Abstract
Nonreceptor tyrosine kinases have an increasingly appreciated role in cardiac injury and protection. To investigate novel tasks for members of the Tec family of nonreceptor tyrosine kinases in cardiac phenotype, we examined the behavior of the Tec isoform in myocardial ischemic injury. Ischemia-reperfusion, but not cardiac protective agents, induced altered intracellular localization of Tec, highlighting distinct actions of this protein compared with other isoforms, such as Bmx, in the same model. Tec is abundantly expressed in cardiac myocytes and assumes a diffuse intracellular localization under basal conditions but is recruited to striated structures upon various stimuli, including ATP. To characterize Tec signaling targets in vivo, we performed an exhaustive proteomic analysis of Tec-binding partners. These experiments expand the role of the Tec family in the heart, identifying the Tec isoform as an ischemic injury-induced isoform, and map the subproteome of its interactors in isolated cells.
Keywords: ischemia, tyrosine kinase, proteomics
nonreceptor tyrosine kinases are key regulatory molecules in the heart. Src, the prototypical nonreceptor tyrosine kinase, participates in numerous signaling events in the heart: it is a critical component of cardiac protection against ischemia (9, 27) as well as physiological responses to ANG II receptor stimulation in the cardiovascular system (7). Focal adhesion kinase (FAK) has an established role in cardiac hypertrophy (5), in response to angiogenic stimuli (22), or in the setting of ischemic injury (8). Likewise, the roles of protein tyrosine kinase PYK2 in hypertrophy (11) and JAK in ischemic injury and cardiac protection have been previously described (31).
In contrast, the actions of the Tec family of nonreceptor kinases in the heart are less well characterized. Tec family members are known to govern lymphocyte proliferation and the responses of these cells to various forms of receptor stimulation. The Tec isoform, in particular, couples T cell receptor (TCR) activation to Ca2+ release and the mobilization of stress kinases (19). Recent studies have implicated another isoform of the Tec family, Bmx, in angiogenesis and stress response. Specifically, genetic loss-of-function studies have shown that Bmx participates in wound healing (18), promotes angiogenesis and prevents cell death in ischemic skeletal muscle (10), and is a necessary component of compensatory cardiac hypertrophy at the cell and organ level after transverse aortic banding (14). While these observations support a stress-activated role for the Bmx isoform, a role unique to the Tec isoform in the heart has not been characterized.
In T cells, Tec is activated by TCR/CD3 or CD28 ligation and interacts with the CD28 receptor via its SH3 domain (24). While its expression in primary T cells is lower than that of IL-2-inducible T cell kinase (Itk), Tec protein levels are significantly elevated upon T cell activation, and this change is sufficient to induce phospholipase C-γ phosphorylation and nuclear factor of activated T cells (26). Some investigations from noncardiac systems have revealed physical interactions between Tec and other signaling molecules. Tec interacts with c-Kit under the influence of stem cell factor in MO7E cells (24) and other nonreceptor tyrosine kinases such as Lyn, which phosphorylates Tec in vitro (13). JAK1/2, which coprecipitates with and phosphorylates Tec in 293T cotransfected cells (21), has been shown to regulate Tec in heterologous and cell-free systems. The SHIP family of inositol phosphatases can inhibit Tec kinase activity and prevent Tec membrane localization in Jurkat cells (25). Yet, to our knowledge, no unbiased analysis of Tec signaling partners has been carried out in any system.
In support of a role outside of the immune system, an examination of Tec tissue distribution via the Human Protein Atlas (3) revealed a broad tissue distribution, including expression in heart muscle and cardiac myocytes, in contrast to other Tec family members, which exhibited more restricted tissue distribution. The ramifications of Tec signaling in the intact heart, however, are unknown. To investigate the role of Tec in cardiac signaling and to evaluate whether, like Bmx, it is a stress-activated kinase in this setting, we undertook an extensive characterization of its intracellular actions and signaling partners. Our data reveal the subproteome of Tec interactors and demonstrate the activation of Tec after cardiac stress, setting the groundwork for further investigations of the signaling actions of this family of kinases in the cardiovascular system.
METHODS
Cloning and transfection.
Mouse TecIV cDNA (94% amino acid homology) (13) was subcloned into pcDNA3 with and without the COOH-terminal FLAG tag (DYKDDDDK). Nontagged Tec was used as a negative control for all subsequent steps. Transfections of human embryonic kidney (HEK)-293 cells were performed with Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol in 100-mm dishes. Cells were harvested 48 h after transfection and lysed with immunoprecipitation buffer containing (in mM) 150 NaCl, 20 Tris·HCl (pH 7.4), 1 EDTA, 1 EGTA, 2.5 sodium pyrophosphate, 1 β-glycerophosphate, 1 Na3VO4, 1 PMSF, and 1 NaF with protease inhibitor cocktail (Roche) and 1% Triton X-100 alone or with 0.6% CHAPS.
Purification of Tec-associated proteins.
Transfected cell lysates were sonicated three times for 10 s and centrifuged at 16,000 g for 10 min. The supernatant was precleared with protein A/G beads (Santa Cruz Biotechnology) and nonimmune mouse IgG (Santa Cruz Biotechnology) for 1 h at 4°C. Two different methods were used to purify Tec complexes, with both methods using nontagged Tec as the negative control. For immunoprecipitation, precleared lysates were incubated with protein A/G beads and anti-FLAG-M2 antibody (Sigma) overnight at 4°C. After three washes with immunoprecipitation buffer, beads were resuspended in Laemmli buffer and boiled. For FLAG affinity pulldown, precleared lysates were passed 20 times over a 200-μl column containing anti-FLAG-M2 affinity gel (Sigma). After a wash with 10 column volumes, Tec complexes were eluted with 1 ml of 100 μg/ml 3×FLAG peptide (Sigma). The eluate was concentrated to 30 μl with a 3-kDa MWCO Centricon column (Sigma). Samples were run on 7% Tris-glycine gels and stained with Coomassie blue R250.
In-gel trypsin digestion.
