Abstract
The mechanisms of sinoatrial node (SAN) dysfunction in patients with chronically elevated sympathetic tone and reduced pacemaker current (If; such as heart failure) are poorly understood. We simultaneously mapped membrane potential and intracellular Ca2+ in the Langendorff-perfused canine right atrium (RA). Blockade of either If (ZD-7288) or sarcoplasmic reticulum Ca2+ release (ryanodine) alone decreased heart rate by 8% (n = 3) and 16% (n = 3), respectively. Combined treatment of ZD-7288 and ryanodine consistently resulted in prolonged (≥3 s) sinus pauses (PSPs) (n = 4). However, the middle SAN remained as the leading pacemaking site after these treatments. Prolonged exposure with isoproterenol (0.01 μmol/l) followed by ZD-7288 completely suppressed SAN but triggered recurrent ectopic atrial tachycardia. Cessation of tachycardia was followed by PSPs in five of eight RAs. Isoproterenol initially increased heart rate by 75% from baseline with late diastolic intracellular Ca2+ elevation (LDCAE) from the superior SAN. However, after a prolonged isoproterenol infusion, LDCAE disappeared in the superior SAN, the leading pacemaker shifted to the inferior SAN, and the rate reduced to 52% above baseline. Caffeine (2 ml, 20 mmol/l) injection after a prolonged isoproterenol infusion produced LDCAE in the SAN and accelerated the SAN rate, ruling out sarcoplasmic reticulum Ca2+ depletion as a cause of Ca2+ clock malfunction. We conclude that in an isolated canine RA preparation, chronically elevated sympathetic tone results in abnormal pacemaking hierarchy in the RA, including suppression of the superior SAN and enhanced pacemaking from ectopic sites. Combined malfunction of both membrane and Ca2+ clocks underlies the mechanisms of PSPs.
Keywords: sinoatrial node, calcium, sarcoplasmic reticulum, sympathetic nerve activity
spontaneous diastolic depolarization of sinoatrial node (SAN) cells periodically initiates action potentials to set the rhythm of the heart. The mechanism of spontaneous diastolic depolarization has been attributed to the rhythmic activation of the pacemaker current (If) and other membrane ionic currents (“membrane clock”) and the rhythmic spontaneous sarcoplasmic reticulum (SR) Ca2+ release and Na+/Ca2+ exchanger current (INCX) activation (“Ca2+ clock”) (10, 17). It has been proposed that the membrane clock acts as stabilizer of the pacemaker rate to prevent bradycardia (18, 20), whereas the Ca2+ clock is primarily responsible for heart rate acceleration during sympathetic stimulation (16). The SAN is a clinically important structure, as failure of SAN rhythm generation may lead to symptomatic bradycardia, known as sick sinus syndrome. While bradycardia is the mechanism of symptoms such as dizziness and presyncope, the onset of bradycardia in sick sinus syndrome is often immediately preceded by tachycardia. Therefore, sick sinus syndrome is often referred to as tachybradycardia syndrome (12). Patients with heart failure typically have abnormal sinus node function. Cessation of rapid pacing in these patients is followed by a prolonged sinus node recovery time (24). Dogs with pacing-induced heart failure may also develop typical spontaneous episodes of tachybradycardia (21). Because heart failure is known to have both chronically increased circulating catecholamines and downregulated If (6, 29), we hypothesize that If blockade in the presence of prolonged sympathetic stimulation could produce tachybradycardia in intact canine right atrial (RA) preparations. The purpose of the present study was to test this hypothesis.
MATERIALS AND METHODS
Langendorff-perfused canine RA preparation.
The study protocol was approved by the Institutional Animal Care and Use Committee of Indiana University School of Medicine and the Methodist Research Institute and conforms with guidelines of the American Heart Association. We studied isolated canine RAs in 21 mongrel dogs (22–28 kg). Hearts were rapidly excised under general anesthesia, and the right coronary artery was perfused with 37°C Tyrode solution equilibrated with 95% O2-5% CO2 to maintain a pH of 7.4. The composition of Tyrode solution was (in mmol/l) 125 NaCl, 4.5 KCl, 0.25 MgCl2, 24 NaHCO3, 1.8 NaH2PO4, 1.8 CaCl2, and 5.5 glucose. The coronary perfusion pressure was regulated between 50 and 60 mmHg. Both ventricles and the left atrium were removed, and all visible coronary artery branches were tied off. Contractility was inhibited by 10–17 μmol/l blebbistatin, and the motion artifact was negligible even after the isoproterenol infusion (16). The pseudo-ECG was recorded with widely spaced bipolar RA electrodes using ISO-DAM8A (World Precision Instruments). RA preparations were equilibrated for >1 h before baseline measurement (28).
Assessment of SAN function.
SAN recovery time (SNRT) was determined by bipolar pacing with a programmable stimulator (Bloom Associates, Reading, PA) for 30 s at progressively shorter pacing cycle lengths (400, 350, and 300 ms) with the two electrodes placed near the SAN region. The longest time interval from the last paced atrial depolarization to the first spontaneous sinus beat was recorded as the SNRT. The corrected SNRT (cSNRT) was determined by subtracting an average of three sinus cycle lengths before the commencement of atrial pacing from the SNRT.
Dual membrane potential and intracellular Ca2+ recordings.
