Abstract
Brucella strains are exposed to potentially toxic levels of H2O2 both as a consequence of their aerobic metabolism and through the respiratory burst of host phagocytes. To evaluate the relative contributions of the sole catalase KatE and the peroxiredoxin AhpC produced by these strains in defense against H2O2-mediated toxicity, isogenic katE, ahpC, and katE ahpC mutants were constructed and the phenotypic properties of these mutants compared with those of the virulent parental strain B. abortus 2308. The results of these studies indicate that AhpC is the primary detoxifier of endogenous H2O2 generated by aerobic metabolism. KatE, on the other hand, plays a major role in scavenging exogenous and supraphysiologic levels of H2O2, although this enzyme can play a supporting role in the detoxification of H2O2 of endogenous origin if AhpC is absent. B. abortus ahpC and katE mutants exhibit wild-type virulence in C57BL/6 and BALB/c mice, but the B. abortus ahpC katE double mutant is extremely attenuated, and this attenuation is not relieved in derivatives of C57BL/6 mice that lack NADPH oxidase (cybb) or inducible nitric oxide synthase (Nos2) activity. These experimental findings indicate that the generation of endogenous H2O2 represents a relevant environmental stress that B. abortus 2308 must deal with during its residence in the host and that AhpC and KatE perform compensatory roles in detoxifying this metabolic H2O2.
Brucella abortus, a facultative intracellular pathogen, causes abortion and infertility in cattle. Humans can also be infected by ingesting contaminated dairy products, through inhalation of infectious aerosols, or via direct contact with an infected fetus (43). Human brucellosis causes flu-like symptoms with a relapsing fever, and this debilitating disease can persist for months or years without appropriate treatment. Although human brucellosis remains a significant zoonotic disease worldwide (47) and a potential bioterrorism threat (70), there is currently no vaccine to prevent human infection, and antibiotic treatment of these infections remains problematic (2).
Prolonged survival and replication in host macrophages play a critical role in the virulence of the Brucella spp. (34, 57). Experimental evidence indicates that reactive oxygen species (ROS) such as superoxide (O2−) and hydrogen peroxide (H2O2) are important components of the brucellacidal activity of these phagocytes (31). Because the brucellae rely on respiratory metabolism for their energy production (52), these bacteria must also deal with endogenous ROS generated as a by-product of aerobic metabolism (27). Several enzymes that directly detoxify O2− and H2O2 have been identified in Brucella. SodC is a periplasmic Cu-Zn superoxide dismutase (6), and phenotypic evaluation of an isogenic sodC mutant indicates that this enzyme protects B. abortus 2308 from O2− generated by the oxidative burst of host macrophages (22). Brucella strains also produce a single monofunctional catalase that is a structural homolog of Escherichia coli KatE. Although this protein does not possess a standard export signal sequence (63) or a predicted twin arginine transport signal sequence (data not shown), cell fractionation studies with the appropriate controls indicate that this protein resides in the periplasmic compartment (63). B. abortus and Brucella melitensis katE mutants exhibit increased sensitivity to H2O2 compared to their parental strains in in vitro assays (21, 33). A B. melitensis katE mutant retains its virulence in experimentally infected goats (21), and B. abortus katE mutants display wild-type virulence in the mouse model (59). These experimental findings suggest that KatE does not play an indispensable role in protecting the brucellae from oxidative killing by host phagocytes. A gene (BAB1_0591) encoding a Mn superoxide dismutase (SodA) has also been identified in B. abortus 2308. SodA activity increases in a B. abortus sodC mutant, suggesting that SodA works in concert with SodC to protect B. abortus 2308 from oxidative damage (65), but the precise role that SodA plays in resistance to oxidative stress in this bacterium remains to be determined experimentally.