Purifications of FLAG-tagged Tec and nontagged Tec were run in adjacent lanes, and, after Coomassie blue staining, each lane was sectioned into ∼15 bands. Gel bands were washed with 50 mM NH4HCO3-50% acetonitrile twice and once with acetonitrile. Gel bands were dried in a speedvac and incubated sequentially with 10 mM Tris(2-carboxy-ethyl)phosphine hydrochloride-10 mM DTT at 56°C and 100 mM iodoacetamide at room temperature. Afterward, gel bands were incubated with 20 ng/μl trypsin for 18 h. Digestion was halted with 5% trifluoroacetic acid. Gel bands were vortexed and sonicated to extract digested peptides. The final solution was dried and resuspended in mass spectrometry (MS) buffer A (2% acetonitrile and 0.1% formic acid).
MS analysis and protein identification.
Digested peptides were separated by reverse-phase nanoflow liquid chromatography (LC) on an Eksigent HPLC and introduced by electrospray into a ThermoFisher LTQ-Orbitrap mass spectrometer operating in data-dependent mode. Spectra were then searched with the SEQUEST algorithm against the IPI Human version 3.51 database, and proteins with the following criteria underwent further analysis (as discussed in results): ΔCN > 0.1 and Xcorr versus charge state >2 (+1), >3(+2), >4(+3), and >5(+4). No proteins were accepted on the basis of less than two peptides, and all spectra used for identification were manually inspected.
Bioinformatic analysis of Tec-associated proteins.
The molecular weight, isoelectric point, and grand average of hydropathy of Tec-interacting subsets were calculated based on the Swiss-Prot ProtParam tool and in-house software. Disorder prediction was performed using DISOPRED2 software (29). The reported values for each protein are the percentage of the molecule that is disordered.
Myocardial ischemia-reperfusion surgery and infarct size analysis.
All experiments involving animals were conducted following protocols approved by the University of California-Los Angeles Institutional Animal Care and Use Committee. Adult (8–12 wk old) male Balb/c mice were used for all experiments. Anesthesia was induced by an intraperitoneal injection of pentobarbital (50 mg/kg body wt), and mice were intubated and mechanically ventilated with 95% O2-5% CO2 for the duration of the surgical procedure. The animal's temperature was continuously measured rectally and maintained at 36.5–37.5°C. After a left thoracotomy between ribs three and four, the pericardium was opened, and a silk 8-0 suture was passed under the left anterior descending coronary artery (LAD) 1–3 mm from the tip of the left atrium. Ischemia was induced by ligation of this suture over a 2-mm section of polyethylene-10 tubing, which was placed between the suture and the artery. After 30 min of coronary artery occlusion, the suture was removed to allow reperfusion, and the chest wall was closed. After 24 h of coronary artery reperfusion, the animal was anesthetized with a lethal dose of pentobarbital, and the heart was stopped by an injection of a bolus of saturated KCl solution. The infarcted region was determined by retrograde perfusion through the aorta of a 1% solution of 2,3,5-triphenyltetrazolium chloride in phosphate buffer (pH 7.4, 37°C), and the risk region was delineated by 1% solution of blue dye perfusion after reocclusion of the LAD. The heart was then frozen and sectioned, and individual sections were photographed. Infarct size was measured by manual tracing using the ImageJ program and expressed as a percentage of the area at risk. All surgeries and image analyses were conducted by different individuals in a blinded fashion.
Administration of diethylenetriamine/nitric oxide.
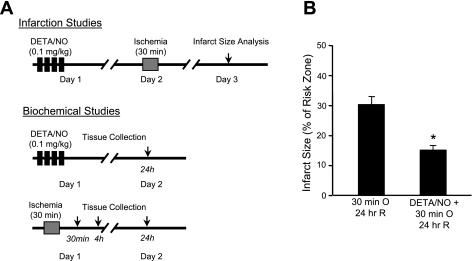
Mice were given the known cardiac protective agent diethylenetriamine (DETA)/nitric oxide (NO) as four consecutive intravenous boluses (0.1 mg/kg each), each separated by 25 min, or PBS (vehicle) (23, 28). Ischemic injury was induced 24 h later, as described above (see Fig. 2A).
Fig. 2.
Model of cardiac injury and protection. A: adult Balb/c mice were subjected to 30 min of left anterior descending coronary artery occlusion in the presence or absence of the cardioprotective reagent diethylenetriamine (DETA)/nitric oxide (NO). After 24 h of reperfusion, infarct size was measured by postmortem tetrazolium chloride staining in one cohort of mice, whereas hearts from the other cohort were excised for biochemical analysis. To investigate temporal changes in protein abundance after myocardial ischemia, tissue samples were also harvested after 30 min and 4 h of reperfusion in separate groups. B: infarct size analyses in control and preconditioned mice. Acute myocardial ischemia and reperfusion [occlusion (O) and reperfusion (R)] produced left ventricular infarction, which was significantly reduced by pretreatment with the NO donor DETA/NO. n = 12 mice/group. *P = 0.013.
Myocyte isolation, culture, and stimulation.
Adult cardiac myocytes were isolated using a protocol by O'Connell and colleagues (16) with minor modifications. Balb/c mice, injected 20 min before with 0.5 ml heparin, were killed, and their hearts were immediately excised and retrograde perfused with Tyrode solution followed by collagenase and type XI protease. The ventricular portion was taken and minced to separate cells. Additional filtering was done by gravity and a brief centrifugation, followed by calcium reintroduction and plating onto laminin-coated coverslips. For the overnight culture, cells were grown in DMEM (with Hanks balanced salts, 10% FBS, and 100 U/ml penicillin-streptomycin; 2% CO2). Hypoxia was induced by placing the culture dishes in an air-tight chamber flushed for 5 min with 95% N2-5% CO2. Room air was reintroduced to induce reoxygenation. For purinergic stimulation, ATP was dissolved directly in PBS, and adenosine was first dissolved in DMSO and then in PBS.
Sucrose gradient ultracentrifugation of mouse hearts.
Mouse hearts were homogenized with a dounce homogenizer in ice-cold immunoprecipation buffer containing 1% Triton X-100 (or with the addition of 0.6% CHAPS and 0.1% deoxycholate; 2 ml buffer/heart). Homogenates were mixed at 1:1.25 with 80% sucrose and laid at the bottom of ultracentrifuge tubes. Equal volumes of 35% sucrose and then 5% sucrose were overlaid. The resulting discontinuous gradient was centrifuged at 240,000 g for 18 h at 4°C, after which samples were immediately separated into eight fractions. Proteins were then chloroform-methanol precipitated and resuspended in Laemmli buffer for subsequent analyses.
