Abstract
Burkholderia species use BimA for intracellular actin-based motility. Uniquely, Burkholderia thailandensis BimA harbors a central and acidic (CA) domain. The CA domain was required for actin-based motility, binding to the cellular Arp2/3 complex, and Arp2/3-dependent polymerization of actin monomers. Our data reveal distinct strategies for actin-based motility among Burkholderia species.
In common with selected species of Listeria, Shigella, Rickettsia, and Mycobacterium, some members of the genus Burkholderia are capable of intracellular actin-based motility (reviewed in reference 9). Such motility promotes cell-to-cell spread in the absence of immune surveillance and, in some cases, escape from autophagy. The melioidosis pathogen Burkholderia pseudomallei forms actin-rich bacterium-containing membrane protrusions (5), in a manner that requires BimA (Burkholderia intracellular motility A) (11). B. pseudomallei BimA exhibits C-terminal homology to the Yersinia autosecreted adhesin YadA and possesses motifs associated with actin binding, including WASP homology 2 (WH2) domains and proline-rich motifs (11). Actin-based motility is also a feature of infection by the glanders pathogen Burkholderia mallei and the avirulent B. pseudomallei-like species Burkholderia thailandensis (10). BimA homologs exist in these species that can compensate for the actin-based motility defect of a B. pseudomallei bimA mutant (10), and mutation of B. mallei bimA results in loss of function (7). BimA homologs from B. mallei and B. thailandensis (BimAth) differ markedly in primary sequence from the B. pseudomallei protein (BimAps) and each other (10), raising the possibility that they may act in distinct ways. Intraspecies conservation of BimA is high in natural populations of B. pseudomallei, with the exception of a geographically restricted B. mallei-like BimA variant (8).
Mechanisms of bacterial actin-based motility converge on activation of the Arp2/3 (actin-related protein 2/3) complex. Activation of Arp2/3 requires cellular nucleation-promoting factors (NPFs) such as Wiskott-Aldrich syndrome protein (WASP) family members, and pathogens capable of actin-based motility often mimic the activity of NPFs or recruit and activate them at the bacterial pole (reviewed in references 3 and 9). Arp2/3 is localized throughout B. pseudomallei-induced actin tails (2); however, the role that it plays in actin-based motility is unclear, as expression of an inhibitory domain of the cellular NPF Scar1 does not interfere with actin-based motility of B. pseudomallei (2). Moreover, BimAps can polymerize actin in vitro in an Arp2/3-independent manner (11).
Recruitment and activation of the Arp2/3 complex by cellular and pathogen-associated NPFs require one or more WH2 domains and an amphipathic central and acidic (CA) domain (3, 6). Analysis of the primary sequence of BimA homologs revealed a CA domain in B. thailandensis BimA that was absent in the BimA proteins of other Burkholderia species and conserved relative to WASP family members, Listeria ActA, and Rickettsia RickA (10). Here we surveyed the conservation of the CA domain and probed its role in actin-based motility and the binding and activation of the Arp2/3 complex.
Primers were designed to amplify an 87-bp region of the CA domain (corresponding to amino acid residues 102 to 130 inclusive) of the bimA gene of the sequenced B. thailandensis strain E264 (5′-AGGCGGGTAATCGACTCA-3′ and 5′-TTCGTCGTCCGACCATGA-3′). These primers, and universal bimA-specific primers (8), were used to screen 203 Burkholderia isolates for bimA and the CA domain by PCR using Platinum Taq DNA polymerase (Invitrogen, Paisley, United Kingdom) and boiled lysates of single colonies as template. The strain collection was described previously (8), supplemented by an additional 52 B. pseudomallei, 23 B. thailandensis, and 19 B. mallei isolates and 10 other isolates representing 5 other Burkholderia species. A bimA amplicon was detected for all isolates of species B. pseudomallei, B. thailandensis, B. mallei, and Burkholderia oklahomensis. Consistent with analysis of sequenced genomes (8), the CA domain was restricted to, and always found in, B. thailandensis isolates. Such a test may prove useful to rapidly discriminate between avirulent B. thailandensis and the closely related biothreat agents B. pseudomallei and B. mallei.
