Abstract
A major difference between experimental ischemic preconditioning (IPC), induced by brief ischemic episodes, and the clinical situation is that patients generally have repetitive episodes of ischemia. We used a swine model to examine differences in genes regulated by classical second-window IPC (SWOP) [two 10-min episodes of coronary artery occlusion (CAO) followed by 24 h reperfusion] compared with repetitive CAO/reperfusion (RCO), i.e., two 10-min CAO 12 h apart, and to repetitive coronary stenosis (RCS), six episodes of 90 min coronary stenosis 12 h apart (n = 5/group). All three models reduced infarct size by 60–85%, which was mediated by nitric oxide in SWOP but not in the other two models. We employed microarray analyses to discover additional molecular pathways intrinsic to models of repetitive ischemia and different from classical SWOP. There was an 85% homology in gene response between the RCO and RCS models, whereas SWOP was qualitatively different. Both RCO and RCS, but not SWOP, showed downregulation of genes encoding proteins involved in oxidative metabolism and upregulation of genes involved in protein synthesis, unfolded protein response, autophagy, heat shock response, protein secretion, and an activation of the NF-κB signaling pathway. Therefore, the regulated genes mediating IPC with repetitive ischemia differ radically from SWOP both quantitatively and qualitatively, showing that a repetitive pattern of ischemia, rather than the difference between no-flow vs. low-flow ischemia, dictates the genomic response of the heart. These findings illustrate new cardioprotective mechanisms developed by repetitive IPC, which are potentially more relevant to patients with chronic ischemic heart disease, who are subjected to repetitive episodes of ischemia.
Keywords: coronary occlusion, gene expression, myocardial infarction, ischemia, preconditioning
ischemic preconditioning (IPC) is the “gold standard” of cardiac cell survival because it is the most powerful mechanism for cardioprotection (16). The first window of IPC confers protection for 1–2 h after ischemia (17), whereas the second window of IPC (SWOP) (12, 14) confers protection during the 24–72 h that follow the preconditioning stimulus (28). The SWOP involves changes in gene expression, including cyclooxygenase-2 and the inducible isoform of nitric oxide (NO) synthase (iNOS) (2, 29). Despite the intense investigation of the mechanisms of IPC and the demonstrated power of the cardioprotection it confers, the clinical translation of IPC remains limited. One possibility to explain this limitation is that IPC is classically induced by brief periods of coronary artery occlusion (CAO)/reperfusion within an hour, whereas patients with ischemic heart disease frequently experience repetitive episodes of ischemia that can result in hibernating myocardium (25). We developed a swine model of repetitive episodes of low-flow coronary stenosis [repetitive coronary stenosis (RCS)], which reproduces the conditions of transient and repeated ischemic episodes found in patients with ischemic heart disease (10, 22). This model demonstrated that a repetitive pattern of ischemia provides IPC at least equal to the SWOP, but through radically different mechanisms (22). First, in contrast to SWOP, the protection conferred by RCS was independent from NO production (22). Second, RCS elicited a genomic response dramatically different from SWOP. In particular, RCS but not SWOP displayed an activation of genes involved in autophagy and endoplasmic reticulum stress, as well as a downregulation of genes involved in mitochondrial oxidative metabolism (22).
It is conceivable that the difference in mechanisms between SWOP and RCS may not be due simply to single episode vs. repetitive ischemia, but also to the fact that SWOP is induced by total CAO, whereas RCS does not involve total CAO. The goal of the present study was to compare DNA microarray results from a model of repetitive episodes of complete CAO (RCO) with the SWOP and RCS models. Our results clearly indicate that the repetitive pattern of ischemia, rather than the difference between no-flow vs. low-flow ischemia, dictates the genomic response of the heart. The molecular mechanisms mediating these models of repetitive ischemia improve our understanding of the cardiac survival mechanisms activated in conditions reproducing the clinical setting.
MATERIALS AND METHODS
Animal models.
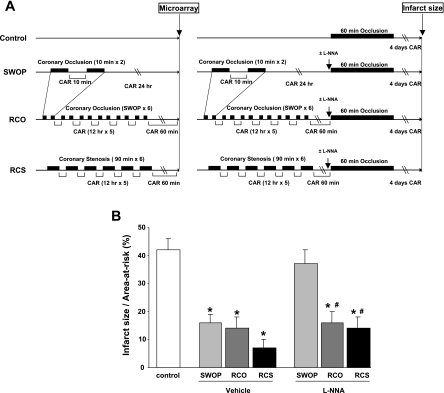
All protocols concerning animal use were approved by the Institutional Animal Care and Use Committee at the New Jersey Medical School. All of the investigations conformed to the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health. All experiments were conducted in the conscious state after the pigs fully recovered from surgery, as reported previously (22). The different experimental protocols are shown in Fig. 1A. Classical SWOP was induced by two 10-min episodes of CAO followed by 24 h reperfusion. RCO was induced by six episodes of SWOP 12 h apart. RCS was induced by six episodes of 90 min coronary stenosis 12 h apart. In the latter model, coronary blood flow was reduced to 55–65% of baseline, and this reduction was maintained stable under the control of a flow probe, as before (10, 22).
Fig. 1.
Cardioprotection by preconditioning following classical second window or chronic ischemia. Description of the experimental protocols (A) used for microarray analysis or for measurement of infarct size in the different groups. Reduction of infarct size (B) by the different protocols of preconditioning and the effect thereupon of nitric oxide (NO) blockade with N-nitro-l-arginine (l-NNA) (n = 4/group) compared with vehicle (n = 5/group), administered intravenously before lethal coronary occlusion. CAR, coronary artery reperfusion; RCO, repetitive coronary occlusion; RCS, repetitive coronary stenosis; SWOP, second window of preconditioning. *P < 0.05 vs. control. #P < 0.05 vs. SWOP + l-NNA.
After this protocol, two procedures were applied on different pigs, one for microarray analysis and one for measurement of infarct size (Fig. 1A). For the genomic analysis by microarrays, tissue samples were harvested from the hearts of pigs (n = 5/group) obtained 24 h after the preconditioning stimulus for the SWOP model, and at 1 h following the completion of the RCO and RCS protocols (22). Non-IPC samples were obtained from sham-operated animals. For measurement of infarct size, CAO was performed for 60 min in additional pigs (n = 5/group), 24 h after the preconditioning stimulus in the SWOP model or 1 h after the last stimulus in the models of RCO and RCS (Fig. 1A). This episode of lethal ischemia was followed by 4 days of reperfusion (Fig. 1A), after which infarct size was determined by triphenyltetrazolium staining and reported as a percentage of the area-at-risk (10, 22). NO blockade (n = 4/group) was performed by intravenous administration of 35 mg/kg N-nitro-l-arginine (l-NNA) 1 h before CAO (22). No mortality and no episode of fatal arrhythmia were observed in any experimental group.