The genes designated BAB2_0531 and BAB2_0532 in the B. abortus 2308 genome sequence are predicted to encode the components of an alkyl hydroperoxide reductase complex (AhpC and AhpD, respectively). Peroxiredoxins of the AhpC family detoxify H2O2, organic peroxides, and peroxynitrite (ONOO−) (9, 48). AhpD and AhpF are peroxiredoxin reductases that use reducing equivalents generated by cellular metabolism to restore the enzymatic activity of AhpC (10, 49). Studies performed with multiple bacterial species indicate that the AhpCD and AhpCF complexes serve as important antioxidants (4, 8, 11, 15, 16, 36, 37, 41, 44, 55, 66), and indeed, work in E. coli suggests that AhpC is the major scavenger of H2O2 generated in the cytoplasm of this bacterium as a by-product of aerobic metabolism (61). AhpC has also been shown to play a role in the virulence of several bacterial pathogens, including Helicobacter pylori (45), Mycobacterium bovis (72), and Staphylococcus aureus (15) but does not appear to be required for the virulence of Salmonella enterica serovar Typhimurium (68), Mycobacterium tuberculosis (64), Legionella pneumophila (51), or Porphyromonas gingivalis (32) in experimental models.
In this report, we present evidence that AhpC is the primary antioxidant used by B. abortus 2308 to detoxify endogenous H2O2 generated by respiratory metabolism during routine aerobic cultivation. KatE, on the other hand, plays a major role in scavenging exogenous and supraphysiologic levels of H2O2, although this enzyme can play a supporting role in the detoxification of H2O2 of endogenous origin if AhpC is absent. Interestingly, AhpC and KatE appear to play complementary roles in protecting B. abortus 2308 from H2O2 of metabolic origin during residence in mice, and the presence of either AhpC or KatE alone is sufficient to allow this strain to maintain a chronic infection.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Brucella abortus 2308 and derivatives of this strain (Table 1) were cultivated on Schaedler agar (SA; Becton, Dickinson and Company) supplemented with 5% defibrinated bovine blood (SBA) at 37°C with 5% CO2 or in brucella broth (Becton, Dickinson and Company) at 37°C with shaking at 165 rpm. Escherichia coli strains were grown in Luria Bertani (LB) broth (58) or on LB agar at 37°C. Chloramphenicol (15 μg/ml for Brucella strains and 30 μg/ml for E. coli strains) (Sigma-Aldrich), kanamycin sulfate (45 μg/ml) (Invitrogen), and ampicillin or carbenicillin (25 μg/ml for Brucella strains and 100 μg/ml for E. coli strains) (Sigma-Aldrich) were added to culture media as necessary for selection of bacterial strains carrying antibiotic resistance markers.
TABLE 1.
Bacterial strains used in this study
| Strain or plasmid | Genotype or description | Reference or source |
|---|---|---|
| Strains | ||
| Escherichia coli DH5α | F− φ80lacZΔM15 Δ(lacZYA-argF)U169recA1endA1hsdR17(rK− mK+) phoAsupE44thi-1gyrA96relA1 λ− | Invitrogen |
| Brucella abortus | ||
| 2308 | Virulent challenge strain | Laboratory stock |
| KH16 | 2308 ahpCD::cat; Cmr | This study |
| KH40 | 2308 ahpCD::bla; Apr | This study |
| KH2 | 2308 katE::cat; Cmr | This study |
| MEK6 | 2308 katE::aph3A; Knr | This study |
| KK9 | 2308 ahpCD::blakatE::aph3A; Apr Knr | This study |
| KK21 | 2308 ahpCD::catkatE::aph3A; Cmr Knr | This study |
| Plasmids | ||
| pGEM-T Easy | ColE1-based cloning vector; Apr | Promega |
| pBC KS+ | ColE1-based cloning vector; Cmr | Stratagene |
| pBBR1MCS-4 | pBBR-based broad-host-range cloning vector; moderate copy no. (10-14 copies per cell); Apr | 35 |
