Abstract
Most LysR-type transcriptional regulators (LTTRs) function as tetramers when regulating gene expression. The nitrogen assimilation control protein (NAC) generally functions as a dimer when binding to DNA and activating transcription. However, at some sites, NAC binds as a tetramer. Like many LTTRs, NAC tetramers can recognize sites with long footprints (74 bp for the site at nac) with a substantial DNA bend or short footprints (56 bp for the site at cod) with less DNA bending. However, unlike other LTTRs, NAC can recognize both types of sites in the absence of physiologically relevant coeffectors, suggesting that the two conformers of the NAC tetramer (extended and compact) are interchangeable without the need for any modification to induce or stabilize the change. In order for NAC to bind as a tetramer, three interactions must exist: an interaction between the two NAC dimers and an interaction between each NAC dimer and its corresponding binding site. The interaction between one dimer and its DNA site can be weak (recognizing a half-site rather than a full dimer-binding site), but the other two interactions must be strong. Since the conformation of the NAC tetramer (extended or compact) is determined by the nature of the DNA site without the intervention of a small molecule, we argue that the coeffector that determines the conformation of the NAC tetramer is the DNA site to which it binds.
The nitrogen assimilation control protein (NAC) of Klebsiella pneumoniae is a LysR-type transcriptional regulator (LTTR) that regulates a variety of genes and operons whose products allow the cell to utilize alternative nitrogen sources in place of ammonium, the preferred source (2, 3, 24). In particular, NAC activates expression of operons whose products catabolize histidine (hut), proline (put), urea (ure), alanine (dad), and cytosine (cod) as nitrogen sources (3, 11, 14, 19). Electrophoretic mobility shift analysis (EMSA) has shown that NAC binds to the promoter region of the hutUH operon (hutUp) and to the promoter region of the ureDABCEFG operon (ureDp) as a dimer (22). Binding of a dimer at these sites generates a DNase I footprint of about 28 bp (9). NAC also generates a DNase I footprint of 25 bp at putPp, again consistent with a dimer of NAC bound at this promoter (9). codBp from Escherichia coli is different in that EMSA shows that NAC binds as a tetramer, and the DNase I footprint of about 56 bp confirms this (19). However, a shorter (and still functional) version of codBp showed a dimer of NAC bound (by EMSA) and a footprint of about 28 bp (19). Centered within the 28-bp region defined by these DNase I footprints, we see a consensus sequence of ATA-N9-TAT (9). Genetic analysis of this sequence within hutUp confirms that these nucleotides are important for NAC binding and function (21).
Like all LTTRs (15, 23), NAC assembles as a dimer of dimers (22). Although wild-type NAC is a tetramer in solution, we have characterized a mutant NAC (NACL111K) that cannot form the tetramer and remains a simple homodimer under all conditions tested (22). The L111K amino acid change lies in the C-terminal (regulatory) domain of NAC and suggests that the C-terminal domain is important for tetramer formation. We also found that NAC mutants containing only the N-terminal, DNA-binding domain (NAC100ter and NAC86ter) are dimeric under the conditions tested, arguing that the C-terminal domain is essential for tetramerization (18). All of these dimeric mutants are able to activate transcription from hutUp and other promoters (18, 22). Thus, the dimer of NAC is the functional form of NAC that activates transcription at many promoters.
In contrast, both EMSA and DNase I footprinting have shown that NAC binds as a tetramer at the promoter of the nac gene (8) and at the codBp promoter of the codBA operon from E. coli (19). EMSA and DNase I footprinting shows that NAC binds to each of two sites in the promoter region of the gdhA gene as a dimer (22). However, tetramerization of NAC is essential for the NAC-mediated repression of gdhA that uses these two sites, presumably by a DNA looping mechanism (22). Virtually all LTTRs give tetramer footprints at most of their binding sites (recently reviewed in reference 15). Also, most LTTRs give two different footprints, depending on the presence or absence of a regulatory coeffector. This coeffector is usually a small molecule related to the function being regulated, but in the case of OxyR, it is a disulfide bond formed within the C-terminal domain (5, 28). In the absence of a coeffector, most LTTRs give a relatively long footprint and elicit some degree of DNA bending; in the presence of the coeffector, the footprint is shorter and the DNA bend angle is shallower. As a result, in the absence of the coeffector, the LTTR recognizes a DNA region that contains two dimer-binding sites with some space between them. In the presence of the coeffector, the LTTR recognizes a region that contains two dimer-binding sites with less space between them. Thus, the DNA sites recognized by LTTRs with and without a coeffector are different, and the presence of the coeffector alters which sites are recognized. In some cases, the longer and shorter sites overlap so that it appears the coeffector shortens the footprint, but in others, the sites are distinct and the coeffector appears to increase the affinity of the LTTR for a shorter site while weakening the affinity for the longer sites.