Microarray data analysis.
cDNA synthesis (SuperScript; Invitrogen) was performed from 10 μg total RNA with a T7-oligo(24)dT primer. The DNA was transcribed into biotin-labeled RNA (Bioarray RNA Labeling Kit, ENZO) and hybridized on the Porcine Genome Array (Affymetrix), which contains 23,256 probe sets targeting 20,201 genes of Sus Scrofa. Samples obtained from each pig heart were processed and analyzed individually. Human genes were used to annotate the porcine array (23). Gene ontology (GO) annotations for genes were obtained from the NCBI Gene database (1). The microarray data were normalized using the Robust Multichip Average method. We discarded probe sets that did not have signals above background in >75% of the arrays for all samples using the Affymetrix MAS5 present/absent calls. The Significance Analysis of Microarrays program was used to select probe sets that have differential signals between comparing groups. A false discovery rate of ∼5% and a median fold change >1.2 were used as the selection criteria for differential expression. GO entries were tested for bias for up- and downregulation of expression using the hypergeometric data distribution as described previously (21). For each GO entry, the significance score was calculated by taking −log10(P value) for upregulation or log10(P value) for downregulation. The redundant GO terms were removed by comparing the overlapped portion between gene sets. Microarray data are available in the GEO repository under accession number GSE21096.
Immunoblotting.
Protein extracts were prepared from the left ventricle using an extraction buffer (pH 7.4) containing 20 mM Tris·HCl, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton, 1 mM sodium orthovanadate, 2.5 mM sodium pyrophosphate, and 1 mM β-glycerophosphate supplemented with 5 μg/ml protease inhibitor cocktail. Protein concentration was determined by the method of Bradford (Bio-Rad). Proteins were separated by SDS-PAGE, transferred to a nitrocellulose membrane, and detected with specific antibodies against the secreted frizzled-related protein 2 (sfrp-2; Abcam) and the heat shock 70-kDa protein A6 (Hsp70BĆ; ProteinTech). Western blotting for glyceraldehyde-3-phosphate dehydrogenase was used to verify equal protein loading of the blots. Primary antibodies were used according to the manufacturer's instructions at a 1:1,000 dilution. The blots were incubated with horseradish peroxidase-labeled secondary antibodies. Immunoreactive bands were detected with chemiluminescence (ECL; Perkin-Elmer Life Sciences). The intensities of the resulting bands were quantified by Quantity One software on a GS-800 densitometer (Bio-Rad).
Quantitative PCR.
Total RNA was extracted by phenol-chloroform extraction (4) from samples of each pig heart. For each measurement, the mRNA of interest was reverse-transcribed from 60 ng of total RNA with the TaqMan RT kit (Applied Biosystems) and used for quantitative PCR (qPCR; 40 cycles of a 10-s step at 95°C and a 1-min step at 60°C) using the SybrGreen PCR mix (Applied Biosystems) on a 7300 ABI-Prizm Sequence Detector (Applied Biosystems). Due to the variation in loading, transcripts were reported to cyclophilin, used as a housekeeping gene (6).
Statistical analysis.
Results are shown as means ± SE for the number of samples indicated in the legends for Figs. 1–7. ANOVA with Bonferroni post hoc correction was used for analysis of more than two groups. A value of P < 0.05 was considered as significant. Student's t-test was used to select up- and downregulated genes (P < 0.05 and fold change >1.2).
Fig. 7.
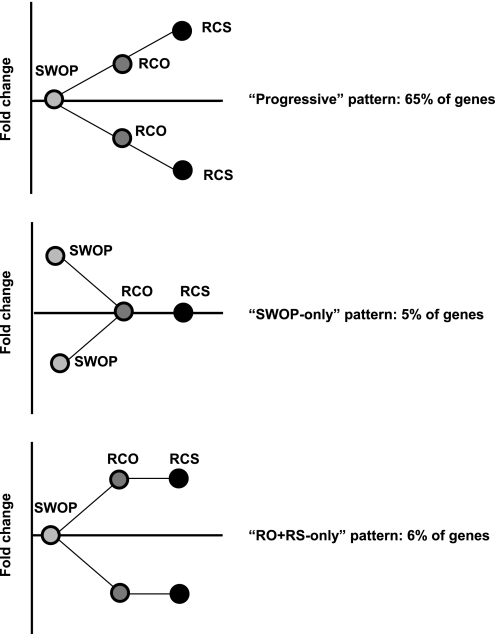
Comparison of the pattern of gene expression among the three models. Sixty-five percent of the gene sets followed a progressive pattern of regulation in which the RCO model was intermediate between SWOP and RCS. Only 5% of the genes showed a regulation that was more important in the model of SWOP compared with both RCO and RCS, and only 6% of genes were regulated in RCS and RCO more than in SWOP.
RESULTS
Characteristics of the three models.
Figure 1A shows the experimental protocol for each model. In Fig. 1B, we report the infarct size, as a percentage of the area-at-risk, in the three models compared with control hearts (n = 5/group), as well as the response to NO blockade with l-NNA (n = 4/group). Briefly, the reduction in infarct size following 60 min CAO and 4 days reperfusion was ∼62% in the SWOP model, 67% in the RCO model, and 84% in the RCS model (all values, P < 0.05 compared with CAO without IPC; Fig. 1B). Importantly, the cardioprotection in the SWOP model was NO-dependent, whereas it was NO-independent in the models of RCO and RCS (Fig. 1B).
Comparison of the global pattern of gene expression.
Total RNA was extracted and prepared as described in materials and methods from five individual pig hearts per group for hybridization to pig microarrays, on which 23,256 genes are represented. Table 1 shows the total number of genes with a significant regulation in each model compared with control samples. The RCS model showed the greatest number of genes regulated, more than double the amount of genes regulated in the SWOP model (Table 1). This is consistent with our prior study (22); however, the numbers are not exactly identical between both studies, since a different analysis was employed in the current investigation to include the RCO model. We had not previously examined the RCO model, which is a major component of the current study. Interestingly, the number of genes regulated in the RCO model was less than with repetitive RCS, and relatively equivalent to SWOP.
Table 1.
Total number of genes regulated in the three models
| Up | Down | Total | |
|---|---|---|---|
| SWOP | 1,745 | 550 | 2,295 |
| RCO | 1,176 | 867 | 2,043 |
| RCS | 3,055 | 2,028 | 5,083 |
SWOP, second window of ischemic preconditioning; RCO, repetitive coronary occlusion; RCS, repetitive coronary stenosis.