To investigate the function of the CA domain, we deleted the region corresponding to residues 96 to 130 of the strain E264 BimA by PCR-ligation-PCR (1) using the cloned bimA gene of B. thailandensis strain E30 as a template (10). Pfu proofreading DNA polymerase was used to separately amplify the region 5′ of the CA domain with primers Bth-comp forward (5′-CATGAATTCCCATGCGTGCAACAGTTGCT-3′) and 5′-CGAGCCGCCCGCGCCTCGCGTGTT-3′ and the region 3′ of the CA domain with Bth-comp reverse (5′-CTTCTCGAGTCACCATTGCCAGCTCATGCCTACGC-3′) and 5′-TCCCCTCCGCCGACGCCGATCGCAA-3′ from pME6032- bimAth (10). The PCR amplicons were ligated, and the desired recombinant was amplified by a further round of PCR with primers Bth-comp forward and Bth-comp reverse. The product was subcloned under the Ptac promoter in pME6032 (4) via EcoRI and XhoI sites incorporated in the primers (underlined), yielding pME6032-bimAth-ΔCA. Faithful amplification and deletion of the CA domain were confirmed by nucleotide sequencing (data not shown).
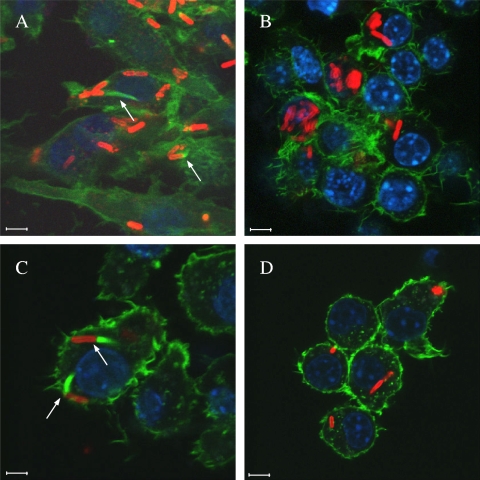
To evaluate the role of the CA domain in actin-based motility, pME6032-bimAth and the ΔCA variant were introduced into a B. pseudomallei strain 10276 bimA::pDM4 mutant (11) by electroporation with selection for tetracycline resistance. Strains were amplified in Luria-Bertani (LB) medium, and J774.2 murine macrophage-like cells were infected at a multiplicity of 10 in RPMI medium containing 10% (vol/vol) fetal calf serum at 37°C in a 5% CO2 atmosphere. After 30 min cells were washed and overlaid with medium containing 250 μg/ml kanamycin to kill extracellular bacteria. During bacterial culture and cell infection, expression of BimA proteins was induced with 0.25 mM isopropyl-β-d-thiogalactoside (IPTG). Eight hours postinfection cells were washed, fixed, permeabilized, and stained for bacteria, polymerized F-actin, and nuclei essentially as described previously (10). Images were captured using a Leica confocal laser scanning microscope with LAS AF v.2.0 software. B. pseudomallei 10276 and 10276 bimA::pDM4 were included as positive and negative controls, respectively (Fig. 1 A and B). As expected B. thailandensis E30 BimA restored the ability of the 10276 bimA::pDM4 mutant to form actin tails (10) (Fig. 1C). Deletion of the CA domain of B. thailandensis BimA abolished this activity (Fig. 1D), indicating that it is required for actin-based motility.
FIG. 1.
Representative confocal laser scanning micrographs of J774.2 cells infected with B. pseudomallei strain 10276 (A), an isogenic bimA::pDM4 mutant (B), or 10276 bimA::pDM4 trans complemented with pME6032-bimAth (C) or pME6032-bimAth-ΔCA (D) and induced to express the proteins under IPTG induction. Bacteria (red) were stained using mouse monoclonal anti-B. pseudomallei lipopolysaccharide antibody (Camlab, Cambridge, United Kingdom) detected with anti-mouse Ig-Alexa Fluor568 (Molecular Probes, Leiden, Netherlands). F-actin (green) was stained with Alexa Fluor488-conjugated phalloidin. DNA (blue) was stained with 4′,6-diamidino-2-phenylindole. Bars, 5 μm. Bacteria forming actin tails are marked with arrows.