Immunoblot analysis and densitometry.
After SDS-PAGE separation, protein samples were transferred onto nitrocellulose membranes. Polyclonal anti-Tec (sc-1109) was purchased from Santa Cruz Biotechnology, and GAPDH was purchased from Abcam (loading control). Detection was performed with horseradish peroxidase-conjugated secondary antibodies along with the ECL detection system (GE Healthcare). Films underwent densitometric analysis with ImageJ.
Immunofluorescence-based confocal microscopy for in situ analysis of Tec localization.
Isolated cardiomyocytes were plated on laminin-coated coverslips, cultured and stimulated as described above, fixed with paraformaldehyde, blocked in 5% milk and 1% serum, and incubated with anti-Tec or anti-caveolin-3 (Santa Cruz Biotechnology) overnight. Secondary antibody was added for 1 h, and, after a wash, coverslips were mounted on slides. Labeling was visualized with an Olympus Fluoview IX70 confocal microscope.
RESULTS
Tec exists in two distinct subcellular locations in cardiomyocytes.
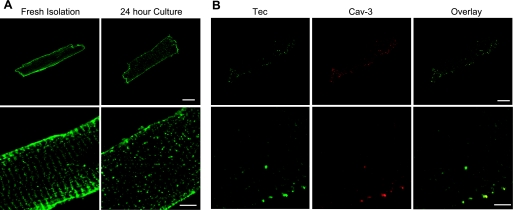
Previous investigations have demonstrated the expression of Tec in the rat heart, but the subcellular localization of this isoform in the mouse heart was unclear. We observed, by Western blot analysis, abundant expression of a single species of Tec protein in whole heart lysates as well as in isolated adult mouse cardiac myocytes. Tec expression was not significantly different in the atria or ventricles or in the base or apex of the heart (data not shown), as will become germane below in the analysis of the injured myocardium. To evaluate Tec subcellular localization in cardiac cells, we used antibody labeling and confocal microscopy. After calcium reintroduction, myocytes were either immediately fixed with paraformaldehyde or cultured for 24 h and then fixed. Interestingly, freshly isolated cardiac cells exhibited a striated Tec expression pattern (Fig. 1A; confirmed by caveolin-3 colocalization in Fig. 1B) that returned, after 24 h of culture, to a diffuse cytoplasmic localization (Fig. 1A). Based on these observations, we reasoned that the transient striated localization may be the result of the stress of isolation and that the cytoplasmic pattern is the endogenous basal state of Tec localization (in agreement with this conclusion, the analysis of Tec localization in cardiac tissue sections revealed a diffuse, nonstriated, cytoplasmic distribution; see Supplemental Fig. 1).1 To further test this possibility, we examined Tec expression in a model of ischemia-reperfusion injury in the mouse as well as in isolated myocytes after culture.
Fig. 1.
Altered intracellular localization of Tec tyrosine kinase in adult cardiac myocytes. A: adult mouse myocytes were isolated by retrograde perfusion with collagenase and examined immediately (fresh isolation) or after 24 h in culture. Cells were fixed, and Tec was visualized by immunodecoration, demonstrating a striated localization pattern that returned to a dispersed, cytoplasmic arrangement after culture. B: colabeling with caveolin (Cav)-3 demonstrated the localization of Tec to T-tubules, a pattern that reverted to the diffuse cytoplasmic distribution after culture. Bar = 10 μM.
Ischemic injury, but not the cardiac protective agent DETA/NO, induces the translocation of Tec.
To explore the role of Tec in normal and diseased hearts, we used an established model of regional myocardial ischemia and pharmacological cardiac protection. Thirty-minute ligation of the LAD followed by 24-h reperfusion produced myocardial infarction in Balb/c mice (31.68 ± 6.2% region at risk; Fig. 2B). Pretreatment with four intravenous boluses of DETA/NO (Fig. 2A) recapitulated the well-documented cardiac protective effect of this NO donor (infarct size was 15.1 ± 2.9% region at risk; Fig. 2B), as previously demonstrated with other mouse strains (28) and originally described in rabbits (23). Region at risk measurements were not significantly different between the two groups (data not shown).
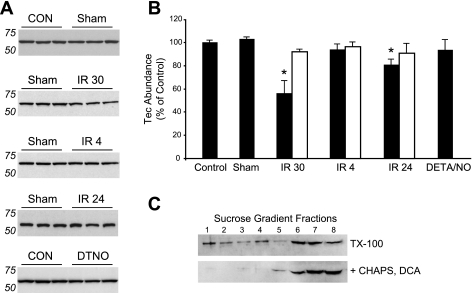
To evaluate Tec abundance, we repeated the ischemic injury experiments in another cohort of mice and divided the hearts postmortem into nonischemic and ischemic regions based on the location of the LAD ligature. As such, the apex region was considered ischemic and the base nonischemic. After 30 min of ischemia and 24 h of reperfusion (the time of infarct size analysis), there was a modest (19.2%) but significant decrease in Tec abundance (blots in Fig. 3A and quantification in Fig. 3B). However, we observed a much greater decrease (43.9%) in Tec abundance after only 30 min of reperfusion, whereas there was no difference from control after 4 h of reperfusion or after treatment with DETA/NO. Strikingly, these changes took place only in the ischemic region (open bars) and not in the nonischemic region (solid bars) of the heart (Fig. 3B).
Fig. 3.
