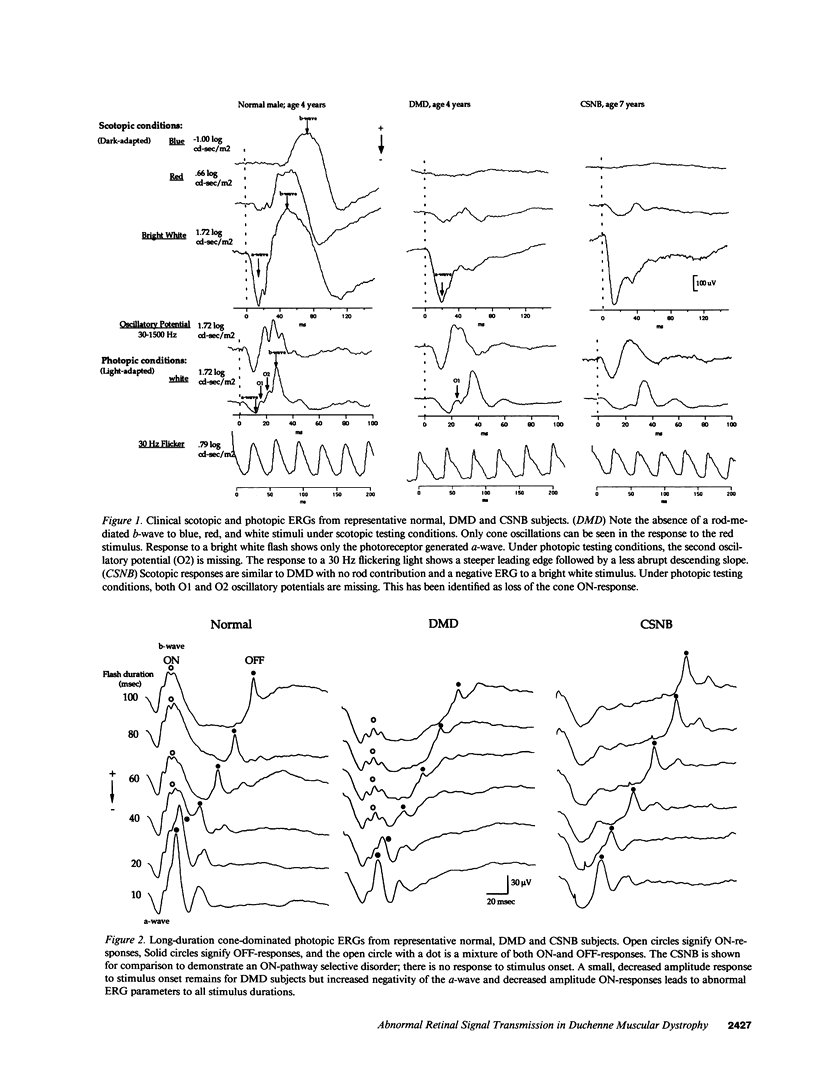
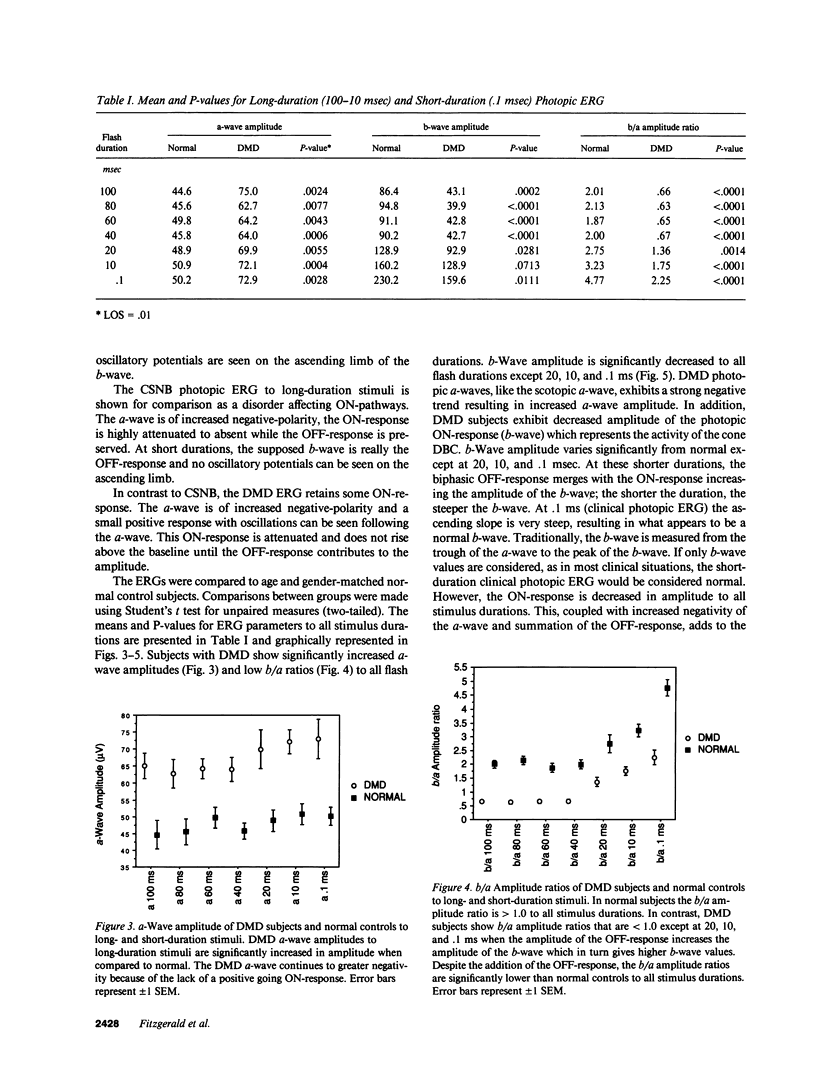
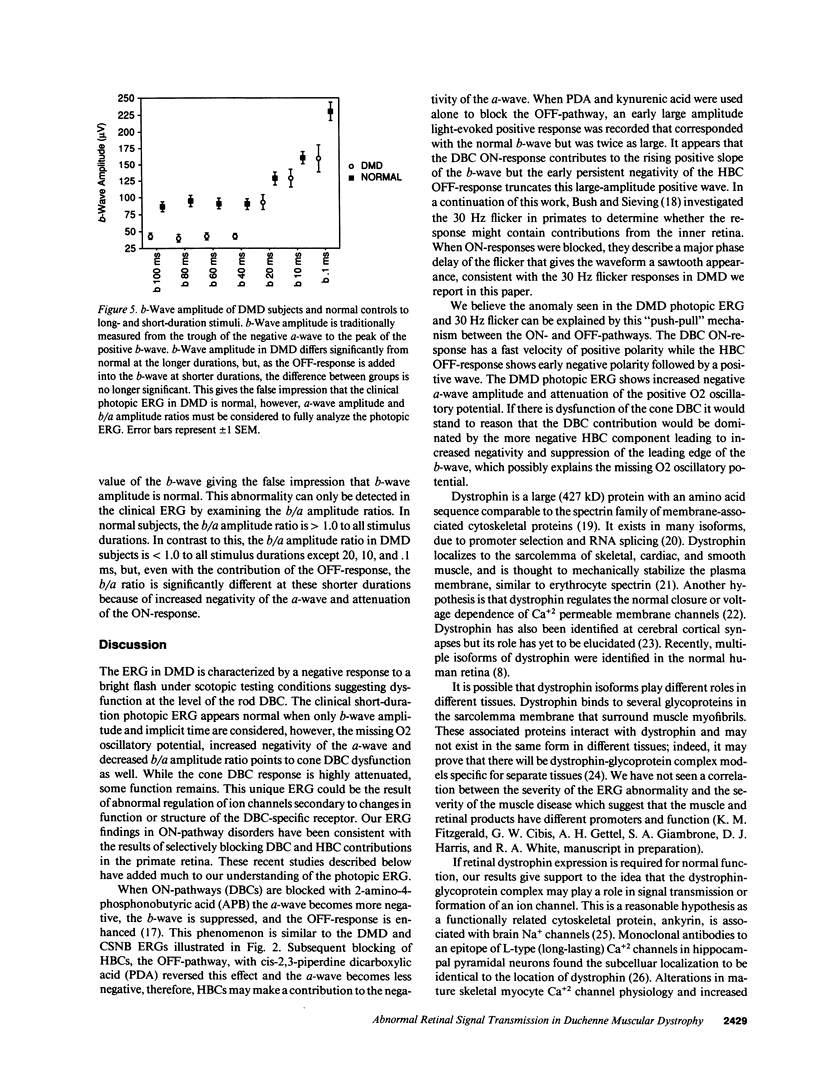
Abstract
There have been reports of abnormal retinal neurotransmission determined by electroretinography in boys with Duchenne and Becker muscular dystrophy. Dystrophin may play a role in transmitting signals between photoreceptors and the excitatory synapse of the ON-bipolar cell. These electroretinographic changes appeared to be limited to the rod ON-pathway but we felt there was also similar abnormality in the cone ON-pathway. We used long-duration stimuli to separate ON-(depolarizing bipolar cell) and OFF (hyperpolarizing bipolar cell) contributions to the cone-dominated ERG to better understand how the retina functions in boys with Duchenne muscular dystrophy. We recorded the electroretinograms of 11 boys with Duchenne muscular dystrophy and found abnormal signal transmission at the level of the photoreceptor and ON-bipolar cell in both the rod and cone generated responses. The OFF-bipolar cell that responds to the offset of the stimulus continues to function normally. The results support our hypothesis that retinal dystrophin plays a role in receptor function or controlling ion channels at the level of the photoreceptor and depolarizing bipolar cell.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander K. R., Fishman G. A., Peachey N. S., Marchese A. L., Tso M. O. 'On' response defect in paraneoplastic night blindness with cutaneous malignant melanoma. Invest Ophthalmol Vis Sci. 1992 Mar;33(3):477–483. [PubMed] [Google Scholar]
