Abstract
Atherosclerosis is an inflammatory process leading to enhanced cellular proliferation, apoptosis, and vasa vasorum (VV) neovascularization. While both diabetes mellitus (DM) and hypercholesterolemia (HC) predispose to atherosclerosis, the precise interaction of these risk factors is unclear. Akt is a central node in signaling pathways important for inflammation, and we hypothesized that DM/HC would lead to aberrant Akt signaling and advanced, complex atherosclerosis. DM was induced in pigs by streptozotocin and HC by a high-fat diet. Animals were randomized to control (non-DM, non-HC), DM only, HC only, and DM/HC groups. Coronary artery homogenates were analyzed by immunoblotting for proteins involved in the Akt pathway, including phosphorylated (p)-Akt (Ser473), p-GSK-3β (Ser9), activated NF-κB p65, and VEGF. Immunohistochemical staining for Ki67 (cell proliferation), terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) (apoptosis), and von Willebrand factor (vWF) (neovascularization) was performed. Neovascularization was visualized with micro-computerized tomography (CT). Only DM/HC animals developed advanced atherosclerosis and showed decreased p-Akt (Ser473) and p-GSK-3β (Ser9) levels (P < 0.01 and P < 0.05, respectively). DM/HC arteries demonstrated increased cellular proliferation (P < 0.001), apoptosis (P < 0.01), and activation of NF-κB p65 (P < 0.05). Induction of DM/HC also resulted in significant VV neovascularization by enhanced VEGF expression (P < 0.05), increased vWF staining (P < 0.01), and increased density by micro-CT. In conclusion, DM and HC synergistically resulted in complex atherosclerosis associated with attenuated p-Akt (Ser473) levels. Aberrant Akt signaling correlated with increased inflammation, cellular proliferation, apoptosis, and VV neovascularization. Our results revealed a synergistic effect of DM and HC in triggering abnormal Akt signaling, resulting in advanced atherosclerosis.
Keywords: atherosclerosis, vasa vasorum neovascularization, vascular endothelial growth factor, glycogen synthetase kinase-3β, nuclear factor-κB, Akt
the combination of diabetes mellitus (DM) and hypercholesterolemia (HC) greatly increases the risk for the development and complications of coronary artery disease (CAD) (23, 48). At any given serum cholesterol level the mortality rates from CAD are three- to fivefold greater in diabetics than nondiabetics (51), and over 80% of diabetics will die of vascular related causes. However, the molecular mechanisms driving the pathogenic vascular processes are not fully elucidated, and there are limited data illustrating how DM and HC act in concert to affect disease development and progression.
The serine/threonine kinase Akt [or protein kinase B (PKB)] is a central signaling node involved in cell growth, proliferation, differentiation, apoptosis, angiogenesis, and inflammation (36, 49). A growing number of disease processes have been linked to aberrant loss or gain of Akt activation, including numerous cancers and type 2 DM (29, 35). Many of the cellular processes regulated by Akt also play a central role in atherosclerosis, and so Akt signaling dysfunction may affect atherosclerosis development and progression.
Akt activity is induced after activation of phosphatidylinositol 3-kinase (PI3K) by a variety of growth factors. For example, insulin binds to growth factor receptor tyrosine kinases (RTKs), which results in the insulin receptor substrate (IRS) family of adaptor molecules engaging and activating PI3K at the plasma membrane. PI3K phosphorylates phosphoinositides to produce the lipid second messenger phosphatidylinositol 3,4,5-trisphosphate (PIP3), which binds to Akt, recruiting the molecule to the plasma membrane. Akt is subsequently phosphorylated at the Thr308 position by 3-phosphoinositide-dependent kinase (PDK1) and at the Ser473 position by the mammalian target of rapamycin-rictor kinase complex (mTORc), resulting in the activated, phosphorylated form of Akt, p-Akt (10, 14, 19, 49, 50, 54).
When activated, Akt phosphorylates GSK-3β (p-GSK-3β), which in turn inactivates this kinase, leading to increased cell survival by blocking the function of proapoptotic proteins such as Bad and decreasing the transcription of death genes by phosphorylation of the forkhead transcription factors (10). By inactivating GSK-3β, p-Akt also prevents the degradation of the antiapoptotic substrate myeloid cell leukemia-1 (Mcl-1) (61). The effects of Akt-dependent phosphorylation of GSK-3β on cellular proliferation are not completely understood. Activated Akt has been shown to enhance the stability of proteins responsible for the G1-to-S phase cell cycle transition, such as cyclin D, cyclin E, c-jun, and c-myc (36). However, p-Akt has also been shown to block cell cycle progression by phosphorylating and inhibiting p21 (24, 38, 62). Akt plays a direct role in NF-κB activation and subsequent inflammation by enhancing the degradation of the NF-κB inhibitor IκB (28) and is involved in modulating the chemotaxic response of neutrophils and macrophages to inflammatory foci (30). Finally, Akt plays an important role in angiogenesis by causing increased production of hypoxia-inducible factor (HIF-1α and HIF-2α) transcription factors, leading to increased expression and secretion of VEGF (36). In summary, while activated Akt appears to play a major role in maintaining cellular homeostasis and is considered antiatherosclerotic, hypoactivation of Akt may help drive the development of atherosclerosis.
The role of the Akt signaling pathway and CAD has not been defined. Since patients with DM and HC have more complex CAD (41), we hypothesized that DM and HC synergistically effect Akt signaling and are associated with the development of complex atherosclerosis. We evaluated this association by comparing the Akt signaling pathway in DM/HC animals, which develop complex disease (20, 40), to Akt signaling in control, DM-only, and HC-only animals, which do not.
MATERIALS AND METHODS
Animals and experimental protocol.