| pMR10-Ap | RK2-based broad-host-range cloning vector; low copy no. (2-4 copies per cell); Apr | 69 |
| pMR10 | RK2-based broad-host-range cloning vector; low copy no. (2-4 copies per cell); Knr | 22 |
| pBlue-CM2 | 656-bp cat gene from pBC cloned into the EcoRV site of pBluescript KS+ | 54 |
| pKS + Kan | 794-bp aph3A gene from TnphoA cloned into SalI-HindIII-digested pBluescript II KS+ | 35 |
| pMWV19 | 2,072-bp genomic DNA fragment from B. abortus 2308 containing ahpCD (PCR primers ahpCD-3F/ahpCD-3R) cloned into pGEM-T Easy | This study |
| pMEK21 | Derivative of pBBR1MCS-4 carrying the katE gene from B. abortus S19 | 21 |
| pMWV77 | 2,094-bp NotI fragment from pMWV19 containing ahpCD cloned into pMR10-Ap | This study |
| pKHS6 | 1,382-bp genomic DNA fragment from B. abortus 2308 containing ahpCD (PCR primers ahpCD-2F/ahpCD-2R) cloned into pBBR1MCS-4 | This study |
| pKHS2 | 2,499-bp genomic DNA fragment from B. abortus 2308 containing ahpCD (PCR primers ahpCD-1F/ahpCD-1R) cloned into pGEM-T Easy | This study |
| pKHS3 | Derivative of pKHS2 in which a 673-bp BsmBI/HindIII fragment internal to the ahpC and ahpD coding regions was replaced with the cat gene from pBlue-CM2 | This study |
| pKHS4 | 2,534-bp fragment from pKHS2 containing ahpCD cloned into pBC KS+ | This study |
| pKHS5 | Derivative of pKHS4 in which a 950-bp HindIII/HincII fragment internal to the ahpC and ahpD coding regions was replaced with the bla gene from pGEM-T Easy | This study |
| pKHS4 | 2,534-bp fragment from pKHS2 containing ahpCD cloned into pBC KS+ | This study |
| pMEK7-9 | 1,917-bp genomic DNA fragment from B. abortus S19 containing katE cloned into the PvuII site of pUC18 | 21 |
| pMEK7-9c | Derivative of pMEK7-9 in which a 1-kb PflMI/EcoRI fragment internal to the katE coding region was replaced with the cat gene from pBlue-CM2 | This study |
| pMEK7-9k | Derivative of pMEK7-9 in which a 1-kb PflMI/EcoRI fragment internal to the katE coding region was replaced with the aph3A gene from pKS + Kan | 21 |
Recombinant DNA techniques.
Standard methods were employed for the manipulation of recombinant DNA molecules and amplification of DNA by PCR (Table 2) (3, 58). Plasmid DNA was introduced into Brucella strains by electroporation (19).
TABLE 2.
Oligonucleotide primers used for PCR in this study
| Designation | Sequence |
|---|---|
| ahpCD-1F | 5′-GCCAGAACCAGCGAACGGAA-3′ |
| ahpCD-1R | 5′-TGGGCTGATGGGCATGACCT-3′ |
| ahpCD-2F | 5′-CCAGTGCGAGAAAATAGTGAAGCTG-3′ |
| ahpCD-2R | 5′-GATCAAAACGGATCGCTTATTCAGT-3′ |
| ahpCD-3F | 5′-GGCAGAACCTTGGGCAGAAG-3′ |
| ahpCD-3R | 5′-CATCGTCACCGTGCTGATCG-3′ |
Construction of B. abortus mutants.
A previously described gene replacement strategy (19) was used to introduce defined mutations into the genome of B. abortus 2308. ColE1-based plasmids containing cat-disrupted versions of the ahpCD (pKHS3) and katE (pMEK7-9c) loci and a bla-disrupted ahpCD locus (pKHS5) (Table 1) were independently introduced into B. abortus 2308 by electroporation, and transformants were selected on SBA containing chloramphenicol or ampicillin. Putative ahpCD-cat (designated KH16), ahpCD-bla (designated KH40), and katE-cat (designated KH2) mutants were selected for further evaluation based on their failure to grow on SBA supplemented with ampicillin (KH16 and KH2) or chloramphenicol (KH40). The genotypes of KH16, KH40, and KH2 were confirmed by PCR analysis of genomic DNA from these strains by use of ahpCD-, katE-, cat-, and bla-specific primer sets as appropriate and Southern blot analysis with probes for ahpCD, cat, and bla.