Tec translocates to Triton X-100 (TX-100)-insoluble fractions after ischemia and reperfusion. A: Tec abundance was measured by Western blot analysis in ischemic and nonischemic regions of the same heart after solublization in Triton-X100 lysis buffer, which leaves lipid rafts insoluble. A significant decrease in Tec levels was detected after 30 min of ischemia and 30 min of reperfusion (IR 30 group) in the ischemic zone, whereas Tec was unchanged after 4 h of reperfusion (IR 4 group) and showed a smaller decrease after 24 h of reperfusion (IR 24 group). CON, control; Sham, mice subjected to sham operation; DTNO, DETA/NO treatment. n = 3 mice/group. B: quantified results from A. Importantly, these changes were only witnessed in the ischemic region of the heart (solid bars), not in the nonischemic region (open bars). No differences in basal Tec expression were detected between the base and apex of the heart (data not shown). DETA/NO treatment alone had no effect on Tec levels. *P < 0.05 vs. control. C: use of zwitterionic (CHAPS) and ionic [deoxycholate (DCA)] detergents and ultracentrifugation demonstrated the raft association of Tec. Fractions 1–8 were collected from the sucrose gradient representing the lowest to highest density. Note that in the absence of CHAPS and DCA, Tec was distributed across low-density fractions (top blot), indicative of insolubility, whereas additional detergents effectively extracted Tec from these low-density fractions (bottom blot).
To our knowledge, there is no evidence of a tyrosine kinase undergoing such rapid degradation, and, in support of this, cardiac injury did not produce any lower-molecular-weight species of Tec protein as detected by Western blot analyis. Our initial experiments (Fig. 3, A and B) were performed in Triton X-100, in which lipid rafts are insoluble. To test whether Tec may be localizing to these rafts, which have been shown to be important compartments for cardiovascular signaling (17), hearts were homogenized in Triton X-100 lysis buffer with or without the addition of 0.6% CHAPS and 0.1% deoxycholate. Lysates were mixed with 80% sucrose, on top of which equal volumes of 35% and 5% sucrose were successively layered. After ultracentrifugation (18 h at 240,000 g), each tube was divided into eight fractions, where the first fraction was the lowest density and the eighth fraction was the highest density. Immunoblot analysis with anti-Tec demonstrated two populations of Tec: one associated with lighter membrane-enriched fractions and the other associated with denser protein-rich fractions (Fig. 3C, top). The addition of CHAPS and deoxycholate, detergents known to solubilize membrane proteins, resulted in the loss of the membrane-associated Tec population (Fig. 3C, bottom).
Purinergic stimulation, but not hypoxia, induces translocation of Tec in cardiac cells.
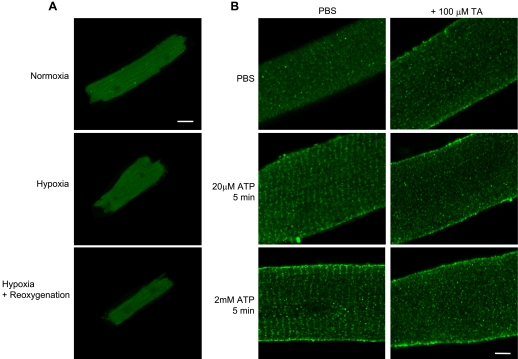
Having established the activation of Tec in the ischemic myocardium, we sought to further explore the triggers for its translocation at the cellular level. Because a major injurious component of ischemia is hypoxia, we first examined the effect of this stress on Tec localization. Myocytes were isolated as described above, cultured for 24 h, and placed in a hypoxic chamber flushed with 95% N2-5% CO2 for 1.5 h with or without 1.5 h of reoxygenation (room air). Hypoxia alone or with reoxygenation had no effect on Tec localization (Fig. 4A), although this same stimulus induced the nuclear localization of hypoxia-inducible factor (HIF)-1α in neonatal rat ventricular myocytes (Supplemental Fig. 2) and cell death, demonstrating that the hypoxic stimulus was sufficient to cause injury. Treatment with H2O2, to reproduce the ROS present during ischemia, also had no effect on Tec localization in the time courses we examined (Supplemental Fig. 3). However, purinergic stimulation by ATP (Fig. 4B) or adenosine (Supplemental Fig. 3) induced Tec translocation to striated structures resembling T-tubules. This translocation was inhibited by terreic acid, a Tec family PH domain inhibitor, implicating this domain of Tec in the interaction and supporting the role of lipid membranes in the altered association.
Fig. 4.
Tec exhibits a striated localization pattern after adenine nucleotide stimulation. Adult mouse myocytes were isolated and cultured for 24 h, after which they were subjected to hypoxia alone (95% N2-5% CO2 for 1.5 h), hypoxia plus 1.5 h of reoxygenation, or ATP/adenosine stimulation. Tec localization was then evaluated by immunolabeling and confocal microscopy. A: hypoxia alone or with reoxygenation produced no discernable alterations in Tec localization. B: ATP stimulation induced Tec translocation to structures resembling T-tubular striation (left; adenosine treatment produced similar effects, not shown). PBS vehicle was used as a control. Inhibition of Tec with terreic acid (TA) blocked this translocation (right panels). Bar = 5 μm. Please see Supplemental Fig. 5 for lower-magnification images.
Unbiased mapping of Tec-interacting proteins by affinity isolation and LC/MS/MS.
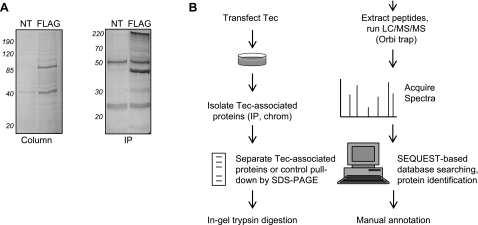
To better understand the in vivo signaling roles of the Tec family of tyrosine kinases, we used proteomic MS to examine proteins associated with the Tec isoform. Because it is abundantly expressed in the heart, we first sought to capture endogenous Tec interactors via immunoprecipitation from mouse hearts using an anti-Tec antibody. After MS analyses, this approach revealed many proteins specific to the Tec pulldown compared with the IgG control; however, the target itself (Tec) was not recovered at levels detectable by MS. We obtained similar results after immunoprecipitations for endogenous Tec from isolated mouse cardiac myocytes and fibroblasts. From a standpoint of protein interaction stoichiometry, we reasoned that while some of these interactors may be real, the isolation-detection approach was inappropriately selecting for abundant proteins. To overcome this limitation, we shifted our analyses to a HEK-293 cell-based system using a pcDNA3 vector containing Tec with and without a COOH-terminal FLAG tag. This approach afforded two methods of purification (Fig. 5): 1) immunoprecipitation with anti-FLAG-M2-bound protein A/G beads and elution by boiling and 2) column purification with FLAG affinity beads and competitive elution by 3× FLAG peptide. As a negative control, both types of FLAG purification were carried out on cells transfected with untagged Tec. Both methods of FLAG purification were performed in triplicate, and protein bands were examined along the continuum of the gel from experimental and control lanes. Importantly, this method allowed for MS identification of the target protein (Tec) at stoichiometric levels similar to the novel interactors we report below. Thus, while this method for protein identification was quite stringent and may have missed transient interactions, we feel that the rigor of this approach enhanced the validity of the results.