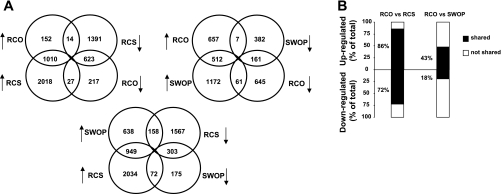
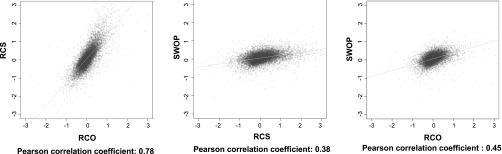
Figure 2 shows the global comparison of gene expression among the three models by Venn diagrams and by quantification of similarities. Clearly, the RCO model showed much more similarity in gene expression profile with RCS than with the SWOP (Fig. 2A). For example, 86% of all the upregulated genes and 72% of all the downregulated genes in the RCO model were shared with the RCS model, whereas these ratios were only 43 and 18%, respectively, when comparing RCO with the SWOP (Fig. 2B). When regrouping the genes on a scatter plot, the Pearson correlation coefficient was 0.78 between RCO and RCS, whereas it was only 0.45 between RCO and SWOP and 0.38 between RCS and SWOP (Fig. 3).
Fig. 2.
Comparison of individual gene expression among the three models. Analysis by Venn diagrams (A) and quantification of the similarities (B) showing a high similarity between RCO and RCS, and marked differences of both models compared with SWOP. Changed genes were selected if fold change (FC) >1.2 and P < 0.05 by t-test. In B, stacked columns compare the percentage that commonly regulated genes contribute to total regulated genes in two groups.
Fig. 3.
Correlation of individual gene expression among the three models. The correlation between gene expression profiles is represented in a scatter plot using the Pearson correlation coefficient.
Identity of the regulated genes.
The identity of the individual genes regulated in the three models was further investigated. Tables 2, 3, and 4 show the 20 genes with the most significant change in expression, either upregulation or downregulation, in the three models of SWOP, RCO, and RCS, respectively. Remarkably, among these 40 genes, 14 of them (10 upregulated and 4 downregulated) were shared between RCO and RCS, whereas only four genes were shared between RCS and SWOP. These shared genes are highlighted in Tables 2 and 3. In addition, only five genes were shared between RCO and SWOP. These results demonstrate that, in addition to the large similarity in the global pattern of gene regulation (Figs. 1 and 2), the models of RCO and RCS also share the most significant regulation of specific genes. Among these shared genes, several of them correspond to secreted proteins (such as the sfrp-2 and the secreted phosphoprotein 1). These secreted proteins can activate autocrine/paracrine mechanisms and might thereby play a role in the phenomenon of remote preconditioning. The complete list of genes regulated in the three models is available from the GEO repository (see materials and methods).
Table 2.
Genes showing the most significant regulation in the SWOP model
| GeneID | Gene Symbol | Description | Fold Change | P Value |
|---|---|---|---|---|
| Upregulated | ||||
| 8483 | CILP | Nucleotide pyrophosphohydrolase | 3.5 | 8.91E-03 |
| 406 | ARNTL | Aryl hydrocarbon receptor nuclear translocator-like | 3.0 | 9.83E-04 |
| 56267 | CCBL2 | Cysteine conjugate-β-lyase 2 | 2.8 | 3.05E-02 |
| 1264 | CNN1 | Calponin 1, basic, smooth muscle | 2.8 | 3.64E-04 |
| 7037 | TFRC | Transferrin receptor (p90, CD71) | 2.5 | 5.72E-04 |
| 5723 | PSPH | Phosphoserine phosphatase | 2.3 | 6.32E-03 |
| 23424 | TDRD7 | Tudor domain containing 7 | 2.3 | 2.32E-02 |
| 8884 | SLC5A6 | Solute carrier family 5, member 6 | 2.2 | 9.64E-04 |
| 7060 | THBS4 | Thrombospondin 4 | 2.2 | 3.95E-02 |
| 3939 | LDHA | Lactate dehydrogenase A | 2.1 | 9.67E-04 |
| 6309 | SC5DL | Sterol-C5-desaturase-like | 2.1 | 4.63E-03 |
| 7466 | WFS1 | Wolfram syndrome 1 | 2.1 | 6.33E-03 |
| 582 | BBS1 | Bardet-Biedl syndrome 1 | 2.1 | 5.68E-03 |
| 1033 | CDKN3 | Cyclin-dependent kinase inhibitor 3 | 2.0 | 9.12E-03 |
| 10077 | TSPAN32 | Tetraspanin 32 | 2.0 | 1.50E-03 |
| 1407 | CRY1 | Cryptochrome 1 | 2.0 | 1.44E-03 |
| 222643 | UNC5CL | Unc-5 homolog C-like | 2.0 | 1.54E-03 |
| 219736 | STOX1 | Storkhead box 1 | 2.0 | 1.25E-02 |
| 7424 | VEGFC | Vascular endothelial growth factor C | 2.0 | 3.53E-03 |
| 147463 | ANKRD29 | Ankyrin repeat domain 29 | 1.9 | 6.21E-03 |
| Downregulated | ||||
| 8013 | NR4A3 | Nuclear receptor subfamily 4, group A, member 3 | −12.0 | 2.32E-02 |
| 8322 | FZD4 | Frizzled homolog 4 | −4.3 | 1.71E-02 |
| 1191 | CLU | Clusterin | −2.7 | 2.64E-03 |
| 8864 | PER2 | Period homolog 2 | −2.7 | 1.60E-02 |
| 8462 | KLF11 | Kruppel-like factor 11 | −2.5 | 3.55E-02 |
| 1545 | CYP1B1 | Cytochrome P-450, family 1B1 | −2.5 | 4.75E-02 |
| 280 | AMY2B | Amylase, α2B | −2.3 | 1.40E-02 |
| 55603 | FAM46A | Family with sequence similarity 46, member A | −2.2 | 1.23E-03 |
| 9020 | MAP3K14 | Mitogen-activated protein kinase kinase kinase 14 | −2.2 | 3.98E-04 |
| 8863 | PER3 | Period homolog 3 | −2.1 | 9.69E-03 |
| 83894 | TTC29 | Tetratricopeptide repeat domain 29 | −2.0 | 2.35E-04 |
| 3725 | JUN | Jun oncogene | −2.0 | 3.28E-02 |
| 8499 | PPFIA2 | Protein tyrosine phosphatase interacting protein | −1.9 | 2.72E-04 |
| 7078 | TIMP3 | TIMP metallopeptidase inhibitor 3 | −1.9 | 2.10E-03 |
| 135138 | PACRG | PARK2 coregulated | −1.9 | 1.62E-02 |
| 8870 | IER3 | Immediate early response 3 | −1.8 | 1.92E-02 |
| 6470 | SHMT1 | Serine hydroxymethyltransferase 1 | −1.8 | 2.05E-02 |
| 5499 | PPP1CA | Protein phosphatase 1, catalytic subunit, α | −1.8 | 4.42E-03 |
| 4915 | NTRK2 | Neurotrophic tyrosine kinase, receptor, type 2 | −1.8 | 2.32E-02 |
| 5325 | PLAGL1 | Pleiomorphic adenoma gene-like 1 | −1.8 | 3.64E-02 |
Genes in bold are shared with Table 4.
Table 3.