All animal procedures conformed to U.S. Department of Agriculture regulations and requirements and were approved by the University of Pennsylvania Animal Care and Use Committee. Yorkshire domestic male swine weighing 20–25 kg (Archer Farms, Darlington, MD) were randomized into one of four groups: control (non-DM, non-HC, n = 9), DM only (n = 5), HC only (n = 5), and DM/HC (n = 10). An additional four DM/HC animals were used to evaluate the temporal effects of DM/HC on Akt signaling. DM was induced by the intravenous administration of 125 mg/kg of streptozotocin (Sicor Pharmaceuticals, Irvine, CA), while HC was induced by an atherogenic diet, which was continued until death (0.5% cholesterol, 10% lard, and 1.5% sodium cholate; Animal Specialties, Quakertown, PA) (20, 40, 57). Exogenous insulin was administered via a sliding scale to ensure that glucose levels did not exceed 500 mg/dl for prolonged periods of time. Insulin treatment was discontinued 1 wk before animal death.
Animals were euthanized with Euthasol ∼4 wk, 12 wk, or 24 wk after disease induction, and the coronary arteries were harvested under sterile conditions. After a thoracotomy, the heart was quickly removed and the coronary arteries were isolated. Saline pressure perfusion of the arteries was performed to remove any residual blood. The three coronary arteries (total: 87 arteries) were then sectioned in 5-mm pieces, labeled and immediately frozen at −80°C. A single 5-mm section from the proximal, middle, and distal artery was fixed in neutral buffered formalin (10%) for 16 h and embedded in paraffin for histological and immunohistochemical evaluation.
Antibodies.
The following primary antibodies were used for Western blot analysis: rabbit polyclonal antibody to Akt (1:1,000; Cell Signaling, Danvers, MA), rabbit monoclonal antibody to p-Akt (Ser473, 1:1,000; Cell Signaling), rabbit monoclonal antibody to GSK-3β (1:1,000; Cell Signaling), rabbit monoclonal antibody to p-GSK-3β (Ser9, 1:1,000; Cell Signaling), mouse monoclonal antibody to VEGF (1:1,000, Abcam, Cambridge, MA), rabbit polyclonal antibody to p-NF-κB, specific for the phosphorylated, active form of the p65 NF-κB monomer (Ser276, 1:1,000; Abcam), and horseradish peroxidase (HRP)-conjugated mouse monoclonal antibody to β-actin (1:5,000; Abcam). The following antibodies were used for immunohistochemical staining: rabbit polyclonal antibody to von Willebrand factor (vWF) (1:300; Abcam) and rabbit polyclonal antibody to Ki67 (1:200; Abcam).
Western blot analysis.
Coronary artery samples were washed twice with ice-cold phosphate-buffered saline (PBS), followed by lysis and homogenization in tissue lysis buffer [62.5 mM Tris·HCl, pH 6.8 at 25°C, 20% (wt/vol) SDS, 10% glycerol, 50 mM DTT, and protease cocktail]. Samples were then centrifuged for 5 min at 13,000 rpm. Protein concentration was determined by Bio-Rad Protein Assay (Bio-Rad, Hercules, CA) according to manufacturer's instructions. Protein samples (20 μg) were separated by SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes (Bio-Rad). The blots were incubated for 16 h at 4°C in Blotto blocking solution (PBS with 5% dry milk), followed by incubation with primary antibody for 3 h at room temperature. Blots were then washed three times for 15 min in PBS with 0.05% Tween 20 and incubated with secondary HRP-conjugated antibody for 1 h at room temperature. The blots were then washed three times for 15 min in PBS with 0.05% Tween 20 and incubated in enhanced chemiluminescence developing substrate (Amersham). The blots were exposed to Kodak RX film. The size of the detected protein was determined by comparison to high-molecular-mass standard markers (range 15–220 kDa; Amersham). For each analysis coronary arteries from at least four animals/group were used. All analyses were performed in duplicate.
Histology/immunohistochemistry.
Movat pentachrome staining was performed on paraffin-embedded 5-μm sections. For immunohistochemistry, after deparaffinizing and two 5-min washes in PBS, the sections were blocked for 20 min with a solution of 2% unconjugated goat anti-mouse IgG in PBS. Indicated dilutions of the primary antibodies were added to the slides and incubated for 2 h at room temperature, followed by two 5-min washes with PBS. Biotinylated secondary IgG diluted at 1:100 was added to the slides and incubated for 30 min. After two additional 5-min PBS washes, the tissue sections were treated with a 3% solution of H2O2 to quench endogenous peroxidase activity. After two additional 5-min washes, the slides were incubated for 30 min in solution containing the avidin-biotin-peroxidase complex. Diaminobenzidine/H2O2 substrate was allowed to react with the peroxidase-labeled tissue sections for 20 min. After two 1-min rinses in distilled water, the tissue sections were counterstained with hematoxylin and eosin, cleared, and dehydrated by successive gradations through 70%, 95%, and 100% ethanol, followed by a final passage in xylene. The slides were mounted and analyzed by bright field microscopy with a Leica DM4000 B microscope (Bannockburn, IL) and a Leica DC 480 camera.
Proliferating cells were detected by staining with an antibody to Ki67. Quantification of Ki67-positive staining was performed by morphometric analysis of slides at ×400 magnification by two technicians in a blinded manner. The area of the tissue that exhibited immunostaining was quantified within the arterial medial and intimal layers, and the percentage of Ki67-positive cells was determined with Image-Pro Plus (MediaCybernetics) software.
Apoptosis was evaluated with the terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) TACS Blue Label kit (R&D Systems, Minneapolis, MN). Briefly, 5-μm sequential sections were deparaffinized as described for the immunohistochemical analysis and treated with proteinase K. After quenching of endogenous peroxidase activity with H2O2, the slides were washed with PBS. TUNEL reaction mixture was then added to tissue sections, followed by incubation in a humidified chamber for 60 min at 37°C. The slides were then rinsed in PBS, color developed, and counterstained with nuclear fast red. Analysis of the slides was performed under a bright field microscope with Image-Pro Plus for quantification.
Quantification of vasa vasorum (VV) area and density was made on arterial segments (n = 6/artery) stained with vWF antibody. Computerized analysis with Image-Pro Plus under ×50 magnification was performed by an investigator in a blinded manner, and results were independently verified by a second investigator. The area bordered by the external elastic lamina and the outer adventitia, excluding muscle, adipose tissue, and fibrous tissue, was analyzed with a technique described by Edelman et al. (13). The concentration (total number of vasa/mm2 of vessel wall) and area density (area of vasa/mm2 of vessel wall) of VV for each section were then calculated.