Fig. 5.
Proteomic dissection of Tec tyrosine kinase signaling. A: Tec-associated proteins were purified by FLAG tag-based chromatography (left) or immunoprecipitation (IP; right). Cells transfected with nontagged Tec (NT) were used as a negative control. Proteins were separated by SDS-PAGE, and gels were stained with SYPRO. B: workflow for proteomic analyses. Human embryonic kidney-293 cells were transfected with pcDNA3 containing the Tec-COOH-FLAG insert (or pcDNA3 with untagged Tec as a control). Forty-eight hours later, cells were lysed and subjected to either FLAG IP using anti-FLAG-M2 antibody or column purification with FLAG affinity beads. Tec-associated proteins were separated by SDS-PAGE and stained with Coomassie blue. Protein bands were excised, digested with trypsin, and analyzed with liquid chromotography (LC)/mass spectrometry (MS)/MS on an Orbi-trap. Protein identification was carried out by database searching using the Sequest algorithm. Results from both purification processes were merged and then filtered.
Tec interactors were filtered based on peptide count and analyzed with bioinformatics.
Protein bands from Tec isolations (or untagged controls) were digested with trypsin and peptides analyzed by LC/MS/MS. Peptide spectra were searched against the human IPI database using the SEQUEST algorithm. To decipher distinct tiers of proteins interacting with Tec, we examined those detected with greater than or equal to five peptides or with greater than or equal to two peptides as indicators of the relative abundance of the protein in the Tec complexes (see the Supplemental Table for extensive experimental parameters on Tec-interacting proteins, including domain and functional annotation). In each case, we required that the protein was identified with the given number of peptides in more than one biological replicate (all identifications come from at least 2 technical and 2 biological replicates) and that the protein was not detected in the parallel negative control run. These experiments are the first to examine the signaling network of a member of the Tec family of tyrosine kinase using a nonbiased proteomic approach and provide the foundation for further studies of newly identified members of this network.
DISCUSSION
Since its discovery more than a decade ago, Tec tyrosine kinase has been the focus of extensive investigations in the immune system. Tec participates in proliferative signaling and differentiation in lymphocytes, whereas other members of the Tec family have been attributed specific signaling tasks in intracellular processes as varied as calcium signaling and apoptosis (19). Outside of the lymphocyte, however, the actions of Tec are less clear.
Several lines of evidence have pointed to a conserved role of nonreceptor tyrosine kinase signaling in cardiac growth and protection. However, the actions of the Tec family, in particular, have remained unexplored. One key study (4) has shown that Tec is activated in rat neonatal cardiomyocytes, whereas Bmx has been shown to participate in NO-induced cardiac protection (32) and pressure-overload-induced cardiac hypertrophy (14). We therefore hypothesized that Tec may be a stress-activated tyrosine kinase in the heart and have conducted extensive characterization of this isoform in the heart and isolated myocytes.
Role of Tec in myocardial ischemia.
Tec protein is abundantly expressed in both the mouse heart and myocytes, in contrast to other members of the family, such as Bmx. To investigate whether the Tec isoform is involved in cardiac stress in vivo, we examined Tec abundance after ischemia-reperfusion in the mouse. Again, in contrast with Bmx (32), Tec was unaltered by NO donors. However, ischemia induced a transient decrease in Tec abundance, and our data suggest that this results from an altered association of the protein with lipid membranes. Willey and others (30) have described a similar membrane translocation by Bmx in the pressure-overloaded feline myocardium. In support of this observation, confocal microscopy clearly revealed two distinct populations of Tec, in the myocyte—diffuse cytoplasmic and striated T-tubular—and showed that after the stress of myocyte isolation, Tec transiently relocates to the latter. It is interesting to speculate that this change at the cellular level recapitulates that changes in Tec abundance seen in the mouse heart, but additional studies with in vivo tracking of Tec over time will be necessary to test this hypothesis. Thus, Tec is clearly involved in the temporal response of the heart to ischemia-reperfusion. Because Tec is also expressed and active in inflammatory cells, an alternative explanation for the increase in Tec abundance that effectively returns the relative levels close to the uninjured baseline is an increased contribution of Tec protein to the myocardial lysate from inflammatory cells that have infiltrated the injured myocardium during reperfusion. Testing this interesting possibility will require future in vivo fate mapping studies that allow us to distinguish different cell types and to correlate Tec abundance in these cells.
Tec is not activated in myocytes stimulated by hypoxia or ROS.
Having demonstrated the involvement of Tec in ischemia-reperfusion injury to the heart, we next sought to further explore the activation at the cellular level. Hypoxia and ROS are two injurious components of ischemic stress commonly used in isolated cells to simulate ischemia. To our surprise, neither hypoxia nor H2O2 in any of the treatment combinations we administered were sufficient to induce alterations in Tec intracellular localization detectable by microscopy. It remains an open question, however, as to how these stresses alter Tec enzymatic activity and association with intracellular signaling partners. It is interesting to note one Tec interactor we discovered is HIF-1α, a critical participant in hypoxic stress. While there have been no previous reports of this interaction, phosphatidylinositol 3-kinase has been shown to independently signal to Tec and lead to HIF-1α protein accumulation (15).
Tec is involved in purinergic stimulation.
Adenosine and other purine nucleotides are released during ischemia-reperfusion (6). Bony et al. (4) made the observation that transfected Tec-green fluorescent protein displayed T-tubule localization in ATP-treated neonatal rat ventricular myocytes. The actions of endogenous Tec in the setting of adult cardiac my ocytes, however, remains unknown. We observe T-tubular localization of endogenous Tec after ATP, similar to the observations of Bony et al., and documented, for the first time, the translocation of endogenous Tec in adult cardiac cells.