Genes showing the most significant regulation in the RCO model
| GeneID | Gene Symbol | Description | Fold Change | P Value |
|---|---|---|---|---|
| Upregulated | ||||
| 3310 | HSPA6 | Heat shock 70 kDa protein 6 (HSP70B′) | 65.8 | 1.48E-03 |
| 6696 | SPP1 | Secreted phosphoprotein 1 | 28.4 | 4.49E-03 |
| 4069 | LYZ | Lysozyme (renal amyloidosis) | 22.2 | 1.53E-03 |
| 3337 | DNAJB1 | DnaJ (Hsp40) homolog, subfamily B, member 1 | 11.2 | 2.63E-03 |
| 6423 | SFRP2 | Secreted frizzled-related protein 2 | 10.5 | 3.40E-02 |
| 8483 | CILP | Nucleotide pyrophosphohydrolase | 8.5 | 6.04E-04 |
| 10808 | HSPH1 | Heat shock 105 kDa/110 kDa protein 1 | 7.9 | 6.91E-04 |
| 3371 | TNC | Tenascin C | 7.6 | 2.27E-02 |
| 467 | ATF3 | Activating transcription factor 3 | 7.0 | 8.94E-03 |
| 3958 | LGALS3 | Lectin, galactoside-binding, soluble, 3 | 6.8 | 4.49E-02 |
| 10457 | GPNMB | Glycoprotein (transmembrane) nmb | 6.3 | 1.18E-02 |
| 5997 | RGS2 | Regulator of G protein signaling 2 (24 kDa) | 5.7 | 8.65E-03 |
| 6277 | S100A6 | S100 calcium binding protein A6 | 5.7 | 6.83E-04 |
| 56267 | CCBL2 | cysteine conjugate-β-lyase 2 | 5.2 | 3.54E-03 |
| 7060 | THBS4 | Thrombospondin 4 | 4.6 | 7.27E-03 |
| 1264 | CNN1 | Calponin 1, basic, smooth muscle | 4.3 | 7.57E-05 |
| 2331 | FMOD | Fibromodulin | 4.3 | 3.69E-02 |
| 4632 | MYL1 | Myosin, light chain 1, alkali; skeletal, fast | 4.3 | 8.44E-03 |
| 4016 | LOXL1 | Lysyl oxidase-like 1 | 4.1 | 1.20E-02 |
| 51186 | WBP5 | WW domain binding protein 5 | 4.0 | 1.87E-03 |
| Downregulated | ||||
| 200539 | ANKRD23 | Ankyrin repeat domain 23 | −6.4 | 5.02E-03 |
| 183 | AGT | Angiotensinogen | −4.3 | 2.16E-03 |
| 119391 | GSTO2 | Glutathione S-transferase omega 2 | −3.5 | 8.58E-03 |
| 30819 | KCNIP2 | Kv channel interacting protein 2 | −2.9 | 1.09E-03 |
| 9771 | RAPGEF5 | Rap guanine nucleotide exchange factor 5 | −2.8 | 6.64E-03 |
| 286133 | SCARA5 | Scavenger receptor class A, member 5 | −2.7 | 3.16E-03 |
| 9020 | MAP3K14 | Mitogen-activated protein kinase kinase kinase 14 | −2.7 | 7.17E-03 |
| 51421 | AMOTL2 | Angiomotin like 2 | −2.6 | 3.04E-04 |
| 137872 | ADHFE1 | Alcohol dehydrogenase, iron containing, 1 | −2.5 | 2.69E-04 |
| 80303 | EFHD1 | EF-hand domain family, member D1 | −2.5 | 1.82E-04 |
| 23301 | EHBP1 | EH domain binding protein 1 | −2.5 | 6.27E-03 |
| 10351 | ABCA8 | ATP-binding cassette, subfamily A, member 8 | −2.4 | 2.99E-03 |
| 145864 | HAPLN3 | Hyaluronan and proteoglycan link protein 3 | −2.4 | 2.54E-03 |
| 10580 | SORBS1 | Sorbin and SH3 domain containing 1 | −2.3 | 1.05E-02 |
| 4023 | LPL | Lipoprotein lipase | −2.3 | 3.10E-03 |
| 254428 | SLC41A1 | Solute carrier family 41, member 1 | −2.3 | 2.67E-02 |
| 83894 | TTC29 | Tetratricopeptide repeat domain 29 | −2.3 | 1.65E-02 |
| 2180 | ACSL1 | Acyl-CoA synthetase long-chain family member 1 | −2.2 | 1.15E-02 |
| 338645 | LUZP2 | Leucine zipper protein 2 | −2.2 | 1.89E-03 |
| 8991 | SELENP1 | Selenium binding protein 1 | −2.2 | 1.88E-03 |
Genes in bold are shared with Table 4.
Table 4.
Genes showing the most significant regulation in the RCS model
| GeneID | Gene Symbol | Description | Fold Change | P Value |
|---|---|---|---|---|
| Upregulated | ||||
| 6423 | SFRP2 | Secreted frizzled-related protein 2 | 250.7 | 1.11E-04 |
| 6696 | SPP1 | Secreted phosphoprotein 1 | 61.0 | 1.95E-05 |
| 4069 | LYZ | Lysozyme | 57.3 | 1.13E-09 |
| 3958 | LGALS3 | Lectin, galactoside-binding, soluble, 3 | 41.9 | 2.18E-05 |
| 2120 | ETV6 | Ets variant 6 | 37.8 | 5.27E-06 |
| 9547 | CXCL14 | Chemokine ligand 14 | 32.0 | 4.64E-06 |
| 10457 | GPNMB | Glycoprotein (transmembrane) nmb | 31.3 | 1.56E-05 |
| 3371 | TNC | Tenascin C | 23.1 | 1.06E-04 |
| 10409 | BASP1 | Membrane attached signal protein 1 | 22.8 | 1.38E-06 |
| 5918 | RARRES1 | Retinoic acid receptor responder | 20.7 | 3.30E-04 |
| 2335 | FN1 | Fibronectin 1 | 20.0 | 6.57E-04 |
| 8483 | CILP | Nucleotide pyrophosphohydrolase | 17.9 | 3.18E-05 |
| 6947 | TCN1 | Transcobalamin I | 14.1 | 3.31E-04 |
| 58527 | C6orf115 | Chromosome 6 open reading frame 115 | 13.8 | 1.28E-06 |
| 3310 | HSPA6 | Heat shock 70 kDa protein 6 (HSP70B′) | 13.6 | 3.95E-03 |
| 167681 | PRSS35 | Protease, serine, 35 | 13.5 | 2.37E-03 |
| 6277 | S100A6 | S100 calcium binding protein A6 | 13.2 | 3.13E-07 |
| 4878 | NPPA | Natriuretic peptide precursor A | 12.6 | 1.47E-04 |
| 1880 | GPR183 | G protein-coupled receptor 183 | 12.5 | 4.41E-07 |
| 56267 | CCBL2 | Cysteine conjugate-β-lyase 2 | 12.2 | 1.50E-04 |
| Downregulated | ||||
| 30819 | KCNIP2 | Kv channel interacting protein 2 | −7.4 | 6.74E-03 |
| 137872 | ADHFE1 | Alcohol dehydrogenase, iron containing, 1 | −7.0 | 2.60E-03 |
| 183 | AGT | Angiotensinogen | −5.8 | 5.30E-04 |
| 201140 | DHRS7C | Dehydrogenase/reductase member 7C | −5.6 | 8.08E-03 |
| 149297 | FAM78B | Family with sequence similarity 78, member B | −5.5 | 3.29E-02 |
| 131034 | CPNE4 | Copine IV | −5.5 | 2.14E-03 |