- Cibis G. W., Fitzgerald K. M., Harris D. J., Rothberg P. G., Rupani M. The effects of dystrophin gene mutations on the ERG in mice and humans. Invest Ophthalmol Vis Sci. 1993 Dec;34(13):3646–3652. [PubMed] [Google Scholar]
- Ervasti J. M., Campbell K. P. Dystrophin and the membrane skeleton. Curr Opin Cell Biol. 1993 Feb;5(1):82–87. doi: 10.1016/s0955-0674(05)80012-2. [DOI] [PubMed] [Google Scholar]
- Feener C. A., Koenig M., Kunkel L. M. Alternative splicing of human dystrophin mRNA generates isoforms at the carboxy terminus. Nature. 1989 Apr 6;338(6215):509–511. doi: 10.1038/338509a0. [DOI] [PubMed] [Google Scholar]
- Franco A., Jr, Lansman J. B. Calcium entry through stretch-inactivated ion channels in mdx myotubes. Nature. 1990 Apr 12;344(6267):670–673. doi: 10.1038/344670a0. [DOI] [PubMed] [Google Scholar]
- Gurevich L., Slaughter M. M. Comparison of the waveforms of the ON bipolar neuron and the b-wave of the electroretinogram. Vision Res. 1993 Dec;33(17):2431–2435. doi: 10.1016/0042-6989(93)90122-d. [DOI] [PubMed] [Google Scholar]
- Kaneko A., Pinto L. H., Tachibana M. Transient calcium current of retinal bipolar cells of the mouse. J Physiol. 1989 Mar;410:613–629. doi: 10.1113/jphysiol.1989.sp017551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig M., Monaco A. P., Kunkel L. M. The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell. 1988 Apr 22;53(2):219–228. doi: 10.1016/0092-8674(88)90383-2. [DOI] [PubMed] [Google Scholar]