Micro-computerized tomography evaluation of VV neovascularization.
To analyze the development of the VV, three arteries from each group were imaged with micro-computerized tomography (CT). After removal of the heart, glass cannulas were tied at the coronary orifices and injected with 500 ml of heparinized saline (0.9% sodium chloride + 5,000 IU unfractionated heparin) for 30 min at physiological pressure to clear the vascular system. A low-viscosity, radiopaque liquid polymer compound (MV-122; Canton Biomedical Products, Boulder, CO) was injected through the cannulas until the injected mass flowed freely from the arterial vent. The heart was then immersed in 10% buffered formalin and refrigerated at 4°C overnight to allow compound polymerization. On the following day, arterial segments 60–80 mm in length were removed and placed in a 95% alcohol solution for 48 h. At successive 24-h intervals, the glycerin concentration was raised from 30%, 50%, 75%, and finally 100% to completely dehydrate the segments. The specimen was rinsed with acetone, left in the open air for 24 h, and embedded in paraffin molds for three-dimensional micro-CT imaging. All samples underwent micro-CT imaging at 24-μm isotropic resolution with an Explore Locus SP specimen scanner (GE Healthcare).
Ex vivo treatment of coronary arteries with insulin.
Coronary arteries from each of the four groups were incubated immediately after collection in DMEM containing 1 mM insulin for 5 min. The arteries were then processed for Western blot analysis with p-Akt (Ser473) and Akt antibodies.
Statistical analysis.
Numerical data are expressed as means ± SD, unless otherwise noted. Comparisons of multiple groups were made by analysis of variance (ANOVA), and if significant the Scheffé method was performed to evaluate intergroup differences. SPSS version 12 was used for statistical analysis. A P value < 0.05 was considered significant.
RESULTS
In total 33 animals were used in this study, of which 29 were killed at ∼24 wk after induction, 2 DM/HC animals at 1 mo after induction, and 2 DM/HC animals at 3 mo. At baseline, serum glucose levels averaged 65.0 ± 5.4 mg/dl and cholesterol levels 91.0 ± 5.2 mg/dl. At death, blood glucose levels were significantly higher in the DM (441.3 ± 33.3 mg/dl) and DM/HC (415.0 ± 50.0 mg/dl) groups compared with control (64.7 ± 5.4 mg/dl) and HC (85.2 ± 30.2 mg/dl) groups (P < 0.001). Both control and HC animals rapidly gained weight during the course of the experiment (77.8 ± 4.5 kg and 85.2 ± 30.2 kg, respectively, at death), while DM and DM/HC animals had significantly reduced body weight gain (31.5 ± 1.6 kg and 44.1 ± 1.7 kg, respectively; P < 0.01). Despite identical diets and feeding protocols, serum cholesterol levels were increased in the DM/HC group (619 ± 100 mg/dl) compared with the HC group (326 ± 54 mg/dl) (P < 0.01), indicating an effect of DM on subsequent serum cholesterol levels.
Histology of coronary lesions.
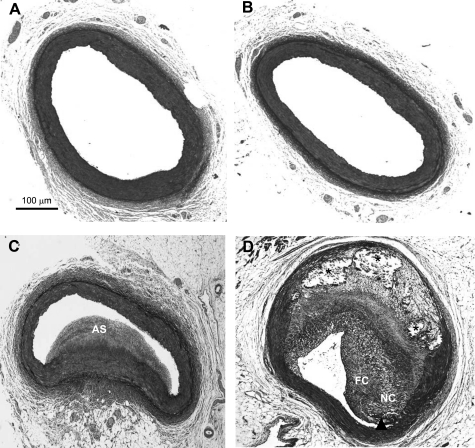
Control and DM animals did not develop atherosclerotic lesions (Fig. 1, A and B). HC pigs (Fig. 1C) exhibited moderate coronary atherosclerosis, which was relatively homogeneous in appearance and characterized as pathological intimal thickening (55). Sections collected from DM/HC pigs exhibited extensive complex atherosclerosis demonstrating a range of high-risk lesions including fibroatheromas, thin-fibrous cap atheromas, and fibrocalcific lesions (Fig. 1D). This observation is consistent with previously published data by our group (40, 57) and demonstrates a synergistic effect of DM and HC in the development of complex coronary atherosclerosis. By morphometry the percent stenosis for HC animals averaged 27.5 ± 23.9%, intimal area 0.79 ± 1.41 mm2, and intimal-to-medial ratio 0.67 ± 1.54. For DM/HC animals the percent stenosis was similar at 27.7 ± 27.8%, intimal area 0.74 ± 1.45 mm2, and intimal-to-medial ratio 0.78 ± 1.20. The extent of disease is similar in both HC and DM/HC animals.
Fig. 1.
Induction of both diabetes mellitus (DM) and hypercholesterolemia (HC) results in advanced atherosclerosis. Representative histological samples: control (A), DM only (B), HC only (C), and DM/HC (D). While HC only produces atherosclerosis (AS), only the DM/HC group developed complex lesions as evidenced by a large necrotic core (NC), calcifications (asterisks), and a thin fibrous cap (arrowhead). FC, fibrous cap. Movat pentachrome staining.
Effect of DM/HC on Akt pathway.
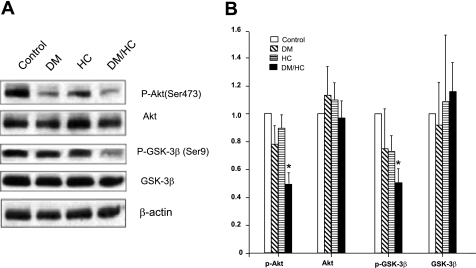
Previous studies using mouse models have shown that Akt exerts vascular protection against atherosclerosis (16), so we hypothesized that the complex atherosclerosis observed in the DM/HC group would be associated with aberrant Akt signaling. Indeed, induction of DM and HC led to significantly decreased p-Akt (Ser473) levels (P < 0.01), whereas DM or HC alone did not (Fig. 2). This suggests that DM and HC may act synergistically to modulate the Akt pathway.