NAC bound to the nac promoter produces a DNase I footprint of about 74 bp and induces a significant DNA bend with significant DNase I hypersensitivity at the site of the bend (8, 22). NAC bound to codBp produces a DNase I footprint of about 56 bp and very little DNase I hypersensitivity, perhaps suggesting less DNA bending (19). In other words, NAC appears to be able to produce footprints characteristic of both the modified and unmodified forms of LTTRs. However, there is no physiologically relevant coeffector for NAC (25). At every DNA site tested, NAC binds equally well in the presence or absence of any small molecule tested and regardless of whether the NAC was purified from cells grown under nitrogen excess or nitrogen limitation (9). Similarly, activation of hut and ure, as well as repression of gdh, occurs equally in strains with excess or limiting nitrogen if NAC is produced from an isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible promoter (25). If no coeffector exists and if NAC can function as either a dimer or a tetramer, two questions are raised. What determines whether NAC will bind as a tetramer? And what determines whether a NAC tetramer recognizes a longer site (typical of a coeffector-free LTTR) or a shorter site (typical of a coeffector-bound LTTR) if NAC has no coeffector?
Experiments with the NACL111K mutant suggested that the single tetramer-binding site at nac might actually be two adjacent dimer-binding sites (22). The data from gdhA expression also suggest that two dimer-binding sites, even when separated by 146 bp, might be able to interact in a way that mediates tetramer formation (10, 22). However, we also noted that neither nac nor gdhAp contained the consensus ATA-N9-TAT (10, 22). Each of the three DNase I footprints did, however, include an appropriately located sequence, ATA-N9-GAT (or its equivalent, ATC-N9-TAT). So we set out to determine what is required for NAC to bind as a tetramer. We were also interested in determining whether there are two fundamentally different kinds of NAC tetramers, one able to bind to the longer DNA sites and one able to bind to the shorter sites, or whether NAC was a more flexible tetramer than other LTTRs and could assume either or both conformations without the need for a coeffector to stabilize one conformer or switch from one to another.
MATERIALS AND METHODS
PCR.
PCR was carried out with plasmid DNA containing either the nac promoter (pJF200), the cod promoter (pCB816), or the hut promoter (pOS1) as a template (8, 19, 20). Reactions were carried out using ca. 10 ng of template DNA and a final concentration of 0.8 μM for each primer in 50 μl of Platinum PCR super mix (Invitrogen Corp.). PCR amplification was performed using a standard PCR program: initial template denaturation (94°C for 5 min) was followed by 35 cycles of amplification (template denaturation at 94°C for 1 min, primer annealing at 51°C for 1 min, and primer extension at 72°C for 1 min), and finally, the program was terminated with a final extension at 72°C for 2 min. For mutations that could not be generated by a single primer pair, a two-step PCR was performed as described elsewhere to obtain the desired double-stranded fragment (1). Briefly, the first step was performed by generating two fragments with 10 to 20 bp of overlapping complementary strands at the 3′ ends of the DNA. These fragments were isolated by Qiaquick gel extraction (Qiagen Inc.). These fragments were used as a template in a second PCR with primers that annealed to the 3′ ends most distant from the overlapping region to generate a single fragment composed of both initial fragments.
Protein purification.
C-terminally His-tagged versions of NACWT and NACL111K (henceforth referred to as NACWT and NACL111K) were purified as previously described (12). Purity was monitored by SDS-PAGE followed by Coomassie blue staining, using the method of Fairbanks et al. (6). Using band densitometry of the Coomassie-stained gels, the average purity of NACWT was 95%, and that of NACL111K was 89%. The activity of NACWT was approximately 24%, and the activity of NACL111K was approximately 4%. The protein concentration was determined using the method of Lowry et al. and a bovine serum albumin (BSA) standard (13). Purified protein was stored in 50% (vol/vol) glycerol at −20°C.
EMSA.
