ca2+ exchange. Circ Res 65: 1045–1056, 1989 [DOI] [PubMed] [Google Scholar]
- 39.Tanno M, Bassi R, Gorog DA, Saurin AT, Jiang J, Heads RJ, Martin JL, Davis RJ, Flavell RA, Marber MS. Diverse mechanisms of myocardial p38 mitogen-activated protein kinase activation: evidence for MKK-independent activation by a TAB1-associated mechanism contributing to injury during myocardial ischemia. Circ Res 93: 254–261, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Valtcheva N, Nestorov P, Beck A, Russwurm M, Hillenbrand M, Weinmeister P, Feil R. The commonly used cGMP-dependent protein kinase type I (cGKI) inhibitor Rp-8-Br-PET-cGMPS can activate cGKI in vitro and in intact cells. J Biol Chem 284: 556–562, 2009 [DOI] [PubMed] [Google Scholar]
- 41.van Echteld CJ, Kirkels JH, Eijgelshoven MH, van der Meer P, Ruigrok TJ. Intracellular sodium during ischemia and calcium-free perfusion: a 23Na NMR study. J Mol Cell Cardiol 23: 297–307, 1991 [DOI] [PubMed] [Google Scholar]
- 42.Walaas SI, Czernik AJ, Olstad OK, Sletten K, Walaas O. Protein kinase C and cyclic AMP-dependent protein kinase phosphorylate phospholemman, an insulin and adrenaline-regulated membrane phosphoprotein, at specific sites in the carboxy terminal domain. Biochem J 304: 635–640, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.William M, Vien J, Hamilton E, Garcia A, Bundgaard H, Clarke RJ, Rasmussen HH. The nitric oxide donor sodium nitroprusside stimulates the Na+-K+ pump in isolated rabbit cardiac myocytes. J Physiol 565: 815–825, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilson LS, Elbatarny HS, Crawley SW, Bennett BM, Maurice DH. Compartmentation and compartment-specific regulation of PDE5 by protein kinase G allows selective cGMP-mediated regulation of platelet functions. Proc Natl Acad Sci USA 105: 13650–13655, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wood JS, Yan X, Mendelow M, Corbin JD, Francis SH, Lawrence DS. Precision substrate targeting of protein kinases. The cGMP- and cAMP-dependent protein kinases. J Biol Chem 271: 174–179, 1996 [DOI] [PubMed] [Google Scholar]
- 46.Yang L, Liu G, Zakharov SI, Bellinger AM, Mongillo M, Marx SO. Protein kinase G phosphorylates Cav1.2 alpha1c and beta2 subunits. Circ Res 101: 465–474, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med 357: 1121–1135, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Zhao L, Long L, Morrell NW, Wilkins MR. NPR-A-Deficient mice show increased susceptibility to hypoxia-induced pulmonary hypertension. Circulation 99: 605–607, 1999 [DOI] [PubMed] [Google Scholar]
- 49.Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol 285: H579–H588, 2003 [DOI] [PubMed] [Google Scholar]