Plasmid pMEK7-9k, which contains an aph3A-disrupted version of katE (21), was introduced into the B. abortus ahpCD mutant KH16 by electroporation, and transformants were selected on SBA supplemented with kanamycin. A putative B. abortus ahpCD katE double mutant (designated KK21) was selected for further evaluation based on its resistance to kanamycin and chloramphenicol and its sensitivity to ampicillin. A two-step process was also used to construct a second B. abortus ahpCD katE double mutant, designed to meet the regulatory requirement that Brucella strains engineered to possess resistance to chloramphenicol not be introduced into experimentally infected animals. First, pMEK7-9k was used in a gene replacement strategy as described above to construct a katE mutant (MEK6) from B. abortus 2308. Plasmid pKHS5 was then used to introduce a bla-disrupted version of the ahpCD locus into MEK6, resulting in the construction of the B. abortus ahpCD katE double mutant KK9. The genotypes of B. abortus strains KK21 and KK9 were confirmed by PCR analysis of genomic DNA from these strains by use of ahpCD-, katE-, cat-, bla-, and aph3A-specific primer sets as appropriate and Southern blot analysis with probes for ahpCD, cat, bla, and aph3A.
Crystal violet exclusion was used to verify that the B. abortus ahpCD and katE mutants and ahpCD katE double mutants retain their smooth lipopolysaccharide phenotypes (1). A solution of 3% H2O2 was also placed on bacterial colonies grown on Schaedler agar to verify the presence or absence of visible catalase activity in the B. abortus strains used in this study.
Quantification of peroxide levels in Brucella abortus cell suspensions.
B. abortus strains grown on SBA supplemented with the appropriate antibiotics for 48 h were inoculated into 3 ml brucella broth in 17- by 100-mm tubes and incubated at 37°C with shaking at 165 rpm. Following overnight incubation, the bacterial cells were harvested by centrifugation and resuspended in phosphate-buffered saline (PBS) to an optical density at 600 nm (OD600) of 1.0. A commercial version of the xylenol orange/ferrous iron-based hydrogen peroxide assay originally described by Wolff (73) (National Diagnostics) was used to measure the amount of peroxides in the cell suspensions in accordance with the manufacturer's directions. Briefly, 100 μl of bacterial cell suspension was added to 900 μl of the assay reagent, the reaction mixtures were incubated at room temperature for 30 min, and the absorbance of the reaction mixtures was measured with a spectrophotometer at 560 nm. Cell-free H2O2 standards (0 μM, 1 μM, 2 μM, 4 μM, and 8 μM) were used to construct a standard curve, and the levels of peroxides in the cell suspensions were determined by comparison to the standard curve.
This assay was also used to measure the degradation of H2O2 by B. abortus strains after an exogenous exposure. B. abortus strains grown on SBA supplemented with appropriate antibiotics for 48 h were inoculated into 5 ml brucella broth in 17- by 100-mm tubes and incubated at 37°C with shaking at 165 rpm. Following overnight incubation, the bacterial cells were harvested by centrifugation (12,100 × g for 10 min at room temperature) and resuspended in PBS to an OD600 of 1.0 in three 17- by 100-mm tubes, designated “no exposure,” “immediately after exposure,” and “time after exposure.” For the “immediately after exposure” tubes, cultures were vortexed for 5 s after the addition of hydrogen peroxide, and 100 μl of bacterial cell suspension was removed and immediately added to 900 μl of the assay reagent. For the “time after exposure” tubes, cultures were vortexed for 5 s after the addition of H2O2 and allowed to incubate at 37°C with shaking at 165 rpm for the appropriate time. Following incubation, 100 μl of the bacterial cell suspension was removed and added to 900 μl of the assay reagent.
Disk assay for measuring the sensitivity of B. abortus strains to PQ and H2O2.
B. abortus strains were grown on SBA supplemented with the appropriate antibiotics for 2 days, harvested into brucella broth, and adjusted to a cell density of 109 CFU per ml (OD600 = 0.15). Six hundred microliters of each cell suspension was then added to 18 ml prewarmed (55°C) brucella broth supplemented with 0.7% agar, and 3-ml portions of the resulting cell suspensions were plated onto three Schaedler agar (SA) plates and three SA plates containing 7,800 U/ml bovine catalase (Sigma). A sterile 7-mm Whatman no. 3 filter paper disk was placed in the center of each plate, and 10 μl of a fresh 0.5 M solution of paraquat (PQ; Acrōs Organics) was added to each disk. Plates were incubated for 3 days, and the zones of inhibition surrounding each disk were measured in millimeters.
This same assay was also used to measure to sensitivity of B. abortus strains to H2O2, with the exceptions that 10 μl of a 30% solution of H2O2 was added to the filter disks instead of PQ, and SA plates supplemented with bovine catalase were not used.
Growth characteristics of the Brucella abortus strains in rich and nutrient-limited media.