An electrophoretic mobility shift assay (EMSA) was performed as previously described (9). Briefly, protein was diluted in buffer 6 (50% [vol/vol] glycerol, 125 mM NaCl, 50 mM NaH2PO4 [pH 7.0], 1.25 mM MgCl2, 0.5 mM 2-mercaptoethanol, 1 mg/ml BSA), and DNA fragments were diluted in water. The concentration of protein and DNA added to each reaction is listed in the figure legends. These reactions did not contain nonspecific competitor DNA for two reasons: (i) nonspecific competitor DNA is incompatible with ethidium bromide staining, and (ii) the relatively large amount of DNA that was present in these reactions diminished the need for competitor DNA. Twelve μl of the desired DNA dilution was mixed with either 8 μl of buffer 6 alone or 8 μl of the desired protein dilution and incubated at room temperature for 20 min. A 1.5-μl volume of loading buffer (40 mM Tris-HCl [pH 8.4], 4 mM EDTA, 0.2% [wt/vol] bromophenol blue, 0.2% [wt/vol] xylene cyanol, 25% [vol/vol] glycerol) was added to each sample, and the sample was loaded onto a 6% gel (polyacrylamide buffered with 0.5× Tris-borate-EDTA [TBE]) that had been prerun at 20 V/cm for 30 min (Invitrogen). NAC-DNA complexes were separated by electrophoresis at 20 V/cm for 1 h at room temperature. Gels were stained in ethidium bromide solution (40 μg/ml) for 10 min at room temperature and then destained in water for 10 min with agitation. DNA was visualized by UV fluorescence.
Competition assay.
The PCR product from the codB promoter of E. coli was end labeled with [32P]dATP by end filling with Klenow fragment as described previously (16). Labeled DNA was separated from unincorporated nucleotide using Qiagen nucleotide removal columns (Qiagen corp.). For each reaction, 0.16 pmol of NAC in 4 μl of buffer 6 was mixed with 0.08 pmol of labeled codBp DNA and 0, 0.08, 0.16, or 0.32 pmol of unlabeled competitor DNA in 6 μl of double-distilled water (ddH2O) for a total reaction volume of 10 μl. Reaction mixtures were incubated at room temperature for 20 min. A 1-μl volume of loading buffer was added to each sample, and the samples were loaded onto a prerun 5% acrylamide gel buffered with 0.5× TBE. Unbound DNA and NAC-DNA complexes were separated by electrophoresis at 10 V/cm for 60 min. Gels were transferred to filter paper and exposed to RX-B X-ray film (Marsh Bioproducts Inc.) for 60 min. Quantitation was performed using the densitometry feature of the Photoshop software program (Adobe Inc.).
DNA bending assay.
The DNA bending assay was performed as previously described (27). DNA fragments (150 bp in length) were amplified as described above using the two-step PCR process. Fragments for “codWT” and “nacWT” had the binding site at 43 bp, 64 bp, or 75 bp from one end of the fragment. Fragments for “cod+10” had the binding site at 48 bp, 69 bp, or 80 bp from one end of the fragment. Fragments for “nac−10” had the binding site at 39 bp, 59 bp, or 70 bp from one end of the fragment. EMSA on these fragments was performed as described above except the concentration of NACWT used was adjusted to shift ca. 50% of the DNA for each fragment.
RESULTS
For most LTTRs, the ability to recognize binding sites of two different lengths depends on the presence or absence of a coeffector (15). In general, the free LTTR binds the longer site but not the shorter site. In the presence of the coeffector, the LTTR is altered such that the shorter site is recognized, but not the longer site. In contrast, NAC recognizes both a long (74-bp) site and a short (56-bp) site in cells growing under the same condition, namely, nitrogen limitation (summarized in Fig. 1). This might suggest that there exist two different isoforms of NAC, one of which is assembled as an extended tetramer that recognizes the longer binding sites and the other of which is assembled as a compact tetramer that recognizes the shorter binding sites. Or it might suggest that a single pool of NAC tetramers is capable of freely converting from one conformation to the other without the need for a coeffector to drive the transition. Such a conversion might be direct or might involve dissociation to dimers as an intermediate step.
FIG. 1.
Sequence of the hutU, codB, and nac promoters. The matches to the consensus binding sites are boxed. For hut, the boxed region is centered at −64 relative to the start of transcription, for cod, it is centered at −59, and for nac, it is centered at −75. Regions protected from DNase I digest by NAC at each promoter are indicated by a bold line over the sequence and represent the farthest edges of the NAC footprint at these promoters. Arrowheads above the sequence indicate regions of DNase I hypersensitivity in the presence of NAC. The ATA half-site at the codB promoter and the secondary site of the nac promoter (the “nac* site”) are underlined. Mutants made for analysis are indicated underneath the appropriate sequence and indicate the nucleotides changed, deleted, or inserted. The numbers under the cod and nac promoter changes correspond to the labels in Fig. 5. “hutATA(42)” and “hutATA(32)” correspond to labels in Fig. 6. The insertion of 10 bp in the cod promoter and the removal of 10 bp from the nac promoter refer to labels in Fig. 7.
The EMSA experiment in Fig. 2 (lanes 1 and 2) shows that NAC binds to a radiolabeled DNA fragment containing the short (56 bp) NAC-binding site from the cod promoter region. If cold competitor DNA carrying a long (74-bp) NAC-binding site from the nac promoter was present in the binding reaction, it was fully able to compete for the NAC (lanes 3 to 5). When a fragment known to contain no NAC-binding site (lanes 6 to 8) was used, no competition was seen. The ability of the long site to compete with the short site argues strongly that there is only one pool of NAC that can freely exchange between the forms that bind as a tetramer to the long and short sites.