Hearts were stained with rhod-2 AM and RH237 (Molecular Probes, Eugene, OR) and excited with laser light at 532 nm (16). Fluorescence was collected using two cameras (MiCAM Ultima, BrainVision, Tokyo, Japan) at 1 ms/frame and 100 × 100 pixels with a spatial resolution of 0.35 × 0.35 mm2/pixel for 2 s. The SAN area was identified by spontaneous diastolic depolarization and confirmed by histological experiments (trichrome) and by immunostaining of anti-K+/Na+ hyperpolarization-activated cyclic nucleotide-gated channel 4 (HCN4). After baseline spontaneous beats had been mapped, the pharmacological interventions were as follows: ZD-7288 (3 μmol/l) infusion (n = 3) (8, 16), ryanodine (3 μmol/l) infusion (n = 3), ZD-7288 plus ryanodine infusion (n = 4), low-dose isoproterenol (0.01 μmol/l) alone infusion for 140 min (n = 3), and low-dose isoproterenol (0.01 μmol/l) infusion for 1 h and followed by ZD-7288 (n = 8), including four RAs in which caffeine (20 mmol/l, 2 ml) was given as a bolus injection before and after the prolonged isoproterenol infusion.
After the combined ZD-7288 and ryanodine infusion, a marked sinus pause was consistently produced. Therefore, to record the first and last spontaneous beats between pauses, only membrane potential (Vm) was recorded at 10 ms/frame for 20 s. The fluorescence induced by the laser illumination was obtained through a common lens, separated with a dichroic mirror (650-nm cutoff wavelength), and directed to the respective camera with additional filtering [715-nm long-pass filter for Vm and 580 ± 20 nm for intracellular Ca2+ (Cai2+)].
Data analysis.
We defined tachybradycardia as the alternation of paroxysms of rapid (>100 beats/min) regular or irregular atrial tachyarrhythmias followed periods of prolonged sinus pauses (PSPs) that exceeded 3 s. Cai2+ and Vm traces were normalized to their respective peak-to-peak amplitude for a comparison of timing and morphology. The onset of late diastolic Cai2+ elevation (LDCAE) was defined by the time of the transition between negative to positive values in the dCai2+/dt curve. The slopes of LDCAE were measured from the onsets to peak levels of LDCAE. The “90% Cai2+ relaxation time” was measured from the maximum systolic Cai2+ to 90% reduction of Cai2+ (16). Data are expressed as means ± SE. Student's t-tests with Bonferroni's correction were used to compare the means of two numeric values. ANOVA with Fisher's least-significant-difference post hoc test was used to compare the means among three and four groups. Pearson's χ2-tests were used to compare two categorical variables. P values of ≤0.05 were considered significant.
RESULTS
Effects of membrane clock and Ca2+ clock inhibition in the absence of an isoproterenol infusion.
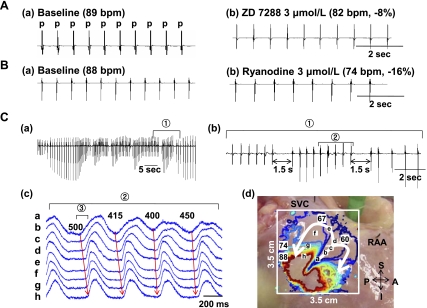
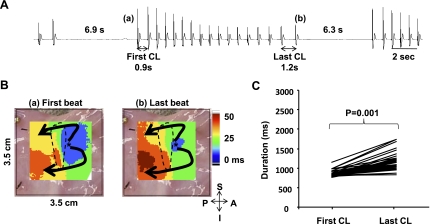
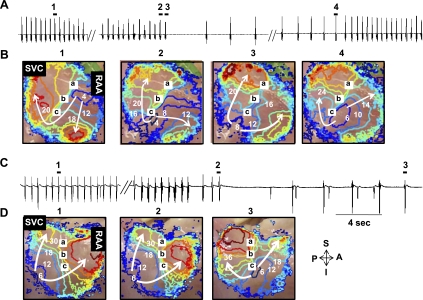
The baseline heart rate of the 21 RA preparations was 88 ± 10 beats/min (76–110 beats/min). Figure 1A shows that the infusion of ZD-7288 for 30 min deceased heart rate from 89 to 82 beats/min (−8%). The infusion of ZD-7288 (3 μmol/l) reduced heart rate by 8% to 80 ± 4 beats/min (n = 3). The infusion of ryanodine for 30 min, which suppresses the Ca2+ clock by the inhibition of cardiac ryanodine receptor 2 (RyR2), decreased heart rate from 89 to 74 beats/min (−16%; Fig. 1B). The ryanodine infusion decreased heart rate by 16% (73.9 ± 8.3 beats/min, n = 3; Fig. 1B). The ryanodine infusion in two of three RAs intermittently impaired the regularity of sinus rhythm, producing short and recurrent sinus pauses (Fig. 1B). These pauses were preceded by transient tachycardia caused by the acceleration of pacemakers in the inferior SAN (Fig. 1C). Neither ZD-7288 nor ryanodine produced sinus pauses that exceeded 3 s. However, simultaneous use of these two drugs produced PSPs. Fig. 2 shows the effect of a combined infusion of both ZD-7288 (3 μmol/l) and ryanodine (3 μmol/l) without isoproterenol infusion. Figure 2A shows sinus pauses of 6.9 and 6.3 s. In all 4 dogs treated with both ZD-7288 and ryanodine, 59 episodes of PSPs with a mean duration of 6.4 ± 1.9 s (range: 3–18 s) were produced. Figure 2B shows isochronal maps of the first (a) and last (b) spontaneous beats between the pauses. Both beats originated from the middle SAN. All 171 beats recorded between pauses in this RA originated from the middle SAN. Similarly, in all RAs mapped, the leading pacemakers during combined ZD-7288 and ryanodine treatment were located in the middle SAN and not from the inferior SAN or ectopic sites. The mean of the first cycle length after the pause was significantly shorter than that of the last cycle length (857.8 ± 71.7 vs. 1160.5 ± 212.4 ms, P = 0.001; Fig. 2C). Although a pause (>3 s) was successfully generated by blockade of both If and SR Ca2+ release, typical episodes of tachycardia (>100 beats/min) in the tachybradycardia syndrome were not observed. The mean cycle length between the pause was 804 ± 135 ms (range: 710–1,171 ms).