| 55331 | ACER3 | Alkaline ceramidase 3 | −5.4 | 5.68E-04 |
| 140456 | ASB11 | Ankyrin repeat and SOCS box-containing 11 | −5.0 | 7.27E-03 |
| 8322 | FZD4 | Frizzled homolog 4 | −4.6 | 1.21E-02 |
| 286133 | SCARA5 | Scavenger receptor class A, member 5 | −4.6 | 1.15E-04 |
| 92196 | DAPL1 | Death associated protein-like 1 | −4.3 | 1.47E-02 |
| 1803 | DPP4 | Dipeptidyl-peptidase 4 | −4.3 | 2.75E-04 |
| 4504 | MT3 | Metallothionein 3 | −4.2 | 1.64E-03 |
| 4051 | CYP4F3 | Cytochrome P-450, family 4F3 | −4.0 | 8.07E-03 |
| 23057 | NMNAT2 | Nicotinamide nucleotide adenylyltransferase 2 | −3.9 | 4.09E-03 |
| 54765 | TRIM44 | Tripartite motif-containing 44 | −3.6 | 6.43E-04 |
| 9172 | MYOM2 | Myomesin 2, 165 kDa | −3.6 | 5.10E-03 |
| 84239 | ATP13A4 | ATPase type 13A4 | −3.4 | 6.47E-03 |
| 6258 | RXRG | Retinoid X receptor, γ | −3.4 | 9.39E-03 |
| 8462 | KLF11 | Kruppel-like factor 11 | −3.4 | 1.28E-02 |
Validation of the microarrays.
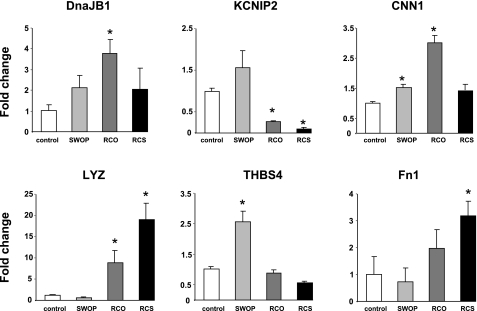
Validation of the microarrays shown on Fig. 4 was performed by qPCR, normalized per cyclophilin transcript, in frozen samples from swine hearts subjected to each of the three protocols and compared with sham-operated animals for specific target genes showing significant change in expression in any of the three groups studied (Tables 2–4). For most of the validated genes, the expression profile measured by qPCR followed the one found by microarrays, with few exceptions. For example, thrombospondin 4 showed a significant upregulation in the SWOP group, in agreement with the microarray data (Table 2), but not in the RCO group, at the opposite of the regulation shown in Table 3, and was significantly downregulated in the RCS model (Fig. 4). Although the global profile was very similar between microarrays and qPCR, the quantitative fold change in expression showed some differences, possibly because of the different sensitivity of both techniques.
Fig. 4.
Validation of the microarray data by quantitative PCR. Absolute transcript values for each gene of interest measured in individual RNA samples (n = 3/group) were reported per cyclophilin transcript to correct for sample-to-sample loading variations and subsequently reported as a fold change vs. controls for easier comparison with the microarray data. The abbreviations correspond to those shown in Tables 2–4. DnaJB1, DnaJ (40-kDa heat shock protein) homolog, subfamily B, member 1; KCNIP2, voltage-gated K+ channel interacting protein 2; CNN1, calponin 1; LYZ, lysozyme; THBS4, thrombospondin 4; Fn1, fibronectin 1. *P < 0.05 vs. control.
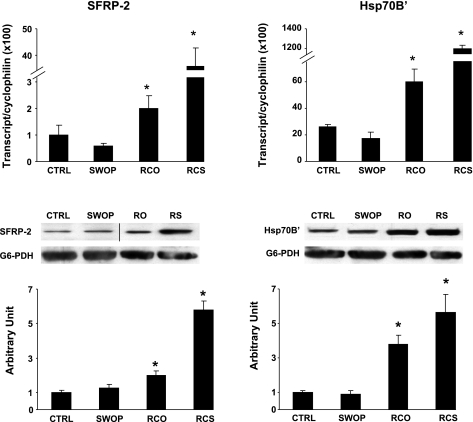
We further validated the expression of the genes showing the most important regulation in RCO and RCS by both qPCR and by Western blotting (normalized by glucose-6-phosphate dehydrogenase), as shown in Fig. 5. The transcript level of both the sfrp-2 and Hsp70BĆ were significantly upregulated in both models of RCO and RCS but not in SWOP, in agreement with the microarray data (Fig. 5). However, the extent of upregulation of these transcripts was very different between both models, with a 2- to 3-fold increase in RCO compared with a 40- to 60-fold increase in RCS (Fig. 5). The expression of the corresponding proteins increased to the same extent as the transcripts in the RCO model (Fig. 5). However, the increased protein abundance of sfrp-2 and Hsp70BĆ in the RCS model was about fivefold, which is 10 times less than the increased concentration of the corresponding transcripts (Fig. 5), suggesting the possibility of defects in posttranscriptional mechanisms in the RCS condition.
Fig. 5.
Validation of genes and proteins specifically upregulated by a repetitive pattern of ischemia. Quantitative PCR and Western blotting is shown for secreted frizzled-related protein 2 (sfrp-2) and the heat shock 70-kDa protein A6 (Hsp70BĆ) gene and protein expression, using cyclophilin transcript and glucose-6-phosphate dehydrogenase (G6-PDH) as normalizers, respectively. *P < 0.05 vs. sham. The bar graph for the immunoblottings represents the average ± SE for n = 4/group. One representative example of the immunoblottings is shown for each protein measured. The black line on the immunoblotting of sfrp-2 indicates that two separate areas of the film were chosen for representative illustration.
Comparison by GO.
It is also our hypothesis that the nature of the preconditioning stimulus will dictate the amplitude of the gene response. To address that hypothesis, we compared by cluster analysis all of the genes showing a significant regulation in the three models, as shown in Fig. 6. This cluster analysis confirmed that, for a vast majority of genes, the regulation of expression found in the RCO model was intermediate between that found in SWOP and in RCS models (Fig. 6). This analysis also showed that the RCS and SWOP models actually show a reciprocal pattern of regulation for many genes (Fig. 6).