Tec is inhibited by terreic acid.
Terreic acid was originally characterized as a direct Btk PH domain inhibitor (12). Although Itk inhibition has been concurrently reported at a higher inhibitor concentration, to our knowledge, the present study is the first to demonstrate that terreic acid inhibits Tec membrane translocation in isolated cells. Because the Tec and Btk PH domains are nearly identical, it is likely that this domain is necessary for Tec membrane localization. However, protein structure data on the Tec family remain sparse, and further research in this area is required to fully understand the mechanism of terreic acid inhibition of the Tec family kinase PH domain.
Defining the intracellular signaling actions of Tec.
The FLAG-based affinity isolation approach presented here is reproducible, specific, and does not disrupt interactors that may be affected by antibody binding, as in the case with immunoprecipitation. The drawbacks of this approach include the use of a heterologous system, and so the observations we made in the present study for Tec interactors must be confirmed in distinct cell types of interest in the future. Another concern is that some interactors may have escaped our rigorous identification criteria either because they are present at substoichiometric levels (such as the known interactor Src, which was only identified with lower threshold search criteria) and/or have a transient association with Tec. The higher threshold list reported in the present study was arrived at by an unbiased analysis of the data from multiple independent biological and technical replicates, decreasing the likelihood of false positive identifications (see the Supplemental Table for detailed domain and functional annotation of Tec interactors). Additionally, while several of the Tec-interacting proteins in subsets A and B are shared, such as ArfGAP with coiled-coil, ankyrin repeat and PH domains (ACAP)-2 (a PH domain containing GTPase) and tropomodulin (an actin regulatory protein), most of these proteins are distinct between the two subsets. We interpret this observation as a result of the distinct identification criteria selecting for proteins with different types of association with Tec. Subset A is more stringent from the standpoint of requiring a greater level of total peptides to be included for positive identification (higher inclusion threshold) and thus would select based on the total abundance of the protein. In contrast, subset B is more stringent from the standpoint of requiring fewer peptides in a control run to eliminate the protein (lower exclusion threshold in negative controls) and thus would be expected to select for proteins with a greater specificity in their interaction with Tec (although perhaps with lower abundance). Future in vitro protein interactions studies will be required to test this conjecture, but these distinct levels of interactions are presented here to give the most information about Tec interactors along with the appropriate level of experimental confidence.
HIF-1α, identified as a Tec interactor in this study, is an important mediator of adenosine nucleoside-induced neuroprotection in hypoxia-stimulated PC12 cells (34). Since Tec is activated by adenosine nucleotide stimulation in cardiomyocytes, it may participate in HIF-1α signaling during cardiac protection. Another interactor, CDC37, is an emerging heat shock protein 90 cochaperone whose client proteins are predominantly kinases (20). Also among the interactors we found was Rho kinase 1 (ROCK1), an immediate downstream kinase effector of RhoA GTPase. A recent knockout study (33) has indicated a critical role for ROCK1 in reactive fibrosis during pressure-overload hypertrophy. The specifics of how Tec may contribute to this process are unclear, but this observation is consistent with Tec's proliferative role in cell signaling and its abundant expression in fibroblasts. Finally, we found many structural proteins, such as ankyrin, spectrin, and clathrin, and many muscle contraction-associated proteins.
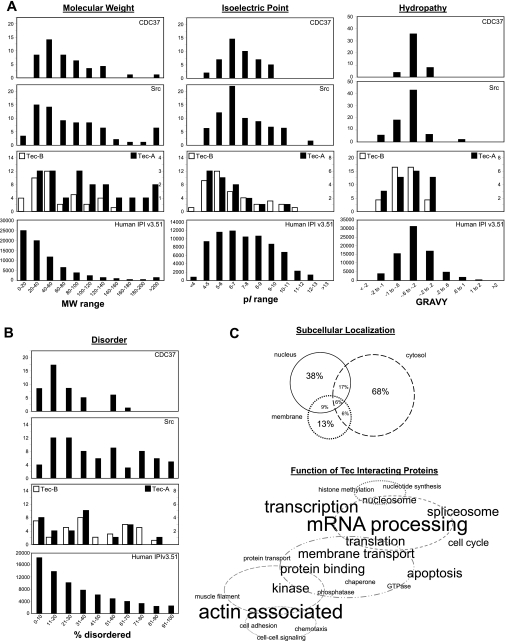
To mine for bioinformatic insights into the Tec subproteome, we examined how the physical-chemical properties of Tec interactors compare with the entire tandem MS-detectable proteome (i.e., the IPI database). The distributions of Tec interactors were not dramatically different from the IPI database for the metrics of molecular weight, isoelectric focusing point, and hydropathy score; however, Tec interactors differed significantly from the IPI database proteome in the area of protein disorder (Fig. 6), in which the Tec subproteome was populated by a significant number of proteins with extensive regions of disorder. Some investigators (29) have postulated that protein disorder is correlated with multifunctionality for protein interaction surfaces–the exact type used in signal transduction. It is worthwhile to note that Src (a homologous tyrosine kinase from a different family) and CDC27 (a Tec interactor identified in this study) subproteomes also had similar bias toward disorder.
Fig. 6.
Subsets of interactors within the Tec signaling network. MS data were acquired on proteins associating with Tec (Fig. 5) and, after an analysis with distinct protein identification criteria, revealed two subsets of Tec interactors. Subset A was defined as interactors with 1) identification by ≥5 peptides in ≥2 purification runs and 2) never being identified (with 5 peptides) in a control run. Subset B was defined as interactors with 1) identification by ≥2 peptides in ≥2 purification runs and 2) never being identified (with 2 peptides) in a control run. A: physicochemical analysis of each tier of Tec interactor compared with the entire tandem MS-detectable proteome (as calculated from human IPI database version 3.51). Two other published experimental subproteomes with relevance to Tec signaling, Src (2) and CDC37 (20), are shown for comparison. MW, molecular weight; pI, isoelectric point; GRAVY, grand average of hydropathy. B: percentages of disordered regions in individual proteins from the various proteomes in our study as predicted by the DISOPRED2 algorithm. C: based on the UniProt annotations of subset A and B proteins, subcellular localization and protein function were visually represented. In the Venn diagram, percentages correspond to the portion of interactors with annotated localization in the three cellular compartments enriched in this data set. In the word cloud, proteins are grouped with relevance to cellular processes, and the font size corresponds directly to the number of proteins with the given annotation.