Fig. 1.
Effects of ZD-7288 (ZD; n = 3) or ryanodine alone (n = 3) on the sinoatrial node (SAN) in the absence of isoproterenol (Iso). Shown are pseudo-ECGs of the canine isolated right atrium (RA). A,a: Baseline. Regular atrial activations (p) were found on the pseudo-ECG. b, ZD-7288 (3 μmol/l) infusion for 30 min. B,a: baseline. b, Ryanodine (3 μmol/l) infusion for 30 min in the first dog reduced heart rate (HR) by 16%. C: ryanodine (3 μmol/l) infusion for 30 min in the second dog caused an irregular sinus rhythm. a, Intermittent sinus pause induced by ryanodine infusion. b, Extended view of period 1 in C,a. c, Membrane voltage (Vm) tracings recorded from the inferior (traces a and b), middle (trace c), and superior (trace d) SAN and RA (traces f–h) during period 2 in C,b. Numbers on Vm tracings show the cycle length (CL; in ms). d, Isochronal map of period 3 in C,c. The isochronal lines are shown in colors. The time of each isochrone is marked by a number (in ms), with the time of the first activation as time 0. Numbers on Vm isochronal maps are in milliseconds. SVC; superior vena cava, RAA; right atrial appendage, A, anterior; P, posterior; S, superior; I, inferior; bpm, beats/min.
Fig. 2.
Effects of a simultaneous infusion of ZD and ryanodine in the absence of Iso (n = 4). A: pseudo-ECG during simultaneous ZD and ryanodine infusion for 30 min showing 2 episodes of sinus pauses. B: isochronal maps of the first (a) and last (b) spontaneous beats originated from the middle SAN. The SAN is outlined with a dashed line. C: comparison of the first and last CLs between pauses. The mean CL of the first beat after the pause was significantly shorter than that of the last beat (P = 0.001).
Effect of a prolonged low-dose isoproterenol infusion on pacemaker hierarchy.
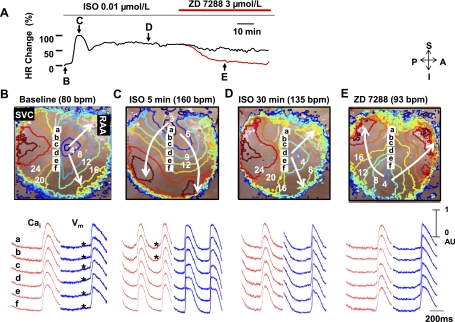
Figure 3A shows a typical episode of heart rate change by a prolonged low-dose isoproterenol infusion of 0.01 μmol/l followed by a ZD-7288 infusion of 3 μmol/l. Figure 3B shows the baseline rhythm generation. The heart rhythm was generated from the middle SAN (sites c and d in Fig. 3B), and diastolic depolarization was observed from SAN regions (asterisks in Fig. 3B). Figure 3C shows the response to the isoproterenol infusion during maximum heart rate. The leading pacemaker site was shifted to the superior SAN with LDCAE (asterisks in Fig. 3C, bottom). In a total of 11 intact canine RAs, low-dose (0.01 μmol/l) isoproterenol increased the mean maximum heart rate to 145 ± 21 beats/min (71%) from a baseline of 85 ± 12 beats/min (P = 0.004). However, during the prolonged isoproterenol infusion (>1 h), heart rate was reduced to 129 ± 19 beats/min (52% from baseline) with disappearance of LDCAE (site d in Fig. 3D). The reduced heart rate was maintained for 140 min (n = 3, 52% from baseline). The earliest activation site also changed to the inferior SAN (site e in Fig. 3D). The downward shift of the leading pacemaking site in the SAN was a consistent finding of all RAs studied. These findings indicate that a prolonged low-dose isoproterenol infusion selectively inhibits the superior SAN and changes the hierarchy of pacemaking sites in the SAN. The ZD-7288 (3 μmol/l) infusion at this time further decreased heart rate to 92 ± 13 beats/min (8% from baseline; site e in Fig. 3D) with more inferior shift of the leading pacemaker site (site f in Fig. 3E). The difference of heart rate between site d (129 ± 19 beats/min) and site e (92 ± 13 beats/min) in Fig. 3A was statistically significant (P = 0.003).