Fig. 2.
DM/HC pigs exhibit aberrant Akt signaling including hypoactivation of Akt and activation of GSK-3β. A: Western blot analyses of whole coronary artery lysates from animals randomized into 4 experimental groups for 24 wk with antibodies against phosphorylated (p)-Akt (Ser473), Akt, p-GSK-3β (Ser9), and GSK-3β. B: densitometry results are expressed as a fold change compared with the control group, with β-actin as the internal loading control. *P < 0.01 for p-Akt, P < 0.05 for p-GSK-3β for DM/HC group.
Phosphorylation of Akt results in subsequent phosphorylation of GSK-3β at the Ser9 site, thereby inactivating the enzyme. GSK-3β regulates important cellular processes involved in atherosclerosis such as cellular survival and cellular proliferation (36), so we investigated the effect of DM/HC on GSK-3β kinase phosphorylation. Induction of DM and HC acted synergistically to decrease the phosphorylation of GSK-3β (Ser9) (P < 0.05, Fig. 2), thus leading to activation of the enzyme. Control, DM, and HC groups did not display this aberrant signaling (Fig. 2). Hence, the combination of DM and HC was associated with enhanced activation of GSK-3β, thus implicating a potential role of aberrant downstream Akt signaling in the development of advanced atherosclerosis.
Effects of hypophosphorylated Akt on markers of inflammation, including cellular proliferation, apoptosis, and activation of NF-κB.
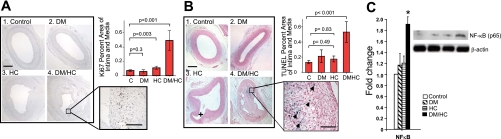
DM/HC arteries demonstrated a highly significant increase in cellular proliferation as demonstrated by Ki67 staining (P < 0.001) compared with other experimental groups (Fig. 3A). Hypophosphorylation of Akt was also associated with increased levels of cellular apoptosis as shown by the results of TUNEL staining (P < 0.01, Fig. 3B). Interestingly, apoptotic cells were generally located either within the fibrous cap or within the necrotic core (Fig. 3B), suggesting increased plaque vulnerability. Finally, we investigated the synergistic effect of DM and HC on the activation of the proinflammatory and prosurvival transcription factor NF-κB, which is activated in atherogenesis (11, 58). The results showed that while DM or HC alone had no effect on NF-κB p65 activation, the combination of DM and HC resulted in a significant increase in enzyme activation (P < 0.05, Fig. 3C).
Fig. 3.
Aberrant Akt signaling in DM/HC animals is associated with increased markers of inflammation, including proliferation, apoptosis, and increased activation of NF-κB p65. A: increased proliferation rate in the DM/HC group. C, control. B: increased cellular apoptotic rate by terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) staining in the DM/HC group. +, side branch. Blue staining shows TUNEL-positive cells. Arrowheads show TUNEL and examples. C: increased NF-κB p65 (Ser276) activation in the DM/HC group (*P < 0.05). Bar indicates 100 μm at low magnification and 25 μm at high magnification.
Effect of DM/HC on vasa vasorum neovascularization.
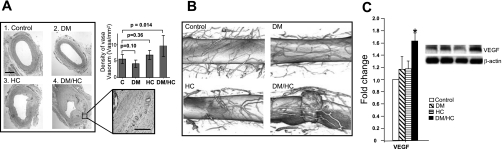
Enhanced VV neovascularization has been implicated in the development of atherosclerosis by providing inflammatory cells access to sites of inflammation (22, 31), and insofar as Akt signaling pathway plays a key role in regulating angiogenesis, we determined the effect of DM/HC on plaque neovascularization. DM/HC animals demonstrated a significantly increased density of VV by vWF staining (P < 0.01) compared with other groups (Fig. 4A), a finding confirmed by direct visualization of the VV by micro-CT (Fig. 4B). Enhanced VV neovascularization was associated with increased expression of VEGF in the DM/HC group (P < 0.05, Fig. 4C).
Fig. 4.
Aberrant Akt signaling in DM/HC animals is associated with increased angiogenesis. A: increased density of vasa vasorum (VV) in the DM/HC group. Bar indicates 100 μm. B: micro-computerized tomography (CT) images showing VV neovascularization. Data have been volume rendered in 3 dimensions to visualize the spatial distribution of the VV network. The DM/HC image shows an intramural hemorrhage with a corresponding high local density of VV. C: Western blot analysis and densitometry showing increased VEGF expression in the DM/HC group (*P < 0.05).
p-Akt and p-GSK-3β levels early after DM/HC induction.
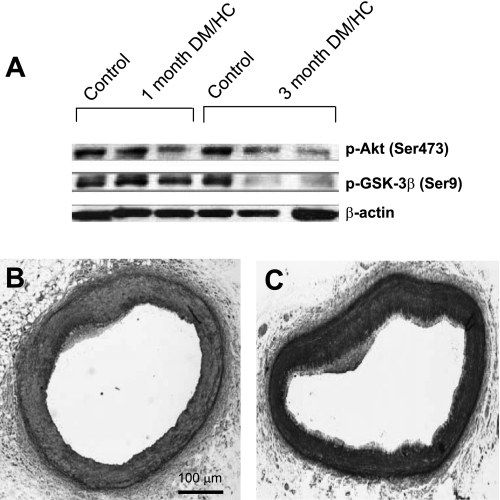
To assess the temporal effect of DM/HC on Akt and GSK-3β phosphorylation we determined their levels 1 and 3 mo after DM/HC induction. The results demonstrated a mild effect on Akt hypoactivation at 1 mo and a greater effect at 3 mo (Fig. 5).
Fig. 5.
Hypoactivation of Akt occurs soon after DM/HC induction. A: Western blot analyses of whole coronary artery lysates obtained from control or DM/HC animals 1 or 3 mo after induction. Decreased p-Akt is observed in 1 of 2 animals at 1 mo and both animals at 3 mo. B and C: representative histological samples from a coronary artery 1 mo (B) and 3 mo (C) after the induction.