B. abortus strains were grown overnight in 3 ml brucella broth in 17- by 100-mm culture tubes incubated at 37°C with shaking at 165 rpm. The resulting cultures were inoculated into either 500-ml flasks containing 100 ml of brucella broth at a cell density of approximately 103 CFU per ml or 500-ml flasks containing 100 ml Gerhardt's minimal medium (GMM) (23) at a cell density of 108 CFU per ml and the flasks incubated at 37°C with shaking at 165 rpm. The number of viable brucellae in these cultures was determined at selected time points after inoculation by serial dilution and plating on SBA or SBA containing the appropriate antibiotic.
Peroxynitrite resistance assay.
B. abortus strains were grown on SBA at 37°C with 5% CO2 for 48 h. Bacterial cells were harvested and resuspended to a cell density of 108 in 1 ml PBS in 17- by 100-mm culture tubes. The peroxynitrite generator SIN-1 (3-morpholinosydnonimine HCl; Sigma Aldrich) at a final concentration of 15 mM and 1,000 U/ml of bovine catalase were added to the cell suspensions and the mixtures incubated for 1 h at 165 rpm at 37°C. The numbers of viable brucellae in these cultures and in parallel cultures that were not exposed to SIN-1 were then determined by serial dilution and plating on SBA.
Experimental infection of cultured murine macrophages.
A modification of the methods described by Gee et al. (22) was used to evaluate the capacity of the B. abortus strains to survive and replicate in cultured murine resident peritoneal macrophages. Briefly, macrophages obtained from 6- to 8-week-old female BALB/c mice were seeded at a density of 1.5 × 105 cells per well in sterile 96-well plates in Dulbecco's modified Eagle's medium (ATCC) with fetal calf serum (DMEM-FCS) containing 20 μg/ml gentamicin. After an overnight incubation, 100 U/ml gamma interferon (IFN-γ; Peprotech) was added to cultured macrophages for 30 min before macrophages were washed and coincubated with the Brucella strains (multiplicity of infection [MOI], 50:1) that were previously opsonized with a subagglutinating concentration (1:1,000) of hyperimmune mouse serum. After allowing phagocytosis to occur for 2 h, extracellular bacteria were removed by incubating the macrophage monolayer with DMEM-FCS containing 50 μg/ml gentamicin for 1 h. The cell culture medium was then replaced with DMEM-FCS containing 20 μg/ml gentamicin, and this medium was replaced at 24-h intervals for incubation times that extended to 48 h. At 2, 24, and 48 h postinfection (p.i.), the phagocyte monolayer was washed with PBS-FCS and lysed with 0.1% deoxycholate, and the number of viable intracellular brucellae present was determined by serial dilution and plating on SBA. Triplicate wells of phagocytes infected with each strain were evaluated at every time point.
Experiments were also performed to evaluate the effects of treatment of the cultured macrophages with the NADPH oxidase inhibitor apocynin (Sigma) (42) and the inducible nitric oxide synthase (iNOS) inhibitor NG-methyl-l-arginine (L-NMMA; Fisher) (18). The protocol described above was used, except that 500 μM apocynin, L-NMMA, or both were included in the DMEM-FCS throughout the experiment. Microscopic analysis of nitroblue tetrazolium reduction was used to monitor the oxidative burst capacity of the cultured macrophages (7), and control experiments were performed to ensure that apocynin and/or L-NMMA at this concentration in DMEM was not toxic for the Brucella strains.
Experimental infection of mice.
The procedures previously described by Robertson and Roop (53) were used to evaluate the spleen colonization profiles of the B. abortus strains in C57BL/6 and BALB/c mice from Harlan Laboratories. These methods were also used to compare the virulence of B. abortus strains in C57BL/6J mice and derivatives of these mice that lack a functional phagocyte NADPH oxidase (B6.129S6-Cybbtm1Din/J) or inducible NO synthase (B6.129P2-Nos2tm1lau/J) obtained from Jackson Laboratories. Briefly, mice were infected with 5 × 104 brucellae via the intraperitoneal route, and at each sampling point postinfection, the mice were euthanized, their spleens aseptically removed, and spleen homogenates serially diluted and plated on SBA to determine the number of viable brucellae present.