Fig. 3.
Effect of a prolonged low-dose Iso infusion (n = 8). A: HR trends after a prolonged (>1 h) isoproterenol infusion of 0.01 μmol/l. HR was increased to a maximum at 5 min, decreased after 10 min, and maintained for 140 min (black line, n = 3). ZD infusion (3 μmol/l) decrease HR close to the baseline level (red line, n = 8). B–E: Vm isochronal maps of the SAN and surrounding RA (top) and intracellular Ca2+ (Cai2+; red) and Vm (blue) tracings from the superior (sites a and b), middle (sites c and d), and lower (sites e and f) SAN. B: baseline. Note the diastolic depolarization (*) in the SAN. C: Iso infusion for 5 min. Note the late diastolic Cai2+ elevation (LDCAE; *) from the superior SAN. D: Iso infusion for 1 h. Note the disappearance of LDCAE with shifting of the leading pacemaker site to the inferior SAN (site e). E: ZD infusion for 30 min. The pacemaker site shifted to the more inferior SAN (site f). Numbers on the Vm isochronal maps are in milliseconds. AU, arbitrary units.
Prolonged isoproterenol and ZD-7288 infusion produced tachybradycardia.
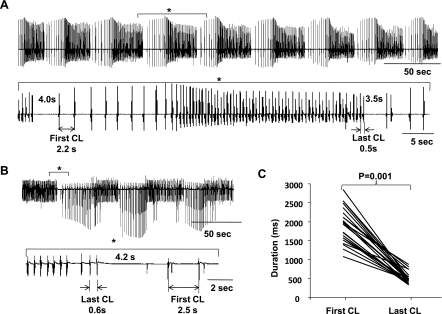
We were able to produce an in vitro model of tachybradycardia after prolonged (>1 h) exposure to low-dose (0.01 μmol/L) isoproterenol and subsequent blockade of If by ZD-7288 in five (63%) of eight RA preparations. Figure 4 shows examples of tachybradycardia recorded from two different canine RAs. Figure 4A shows eight episodes of tachybradycardia. The RA shown in Fig. 4B demonstrated three episodes of tachybradycardia. During 29 episodes of tachybradycardia, the mean of the first cycle length after the pause was significantly longer than that of the last cycle length (1,917.8 ± 457.5 vs. 505.4 ± 148.9 ms, P = 0.001; Fig. 4C). Acceleration of the heart rate leading to eventual tachycardia can be observed after the PSP in these episodes. Importantly, prolonged isoproterenol infusion was essential in all episodes to produce tachybradycardia. Tachybradycardia was not produced by ZD-7288 alone, without a previous prolonged isoproterenol infusion (n = 5). Note that tachycardia (>100 beats/min) appeared in the results shown in Fig. 4 but not in Fig. 2.
Fig. 4.
Tachybradycardia produced by prolonged isoproterenol and ZD infusion. Shown are pseudo-ECGs from the canine isolated RA. A: tachybradycardia from the first dog. Top, eight episodes of tachybradycardia; bottom, expanded view of the indicated section (*) showing 4.0- and 3.5-s pauses. B: tachybradycardia from a second RA. Top, three episodes of tachybradycardia; bottom, expanded view of the indicated section (*) showing a 4.2-s pause. C: comparison of the first and last CLs between pauses.
The origin of heart beat during tachybradycardia.
Figure 5, A and B, shows optical recordings of the tachybradycardia episode shown in Fig. 4A. During the beginning of tachycardia, the activation originated from the ectopic site of the anterosuperior RA (Fig. 5B). Before the termination of tachycardia, alternation of heart rhythm was caused by the activation from the lower SAN (beat 2 in Fig. 5B) and ectopic sites from the inferior RA (beat 3 in Fig. 5B). After the sinus pause, a spontaneous beat was generated from the inferior SAN without LDCAE (beat 4 in Fig. 5B). Optical recordings of the RA preparation shown in Fig. 4B are shown in Fig. 5, C and D. Tachycardia originated from the ectopic site from the inferoposterior RA (beat 1 in Fig. 5D). The ectopic site from the inferoposterior RA served as the leading pacemaker site until the termination of tachycardia (beat 2 in Fig. 5D). After a pause of the ectopic beat, the spontaneous beat was produced from the inferior SAN without LDCAE (beat 3 in Fig. 5D).
Fig. 5.
Comparison of activation sites during tachybradycardia. A: pseudo-ECG of one episode of tachybradycardia from Fig. 4A. B: isochronal maps of the beats marked in A. Regular tachycardia with a CL of 480 ms originated from the anterosuperior RA (beat 1). Irregular tachycardia with alternans was observed before the prolonged pause (beats 2 and 3). Beats 2 and 3 were generated from the inferior SAN and inferior RA, respectively. The slow rhythm after a sinus pause originated from the inferior SAN (beat 4). C: pseudo-ECG of one episode of tachybradycardia from Fig. 4B. D: isochronal maps of the beats marked in C. Regular tachycardia with a CL of 475 ms originated from the inferoposterior RA (beats 1 and 2). The slow rhythm after a sinus pause originated from the inferior SAN (beat 3). Numbers on the Vm isochronal maps are in milliseconds.