We recognize that some of the most interesting aspects of the subproteome of Tec-associated signaling proteins remain unknown, including how it changes with stimulation and subcellular location. Indeed, these end points are the focus of ongoing work. However, the present study presents the first nonbiased analysis of Tec interactors in any cell type and sets the groundwork for the future investigation of cell- and stimulus-specific changes. Furthermore, we presented a rigorous and detailed methodological description of how protein interactions were determined (and false positives ruled out), which will inform future investigations of Tec and other signaling proteins via proteomics.
Summary and outlook.
This study demonstrates the activation of endogenous Tec tyrosine kinase in mouse cardiac cells, illustrating similarities and differences with other Tec family members (Bmx in particular) as well as other nonreceptor tyrosine kinases from other protein families in myocardial ischemia and protection. This investigation provides the first global map of Tec-interacting proteins in any cell type. A model that emerges from this and other work is one in which a negative regulatory process (such as a tyrosine phosphatase) physically interacts with Tec kinase in an autoregulatory mechanism (Fig. 7) (25). Release from this cycle by stress stimuli allows Tec to associate with lipid membranes and regulate target proteins. Future studies will be necessary to test this model in detail in cardiac myocytes as well as to reveal the nuances of how Tec signals to different in vivo cellular targets.
Fig. 7.
Proposed mechanism of Tec signaling in the myocardium. A previous study (1) and our data suggest that stress-induced Tec activation is concomitant with a loss of regulation by phosphatases, followed by membrane localization. We propose that this translocation brings Tec into apposition with signaling partners, some of which have been identified in the present study.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute (NHLBI) Grants HL-087132 and HL-096041 (to T. M. Vondriska), HL-088640 (to E. Stefani), and HL-080111 (to P. Ping) and the Laubisch Endowment of the University of California-Los Angeles (to T. M. Vondriska). M. J. Zhang received a scholarship from the University of California-Los Angeles Undergraduate Research Center. S. Franklin is the recipient of NHLBI Ruth Kirschstein Postdoctoral Fellowship F32-HL-091673.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Footnotes
Supplemental Material for this article is available online at the American Journal of Physiology-Heart and Circulatory Physiology website.
REFERENCES
- 1.Aoki N, Ueno S, Mano H, Yamasaki S, Shiota M, Miyazaki H, Yamaguchi-Aoki Y, Matsuda T, Ullrich A. Mutual regulation of protein-tyrosine phosphatase 20 and protein-tyrosine kinase Tec activities by tyrosine phosphorylation and dephosphorylation. J Biol Chem 279: 10765–10775, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Aranda B, Achuthan P, Alam-Faruque Y, Armean I, Bridge A, Derow C, Feuermann M, Ghanbarian AT, Kerrien S, Khadake J, Kerssemakers J, Leroy C, Menden M, Michaut M, Montecchi-Palazzi L, Neuhauser SN, Orchard S, Perreau V, Roechert B, van Eijk K, Hermjakob H. The IntAct molecular interaction database in 2010. Nucleic Acids Res 38: D525–D531, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berglund L, Bjorling E, Oksvold P, Fagerberg L, Asplund A, Szigyarto CA, Persson A, Ottosson J, Wernerus H, Nilsson P, Lundberg E, Sivertsson A, Navani S, Wester K, Kampf C, Hober S, Ponten F, Uhlen M. A genecentric Human Protein Atlas for expression profiles based on antibodies. Mol Cell Proteomics 7: 2019–2027, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Bony C, Roche S, Shuichi U, Sasaki T, Crackower MA, Penninger J, Mano H, Puceat M. A specific role of phosphatidylinositol 3-kinase gamma. A regulation of autonomic Ca2+ oscillations in cardiac cells. J Cell Biol 152: 717–728, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eble DM, Strait JB, Govindarajan G, Lou J, Byron KL, Samarel AM. Endothelin-induced cardiac myocyte hypertrophy: role for focal adhesion kinase. Am J Physiol Heart Circ Physiol 278: H1695–H1707, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Fox AC, Reed GE, Glassman E, Kaltman AJ, Silk BB. Release of adenosine from human hearts during angina induced by rapid atrial pacing. J Clin Invest 53: 1447–1457, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haendeler J, Berk BC. Angiotensin II mediated signal transduction. Important role of tyrosine kinases. Regul Pept 95: 1–7, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Hakim ZS, DiMichele LA, Rojas M, Meredith D, Mack CP, Taylor JM. FAK regulates cardiomyocyte survival following ischemia/reperfusion. J Mol Cell Cardiol 46: 241–248, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hattori R, Otani H, Uchiyama T, Imamura H, Cui J, Maulik N, Cordis GA, Zhu L, Das DK. Src tyrosine kinase is the trigger but not the mediator of ischemic preconditioning. Am J Physiol Heart Circ Physiol 281: H1066–H1074, 2001 [DOI] [PubMed] [Google Scholar]
- 10.He Y, Luo Y, Tang SB, Rajantie I, Salven P, Heil M, Zhang R, Luo DH, Li XH, Chi HB, Yu J, Carmeliet P, Schaper W, Sinusas AJ, Sessa WC, Alitalo K, Min W. Critical function of Bmx/Etk in ischemia-mediated arteriogenesis and angiogenesis. J Clin Invest 116: 2344–2355, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirotani S, Higuchi Y, Nishida K, Nakayama H, Yamaguchi O, Hikoso S, Takeda T, Kashiwase K, Watanabe T, Asahi M, Taniike M, Tsujimoto I, Matsumura Y, Sasaki T, Hori M, Otsu K. Ca2+-sensitive tyrosine kinase Pyk2/CAK beta-dependent signaling is essential for G-protein-coupled receptor agonist-induced hypertrophy. J Mol Cell Cardiol 36: 799–807, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Kawakami Y, Hartman SE, Kinoshita E, Suzuki H, Kitaura J, Yao L, Inagaki N, Franco A, Hata D, Maeda-Yamamoto M, Fukamachi H, Nagai H, Kawakami T. Terreic acid, a quinone epoxide inhibitor of Bruton's tyrosine kinase. Proc Natl Acad Sci USA 96: 2227–2232, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mano H, Yamashita Y, Miyazato A, Miura Y, Ozawa K. Tec protein-tyrosine kinase is an effector molecule of Lyn protein-tyrosine kinase. FASEB J 10: 637–642, 1996 [DOI] [PubMed] [Google Scholar]