RESULTS
Identification of an alkyl hydroperoxide reductase complex (AhpCD) in B. abortus 2308.
The genes designated BAB2_0531 and BAB2_0532 in the B. abortus 2308 genome sequence are annotated as ahpC and ahpD, respectively. The products of these two genes are predicted to be components of the alkyl hydroperoxide reductase complex AhpCD. In many bacteria, the peroxiredoxin AhpC serves as an important antioxidant that detoxifies hydrogen peroxide, organic peroxides, and/or peroxynitrite (9, 26, 61). AhpD is a peroxiredoxin reductase that uses reducing equivalents generated by cellular metabolism to recycle the enzymatic activity of AhpC (10, 26). The Brucella AhpC shares 47% amino acid identity with the Mycobacterium tuberculosis AhpC, and the Cys-61, Cys-174, and Cys-176 residues that have been shown to be important for activity in the latter protein (12, 26) are conserved as Cys-57, Cys-171, and Cys-173 in the Brucella AhpC ortholog. Likewise, Brucella AhpD displays 44% amino acid identity with its M. tuberculosis counterpart, and amino acid sequence alignment indicates that the Cys-131 and Cys-134 residues in this protein are equivalent to the Cys-130 and Cys-133 residues that are required for the peroxiredoxin reductase activity of mycobacterial AhpD (10, 26). Reverse transcriptase PCR analysis indicates that the ahpC and ahpD genes in B. abortus 2308 are cotranscribed as an operon (data not shown), which is consistent with the predicted function of their products in an enzymatic complex and the genetic organization of the ahpCD operons in other bacteria (10, 26).
A B. abortus ahpCD mutant exhibits higher levels of endogenous cellular peroxides than the parental strain.
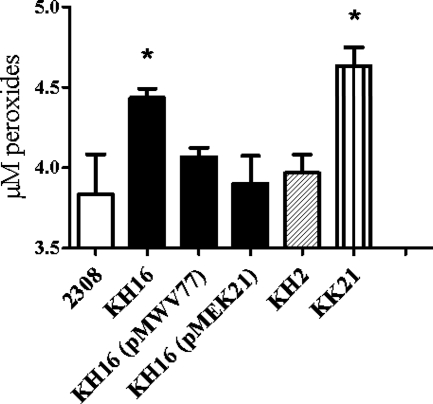
Studies performed with E. coli indicate that AhpC plays a major role in removing the H2O2 that is generated in the cytoplasm of this bacterium as a by-product of aerobic metabolism (61). Phenotypic analysis of the B. abortus mutant ahpCD KH16 suggests that Brucella the AhpC performs a similar function. Significantly higher levels of endogenous peroxides are detected in KH16 than in the parental 2308 strain following aerobic growth (Fig. 1), and the levels of these ROS are significantly diminished in a derivative of the ahpCD mutant carrying a plasmid-borne copy of the ahpCD locus. Endogenous peroxide levels also return to approximately wild-type levels in a derivative of KH16 carrying a plasmid that overexpresses katE (Fig. 1). Because monofunctional catalases such as the Brucella KatE detoxify H2O2 but not organic peroxides (13, 38), these findings indicate that the elevated levels of endogenous peroxides detected in the ahpC mutant are predominantly made up of H2O2. It is also important to note that overexpression of katE or the addition of exogenous catalase reduces the levels of endogenous peroxides detected in B. abortus 2308 cell suspensions below the baseline levels shown in Fig. 1 (data not shown), indicating that this assay provides a reliable indication of the levels of endogenous H2O2 generated by the Brucella strains examined in this study.
FIG. 1.
B. abortus ahpC mutants exhibit increased levels of endogenous peroxides. The levels of endogenous peroxides present in B. abortus 2308, KH16 (2308 ahpCD), KH2 (2308 katE), KK21 (2308 ahpCD katE), KH16 (pMWV77), and KH16 (pMEK21) cell suspensions following aerobic cultivation were determined using a xylenol orange/ferrous iron-based hydrogen peroxide assay (73). The data presented are means and standard deviations for triplicate determinations for a single strain in a single experiment. The data presented here are representative of multiple (≥6) experiments performed from which equivalent results and statistical trends were obtained. Statistical significance (P ≤ 0.05) as determined by the Student two-tailed t test for the comparison of 2308 versus the other strains is represented by an asterisk (*).