Monoclonal antibodies raised against BimAps (11) failed to recognize BimAth on the bacterial pole (data not shown); therefore, we were unable to conclude that loss of actin-based motility upon deletion of the CA domain may be a consequence of failed secretion or polar localization. To investigate the role of the CA domain in actin binding and polymerization, the ΔCA variant of B. thailandensis BimA was PCR amplified from pME6032-bimAth-ΔCA with primers 5′-GGGCCCGGATCCGCCGCTGACGAGACG-3′ and 5′-GGGCCCGAATTCTCACGCTCGCGCGTCG-3′. The product was first cloned by a topoisomerase-mediated process into pCR2.1-TOPO (Invitrogen, Paisley, United Kingdom) and then subcloned as a BamHI and EcoRI fragment into similarly digested pGEX-2T-1 (Amersham Biosciences, Buckinghamshire, United Kingdom), creating a fusion to the carboxyl terminus of glutathione S-transferase (GST). The subcloned region corresponds to amino acids 47 to 386 of BimAth and was confirmed by sequencing to be identical to the pGEX-BimAth plasmid described previously (10), except for the deleted CA region. The region encoding B. pseudomallei BimA residues 54 to 455 (lacking the signal peptide and membrane anchor) was amplified from pME6032-bimAps (10) with primers 5′-GCGCGCGGATCCATGAATCCCCCCGAACCGCCGGGC-3′ and 5′-GCGCGCGAATTCTTAGCGCGCGGTGTCGGTG-3′ and fused to GST in pGEX-4T-1 as described above. Plasmids encoding GST or GST fusions to BimAps, BimAth, or BimAth-ΔCA domain were separately introduced into Escherichia coli K-12 Rosetta2(DE3)pLysS cells expressing rare aminoacyl tRNAs (Merck Biosciences, Nottingham, United Kingdom). LB cultures were induced to express the proteins at late logarithmic phase for 3 h at ambient temperature, which proved optimal for recovery of intact proteins using glutathione Sepharose 4B beads (Fig. 2A).
FIG. 2.
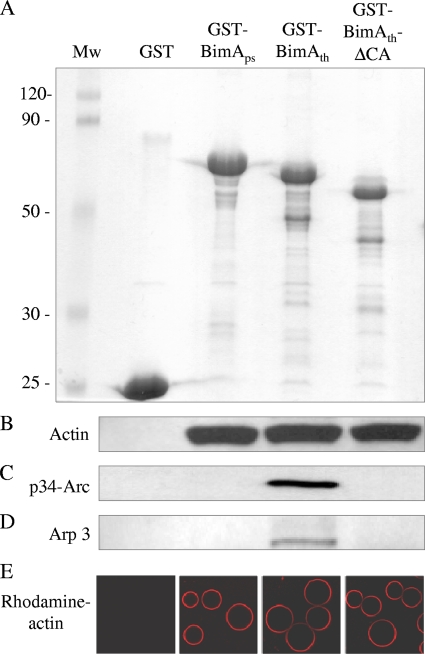
SDS-PAGE analysis of fusions of BimAps (residues 54 to 455), BimAth (residues 47 to 386), or BimAth lacking the CA domain to the carboxyl terminus of GST (A). Sepharose beads coated with these proteins, or GST alone, were examined for their ability to sequester actin (B), p34-Arc/ARPC2 (C), or Arp3 (D) from murine splenic extracts by Western blotting of bead-associated proteins using specific antibodies. Coated beads were also examined for their ability to bind highly purified rhodamine-labeled actin in PBS (red) by confocal laser scanning microscopy (E). Mw, molecular weights in thousands.
Beads coated with GST, GST-BimAps, GST-BimAth, or GST-BimAth-ΔCA were incubated with murine splenic cell lysate as described previously (10). After 15 min of incubation at ambient temperature, beads were washed with ice-cold Tris-buffered saline and analyzed by sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis and Western blotting with antiactin antibody (10). As reported previously, beads coated with GST-BimAps or GST-BimAth, but not GST, sequestered actin from the splenic extract (Fig. 2B) (10). Deletion of the CA domain did not impair actin binding, consistent with the fact that a predicted WH2 domain remains intact (10). It may be inferred that such binding is direct, since coated beads also bound highly purified rhodamine-labeled actin in phosphate-buffered saline (PBS) as assessed by confocal microscopy of beads treated as described previously (Fig. 2E) (10). The ability of coated beads to sequester the Arp2/3 complex from murine splenic extracts was examined using rabbit anti-p34-Arc/ARPC2 (Millipore, Watford, United Kingdom) and goat anti-Arp3 (Autogen Bioclear, Wiltshire, United Kingdom) detected with species-specific Ig-horseradish peroxidase conjugates by a chemoluminescence method (Amersham Biosciences, Buckinghamshire, United Kingdom). GST-BimAth, but not GST-BimAps, sequestered p34-Arc/ARPC2 (Fig. 2C) and Arp3 (Fig. 2D). Deletion of the CA domain abolished the ability to sequester Arp2/3 components (Fig. 2C and D), indicating that it plays a role in recruitment of the complex.