Effect of ex vivo insulin treatment of coronary arteries on Akt signaling.
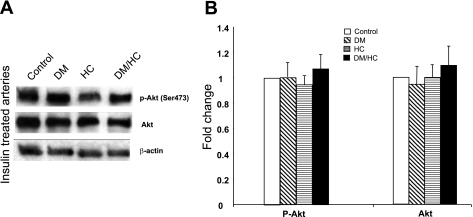
We tested the hypothesis that insulin therapy may normalize aberrant Akt signaling associated with the induction of DM and HC by treating ex vivo arteries with insulin and analyzing Akt phosphorylation. The results demonstrate that p-Akt (Ser473) levels were restored to normal levels in DM/HC arteries treated with insulin (Fig. 6).
Fig. 6.
Ex vivo insulin treatment of coronary arteries normalizes Akt signaling in the DM/HC group. A: Western blot analyses. B: densitometry results.
DISCUSSION
Patients with both DM and HC have an increased risk of developing CAD, but more importantly, an increased risk of developing complications of CAD, i.e., death, myocardial infarction, and stroke. Increased oxidative stress, inflammation, and transvascular LDL transport appear to play critical roles in diabetic cardiovascular disease, resulting in larger necrotic cores and a greater influx of macrophages and T lymphocytes (5, 41, 46). In the DM/HC pig model, complex atherosclerosis was associated with hypoactivation of the Akt signaling pathway. This abnormal signaling was associated with reduced phosphorylation of GSK-3β (disinhibiting GSK-3β), increased activation of NF-κB p65, and increased expression of VEGF. As a result, DM/HC animals exhibited increased cellular proliferation, apoptosis, inflammation, and VV neovascularization. The effects of DM/HC on reducing Akt activation were noted early after DM/HC induction and could be rapidly reversed by the administration of exogenous insulin. In addition to shedding light on the pathophysiology of DM/HC-induced atherosclerosis, these results suggest that the importance of maintaining physiological insulin levels extends beyond glycemic control.
Our results demonstrate that decreased activation of Akt plays a role in the development of severe atherosclerosis. Hypophosphorylation of Akt inactivates the enzyme, thereby effecting important downstream processes regulating atherosclerosis and inflammation. In the double-knockout (ApoE−/−/Akt1−/−) mouse models, loss of Akt leads to severe atherosclerosis and occlusive CAD, as activated Akt is thought to exert vascular protection against atherosclerosis (16). In addition, macrophages from the double-knockout mice are more susceptible to oxidized LDL-induced apoptosis than control cells, indicating that the Akt pathway is important for macrophage survival and macrophage-dependent vascular inflammation (16). The finding in endotoxemic mice that reduced Akt activity resulted in enhanced inflammation (6, 33) is of particular importance given the role of oxidized LDL in inducing the initial inflammatory vascular response that progresses to atherosclerosis (56). Furthermore, the neointima of stented arteries from diabetic rats (both type 1 and type 2) exhibit attenuated p-Akt levels, which in turn correlate with increased neointimal area (27). While data from rodent models have linked attenuated p-Akt levels with the development of atherosclerosis, the present studies show that hypoactivated Akt was associated with complex, advanced CAD, the phenotype thought responsible for acute ischemic cardiac death and acute coronary syndromes. We conclude that the loss of Akt activation results in loss of vascular protection, contributing to increased levels of vascular inflammation and disease progression.
Interestingly, the combination of both risk factors was required to cause significant aberrant Akt signaling, demonstrating a synergistic effect of DM and HC on Akt phosphorylation and the development of complex atherosclerosis. The mechanism by which type 1 DM causes hypophosphorylated Akt has been well studied, as insulin is one growth factor that can activate PI3K by binding to RTKs, leading to the formation of PIP3 and the subsequent phosphorylation of Akt by PDK1 and mTORc (1, 10, 14, 19, 49, 50, 54). However, the mechanism by which hypercholesterolemia leads to hypophosphorylated Akt has only recently been recognized. In studies of endothelial progenitor and bone marrow stem cells, oxidized LDL impacted cellular function primarily by impairing the phosphorylation of Akt at the Ser473 site (8–9, 34). We hypothesize that DM and HC affect Akt phosphorylation by independent mechanisms such that the combination of both risk factors synergistically impacts enzyme signaling.
Downstream aberrant Akt signaling was observed in DM/HC animals by investigating the activity of GSK-3β and NF-κB. Phosphorylation of Akt at Ser473 inhibits GSK-3β by maintaining the enzyme in its inactive phosphorylated state. Hypophosphorylated Akt thus led to disinhibition of GSK-3β (Fig. 2). In regard to apoptosis, activated GSK-3β leads to the phosphorylation and degradation of the antiapoptotic BCL-2 family member Mcl-1, leading to increased apoptosis (37). Furthermore, previous work in mice has shown that the absence of Akt induces increased vascular smooth muscle cell apoptosis (17). In DM/HC pigs, disinhibition of GSK-3β by hypophosphorylated Akt was associated with increased cellular apoptosis consistent with these findings (Fig. 3B). GSK-3β may also have direct effects on atherosclerosis development, as previous studies have demonstrated that GSK-3β participates in activation of NF-κB, an important mediator of cell proliferation and inflammation (25, 45, 53, 59), results confirmed in the present study (Fig. 3, A and C). In hyperglycemic ApoE-deficient mice treatment with an inhibitor of GSK-3β, valproic acid, had antiatherogenic effects (4), providing further evidence that GSK-3β activation may be playing a direct role in the development of advanced atherosclerosis.