- Kramarcy N. R., Sealock R. Dystrophin as a focal adhesion protein. Collocalization with talin and the Mr 48,000 sarcolemmal protein in cultured Xenopus muscle. FEBS Lett. 1990 Nov 12;274(1-2):171–174. doi: 10.1016/0014-5793(90)81356-s. [DOI] [PubMed] [Google Scholar]
- Lidov H. G., Byers T. J., Watkins S. C., Kunkel L. M. Localization of dystrophin to postsynaptic regions of central nervous system cortical neurons. Nature. 1990 Dec 20;348(6303):725–728. doi: 10.1038/348725a0. [DOI] [PubMed] [Google Scholar]
- Mehler M. F., Haas K. Z., Kessler J. A., Stanton P. K. Enhanced sensitivity of hippocampal pyramidal neurons from mdx mice to hypoxia-induced loss of synaptic transmission. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2461–2465. doi: 10.1073/pnas.89.6.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake Y., Yagasaki K., Horiguchi M., Kawase Y. On- and off-responses in photopic electroretinogram in complete and incomplete types of congenital stationary night blindness. Jpn J Ophthalmol. 1987;31(1):81–87. [PubMed] [Google Scholar]
- Pillers D. A., Bulman D. E., Weleber R. G., Sigesmund D. A., Musarella M. A., Powell B. R., Murphey W. H., Westall C., Panton C., Becker L. E. Dystrophin expression in the human retina is required for normal function as defined by electroretinography. Nat Genet. 1993 May;4(1):82–86. doi: 10.1038/ng0593-82. [DOI] [PubMed] [Google Scholar]