The biochemical properties of catalases allow these enzymes to degrade H2O2 across a broad range of concentrations (67). Accordingly, catalases often provide bacteria with a second line of defense against the buildup of endogenous H2O2 of metabolic origin when primary detoxifiers such as AhpC are absent (15, 61). The levels of peroxides detected in the B. abortus katE mutant KH2 are substantially lower than those detected in the isogenic ahpCD mutant and not significantly different from those detected in the parental 2308 strain. Moreover, although the levels of endogenous peroxides detected in the B. abortus ahpCD katE double mutant KK21 are consistently higher than those detected in the isogenic ahpC mutant KH16, these differences are not statistically significant. These experimental findings suggest that KatE plays a limited role in protecting B. abortus 2308 from the buildup of endogenous H2O2 during routine aerobic cultivation.
AhpCD is required for the wild-type resistance of B. abortus 2308 to endogenous H2O2 generated by the redox cycling agent paraquat.
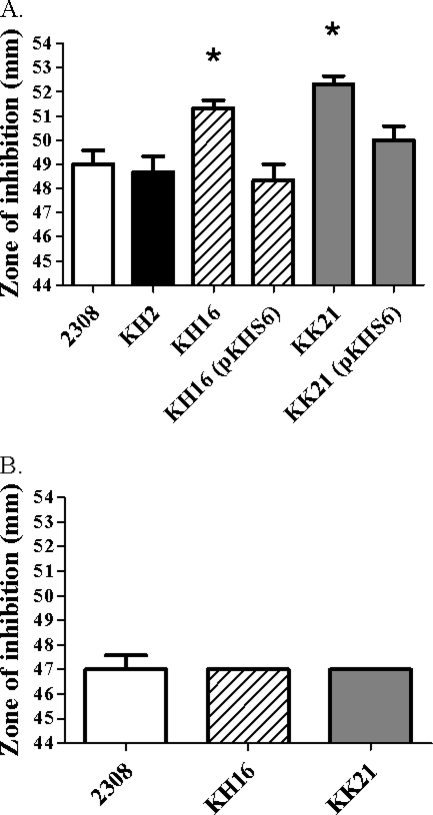
Paraquat (PQ) reacts with components of the respiratory chain in bacterial cells, leading to the univalent reduction of O2 and the generation of O2− in these cells (24). This O2− then serves as a substrate for cytoplasmic superoxide dismutases such as SodA, which can convert this ROS to H2O2 and O2 (20). Spontaneous nonenzymatic dismutation of O2− to H2O2 and O2 also occurs under physiologic conditions (27). Thus, one of the consequences of treating respiring bacterial cells with PQ is the generation of increased intracellular levels of H2O2. The B. abortus ahpCD mutant KH16 and the ahpCD katE mutant KK21 consistently and reproducibly exhibit larger zones of inhibition around disks containing PQ in a disk sensitivity assay than does the parental 2308 strain or the isogenic katE mutant (Fig. 2A). Introduction of a plasmid-borne copy of the ahpCD locus reduces the sensitivity of KH16 and KK21 to PQ to approximately the same levels displayed by B. abortus 2308.
FIG. 2.
B. abortus ahpCD mutants display an increased sensitivity to endogenous H2O2 produced by the redox cycling activity of paraquat. Zones of inhibition for B. abortus 2308, KH2 (2308 katE), KH16 (2308 ahpCD), KK21 (2308 ahpCD katE), KH16 (pKHS6), and KK21 (pKHS6) around disks containing 0.5 M paraquat on Schaedler agar (A) or Schaedler agar supplemented with 7,800 U/ml bovine catalase (B). The data presented are means and standard deviations for triplicate determinations for each strain in a single experiment. The data presented here are representative of multiple (≥4) experiments performed from which equivalent results and statistical trends were obtained. Statistical significance (P ≤ 0.05) as determined by the Student two-tailed t test for comparisons of 2308 versus the other strains is represented by an asterisk (*).