The DM/HC state also affected the major proangiogenic signaling mediator VEGF, leading to increased neovascularization within lesions. Multiple studies have demonstrated the importance of VV neovascularization in the development of atherosclerosis (2, 18, 22, 31, 32, 42, 44), and VEGF-induced neovascularization has been postulated to be a therapeutic target to reduce atherosclerosis (21, 43). An interesting finding from our experiments is that VEGF-induced neovascularization was present despite hypoactivation of Akt. Previous studies primarily performed on cell culture models have shown that Akt activation is both necessary and sufficient to enhance VEGF expression and angiogenesis (12, 26, 39) and that inhibition of Akt decreases VEGF expression (7, 15, 52). However, in our model, VEGF expression and VV neovascularization were induced in the setting of hypophosphorylated Akt. There are potential explanations for this apparent uncoupling of Akt and VEGF signaling. Akt signaling is complicated by multiple negative feedback loops and redundant pathways, and other AGC kinase family members have been shown to activate pathways previously thought to be unique Akt targets (for review see Ref. 35). For example, S6 kinase 1 (S6K1) can phosphorylate GSK-3 on its Akt site under conditions of elevated mTORc1-S6K1 signaling, triggering a negative feedback loop that blocks activation of Akt (36, 60). It is feasible that the induction of DM and HC may activate an alternative AGC kinase that drives the expression of VEGF and a negative feedback mechanism leading to hypophosphorylated Akt. Another possibility is that an Akt-independent mechanism for angiogenesis exists in the setting of DM and HC, as oxidized LDL can directly induce VEGF expression and angiogenesis (3, 47). Further studies are needed to clarify this finding.
There are some potential limitations to our study. The signaling pathways under Akt control are numerous and redundant. While we investigated those pathways we deemed important for the development of complex atherosclerosis, the evaluated pathways are only a selection of Akt-dependent pathways. Nonetheless, the results establish that the Akt signaling pathway plays an important role in the development of complex atherosclerosis in a model that more closely resembles the human condition. We analyzed arterial samples and so did not identify the exact cell type(s) responsible for the observed phenomenon. This may be of importance both in understanding cell type-specific events and cell-to-cell interactions and in designing novel treatments. However, these potential limitations do not deter us from the observation that the intravascular Akt signaling pathway may be a vital mechanistic cause of complex atherosclerosis.
In conclusion, our results show that the combination of DM and HC results in the development of complex coronary atherosclerosis in association with hypoactivation of the Akt signaling pathway. DM/HC animals displayed increased inflammation, cellular proliferation, apoptosis, and VV neovascularization associated with aberrant Akt signaling. The observation that the addition of physiological concentrations of insulin to arterial sections restored Akt phosphorylation suggests that maintaining physiological levels of insulin in type 1 diabetic patients may be important in preventing atherosclerosis by normalizing Akt signaling independent of its effect on blood glucose levels. Indeed, strategies designed to increase Akt phosphorylation early may prove helpful in the primary prevention of CAD in patients with diabetes.
GRANTS
M. J. Birnbaum's participation was funded in part by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-56886.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We gratefully acknowledge the assistance of Harrilla Profka in the care of the animals and the performance of the procedures.
REFERENCES
- 1.Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J 15: 6541–6551, 1996 [PMC free article] [PubMed] [Google Scholar]
- 2.Barger AC, Beeuwkes R, Lainey LL, 3rd, Silverman KJ. Hypothesis: vasa vasorum and neovascularization of human coronary arteries. A possible role in the pathophysiology of atherosclerosis. N Engl J Med 310: 175–177, 1984 [DOI] [PubMed] [Google Scholar]
- 3.Bochkov VN, Philippova M, Oskolkova O, Kadl A, Furnkranz A, Karabeg E, Afonyushkin T, Gruber F, Breuss J, Minchenko A, Mechtcheriakova D, Hohensinner P, Rychli K, Wojta J, Resink T, Erne P, Binder BR, Leitinger N. Oxidized phospholipids stimulate angiogenesis via autocrine mechanisms, implicating a novel role for lipid oxidation in the evolution of atherosclerotic lesions. Circ Res 99: 900–908, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Bowes AJ, Khan MI, Shi Y, Robertson L, Werstuck GH. Valproate attenuates accelerated atherosclerosis in hyperglycemic apoE-deficient mice: evidence in support of a role for endoplasmic reticulum stress and glycogen synthase kinase-3 in lesion development and hepatic steatosis. Am J Pathol 174: 330–342, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burke AP, Kolodgie FD, Zieske A, Fowler DR, Weber DK, Varghese PJ, Farb A, Virmani R. Morphologic findings of coronary atherosclerotic plaques in diabetics: a postmortem study. Arterioscler Thromb Vasc Biol 24: 1266–1271, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Calippe B, Douin-Echinard V, Laffargue M, Laurell H, Rana-Poussine V, Pipy B, Guery JC, Bayard F, Arnal JF, Gourdy P. Chronic estradiol administration in vivo promotes the proinflammatory response of macrophages to TLR4 activation: involvement of the phosphatidylinositol 3-kinase pathway. J Immunol 180: 7980–7988, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Cao Z, Fang J, Xia C, Shi X, Jiang BH. Trans-3,4,5′-trihydroxystibene inhibits hypoxia-inducible factor 1alpha and vascular endothelial growth factor expression in human ovarian cancer cells. Clin Cancer Res 10: 5253–5263, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Chavakis E, Dernbach E, Hermann C, Mondorf UF, Zeiher AM, Dimmeler S. Oxidized LDL inhibits vascular endothelial growth factor-induced endothelial cell migration by an inhibitory effect on the Akt/endothelial nitric oxide synthase pathway. Circulation 103: 2102–2107, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Chu L, Hao H, Luo M, Huang Y, Chen Z, Lu T, Zhao X, Verfaillie CM, Zweier JL, Liu Z. Ox-LDL modifies the behavior of bone marrow stem cells and impairs their endothelial differentiation via inhibition of Akt phosphorylation. J Cell Mol Med (October23, 2009). doi:10.1111/j.1582-4934.2009.00948.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev 13: 2905–2927, 1999 [DOI] [PubMed] [Google Scholar]