Contrary to that found at baseline (before isoproterenol), the leading pacemaking sites after the prolonged isoproterenol infusion were mostly located either at an ectopic site or in the inferior edge of the SAN. Cai2+ and Vm were simultaneously recorded in total 90 beats, including 72 fast beats and 18 slow beats during tachybradycardia. All 18 slow beats originated from the inferior SAN. Among the 72 fast beats, 68 beats (94%) originated from ectopic foci. The remaining four beats were initiated from either the inferior SAN (3 beats) or the superior SAN (1 beat).
Preserved SR Ca2+ content after a prolonged low-dose isoproterenol infusion.
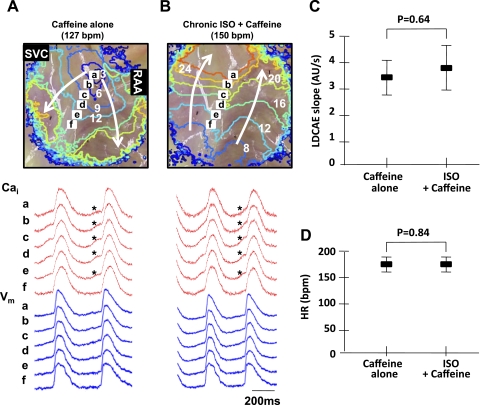
To evaluate the cause of impaired superior SAN function after a prolonged low-dose isoproterenol infusion, we injected 2 ml caffeine (20 mmol/l) for 1 s in four RAs. Caffeine produced LDCAE in the SAN without (asterisks in Fig. 6A) and with a prolonged low-dose isoproterenol infusion (asterisks in Fig. 6B). Before the prolonged isoproterenol infusion, the leading pacemaker site during caffeine infusion was at the superior SAN in all four RAs. However, after the prolonged isoproterenol infusion, it changed to ectopic foci in all four RAs (P = 0.03). The LDCAE slopes after the prolonged isoproterenol infusion (3.88 ± 0.88 arbitrary units/s) showed no significant differences compared with those before the isoproterenol infusion (3.60 ± 0.74 arbitrary units/s, P = 0.64; Fig. 6C). Heart rates after the prolonged isoproterenol infusion (172 ± 7 beats/min) showed no significant differences compared with those before the isoproterenol infusion (174 ± 8 beats/min, P = 0.84; Fig. 6D). This finding suggests that the SR Ca2+ content is preserved after a prolonged low-dose isoproterenol infusion.
Fig. 6.
Response to a bolus caffeine injection after a prolonged low-dose Iso infusion (n = 4). A: effect of a bolus caffeine (20 mmol/l) injection in the normal RA. Note the superior shift of the leading pacemaker site with robust LDCAE (*) with a HR of 127 beats/min. B: effect of a bolus caffeine injection after a prolonged low-dose Iso infusion. Note the robust LDCAE (*) from the superior SAN. The leading pacemaker site was the inferior RA with a HR of 150 beats/min. C: comparison of LDCAE slopes during caffeine alone and Iso plus caffeine infusion. D: comparison of HRs during caffeine alone and Iso plus caffeine infusion.
Comparison of SNRT, LDCAE slope, and Cai2+ relaxation time.
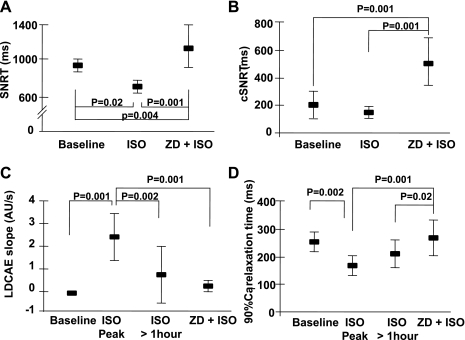
SNRT was 912 ± 212, 684 ± 45, and 1,144 ± 295 ms and cSNRT was 208 ± 110, 152 ± 38, and 502 ± 203 ms at baseline, after the 5-min isoproterenol infusion, and after the combined ZD-7288 and prolonged isoproterenol infusion, respectively (Fig. 7, A and B, respectively). Compared with baseline, isoproterenol initially reduced SNRT, but the combined use of prolonged isoproterenol infusion and ZD-7288 significantly increased both SNRT (Fig. 7A) and cSNRT (Fig. 7B) compared with baseline. Figure 7C shows that the LDCAE slope increased significantly after 5 min of the low-dose isoproterenol infusion, when the isoproterenol effects on heart rate reached their peak. The LDCAE slope then decreased toward baseline after the prolonged isoproterenol infusion and after the addition of ZD-7288 (Fig. 7C). Figure 7D shows the changes of 90% Cai2+ relaxation time. Initially, isoproterenol reduced the relaxation time. However, after the prolonged isoproterenol infusion and after the addition of ZD-7288, the relaxation time returned to the baseline level.
Fig. 7.
A and B: change of SAN recovery time (SNRT; A) and corrected SNRT (cSNRT; B) after a prolonged Iso infusion and ZD infusion. C and D: change of LDCAE slope (C) and 90% Cai2+ relaxation time (D) at the peak and 1 h after Iso and combined Iso and ZD infusion.