FIG. 2.
Competition between long and short sites for NAC binding. A radiolabeled DNA fragment containing the short NAC-binding site from the codB promoter (lane 1) was mixed with purified NAC in a 1:1 ratio (lanes 2 to 8) and analyzed by electrophoretic mobility shift assay. In lanes 3 to 8, unlabeled competitor DNA was added at the ratio indicated. In lanes 3 to 5, the competitor contained the long NAC-binding site from the nac promoter. In lanes 6 to 8, the competitor contained a promoter which had a scrambled NAC binding site. The band corresponding to unbound codB is indicated by a U, and the band representing a tetramer of NAC bound to codB is indicated by a T.
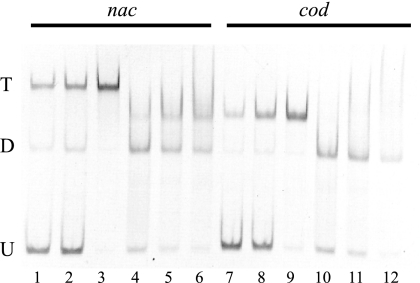
At some sites (e.g., the hut promoter), NAC has been shown to bind as a dimer and to protect a 26-bp region of the DNA, as illustrated in Fig. 1 (9, 22). This raises the question of what features of the binding site determine whether a dimer or tetramer will bind. At sites where NAC activates expression (viz. hut, put, ure, cod, and dad), we have identified a consensus sequence of ATA-N9-TAT that includes many of the most important determinants for binding (9). At sites where NAC represses expression, the corresponding sequence is ATC-N9-TAT or its equivalent, ATA-N9-GAT (10, 22). The NAC-protected region in the nac promoter includes one match to the repression consensus (boxed in Fig. 1 and hereinafter called the nac site) and a second region, ATA-N9-GCT (underlined in Fig. 1 and hereinafter called the nac* site), that differs by only one nucleotide from the repression consensus. This suggested that the nac promoter might contain two dimer-binding sites (22). To test this, we compared the binding of wild-type (tetrameric) NAC with the binding of a mutant, NACL111K, that is unable to form tetramers but retains all known activities associated with dimeric NAC (22). Wild-type NAC has been shown to bind to the site at nac primarily as a tetramer that induces a significant bend in the DNA (8), resulting in a greatly retarded mobility of the bound fragment in a gel shift assay (Fig. 3, lanes 1 to 3). When NACL111K was used in the assay, two shifted species were detected, one corresponding to a bound dimer and one with a mobility corresponding to two bound dimers (22), as seen in Fig. 3, lanes 4 to 6. This is consistent with our previous suggestion that the NAC-binding site at nac contains two dimer-binding sites.
FIG. 3.
Interaction of NACWT and NACL111K with the nac and cod promoters. DNA fragments (ca. 0.3 to 0.45 pmol) containing either the nac (lanes 1 to 6) or cod (lanes 7 to 12) promoter NAC binding regions were incubated with 0.25, 0.5, or 1.1 pmol purified NACWT (lanes 1 to 3 and lanes 7 to 9, respectively) or 6, 12, or 18 pmol purified NACL111K (lanes 3 to 6 and 10 to 12, respectively). The bands representing the unbound promoter fragments are indicated by a U, bands representing promoters bound by a dimer of NAC are indicated by a D, and bands representing promoters bound by a tetramer of NAC are indicated by a T.
In contrast, when the shorter NAC-binding site from the codBA promoter (19) was used in this experiment, two differences were immediately apparent. First, wild-type NAC gave a mobility consistent with either two dimers of NAC or one tetramer that did not bend the DNA significantly (Fig. 3, lanes 7 to 9). When the dimeric mutant NACL111K was used, the only species detected had the mobility of a bound dimer (lanes 10 to 12). Thus, the site at codBp does not appear to have a second dimer-binding site. This is also reflected in the DNA sequence. Within one side of the codBp footprint, we see an activation consensus “the cod site” (boxed in Fig. 1), but the other side, the cod* site, has only a half-consensus sequence, ATA-N9-cca (underlined in Fig. 1).
To confirm the existence of a separate dimer-binding site at nac but not at cod, we generated DNA fragments corresponding to the regions upstream from the consensus dimer-binding sites from hut, cod, and nac. It is known that this region from hut, hut*, does not bind NAC (9). The nac* site did bind NAC as a dimer, but cod* did not (Fig. 4, lanes 1 to 7 and 8 to 14). Thus, the cod promoter does not contain a second independent dimer-binding site. Taken together, these data suggest that NAC binds to the codBp region as a tetramer with little or no bending of the DNA.