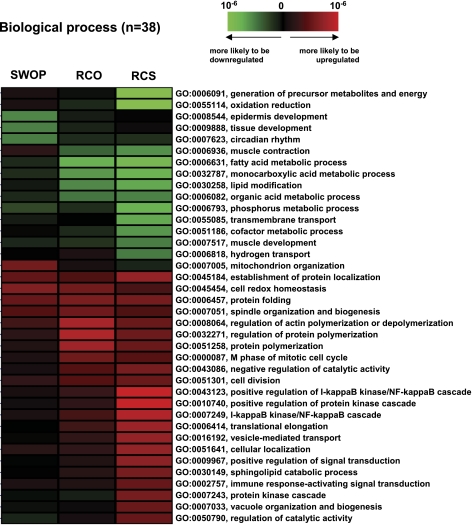
Fig. 6.
Gene ontology (GO) analysis. Genes regulated in the three models were regrouped by GO based on biological processes. Thirty eight GO categories showed a significant regulation in the three models, among which 68% presented a pattern of “progressive regulation” between SWOP, RCO, and RCS. The significance of association was statistically assessed using the hypergeometric test. The color intensity of each cell represents the level of GO association with a group of up/downregulated genes. As shown in the color scale, GO association with upregulated genes was marked by red, and that with downregulated genes was marked by green.
To further characterize these differences, all of the genes regulated in the three models were regrouped by GO based on biological processes (Fig. 6). Out of the 38 GO categories showing a significant regulation in the three models, 68% of GO terms presented a pattern of “progressive regulation” in which the amplitude of change in gene expression (either upregulation or downregulation) progressively increased when comparing SWOP, RCO, and RCS (Fig. 6). In particular, out of the 20 GO groups showing the most significant regulation in the RCS model (either up- or downregulation), 80% of them reproduced this progressive pattern. Clustering the genes by GO (Fig. 6) also revealed that, in the RCO and RCS models but not in the SWOP, the categories showing the most significant downregulation mainly involve the genes participating in oxidative reduction, whereas the most significantly upregulated GO categories involve genes participating in protein turnover (folding, polymerization, elongation, transport, unfolded protein response) and in signal transduction (in particular, the NF-κB signaling pathway). Interestingly, the upregulation of the GO category participating in vesicle-mediated protein transport (Fig. 6) correlates with the upregulation of genes encoding secreted proteins, as shown above. Similarly, the upregulation of the category involved in vacuole organization and biogenesis correlates with the activation of the cytoprotective mechanism of autophagy, which we described previously in the model of RCS (27).
We further considered all the individual genes regrouped by GO analysis in Fig. 6. Individually, 65% of all these genes (i.e., 805 individual genes out of 1,245) followed this progressive pattern of regulation in which the RCO model was intermediate between SWOP and RCS (Fig. 7). Reciprocally, only 5% of the genes showed a regulation that was more important in the model of SWOP compared with both RCO and RCS, whereas only 6% of genes were regulated in RCS and RCO more than in SWOP (Fig. 7). Therefore, the vast majority of genes regulated in the three models show a pattern of regulation that reproduces the intensity of the ischemic stimulus, i.e., the lowest amplitude of changes in the SWOP group and the highest in the RCS group, with RCO in between. This progressive pattern may help explain why several genomic mechanisms are activated in the “repetitive” models but not in the SWOP. Table 5 shows the identity of the 20 genes showing the most significant regulation (either upregulation or downregulation) among all the genes sharing this progressive pattern of regulation.
Table 5.
Genes showing the most important progressive regulation among the three models
| Gene ID | Gene Symbol | Description | SWOP | RCO | RCS |
|---|---|---|---|---|---|
| Upregulated | |||||
| 6696 | SPP1 | Secreted phosphoprotein 1 | 1.7 | 28.4 | 61.0 |
| 10924 | SMPDL3A | Sphingomyelin phosphodiesterase | 1.0 | 2.3 | 12.0 |
| 258 | AMBN | Ameloblastin | 1.1 | 2.8 | 11.5 |
| 822 | CAPG | Capping protein | 1.3 | 2.9 | 10.5 |
| 26287 | ANKRD2 | Ankyrin repeat domain 2 | 1.4 | 2.4 | 9.3 |
| 217 | ALDH2 | Aldehyde dehydrogenase 2 family | 1.1 | 2.0 | 8.0 |
| 5166 | PDK4 | Pyruvate dehydrogenase kinase, isozyme 4 | −7.5 | −5.1 | −2.4 |
| 4016 | LOXL1 | Lysyl oxidase-like 1 | 1.9 | 4.1 | 6.7 |
| 8564 | KMO | Kynurenine 3-monooxygenase | 1.3 | 2.1 | 6.4 |
| 6195 | RPS6KA1 | Ribosomal protein S6 kinase (90 kDa), peptide 1 | 1.4 | 2.1 | 6.3 |
| 23643 | LY96 | Lymphocyte antigen 96 | 1.1 | 2.5 | 6.3 |
| 85477 | SCIN | Scinderin | 1.1 | 2.3 | 5.7 |
| 6238 | RRBP1 | Ribosome binding protein 1 homolog | 1.8 | 2.1 | 5.4 |
| 1277 | COL1A1 | Collagen, type I, α1 | −1.1 | 1.6 | 5.3 |
| 2876 | GPX1 | Glutathione peroxidase 1 | 1.0 | 2.7 | 5.0 |
| 3756 | KCNH1 | Potassium voltage-gated channel, subfamily H | 1.2 | 1.8 | 4.8 |
| 5831 | PYCR1 | Pyrroline-5-carboxylate reductase 1 | 1.2 | 1.9 | 4.8 |
| 11015 | KDELR3 | Endoplasmic reticulum protein receptor 3 | 1.1 | 2.1 | 4.8 |
| 3939 | LDHA | Lactate dehydrogenase A | 2.1 | 3.1 | 4.5 |
| 5352 | PLOD2 | 2-Oxoglutarate 5-dioxygenase 2 | 1.4 | 1.7 | 4.5 |
| Downregulated | |||||
| 183 | AGT | Angiotensinogen | −1.1 | −4.3 | −5.8 |
| 23057 | NMNAT2 | Nicotinamide nucleotide Adenylyltransferase 2 | −1.4 | −1.9 | −3.9 |
| 9172 | MYOM2 | Myomesin 2 | 1.1 | −1.0 | −3.6 |
| 1593 | CYP27A1 | Cytochrome P-450, family 27, subfamily A1 | −1.1 | −1.8 | −3.3 |
| 65268 | WNK2 | WNK lysine-deficient protein kinase 2 | −1.1 | −1.9 | −3.1 |
| 56034 | PDGFC | Platelet-derived growth factor C | −1.2 | −1.4 | −2.9 |
| 10449 | ACAA2 | Acetyl-CoA acyltransferase 2 | 1.0 | −1.4 | −2.8 |
| 203447 | NRK | Nik-related kinase | −1.1 | −2.1 | −2.6 |
| 57544 | TXNDC16 | Thioredoxin domain containing 16 | −1.1 | −1.7 | −2.6 |
| 65018 | PINK1 | PTEN-induced putative kinase 1 | −1.0 | −2.0 | −2.5 |
| 3712 | IVD | Isovaleryl CoA dehydrogenase | 1.0 | −1.9 | −2.5 |
| 5321 | PLA2G4A | Phospholipase A2, group IVA | −1.1 | −1.6 | −2.5 |
| 10370 | CITED2 | Cbp/p300-interacting transactivator | 1.1 | −2.1 | −2.5 |
| 37 | ACADVL | Acyl-CoA dehydrogenase | −1.2 | −1.6 | −2.5 |
| 10105 | PPIF | Peptidylprolyl isomerase F | 1.3 | −1.3 | −2.4 |
| 91851 | CHRDL1 | Chordin-like 1 | 1.1 | −1.4 | −2.4 |
| 51535 | PPHLN1 | Periphilin 1 | −1.3 | −1.8 | −2.4 |
| 30001 | ERO1L | ERO1-like | 1.1 | −1.2 | −2.3 |
| 5775 | PTPN4 | Protein tyrosine phosphatase | −1.0 | −1.6 | −2.3 |
| 9252 | RPS6KA5 | Ribosomal protein S6 kinase (90 kDa), peptide 5 | 1.1 | −1.8 | −2.2 |
DISCUSSION