DISCUSSION
The primary finding of this study is that ZD-7288 infusion in the presence of a prolonged (>1 h) isoproterenol infusion results in combined malfunction of the Ca2+ clock and membrane voltage clock in the SAN but, in the meantime, facilitates the development of paroxysmal ectopic atrial tachycardia. Due to combined deficiency of the Ca2+ clock and membrane voltage clock of both the SAN and backup pacemakers, abrupt termination of the tachycardia results in PSP. Because the SAN continued to respond to caffeine at the end of the isoproterenol infusion, the Ca2+ clock malfunction was not due to SR Ca2+ depletion.
Mechanisms of tachybradycardia.
A prolonged (∼1 h) isoproterenol infusion simulates persistently elevated sympathetic tone, which is typical in patients with heart failure but can also occur in normal individuals. The persistently elevated sympathetic tone by itself does not induce tachybradycardia. However, If blockade in the presence of prolonged sympathetic stimulation could produce tachybradycardia. This finding highlights the importance of If in maintaining normal SAN function under conditions with persistently increased sympathetic tone, such as heart failure. Similar to the experimental condition, heart failure is associated with both chronically elevated sympathetic tone (2) (21) and a concomitant reduction of If (29). The chronically increased sympathetic tone is known to have profound effects on cardiac contractile function and arrhythmogenesis (7, 22). However, the effects of chronic catecholamine stimulation on the SAN remains poorly understood. Our study is the first to map Ca2+ clock function in the SAN during a prolonged isoproterenol infusion. The LDCAE slope and 90% Cai2+ relaxation time reached peak at 5 ± 2 min after the isoproterenol infusion and decreased after the prolonged infusion. A possible explanation of our finding is isoproterenol induced desensitization of the β-adrenergic receptor (6, 14). However, for this explanation to work, it will require a selective desensitization of the β-adrenergic receptor in the SAN but not the ectopic sites that generate recurrent ectopic atrial tachyarrhythmias during a prolonged isoproterenol infusion.
Isoproterenol induced phosphorylation of RyR2.
An alternative explanation is the accelerated opening and closing of the RyR2 after a prolonged isoproterenol infusion in the superior SAN, which has more robust Cai2+ cycling than the inferior SAN and nonpacemaking cells in the RA and ventricles (16, 27). Sympathetic stimulation can result in the phosphorylation of ICaL and phospholamban, leading to increased SR Ca2+ content (26). It can also phosphorylate RyR2, leading to increased opening probability. A combined increase of SR Ca2+ content and the opening probability of RyR2 is essential in producing arrhythmogenic Ca2+ waves (26). A recent study by Blayney et al. (4) investigated PKA phosphorylation with binding of FK506-binding proteins to RyRs, including both RyR1 and RyR2. The authors phosphorylated RyR1 and RyR2 using the active subunit of PKA over 1 h. The results showed that the percentage of phosphorylation reached its maximum in 1 h. When phosphorylated, RyR2 opens and closes more quickly. We also found that at least 1 h of isoproterenol infusion is needed to produce tachybradycardia in our preparation. This time course is consistent with the time needed for isoproterenol to activate PKA and maximally phosphorylate RyR2. The balance between the opening and closing of RyR2 determines how much Ca2+ is released from the SR to the cytosol. Consistent with the results of a previous study (15), we found no evidence of SR Ca2+ depletion in the SAN at the end of the experiment. This finding suggests that reduced SR Ca2+ reuptake is not primarily responsible for SAN dysfunction after a prolonged isoproterenol infusion. Therefore, it is likely that excessively rapid RyR2 closing in the superior SAN may have prevented sufficient spontaneous SR Ca2+ release after a prolonged isoproterenol infusion, leading to PSPs. Finally, at a given Em, the elevated concentration of Cai2+ slows the recovery of L-type Ca2+ current from inactivation (1). This latter factor may also have contributed to the development of SAN dysfunction.
Ectopic pacemakers.
The pacemaker hierarchy in the RA operates with different mechanisms. The Ca2+ clock in the superior SAN responds robustly to isoproterenol infusion (16). However, if RyR2 is inhibited by ryanodine, the superior SAN became quiescent during isoproterenol infusion, whereas the ectopic pacemaker sites in the crista terminalis continue to actively respond to isoproterenol and accelerate heart rate (25). We found that the ectopic pacemakers are highly active after a prolonged isoproterenol infusion, resulting in intermittent ectopic tachycardia while the superior SAN remains dormant. Therefore, heterogeneous and dynamic responses of the pacemaker hierarchy to chronically elevated sympathetic tone play important roles in the generation of tachybradycardia syndrome. We also found that even without isoproterenol infusion, the combined use of ryanodine and ZD-7288 also produced irregular heart rhythms. However, contrary to that found in tachybradycardia, the latter irregular rhythm originated from the middle of the SAN. The mechanism of these irregular rhythms is mostly likely due to If inhibition, which creates long pauses, allowing the SR to be overfilled with Ca2+. Although RyR2 is partially inhibited by ryanodine, the luminal Ca2+ of the superior SAN eventually exceeded the RyR2 releasing threshold, leading to sinus tachycardia. The gradual decline of intraluminal Ca2+ then causes a lengthening of sinus cycle length and transient termination of sinus rhythm. The cycle of long pauses and accelerated SAN rate repeats itself, causing irregular SAN rhythm.
Tachycardia-induced intracellular Na+ accumulation.