- 11.de Winther MP, Kanters E, Kraal G, Hofker MH. Nuclear factor kappaB signaling in atherogenesis. Arterioscler Thromb Vasc Biol 25: 904–914, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Dong G, Chen Z, Li ZY, Yeh NT, Bancroft CC, Van Waes C. Hepatocyte growth factor/scatter factor-induced activation of MEK and PI3K signal pathways contributes to expression of proangiogenic cytokines interleukin-8 and vascular endothelial growth factor in head and neck squamous cell carcinoma. Cancer Res 61: 5911–5918, 2001 [PubMed] [Google Scholar]
- 13.Edelman ER, Nugent MA, Smith LT, Karnovsky MJ. Basic fibroblast growth factor enhances the coupling of intimal hyperplasia and proliferation of vasa vasorum in injured rat arteries. J Clin Invest 89: 465–473, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet 7: 606–619, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Fang J, Xia C, Cao Z, Zheng JZ, Reed E, Jiang BH. Apigenin inhibits VEGF and HIF-1 expression via PI3K/AKT/p70S6K1 and HDM2/p53 pathways. FASEB J 19: 342–353, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Fernandez-Hernando C, Ackah E, Yu J, Suarez Y, Murata T, Iwakiri Y, Prendergast J, Miao RQ, Birnbaum MJ, Sessa WC. Loss of Akt1 leads to severe atherosclerosis and occlusive coronary artery disease. Cell Metab 6: 446–457, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernandez-Hernando C, Jozsef L, Jenkins D, Di Lorenzo A, Sessa WC. Absence of Akt1 reduces vascular smooth muscle cell migration and survival and induces features of plaque vulnerability and cardiac dysfunction during atherosclerosis. Arterioscler Thromb Vasc Biol 29: 2033–2040, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fleiner M, Kummer M, Mirlacher M, Sauter G, Cathomas G, Krapf R, Biedermann BC. Arterial neovascularization and inflammation in vulnerable patients: early and late signs of symptomatic atherosclerosis. Circulation 110: 2843–2850, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Franke TF. PI3K/Akt: getting it right matters. Oncogene 27: 6473–6488, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Gerrity RG, Natarajan R, Nadler JL, Kimsey T. Diabetes-induced accelerated atherosclerosis in swine. Diabetes 50: 1654–1665, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Gossl M, Herrmann J, Tang H, Versari D, Galili O, Mannheim D, Rajkumar SV, Lerman LO, Lerman A. Prevention of vasa vasorum neovascularization attenuates early neointima formation in experimental hypercholesterolemia. Basic Res Cardiol 104: 695–706, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gossl M, Versari D, Mannheim D, Ritman EL, Lerman LO, Lerman A. Increased spatial vasa vasorum density in the proximal LAD in hypercholesterolemia—implications for vulnerable plaque-development. Atherosclerosis 192: 246–252, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol 6: 508–519, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Heron-Milhavet L, Franckhauser C, Rana V, Berthenet C, Fisher D, Hemmings BA, Fernandez A, Lamb NJ. Only Akt1 is required for proliferation, while Akt2 promotes cell cycle exit through p21 binding. Mol Cell Biol 26: 8267–8280, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoeflich KP, Luo J, Rubie EA, Tsao MS, Jin O, Woodgett JR. Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature 406: 86–90, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Jiang BH, Zheng JZ, Aoki M, Vogt PK. Phosphatidylinositol 3-kinase signaling mediates angiogenesis and expression of vascular endothelial growth factor in endothelial cells. Proc Natl Acad Sci USA 97: 1749–1753, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jonas M, Edelman ER, Groothuis A, Baker AB, Seifert P, Rogers C. Vascular neointimal formation and signaling pathway activation in response to stent injury in insulin-resistant and diabetic animals. Circ Res 97: 725–733, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Kane LP, Shapiro VS, Stokoe D, Weiss A. Induction of NF-kappaB by the Akt/PKB kinase. Curr Biol 9: 601–604, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Kim D, Chung J. Akt: versatile mediator of cell survival and beyond. J Biochem Mol Biol 35: 106–115, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Kolsch V, Charest PG, Firtel RA. The regulation of cell motility and chemotaxis by phospholipid signaling. J Cell Sci 121: 551–559, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kwon HM, Sangiorgi G, Ritman EL, McKenna C, Holmes DR, Jr, Schwartz RS, Lerman A. Enhanced coronary vasa vasorum neovascularization in experimental hypercholesterolemia. J Clin Invest 101: 1551–1556, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Langheinrich AC, Michniewicz A, Sedding DG, Walker G, Beighley PE, Rau WS, Bohle RM, Ritman EL. Correlation of vasa vasorum neovascularization and plaque progression in aortas of apolipoprotein E−/−/low-density lipoprotein−/− double knockout mice. Arterioscler Thromb Vasc Biol 26: 347–352, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Luyendyk JP, Schabbauer GA, Tencati M, Holscher T, Pawlinski R, Mackman N. Genetic analysis of the role of the PI3K-Akt pathway in lipopolysaccharide-induced cytokine and tissue factor gene expression in monocytes/macrophages. J Immunol 180: 4218–4226, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma FX, Zhou B, Chen Z, Ren Q, Lu SH, Sawamura T, Han ZC. Oxidized low density lipoprotein impairs endothelial progenitor cells by regulation of endothelial nitric oxide synthase. J Lipid Res 47: 1227–1237, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Manning BD. Balancing Akt with S6K: implications for both metabolic diseases and tumorigenesis. J Cell Biol 167: 399–403, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell 129: 1261–1274, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maurer U, Charvet C, Wagman AS, Dejardin E, Green DR. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol Cell 21: 749–760, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Mayo LD, Donner DB. A phosphatidylinositol 3-kinase/Akt pathway promotes translocation of Mdm2 from the cytoplasm to the nucleus. Proc Natl Acad Sci USA 98: 11598–11603, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mazure NM, Chen EY, Laderoute KR, Giaccia AJ. Induction of vascular endothelial growth factor by hypoxia is modulated by a phosphatidylinositol 3-kinase/Akt signaling pathway in Ha-ras-transformed cells through a hypoxia inducible factor-1 transcriptional element. Blood 90: 3322–3331, 1997 [PubMed] [Google Scholar]