- 14.Mitchell-Jordan SA, Holopainen T, Ren S, Wang S, Warburton S, Zhang MJ, Alitalo K, Wang Y, Vondriska TM. Loss of Bmx nonreceptor tyrosine kinase prevents pressure overload-induced cardiac hypertrophy. Circ Res 103: 1359–1362, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mottet D, Dumont V, Deccache Y, Demazy C, Ninane N, Raes M, Michiels C. Regulation of hypoxia-inducible factor-1alpha protein level during hypoxic conditions by the phosphatidylinositol 3-kinase/Akt/glycogen synthase kinase 3beta pathway in HepG2 cells. J Biol Chem 278: 31277–31285, 2003 [DOI] [PubMed] [Google Scholar]
- 16.O'Connell TD, Rodrigo MC, Simpson PC. Isolation and culture of adult mouse cardiac myocytes. Methods Mol Biol 357: 271–296, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Patel HH, Insel PA. Lipid rafts and caveolae and their role in compartmentation of redox signaling. Antioxid Redox Signal 11: 1357–1372, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rajantie I, Ekman N, Iljin K, Arighi E, Gunji Y, Kaukonen J, Palotie A, Dewerchin M, Carmeliet P, Alitalo K. Bmx tyrosine kinase has a redundant function downstream of angiopoietin and vascular endothelial growth factor receptors in arterial endothelium. Mol Cell Biol 21: 4647–4655, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwartzberg PL, Finkelstein LD, Readinger JA. TEC-family kinases: regulators of T-helper-cell differentiation. Nat Rev Immunol 5: 284–295, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Smith JR, Workman P. Targeting CDC37: an alternative, kinase-directed strategy for disruption of oncogenic chaperoning. Cell Cycle 8: 362–372, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Takahashi-Tezuka M, Hibi M, Fujitani Y, Fukada T, Yamaguchi T, Hirano T. Tec tyrosine kinase links the cytokine receptors to PI-3 kinase probably through JAK. Oncogene 14: 2273–2282, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Takahashi N, Seko Y, Noiri E, Tobe K, Kadowaki T, Sabe H, Yazaki Y. Vascular endothelial growth factor induces activation and subcellular translocation of focal adhesion kinase (p125FAK) in cultured rat cardiac myocytes. Circ Res 84: 1194–1202, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Takano H, Tang XL, Qiu Y, Guo Y, French BA, Bolli R. Nitric oxide donors induce late preconditioning against myocardial stunning and infarction in conscious rabbits via an antioxidant-sensitive mechanism. Circ Res 83: 73–84, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang B, Mano H, Yi T, Ihle JN. Tec kinase associates with c-kit and is tyrosine phosphorylated and activated following stem cell factor binding. Mol Cell Biol 14: 8432–8437, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tomlinson MG, Heath VL, Turck CW, Watson SP, Weiss A. SHIP family inositol phosphatases interact with and negatively regulate the Tec tyrosine kinase. J Biol Chem 279: 55089–55096, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Tomlinson MG, Kane LP, Su J, Kadlecek TA, Mollenauer MN, Weiss A. Expression and function of Tec, Itk, and Btk in lymphocytes: evidence for a unique role for Tec. Mol Cell Biol 24: 2455–2466, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vondriska TM, Zhang J, Song C, Tang XL, Cao X, Baines CP, Pass JM, Wang S, Bolli R, Ping P. Protein kinase C epsilon-Src modules direct signal transduction in nitric oxide-induced cardioprotection: complex formation as a means for cardioprotective signaling. Circ Res 88: 1306–1313, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Wang G, Liem DA, Vondriska TM, Honda HM, Korge P, Pantaleon DM, Qiao X, Wang Y, Weiss JN, Ping P. Nitric oxide donors protect murine myocardium against infarction via modulation of mitochondrial permeability transition. Am J Physiol Heart Circ Physiol 288: H1290–H1295, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Ward JJ, Sodhi JS, McGuffin LJ, Buxton BF, Jones DT. Prediction and functional analysis of native disorder in proteins from the three kingdoms of life. J Mol Biol 337: 635–645, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Willey CD, Palanisamy AP, Johnston RK, Mani SK, Shiraishi H, Tuxworth WJ, Zile MR, Balasubramanian S, Kuppuswamy D. STAT3 activation in pressure-overloaded feline myocardium: role for integrins and the tyrosine kinase BMX. Int J Biol Sci 4: 184–199, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xuan YT, Guo Y, Han H, Zhu Y, Bolli R. An essential role of the JAK-STAT pathway in ischemic preconditioning. Proc Natl Acad Sci USA 98: 9050–9055, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang J, Ping P, Wang GW, Lu M, Pantaleon D, Tang XL, Bolli R, Vondriska TM. Bmx, a member of the Tec family of nonreceptor tyrosine kinases, is a novel participant in pharmacological cardioprotection. Am J Physiol Heart Circ Physiol 287: H2364–H2366, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Zhang YM, Bo J, Taffet GE, Chang J, Shi J, Reddy AK, Michael LH, Schneider MD, Entman ML, Schwartz RJ, Wei L. Targeted deletion of ROCK1 protects the heart against pressure overload by inhibiting reactive fibrosis. FASEB J 20: 916–925, 2006 [DOI] [PubMed] [Google Scholar]
- 34.zur Nedden S, Tomaselli B, Baier-Bitterlich G. HIF-1 alpha is an essential effector for purine nucleoside-mediated neuroprotection against hypoxia in PC12 cells and primary cerebellar granule neurons. J Neurochem 105: 1901–1914, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]