Having observed major differences in the molecular mechanisms mediating preconditioning induced by classical SWOP vs. RCS (22), the question arises as to whether these differences were due to differences between complete CAO used for SWOP vs. coronary stenosis used for repetitive ischemia, or due to differences resulting from the repetitive pattern of ischemia. The main finding of our study is that the signature of the adaptation of gene expression in response to coronary blood flow reduction is largely dictated by the repetitive pattern of ischemia rather than by the difference between CAO and stenosis. This conclusion is based on the fact that the preconditioning stimulus is exactly the same in both SWOP and RCO models (two cycles of 10 min CAO and 10 min coronary artery reperfusion), yet the RCO model is by far closer to RCS than to SWOP in terms of gene expression. This demonstrates that the repetitive pattern of coronary blood flow reduction, rather than the ischemic stimulus itself, plays a major role in the adaptation of gene expression in the ischemic heart. Therefore, the results from the current investigation show that the importance of a repetitive pattern of ischemia supersedes the importance of a quantitative difference in blood flow reduction between a model where coronary blood flow is totally interrupted (RCO) and a model in which coronary blood flow is partially reduced (RCS).
Our experiments also show that the models of RCO and RCS display several specific mechanisms of cytoprotection that are not activated in SWOP, in addition to the differences due to NO protection discussed earlier. There are five groups of molecular mechanisms that differ from SWOP: autophagy, secreted proteins, heat shock proteins (Hsps), NF-κB signaling, and mitochondrial respiration.
The first of these mechanisms, which we described before (26, 27), is autophagy. Autophagy is a mechanism by which the cell under stress may remove dysfunctional intracellular components, such as protein aggregates or damaged mitochondria, and its cytoprotective effects have been demonstrated both in vitro and in vivo (8, 9). The activation of autophagy at the gene level is confirmed in our study by the GO analysis showing an upregulation of genes participating in vacuole biogenesis (Fig. 5).
A second mechanism illustrated by the individual gene analysis (Tables 3 and 4) is the increased production of secreted proteins. Among them, the major upregulation of the sfrp-2 is of particular importance, since this protein was shown recently to mediate the cytoprotective effects of mesenchymal stem cells on the ischemic heart (13). sfrp-2 is produced downstream of the survival kinase Akt (15) and restores cardiac cell survival by interfering with the Wnt signaling pathway (5, 30). However, the signaling mechanisms of sfrp-2 in the myocardium and the mechanisms controlling its production and release remain largely unknown. Our models of RCO and RCS in the swine are the first to describe an upregulation of sfrp-2 in the ischemic heart in vivo. This represents a potentially novel signaling mechanism of cardiac cell survival in response to repetitive myocardial ischemia, since it is not activated in the SWOP model.
A third mechanism, also found from the individual gene analysis (Tables 3 and 4), is the activation of specific Hsps. As shown in our study, both the RCO and RCS models are characterized by an increased gene expression of specific Hsps, in particular Hsp70BĆ. This isoform is particularly interesting because it is strictly induced by cytotoxic stress (19), it promotes cell survival, and it is expressed predominantly in large mammals (18). As for sfrp-2, the upregulation of Hsp70BĆ responds specifically to repetitive ischemia and is not activated by SWOP. Although it is most likely involved in cardiac cell survival, the exact role of Hsp70BĆ in myocardium remains totally unknown at this point.
A fourth mechanism illustrated by the GO analysis (Fig. 5) is the upregulation of the NF-κB signaling pathway, which is much more prevalent in RCO and RCS than in SWOP. Although NF-κB is involved in the mechanisms of SWOP, especially in terms of increased expression of iNOS (3), it is possible that its further activation in the models of repetitive ischemia amplifies its mechanisms of cytoprotection. The paradox that iNOS expression is not affected in the RCS model (22), despite the marked activation of the NF-κB signaling pathway as shown here, remains to be elucidated.
A fifth mechanism of cardioprotection specific for the models of repetitive ischemia is the marked downregulation of the expression of genes encoding enzymes of mitochondrial oxidation and respiration, as shown in the GO analysis (Fig. 5). This observation has been correlated previously at the protein level (20, 22). Although a decrease in oxidative metabolism may, at first, seem to be deleterious for the contractile heart, it could provide cytoprotection by decreasing the generation of reactive oxygen species (ROS) from mitochondria. Also, the models of RCO and RCS are characterized by a loss in regional contractile function (“chronic stunning”), which thereby decreases energy needs from oxidative metabolism. It has been proposed that the protection conferred by NO during the SWOP is also related to decreased activity of mitochondrial respiratory complexes, and thereby decreased generation of ROS (7). The fact that mitochondrial function is affected at the gene level rather than by NO production may explain why the cardioprotection provided by the models of repetitive ischemia is NO independent.
Although very close from the human condition of ischemic heart disease, the pig model remains descriptive by essence, and further validation of these potential pathways of cell survival will require more mechanistic insight from genetically modified mouse models. It will also remain to be elucidated whether these protective mechanisms may also apply to the more recent concept of ischemic postconditioning (24), which has probably more relevance on a clinical point-of-view than IPC because of the impossibility to predict the occurrence of potentially lethal ischemia in patients (11).