- 40.Mohler ER, 3rd, Sarov-Blat L, Shi Y, Hamamdzic D, Zalewski A, Macphee C, Llano R, Pelchovitz D, Mainigi SK, Osman H, Hallman T, Steplewski K, Gertz Z, Lu MM, Wilensky RL. Site-specific atherogenic gene expression correlates with subsequent variable lesion development in coronary and peripheral vasculature. Arterioscler Thromb Vasc Biol 28: 850–855, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Moreno PR, Murcia AM, Palacios IF, Leon MN, Bernardi VH, Fuster V, Fallon JT. Coronary composition and macrophage infiltration in atherectomy specimens from patients with diabetes mellitus. Circulation 102: 2180–2184, 2000 [DOI] [PubMed] [Google Scholar]
- 42.Moreno PR, Purushothaman KR, Fuster V, Echeverri D, Truszczynska H, Sharma SK, Badimon JJ, O'Connor WN. Plaque neovascularization is increased in ruptured atherosclerotic lesions of human aorta: implications for plaque vulnerability. Circulation 110: 2032–2038, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Moulton KS, Heller E, Konerding MA, Flynn E, Palinski W, Folkman J. Angiogenesis inhibitors endostatin or TNP-470 reduce intimal neovascularization and plaque growth in apolipoprotein E-deficient mice. Circulation 99: 1726–1732, 1999 [DOI] [PubMed] [Google Scholar]
- 44.O'Brien ER, Garvin MR, Dev R, Stewart DK, Hinohara T, Simpson JB, Schwartz SM. Angiogenesis in human coronary atherosclerotic plaques. Am J Pathol 145: 883–894, 1994 [PMC free article] [PubMed] [Google Scholar]
- 45.Ougolkov AV, Fernandez-Zapico ME, Savoy DN, Urrutia RA, Billadeau DD. Glycogen synthase kinase-3beta participates in nuclear factor kappaB-mediated gene transcription and cell survival in pancreatic cancer cells. Cancer Res 65: 2076–2081, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Pennathur S, Heinecke JW. Mechanisms for oxidative stress in diabetic cardiovascular disease. Antioxid Redox Signal 9: 955–969, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Ramos MA, Kuzuya M, Esaki T, Miura S, Satake S, Asai T, Kanda S, Hayashi T, Iguchi A. Induction of macrophage VEGF in response to oxidized LDL and VEGF accumulation in human atherosclerotic lesions. Arterioscler Thromb Vasc Biol 18: 1188–1196, 1998 [DOI] [PubMed] [Google Scholar]
- 48.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med 340: 115–126, 1999 [DOI] [PubMed] [Google Scholar]
- 49.Sale EM, Sale GJ. Protein kinase B: signalling roles and therapeutic targeting. Cell Mol Life Sci 65: 113–127, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307: 1098–1101, 2005 [DOI] [PubMed] [Google Scholar]
- 51.Shah PK. Molecular mechanisms of plaque instability. Curr Opin Lipidol 18: 492–499, 2007 [DOI] [PubMed] [Google Scholar]
- 52.Skinner HD, Zheng JZ, Fang J, Agani F, Jiang BH. Vascular endothelial growth factor transcriptional activation is mediated by hypoxia-inducible factor 1alpha, HDM2, and p70S6K1 in response to phosphatidylinositol 3-kinase/AKT signaling. J Biol Chem 279: 45643–45651, 2004 [DOI] [PubMed] [Google Scholar]
- 53.Steinbrecher KA, Wilson W, Cogswell PC, 3rd, Baldwin AS. Glycogen synthase kinase 3beta functions to specify gene-specific, NF-kappaB-dependent transcription. Mol Cell Biol 25: 8444–8455, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Testa JR, Bellacosa A. Membrane translocation and activation of the Akt kinase in growth factor-stimulated hematopoietic cells. Leuk Res 21: 1027–1031, 1997 [DOI] [PubMed] [Google Scholar]
- 55.Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol 20: 1262–1275, 2000 [DOI] [PubMed] [Google Scholar]
- 56.Wilensky RL, Hamamdzic D. The molecular basis of vulnerable plaque: potential therapeutic role for immunomodulation. Curr Opin Cardiol 22: 545–551, 2007 [DOI] [PubMed] [Google Scholar]
- 57.Wilensky RL, Shi Y, Mohler ER, Hamamdzic D, 3rd, Burgert ME, Li J, Postle A, Fenning RS, Bollinger JG, Hoffman BE, Pelchovitz DJ, Yang J, Mirabile RC, Webb CL, Zhang L, Zhang P, Gelb MH, Walker MC, Zalewski A, Macphee CH. Inhibition of lipoprotein-associated phospholipase A2 reduces complex coronary atherosclerotic plaque development. Nat Med 14: 1059–1066, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilson SH, Caplice NM, Simari RD, Holmes DR, Jr, Carlson PJ, Lerman A. Activated nuclear factor-kappaB is present in the coronary vasculature in experimental hypercholesterolemia. Atherosclerosis 148: 23–30, 2000 [DOI] [PubMed] [Google Scholar]
- 59.Wilson W, 3rd, Baldwin AS. Maintenance of constitutive IkappaB kinase activity by glycogen synthase kinase-3alpha/beta in pancreatic cancer. Cancer Res 68: 8156–8163, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang HH, Lipovsky AI, Dibble CC, Sahin M, Manning BD. S6K1 regulates GSK3 under conditions of mTOR-dependent feedback inhibition of Akt. Mol Cell 24: 185–197, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao Y, Altman BJ, Coloff JL, Herman CE, Jacobs SR, Wieman HL, Wofford JA, Dimascio LN, Ilkayeva O, Kelekar A, Reya T, Rathmell JC. Glycogen synthase kinase 3alpha and 3beta mediate a glucose-sensitive antiapoptotic signaling pathway to stabilize Mcl-1. Mol Cell Biol 27: 4328–4339, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou BP, Liao Y, Xia W, Zou Y, Spohn B, Hung MC. HER-2/neu induces p53 ubiquitination via Akt-mediated MDM2 phosphorylation. Nat Cell Biol 3: 973–982, 2001 [DOI] [PubMed] [Google Scholar]