FIG. 4.
Binding of NACWT or NACL111K to the nac* site and cod* site. Duplex DNA (1.5 pmol) containing the upstream DNA sequences from NAC-binding sites at nac (lanes 1 to 7 and 15 to 21), cod (lanes 8 to 14), or hut (lanes 22 to 28) was mixed with 0, 1.5, 3, or 6 pmol purified NACWT (lanes 1 to 4, 8 to 11, 15 to 18, or 22 to 25, respectively) and 4.5, 9, or 18 pmol purified NACL111K (lanes 5 to 7, 12 to 14, 19 to 21, or 26 to 28, respectively). Unbound and shifted bands are marked as described for earlier figures.
Nevertheless, the full-length cod site can bind a tetramer (or two dimers) of wild-type NAC, while the corresponding region from hut cannot (Fig. 4, lanes 8 to 14 and 22 to 28). So there must be something in the cod* site that allows a second dimer to bind. To identify the elements that allow codBp to bind a tetramer (or two dimers), we mutated sets of trinucleotides in the cod* site to GGG, as indicated in Fig. 1, and asked whether the mutant fragments could still bind NAC tetramers. As a control, we also generated GGG replacements in the nac* site. The alterations focused on the two key triplets of the near-consensus ATA-N9-GCT sequence, as well as several nearby. These are indicated in Fig. 1 by the numbers underneath the various GGG elements.
The data in Fig. 5 show that changing ATA in the nac* site to GGG (no. 1 in Fig. 1) reduced the relative amount of tetramer-bound DNA and yielded relatively more dimer-bound material (Fig. 5A, lanes 4 to 6). Not surprisingly, changing the other key triplet (no. 4 in Fig. 1) also reduced the fraction of tetramer-bound DNA in favor of dimer-bound DNA (Fig. 5A, lanes 16 to 18). Replacement of the triplet preceding this (no. 5 in Fig. 1) also reduced tetramer formation (Fig. 5A, lanes 19 to 21). Thus, within the nac* site, the two key triplets in the region suspected to be a near-match to consensus, ATA-N9-GCT, were both important, as was the triplet preceding GCT. Other triplets in the region had little or no effect on tetramer formation (Fig. 5A).
FIG. 5.
Interaction of NAC with mutant forms of the NAC-binding sites from nac and cod. (A) One-hundred-fifty-base-pair DNA fragments (ca. 0.3 to 0.45 pmol) of the wild-type nac promoter or the nac promoter containing the mutations indicated by the numbers in Fig. 1 were mixed with 0.25, 0.5, or 1.0 pmol of purified NACWT. The asterisk indicates the mobility of a PCR-amplified DNA fragment that did not interact with NACWT. (B) One-hundred-fifty-base-pair DNA fragments (ca. 0.3 to 0.45pmol) of the wild-type cod promoter or the cod promoter containing the mutations indicated by the numbers in Fig. 1 were mixed with 0.25, 0.5, or 1.0 pmol of purified NACWT. The asterisk indicates the mobility of a PCR-amplified DNA fragment that did not interact with NAC. The positions of bands corresponding to unbound (U), dimer-bound (D), and tetramer-bound (T) fragments are indicated.
Within the cod* site, only the first triplet of a consensus was evident by observation (ATA-N9-cca). Changing the first triplet of this pair to GGG (no. 1 in Fig. 1) reduced tetramer formation (Fig. 5B, lanes 4 to 6), but changing the other triplet (no. 6 in Fig. 1) had little or no effect (Fig. 5B, lanes 22 to 24). Most other alterations in the cod* site had little or no effect on tetramer formation except for the triplet in the center, between the position of the 2 key triplets (no. 4 in Fig. 1). Changing TTT (within a stretch of five T residues) to GGG led to a slight increase in dimer-bound DNA relative to tetramer-bound DNA (Fig. 5B, lanes 16 to 18).
The data from the mutagenesis of the cod* site suggested that the ATA triplet was very important for tetramer binding, though other elements of the site might also play some role. To test whether an ATA half-site is sufficient to generate a tetramer-binding site, we placed an ATA sequence upstream from the known dimer-binding site from hut. We placed the ATA sequence at 32 bp upstream from the hut consensus (where the ATA sequence in the cod* site lies) and at 42 bp upstream (where the ATA sequence in the nac* site lies). The sites of these changes are illustrated as “hutATA(32)” and “hutATA(42)” in Fig. 1. The data in Fig. 6 show that the ATA at the cod distance of 32 bp generated a tetramer-binding sequence but the ATA at the nac distance of 42 bp did not.