- Ripps H. Night blindness revisited: from man to molecules. Proctor lecture. Invest Ophthalmol Vis Sci. 1982 Nov;23(5):588–609. [PubMed] [Google Scholar]
- Schiller P. H. Central connections of the retinal ON and OFF pathways. Nature. 1982 Jun 17;297(5867):580–583. doi: 10.1038/297580a0. [DOI] [PubMed] [Google Scholar]
- Srinivasan Y., Elmer L., Davis J., Bennett V., Angelides K. Ankyrin and spectrin associate with voltage-dependent sodium channels in brain. Nature. 1988 May 12;333(6169):177–180. doi: 10.1038/333177a0. [DOI] [PubMed] [Google Scholar]
- Standard for clinical electroretinography. International Standardization Committee. Arch Ophthalmol. 1989 Jun;107(6):816–819. doi: 10.1001/archopht.1989.01070010838024. [DOI] [PubMed] [Google Scholar]
- Tamura T., Yoshioka K., Jinno Y., Niikawa N., Miike T. Dystrophin isoforms expressed in the mouse retina. J Neurol Sci. 1993 Apr;115(2):214–218. doi: 10.1016/0022-510x(93)90227-p. [DOI] [PubMed] [Google Scholar]
- Westenbroek R. E., Ahlijanian M. K., Catterall W. A. Clustering of L-type Ca2+ channels at the base of major dendrites in hippocampal pyramidal neurons. Nature. 1990 Sep 20;347(6290):281–284. doi: 10.1038/347281a0. [DOI] [PubMed] [Google Scholar]
- Wässle H., Yamashita M., Greferath U., Grünert U., Müller F. The rod bipolar cell of the mammalian retina. Vis Neurosci. 1991 Jul-Aug;7(1-2):99–112. doi: 10.1017/s095252380001097x. [DOI] [PubMed] [Google Scholar]