The requirement for Arp2/3 and the CA domain in actin polymerization by B. thailandensis BimA was evaluated in vitro using pyrene-labeled actin. Polymerization of such monomers leads to an emission of fluorescence that can be sensitively recorded over time. Lyophilized pyrene-actin, Arp2/3, and the verprolin-like central and acidic (VCA) domain of WASP were obtained from Cytoskeleton (Universal Biologicals, Cambridge, United Kingdom) and prepared per the manufacturer's instructions. Assay conditions were essentially as described previously (12). Briefly, 90-μl reaction mixtures were assembled in black opaque 96-well plates containing 100 nM GST or GST fusion protein, 2 μM pyrene-actin, and 30 nM Arp2/3 as required. Reactions were initiated by the addition of 10 μl of 10× polymerization buffer (100 mM Tris, pH 7.5, 500 mM KCl, 20 mM MgCl2, 10 mM ATP), and the emission of fluorescence at 407 nm, after excitation at 365 nm, was followed every 30 s for 90 min using a Tecan Infinite M200 fluorescent plate reader with i-control software. The GST, GST-BimAps, GST-BimAth, and GST-BimAth-ΔCA proteins were prepared as described above but eluted from beads with 10 mM reduced glutathione. Triplicate determinations were performed with two independently purified sets of protein. Data from a representative assay are shown in Fig. 3. Rates of polymerization were calculated as the rise in fluorescence units per second during the linear phase of polymerization, and the means of six values per protein ± standards of the means are recorded in Table 1. Results were analyzed by pairwise Student t tests using R software (version 2.11), and P values of ≤0.05 were taken as significant.
FIG. 3.
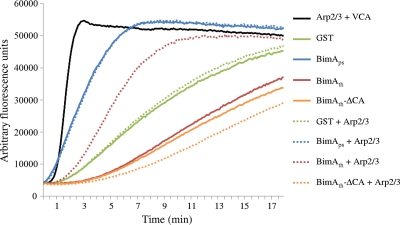
Polymerization of pyrene-labeled actin monomers by GST (green lines), GST-BimAps (blue lines), GST-BimAth (red lines), and GST-BimAth-ΔCA (yellow lines) in the absence (solid lines) or presence (dotted lines) of the Arp2/3 complex. The Arp2/3 complex was confirmed to be active in the presence of the purified VCA domain of WASP (black line). The graph shows the increase in fluorescence units (at 407 nm) over time during a single representative experiment. Triplicate determinations were performed with two sets of independently purified proteins, and the mean rates of fluorescence are recorded in Table 1.
TABLE 1.
Rates of polymerization of pyrene-labeled actin monomers by GST and GST-BimA fusion proteins in the absence or presence of the Arp2/3 complex
| Protein | Fluorescence units/s in: |
Pa | |||
|---|---|---|---|---|---|
| Absence of Arp2/3 |
Presence of Arp2/3 |
||||
| Mean | SEM | Mean | SEM | ||
| GST | 12.12 | 0.42 | 12.88 | 0.27 | 0.0979 |
| GST-BimAps | 25.52 | 1.51 | 29.32 | 2.34 | 0.0572 |
| GST-BimAth | 9.95 | 0.95 | 21.78 | 1.09 | 0.0007 |
| GST-BimAth-ΔCA | 7.27 | 0.66 | 5.40 | 0.70 | 0.0566 |
P values denote the significance of differences in the polymerization rates for the specified proteins in the absence versus the presence of Arp2/3.
Under the assay conditions, pyrene-labeled actin monomers spontaneously polymerize, leading to rising baseline fluorescence over time, as evident in the GST control. The Arp2/3 complex has a low intrinsic ability to stimulate actin polymerization (data not shown) (3) but was activated by 230 nM purified VCA to rapidly polymerize pyrene-labeled actin monomers. Consistent with our earlier studies (11), the GST fusion to residues 54 to 455 of B. pseudomallei BimA exhibited an ability to polymerize pyrene-actin monomers at a rate far greater than that of GST alone. The rate of polymerization by GST-BimAps or GST was not significantly enhanced by addition of the Arp2/3 complex, implying that the latter was not activated. The GST fusion to residues 47 to 386 of BimAth produced a lower rate of polymerization of pyrene-labeled actin monomers than did GST, possibly owing to sequestration of such monomers via the WH2 domain in such a way as to reduce their ability to spontaneously polymerize. Importantly, addition of Arp2/3 markedly enhanced the rate of actin polymerization by BimAth (P = 0.0007), suggesting that BimAth recruits and activates the complex in a way that BimAps does not under the assay conditions. Consistent with the finding that the BimAth CA domain sequesters p34-Arc/ARPC2 and Arp3 in a pulldown assay, the CA domain was required for Arp2/3-dependent polymerization of pyrene-labeled actin monomers by B. thailandensis BimA.