FIG. 6.
An upstream ATA sequence is sufficient to bind a NAC tetramer at hut. One-hundred-fifty-base-pair DNA fragments (ca. 0.3 to 0.45 pmol) containing the hut promoter (lanes 1 to 4), the hut promoter with an ATA half-site positioned 3 helical turns upstream of the NAC-binding site [“hutATA(32)” in Fig. 1] (lanes 5 to 8), and the hut promoter with an ATA half-site positioned 4 helical turns upstream of the NAC-binding site [“hutATA(42)” in Fig. 1] (lanes 9 to 12) were incubated with 0, 0.5, 1.0, or 2.0 pmol purified NACWT (lanes 1 to 12). The asterisk indicates a PCR-amplified DNA fragment that did not interact with NAC. The positions of bands corresponding to unbound (U), Dimer-bound (D), and tetramer bound (T) fragments are indicated.
To test whether the intact cod* site would function at a distance of 42 bp where a simple ATA triplet did not, we inserted 10 bp (CGAGATCTGC) between the cod* site and the cod site as described in Materials and Methods and depicted in Fig. 1. If such an arrangement allowed a tetramer to bind, we expected that the bound tetramer would have to induce a DNA bend similar to that seen when a tetramer binds at the nac site (8, 22). We tested both binding and bending using an EMSA assay involving fragments where the NAC-binding site was located near one end of the fragment, near the middle of the fragment, or halfway in between. As can be seen in Fig. 7, the retardation of the bound fragment containing the wild-type nac site increased as the location of the binding site moved from the edge to the middle of the fragment (lanes 7 to 9). This is diagnostic for bending of the DNA by the bound protein (27). In contrast, the retardation of the bound fragment containing the wild-type cod site did not change (lanes 1 to 3), consistent with the observation that the DNase I footprint of NAC at cod shows much less DNase I hypersensitivity than that at nac (8, 19, 22). However, the retardation of the bound fragment containing the cod site with the extra 10 bp (cod+10 in Fig. 7, lanes 4 to 6) did increase as the location of the binding site moved from the edge to the middle of the fragment. This suggests that NAC binding to the cod+10 site results in a significant DNA bend. To complete the set, we also tested a construct where 10 bp from the wild-type nac site was deleted, generating a “nac−10” site (as depicted in Fig. 1). NAC binding to the nac−10 site did not result in a significant bend (Fig. 7, lanes 10 to 12). These data suggest that the cod* site is stronger than a simple ATA sequence in that it allows NAC to bind to a long site and bend the DNA whereas the ATA half-site can function only at the spacing of the short site.
FIG. 7.
NAC tetramers bend DNA at long sites but not short sites. One-hundred-fifty-base-pair PCR-amplified DNA fragments (ca. 0.7 to 0.8 pmol) that position the NAC-binding region of “codWT” (lanes 1 to 3) or “nacWT” (lanes 7 to 9) either 43 bp (lanes 1 and 7), 64 bp (lanes 2 and 8), or 75 bp (lanes 3 and 9) from one end of the DNA fragment are analyzed. The “cod+10” NAC-binding region was positioned 48, 69, or 80 bp from one end of the fragment (lanes 4 to 6, respectively), and the “nac−10” NAC-binding region was positioned 39, 59, or 70 bp from one end of the fragment (lanes 10 to 12, respectively). Each fragment was incubated with 0.5 pmol of NACWT. The positions of bands corresponding to unbound (U) and tetramer bound (T) fragments are indicated.
DISCUSSION
It is common that an LTTR in cells grown under a particular growth condition recognizes one set of binding sites and that same protein in cells grown under a different set of growth conditions recognizes a different set of binding sites (15, 23). The choice of binding site is usually determined by the arrangement of dimers within the tetramer. Often, in the absence of a coeffector (or a covalent modification), the LTTR binds to a site with a longer footprint and a high-angle bend. In the presence of a coeffector or modification, the footprint is shorter and the bend is reduced (15, 23). NAC tetramers also recognize two kinds of site with these characteristics, but NAC recognizes both kinds of site under a single growth condition. This is not surprising, because NAC responds to its signal (nitrogen limitation) at the transcriptional level and appears not to respond to any physiological signal after its formation (7, 25). Both the short and long NAC-binding sites can titrate all the free NAC, arguing that both conformers of tetrameric NAC must be in equilibrium with each other. This equilibrium might be indirect, with the tetramers of one conformer dissociating into dimers and reassembling into tetramers of the other conformer, either free in solution or when bound to DNA. Or the equilibrium might be direct, with a tetramer of one conformer changing its conformation without dissociating into dimers. Our data are not conclusive on this question; however, we favor the model of direct conformational change. Both the nac site and the cod site are capable of binding dimers of NAC (Fig. 3). So if dissociation were necessary, we might expect that as the concentration of binding sites increased, we would have seen more DNA-dimer complexes and fewer DNA-tetramer complexes. However, we saw no increase in DNA-dimer complexes as the total concentration of sites increased (Fig. 3). In further support of this assumption, we have isolated mutant forms of NAC that can bind only long sites and others that can bind only short sites (21a). By analogy to other LTTRs for which structural data are available (5, 17, 26), these mutations affect regions that should lock NAC in an extended or compact conformation. Put simply, the molecule that determines the tetrameric conformation of NAC is the DNA molecule to which NAC is bound.