An additional possible contributing factor to the occurrence of tachybradycardia is the increased intracellular Na+ load (9, 13). The increased intracellular Na+ may increase the extrusion of Na+ from the cell by Na+-K+-ATPase to produce an increased background repolarizing current, which slows phase 4 diastolic depolarization (5), causing the tachycardia to transiently terminate. Increased intracellular Na+ may also suppress the forward mode of INCX, which prevents Cai2+ from depolarizing the cell membrane, leading to the termination of the tachycardia. The PSP then allowed sufficient Na+ to be extruded through Na+-K+-ATPase and allowed the development of another episode of tachycardia. This additional mechanism is not mutually exclusive with the RyR phosphorylation discussed above. However, tachycardia-induced excessive intracellular Na+ accumulation cannot explain the findings of irregular rhythm during combined treatment of ryanodine and ZD-7288.
PSPs occur only if both membrane and Ca2+ clocks are impaired.
PSPs were produced only after block of both SR Ca2+ release and If. Although Ca2+ clock block alone destroyed the regularity of heart rhythm, PSPs did not develop afterward. Clinically, pharmacological agents that suppress If reduce the heart rate but do not lead to PSPs (11). Similarly, patients with HCN4 mutation that reduces If develop benign familial bradycardia but not PSPs or syncope (19). Patients with RyR2 mutation typically develop bradycardia but not PSPs (3). These findings are consistent with studies (16–18) that have demonstrated interdependence and synergy between the Ca2+ clock and membrane clock in generating heart betas. A mapping study (25) has also shown that the inferior SAN works as the dominant pacemaker even after blockade of both clock mechanisms, suggesting that the different locations of the SAN may have different dependence of these clocks in generating automaticity.
Study limitations.
Because the ectopic foci were not included in the optical mapping field, the exact relationship between Cai2+ and Vm in the generation of ectopic beat cannot be evaluated, but If is likely to be responsible for the initiation of these ectopic beats. Because vagal innervation is absent in the isolated canine RA preparation, the effects of vagal activation on SAN rate control cannot be determined in the present study. The absence of vagal influence may have contributed to some of the findings of the present study.
Conclusions.
Our results from the isolated canine RA show that tachybradycardia occurs by prolonged (>1 h) exposure to β-adrenergic stimulation and a concomitant suppression of If. Maximal RyR2 phosphorylation and intracellular Na+ accumulation in the SAN are possible mechanisms that contribute to the development of tachybradycardia in patients with chronically elevated sympathetic tones.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grants P01-HL-78931, R01-HL-78932, and HL-71140, Faculty Research Grant 6-2009-0176, and Yonsei University College of Medicine CMB-YUHAN Research Grant 7-2009-0583 (to B. Joung), Chinese National Natural Science Foundation Grant 30870659 (to H. Zhang), Medtronic-Zipes Endowments (to P.-S. Chen), and by an American Heart Association Established Investigator Award (to S.-F. Lin).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Lei Lin and Erica Foster for assistance.
REFERENCES
- 1.Altamirano J, Bers DM. Effect of intracellular Ca2+ and action potential duration on L-type Ca2+ channel inactivation and recovery from inactivation in rabbit cardiac myocytes. Am J Physiol Heart Circ Physiol 293: H563–H573, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Anand IS, Fisher LD, Chiang YT, Latini R, Masson S, Maggioni AP, Glazer RD, Tognoni G, Cohn JN. Changes in brain natriuretic peptide and norepinephrine over time and mortality and morbidity in the Valsartan Heart Failure Trial (Val-HeFT). Circulation 107: 1278–1283, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Bhuiyan ZA, Van Den Berg MP, van Tintelen JP, Bink-Boelkens MT, Wiesfeld AC, Alders M, Postma AV, van LI, Mannens MM, Wilde AA. Expanding spectrum of human RYR2-related disease: new electrocardiographic, structural, and genetic features. Circulation 116: 1569–1576, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Blayney LM, Jones JL, Griffiths J, Lai FA. A mechanism of ryanodine receptor modulation by FKBP12/12.6, protein kinase A, and K201. Cardiovasc Res 85: 68–78, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Boyett MR, Fedida D. Changes in the electrical activity of dog cardiac Purkinje fibres at high heart rates. J Physiol 350: 361–391, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bristow MR, Ginsburg R, Minobe W, Cubicciotti RS, Sageman WS, Lurie K, Billingham ME, Harrison DC, Stinson EB. Decreased catecholamine sensitivity and β-adrenergic-receptor density in failing human hearts. N Engl J Med 307: 205–211, 1982 [DOI] [PubMed] [Google Scholar]
- 7.Bristow MR, Krause-Steinrauf H, Nuzzo R, Liang CS, Lindenfeld J, Lowes BD, Hattler B, Abraham WT, Olson L, Krueger S, Thaneemit-Chen S, Hare JM, Loeb HS, Domanski MJ, Eichhorn EJ, Zelis R, Lavori P. Effect of baseline or changes in adrenergic activity on clinical outcomes in the β-blocker evaluation of survival trial. Circulation 110: 1437–1442, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Bucchi A, Barbuti A, Baruscotti M, DiFrancesco D. Heart rate reduction via selective “funny” channel blockers. Curr Opin Pharmacol 7: 208–213, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Cohen CJ, Fozzard HA, Sheu SS. Increase in intracellular sodium ion activity during stimulation in mammalian cardiac muscle. Circ Res 50: 651–662, 1982 [DOI] [PubMed] [Google Scholar]