Taken together, these data imply that Burkholderia species have evolved distinct strategies for actin-based motility. Consistent with the activities assigned to amphipathic central and acidic domains of cellular and pathogen-associated NPFs, we show that the CA domain uniquely found in B. thailandensis BimA is required for actin-based motility, Arp2/3 binding, and Arp2/3-dependent polymerization of actin. One may infer that if the Arp2/3 complex is recruited and activated by other Burkholderia species during intracellular motility, this occurs by an alternative mechanism owing to the absence of the CA domain. The molecular basis of such events is an active focus of our ongoing research.
Acknowledgments
We gratefully acknowledge the support of the National Science and Technology Development Agency (grant BT-B-01-MG-14-5123 to S.K.), the Royal Thai Golden Jubilee studentship scheme (grant PHD0188/2547 to C.S.), and the Biotechnology & Biological Sciences Research Council, United Kingdom (grant BB/E01212/1 to M.P.S.).
We convey sincere thanks to Narisara Chantratita, Sharon Peacock, and other donors of bacterial strains.
Footnotes
Published ahead of print on 6 August 2010.
REFERENCES
- 1.Ali, S. A., and A. Steinkasserer. 1995. PCR-ligation-PCR mutagenesis: a protocol for creating gene fusions and mutations. Biotechniques 18:746-750. [PubMed] [Google Scholar]
- 2.Breitbach, K., K. Rottner, S. Klocke, M. Rohde, A. Jenzora, J. Wehland, and I. Steinmetz. 2003. Actin-based motility of Burkholderia pseudomallei involves the Arp2/3 complex, but not N-WASP and Ena/VASP proteins. Cell. Microbiol. 5:385-393. [DOI] [PubMed] [Google Scholar]
- 3.Campellone, K. G., and M. D. Welch. 2010. A nucleator arms race: cellular control of actin assembly. Nat. Rev. Mol. Cell Biol. 11:237-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heeb, S., C. Blumer, and D. Haas. 2002. Regulatory RNA as mediator in GacA/RsmA dependent global control of exoproduct formation in Pseudomonas fluorescens CHA0. J. Bacteriol. 184:1046-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kespichayawattana, W., S. Rattanachetkul, T. Wanun, P. Utaisincharoen, and S. Sirisinha. 2000. Burkholderia pseudomallei induces cell fusion and actin-associated membrane protrusion: a possible mechanism for cell-to-cell spread. Infect. Immun. 68:5377-5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panchal, S. C., D. A. Kaiser, E. Torres, T. D. Pollard, and M. K. Rosen. 2003. A conserved amphipathic helix in WASP/Scar proteins is essential for activation of Arp2/3 complex. Nat. Struct. Biol. 10:591-598. [DOI] [PubMed] [Google Scholar]
- 7.Schell, M. A., R. L. Ulrich, W. J. Ribot, E. E. Brueggemann, H. B. Hines, D. Chen, L. Lipscomb, H. S. Kim, J. Mrázek, W. C. Nierman, and D. Deshazer. 2007. Type VI secretion is a major virulence determinant in Burkholderia mallei. Mol. Microbiol. 64:1466-1485. [DOI] [PubMed] [Google Scholar]
- 8.Sitthidet, C., J. M. Stevens, N. Chantratita, B. J. Currie, S. J. Peacock, S. Korbsrisate, and M. P. Stevens. 2008. Prevalence and sequence diversity of a factor required for actin-based motility in natural populations of Burkholderia species. J. Clin. Microbiol. 46:2418-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stevens, J. M., E. E. Galyov, and M. P. Stevens. 2006. Actin-dependent movement of bacterial pathogens. Nat. Rev. Microbiol. 4:91-101. [DOI] [PubMed] [Google Scholar]
- 10.Stevens, J. M., R. L. Ulrich, L. A. Taylor, M. W. Wood, D. Deshazer, M. P. Stevens, and E. E. Galyov. 2005. Actin-binding proteins from Burkholderia mallei and Burkholderia thailandensis can functionally compensate for the actin-based motility defect of a Burkholderia pseudomallei bimA mutant. J. Bacteriol. 187:7857-7862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stevens, M. P., J. M. Stevens, R. L. Jeng, L. A. Taylor, M. W. Wood, P. Hawes, P. Monaghan, M. D. Welch, and E. E. Galyov. 2005. Identification of a bacterial factor required for actin-based motility of Burkholderia pseudomallei. Mol. Microbiol. 56:40-53. [DOI] [PubMed] [Google Scholar]
- 12.Yarar, D., J. A. D'Alessio, R. L. Jeng, and M. D. Welch. 2002. Motility determinants in WASP family proteins. Mol. Biol. Cell 13:4045-4059. [DOI] [PMC free article] [PubMed] [Google Scholar]