The data presented here argue that in order for a long site to be fully occupied by two dimers of NAC, three interactions are involved: interaction of one dimer with its binding site on the DNA (e.g., the nac site), interaction of the other dimer with its site (e.g., the nac* site), and interaction between the two dimers. If the two binding sites are strong enough, then even NACL111K, the mutant of NAC that cannot tetramerize, can fill the complete site (Fig. 2). If one of the sites is weak (e.g., the half-site in the cod* site), then the dimer-dimer interaction is required (NACL111K binds as a single dimer). Binding to the complete site requires that two of the three interactions be strong, but bending the DNA seems to require that all three be strong. When the cod* site was positioned with its ATA half-site at −42, the binding of tetrameric NAC was weaker than that with wild-type cod where the ATA is at −32. When an ATA was placed at −42 from the hut site, the effect was even more pronounced (Fig. 6).
In our studies of sites where NAC dimers bind and activate transcription, we defined a consensus sequence of ATA-N6-TNGTAT (9). Mutagenesis studies of the sites at put and hut confirmed the importance of the ATA-N9-TAT consensus and suggested some role for the TNG triplet (4, 21). However, nac, cod, and cod* lack a TNG triplet (Fig. 1), as does the downstream repression site at gdhA (10). Nevertheless, the data presented here argue that a triplet adjacent to the TAT half-site is important for binding. The features that make this triplet region important remain obscure.
When a NAC dimer activates transcription (as it does at hut), the TNG triplet is important for a conformational change that is necessary for NAC's activating ability. Even changes to this trinucleotide that increase NAC's affinity for the hut site reduce hut activation (21). This raises an interesting question: does the conformational change caused when a dimer binds to an asymmetric site (ATAA-N5-TNGTAT in hut) have any effect in determining the tetrameric conformers of NAC or vice versa? Inverting the nac* site had no effect on the binding and bending ability of tetrameric NAC at the nac site (data not shown), so we may be able to exclude gross effects. But our data neither support nor exclude a connection between the effect on the dimeric conformers of NAC caused by the sequence of the DNA in the dimer-binding site and the effect on tetrameric conformers of NAC caused by the spacing between the two dimer-binding sites on the DNA. What is clear is that NAC has evolved to use DNA sequences and spacing (rather than small molecules) to signal conformational changes in both the dimers and the tetramers of the protein.
Acknowledgments
This work was supported by Public Health Service grant GM47156 from the National Institutes of Health to R.A.B.
Footnotes
Published ahead of print on 2 April 2010.
REFERENCES
- 1.Ausubel, F. M., et al. (ed.). 1987. Current protocols in molecular biology. Greene Publishing Associates and Wiley Interscience, New York, NY.