- 10.Difrancesco D. The role of the funny current in pacemaker activity. Circ Res 106: 434–446, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Fox K, Ford I, Steg PG, Tendera M, Robertson M, Ferrari R. Heart rate as a prognostic risk factor in patients with coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a subgroup analysis of a randomised controlled trial. Lancet 372: 817–821, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Gomes JA, Kang PS, Matheson M, Gough WB, Jr, El Sherif N. Coexistence of sick sinus rhythm and atrial flutter-fibrillation. Circulation 63: 80–86, 1981 [DOI] [PubMed] [Google Scholar]
- 13.Graziani AT, Vassalle M. Mechanisms underlying overdrive suppression and overdrive excitation in guinea pig sino-atrial node. J Biomed Sci 13: 703–720, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Hart E, Dawson E, Rasmussen P, George K, Secher NH, Whyte G, Shave R. β-Adrenergic receptor desensitization in man: insight into post-exercise attenuation of cardiac function. J Physiol 577: 717–725, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hussain M, Orchard CH. Sarcoplasmic reticulum Ca2+ content, L-type Ca2+ current and the Ca2+ transient in rat myocytes during β-adrenergic stimulation. J Physiol 505: 385–402, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joung B, Tang L, Maruyama M, Han S, Chen Z, Stucky M, Jones LR, Fishbein MC, Weiss JN, Chen PS, Lin SF. Intracellular calcium dynamics and acceleration of sinus rhythm by β-adrenergic stimulation. Circulation 119: 788–796, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lakatta EG, Maltsev VA, Vinogradova TM. A coupled SYSTEM of intracellular Ca2+ clocks and surface membrane voltage clocks controls the timekeeping mechanism of the heart's pacemaker. Circ Res 106: 659–673, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maltsev VA, Lakatta EG. A novel quantitative explanation for autonomic modulation of cardiac pacemaker cell automaticity via a dynamic system of sarcolemmal and intracellular proteins. Am J Physiol Heart Circ Physiol 298: H2010–H2023, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Milanesi R, Baruscotti M, Gnecchi-Ruscone T, DiFrancesco D. Familial sinus bradycardia associated with a mutation in the cardiac pacemaker channel. N Engl J Med 354: 151–157, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Noble D, Denyer JC, Brown HF, DiFrancesco D. Reciprocal role of the inward currents ib, Na and if in controlling and stabilizing pacemaker frequency of rabbit sino-atrial node cells. Proc Biol Sci 250: 199–207, 1992 [DOI] [PubMed] [Google Scholar]
- 21.Ogawa M, Zhou S, Tan AY, Song J, Gholmieh G, Fishbein MCLH, Siegel RJ, Karagueuzian HS, Chen LS, in SF, Chen PS. Left stellate ganglion and vagal nerve activity and cardiac arrhythmias in ambulatory dogs with pacing-induced congestive heart failure. J Am Coll Cardiol 50: 335–343, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Rubart M, Zipes DP. Mechanisms of sudden cardiac death. J Clin Invest 115: 2305–2315, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanders P, Berenfeld O, Hocini M, Jais P, Vaidyanathan R, Hsu LF, Garrigue S, Takahashi Y, Rotter M, Sacher F, Scavee C, Ploutz-Snyder R, Jalife J, Haissaguerre M. Spectral analysis identifies sites of high-frequency activity maintaining atrial fibrillation in humans. Circulation 112: 789–797, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Sanders P, Kistler PM, Morton JB, Spence SJ, Kalman JM. Remodeling of sinus node function in patients with congestive heart failure: reduction in sinus node reserve. Circulation 110: 897–903, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Shinohara T, Joung B, Kim D, Maruyama M, Luk HN, Chen PS, Lin SF. Induction of atrial ectopic beats with calcium release inhibition: local hierarchy of automaticity in the right atrium. Heart Rhythm 7: 110–116, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Venetucci LA, Trafford AW, Eisner DA. Increasing ryanodine receptor open probability alone does not produce arrhythmogenic calcium waves: threshold sarcoplasmic reticulum calcium content is required. Circ Res 100: 105–111, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Vinogradova TM, Lyashkov AE, Zhu W, Ruknudin AM, Sirenko S, Yang D, Deo S, Barlow M, Johnson S, Caffrey JL, Zhou YY, Xiao RP, Cheng H, Stern MD, Maltsev VA, Lakatta EG. High basal protein kinase A-dependent phosphorylation drives rhythmic internal Ca2+ store oscillations and spontaneous beating of cardiac pacemaker cells. Circ Res 98: 505–514, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Woods WT, Urthaler F, James TN. Spontaneous action potentials of cells in the canine sinus node. Circ Res 39: 76–82, 1976 [DOI] [PubMed] [Google Scholar]
- 29.Zicha S, Fernandez-Velasco M, Lonardo G, L'Heureux N, Nattel S. Sinus node dysfunction and hyperpolarization-activated (HCN) channel subunit remodeling in a canine heart failure model. Cardiovasc Res 66: 472–481, 2005 [DOI] [PubMed] [Google Scholar]