In conclusion, our observations demonstrate that the models of repetitive CAO and stenosis develop mechanisms of cardioprotection radically different from those developed by classical models of SWOP. These models differ, not only in terms of dependence of NO, i.e., upregulation of NO was central to mediating SWOP, but not RCS and RCO, but also in both quantitative and qualitative differences in genes regulated by classical SWOP vs. the two models of repetitive ischemia. In particular, RCS and RCO activate several mechanisms of cell survival that remain dormant in the classical conditions of SWOP. Considering that patients with ischemic heart disease are typically exposed to repetitive bouts of ischemia-reperfusion, our findings are clinically relevant and may be helpful in translating experimental data on cardioprotection to patients.
GRANTS
This work was supported in part by National Institutes of Health Grants AG-027211, HL-033107, HL-059139, HL-69752, HL-095888, HL-069020, AG-023137, and AG-014121.
DISCLOSURES
There are no disclosures.
REFERENCES
- 1.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G. Gene ontology: tool for the unification of biology. Gene Ontol Consortium Nat Genet 25: 25–29, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bolli R. Cardioprotective function of inducible nitric oxide synthase and role of nitric oxide in myocardial ischemia and preconditioning: an overview of a decade of research. J Mol Cell Cardiol 33: 1897–1918, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Bolli R. The late phase of preconditioning. Circ Res 87: 972–983, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159, 1987 [DOI] [PubMed] [Google Scholar]
- 5.Deb A, Davis B, Guo J, Ni A, Huang J, Zhang Z, Mu H, Dzau V. SFRP2 regulqtes cardiomyogenic differentiation by inhibiting a positive transcriptional autofeedback loop of Wnt3a. Stem Cells 26: 35–44, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Depre C, Shipley GL, Chen W, Han Q, Doenst T, Moore ML, Stepkowski S, Davies PJ, Taegtmeyer H. Unloaded heart in vivo replicates fetal gene expression of cardiac hypertrophy. Nat Med 4: 1269–1275, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Halestrap A, Clarke S, Khaliulin I. The role of mitochondria in protection of the heart by preconditioning. Biochim Biophys Acta 1767: 1007–1031, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamacher-Brady A, Brady NR, Gottlieb RA. Enhancing macroautophagy protects against ischemia/reperfusion injury in cardiac myocytes. J Biol Chem 281: 29776–29787, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Hamacher-Brady A, Brady NR, Gottlieb RA, Gustafsson AB. Autophagy as a protective response to Bnip3-mediated apoptotic signaling in the heart. Autophagy 2: 307–309, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Kim SJ, Peppas A, Hong SK, Yang G, Huang Y, Diaz G, Sadoshima J, Vatner DE, Vatner SF. Persistent stunning induces myocardial hibernation and protection: flow/function and metabolic mechanisms. Circ Res 92: 1233–1239, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Kloner R, Rezkalla S. Preconditioning, postconditioning and their application to clinical cardiology. Cardiovasc Res 70: 297–307, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Kuzuya T, Hoshida S, Yamashita N, Fuji H, Oe H, Hori M, Kamada T, Tada M. Delayed effects of sublethal ischemia on the acquisition of tolerance to ischemia. Circ Res 72: 1293–1299, 1993 [DOI] [PubMed] [Google Scholar]
- 13.Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, Dzau VJ. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance in infacrted hearts. Nat Med 9: 1195–1201, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Marber MS, Latchman DS, Walker JM, Yellon DM. Cardiac stress protein elevation 24 hours after brief ischemia or heat stress is associated with resistance to myocardial infarction. Circulation 88: 1264–1272, 1993 [DOI] [PubMed] [Google Scholar]
- 15.Mirotsou M, Zhang Z, Deb A, Zhang L, Gnecchi M, Noiseux N, Mu H, Pachori A, Dzau V. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci USA 104: 1643–1648, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 74: 1124–1136, 1986 [DOI] [PubMed] [Google Scholar]
- 17.Murry CE, Richard VJ, Jennings RB, Reimer KA. Myocardial protection is lost before contractile function recovers from ischemic preconditioning. Am J Physiol Heart Circ Physiol 260: H796–H804, 1991 [DOI] [PubMed] [Google Scholar]
- 18.Noonan E, Giardina C, Hightower L. Hsp70BĆ and Hsp72 form a complex in stressed human colon cells and each contributes to cytoprotection. Exp Cell Res 314: 2468–2476, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Noonan E, Place R, Rasoulpour R, Giardina C, Hightower L. Cell number-dependent regulation of Hsp70BĆ expression: evidence of an extracellular regulator. J Cell Physiol 210: 201–211, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Page B, Young R, Iyer V, Suzuki G, Lis M, Korotchkina L, Patel MS, Blumenthal KM, Fallavollita JA, Canty JM., Jr Persistent regional downregulation in mitochondrial enzymes and upregulation of stress proteins in swine with chronic hibernating myocardium. Circ Res 102: 103–112, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Qiu H, Tian B, Resuello RG, Natividad FF, Peppas A, Shen YT, Vatner DE, Vatner SF, Depre C. Sex-specific regulation of gene expression in the aging monkey aorta. Physiol Genomics 29: 169–180, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Shen YT, Depre C, Yan L, Park JY, Tian B, Jain K, Chen L, Zhang Y, Kudej RK, Zhao X, Sadoshima J, Vatner DE, Vatner SF. Repetitive ischemia by coronary stenosis induces a novel window of ischemic preconditioning. Circulation 118: 1961–1969, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsai S, Cassady JP, Freking BA, Nonneman DJ, Rohrer GA, Piedrahita JA. Annotation of the Affymetrix porcine genome microarray. Anim Genet 37: 423–424, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Vinten-Johansen J, Yellon DM, Opie LH. Postconditioning: a simple, clinically applicable procedure to improve revascularization in acute myocardial infarction. Circulation 112: 2085–2088, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Wijns W, Vatner SF, Camici PG. Hibernating myocardium. N Engl J Med 339: 173–181, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Yan L, Sadoshima J, Vatner DE, Vatner SF. Autophagy: a novel protective mechanism in chronic ischemia. Cell Cycle 5: 1175–1177, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Yan L, Vatner DE, Kim SJ, Ge H, Masurekar M, Massover WH, Yang G, Matsui Y, Sadoshima J, Vatner SF. Autophagy in chronically ischemic myocardium. Proc Natl Acad Sci USA 102: 13807–13812, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yellon DM, Baxter GF. A “second window of protection” or delayed preconditioning phenomenon: future horizons for myocardial protection? J Mol Cell Cardiol 27: 1023–1034, 1995 [DOI] [PubMed] [Google Scholar]
- 29.Yellon DM, Downey JM. Preconditioning the myocardium: from cellular physiology to clinical cardiology. Physiol Rev 83: 1113–1151, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Zhang Z, Deb A, Zhang Z, Pachori A, He W, Guo J, Pratt R, Dzau V. Secreted frizzled related protein 2 protects cells from apoptosis by blocking the effect of canonical Wnt3a. J Mol Cell Cardiol 46: 370–377, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]