- 2.Bender, R. A. 1991. The role of the NAC protein in the nitrogen regulation of Klebsiella aerogenes. Mol. Microbiol. 5:2575-2580. [DOI] [PubMed] [Google Scholar]
- 3.Bender, R. A., P. M. Snyder, R. Bueno, M. Quinto, and B. Magasanik. 1983. Nitrogen regulation system of Klebsiella aerogenes: the nac gene. J. Bacteriol. 156:444-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, L. M., T. J. Goss, R. A. Bender, S. Swift, and S. Maloy. 1998. Genetic analysis, using P22 challenge phage, of the nitrogen activator protein DNA-binding site in the Klebsiella aerogenes put operon. J. Bacteriol. 180:571-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi, H., S. Kim, P. Mukhopadhyay, S. Cho, J. Woo, G. Storz, and S. Ryu. 2001. Structural basis of the redox switch in the OxyR transcription factor. Cell 105:103-113. [DOI] [PubMed] [Google Scholar]
- 6.Fairbanks, G., T. L. Steck, and D. F. Wallach. 1971. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry 10:2606-2617. [DOI] [PubMed] [Google Scholar]
- 7.Feng, J., T. J. Goss, R. A. Bender, and A. J. Ninfa. 1995. Activation of transcription initiation from the nac promoter of Klebsiella aerogenes. J. Bacteriol. 177:5523-5534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng, J., T. J. Goss, R. A. Bender, and A. J. Ninfa. 1995. Repression of the Klebsiella aerogenes nac promoter. J. Bacteriol. 177:5535-5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goss, T. J., and R. A. Bender. 1995. The nitrogen assimilation control protein, NAC, is a DNA binding transcription activator in Klebsiella aerogenes. J. Bacteriol. 177:3546-3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goss, T. J., B. K. Janes, and R. A. Bender. 2002. Repression of glutamate dehydrogenase formation in Klebsiella aerogenes requires two binding sites for the nitrogen assimilation control protein, NAC. J. Bacteriol. 184:6966-6975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janes, B. K., and R. A. Bender. 1998. Alanine catabolism in Klebsiella aerogenes: molecular characterization of the dadAB operon and its regulation by the nitrogen assimilation control protein. J. Bacteriol. 180:563-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janes, B. K., C. J. Rosario, and R. A. Bender. 2003. Isolation of a negative control mutant of the nitrogen assimilation control protein, NAC, in Klebsiella aerogenes. J. Bacteriol. 185:688-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 14.Macaluso, A., E. A. Best, and R. A. Bender. 1990. Role of the nac gene product in the nitrogen regulation of some NTR-regulated operons of Klebsiella aerogenes. J. Bacteriol. 172:7249-7255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maddocks, S. E., and P. C. Oyston. 2008. Structure and function of the LysR-type transcriptional regulator (LTTR) family proteins. Microbiology 154:3609-3623. [DOI] [PubMed] [Google Scholar]
- 16.Maniatis, T., J. Sambrook, and E. F. Fritsch. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 17.Muraoka, S., R. Okumura, N. Ogawa, T. Nonaka, K. Miyashita, and T. Senda. 2003. Crystal structure of a full-length LysR-type transcriptional regulator, CbnR: unusual combination of two subunit forms and molecular bases for causing and changing DNA bend. J. Mol. Biol. 328:555-566. [DOI] [PubMed] [Google Scholar]
- 18.Muse, W. B., and R. A. Bender. 1999. The amino-terminal 100 residues of the nitrogen assimilation control protein (NAC) encode all known properties of NAC from Klebsiella aerogenes and Escherichia coli. J. Bacteriol. 181:934-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muse, W. B., C. J. Rosario, and R. A. Bender. 2003. Nitrogen regulation of the codBA (cytosine deaminase) operon from Escherichia coli by the nitrogen assimilation control protein, NAC. J. Bacteriol. 185:2920-2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Osuna, R., A. Schwacha, and R. A. Bender. 1994. Identification of the hutUH operator (hutUo) from Klebsiella aerogenes by DNA deletion analysis. J. Bacteriol. 176:5525-5529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pomposiello, P. J., B. K. Janes, and R. A. Bender. 1998. Two roles for the DNA recognition site of the Klebsiella aerogenes nitrogen assimilation control protein. J. Bacteriol. 180:578-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21a.Rosario, C. J., B. K. Janes, and R. A. Bender. 2010. Genetic analysis of the nitrogen assimilation control protein from Klebsiella pneumoniae. J. Bacteriol. 192:4834-4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosario, C. J., and R. A. Bender. 2005. Importance of tetramer formation by the nitrogen assimilation control protein for strong repression of glutamate dehydrogenase formation in Klebsiella pneumoniae. J. Bacteriol. 187:8291-8299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schell, M. A. 1993. Molecular biology of the LysR family of transcriptional regulators. Annu. Rev. Microbiol. 47:597-626. [DOI] [PubMed] [Google Scholar]
- 24.Schwacha, A., and R. A. Bender. 1993. The nac (nitrogen assimilation control) gene from Klebsiella aerogenes. J. Bacteriol. 175:2107-2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwacha, A., and R. A. Bender. 1993. The product of the Klebsiella aerogenes nac (nitrogen assimilation control) gene is sufficient for activation of the hut operons and repression of the gdh operon. J. Bacteriol. 175:2116-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smirnova, I. A., C. Dian, G. A. Leonard, S. McSweeney, D. Birse, and P. Brzezinski. 2004. Development of a bacterial biosensor for nitrotoluenes: the crystal structure of the transcriptional regulator DntR. J. Mol. Biol. 340:405-418. [DOI] [PubMed] [Google Scholar]
- 27.Wu, H. M., and D. M. Crothers. 1984. The locus of sequence-directed and protein-induced DNA bending. Nature 308:509-513. [DOI] [PubMed] [Google Scholar]
- 28.Zheng, M., F. Aslund, and G. Storz. 1998. Activation of the OxyR transcription factor by reversible disulfide bond formation. Science 279:1718-1721. [DOI] [PubMed] [Google Scholar]