H2O2 is an uncharged ROS and can readily cross cellular membranes by diffusion. This allows extracellular catalase to serve as an efficient detoxifier of intracellular H2O2 (62). Consequently, since the addition of paraquat results in the generation of both superoxide and hydrogen peroxide, an important control in these assays is the addition of exogenous catalase to the test medium to relieve hydrogen peroxide toxicity. This allows for determination of whether or not the increased susceptibility of the B. abortus ahpCD and ahpCD katE mutants to PQ is due to the increased intracellular accumulation of H2O2. As shown in Fig. 2B, the addition of exogenous catalase to the test medium reduces the zone of inhibition around disks containing PQ exhibited by B. abortus KH16 (ahpCD) and KK21 (ahpCD katE) to the same size as those exhibited by the parental 2308 strain. This indicates that the increased sensitivity of the B. abortus ahpCD and ahpCD katE mutants to PQ is due to the increased intracellular accumulation of H2O2 and not a differential sensitivity of these mutants to O2−. More importantly, these experimental findings further support the contention that AhpC serves as a primary detoxifier of endogenous H2O2 produced by respiratory metabolism in B. abortus 2308, while KatE plays a limited and secondary role in this regard.
The ahpCD locus is required for maintenance of stationary-phase viability of B. abortus 2308 during aerobic growth in a defined minimal medium.
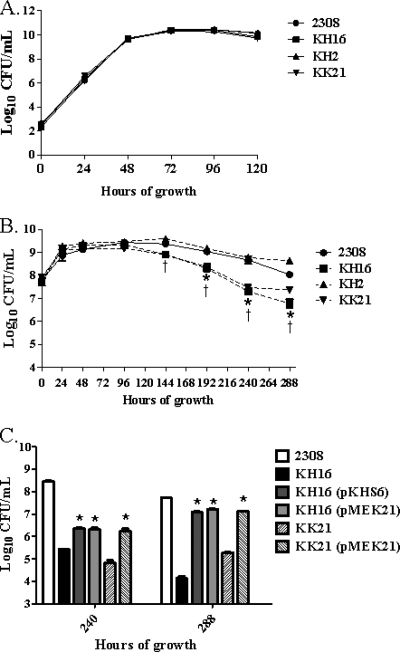
B. abortus 2308, KH16 (ahpCD), KH2 (katE), and KK21 (ahpCD katE) exhibit equivalent growth kinetics and viability during exponential growth and stationary phase when these strains are cultivated aerobically in brucella broth (Fig. 3A). When these strains are grown in Gerhardt's minimal medium (GMM), however, the B. abortus ahpCD mutant KH16 and the isogenic ahpCD katE double mutant KK21 both exhibit a significant loss of stationary-phase viability compared to their respective parental strains 2308 and KH2. Similarly increased levels of endogenous peroxides are present in the B. abortus ahpCD mutant KH16 and the ahpCD katE double mutant KK21 compared to those present in 2308 and the katE mutant KH2 during growth in GMM (data not shown). The loss of stationary-phase viability in GMM exhibited by the B. abortus ahpCD mutant KH16 can also be rescued to a significant degree by the introduction of a plasmid containing either ahpCD or katE into this strain (Fig. 3C), and this phenotype in the B. abortus ahpC katE double mutant KK21 can be rescued by a plasmid carrying katE. These data suggest that AhpC plays a particularly important role in detoxifying endogenous H2O2 generated during stationary phase in B. abortus 2308 during in vitro cultivation under nutrient-limiting conditions.
FIG. 3.
B. abortus ahpCD mutants show loss of stationary-phase viability during cultivation in Gerhardt's minimal medium, and this phenotype can be rescued by either AhpC or KatE. Growth of B. abortus 2308, KH16 (2308 ahpCD), KH2 (2308 katE), and KK21 (2308 ahpCD katE) in brucella broth (A) and Gerhardt's minimal medium (GMM) (B). Part C of this figure shows the viability of B. abortus 2308, KH16, KK21, KH16 (pKHS6), KH16 (pMEK21), and KK21 (pMEK21) following 240 and 288 h of cultivation in GMM. The data presented are means and standard deviations for triplicate determinations for each strain in a single experiment. The data presented here are representative of multiple (≥3) experiments performed from which equivalent results and statistical trends were obtained. In panel B, statistical significance (P ≤ 0.05) as determined by the Student two-tailed t test is represented by an asterisk (*) for the comparison of 2308 versus KH16 and a dagger (†) for the comparison of 2308 versus KK21. For panel C, statistical significance (P ≤ 0.001) for the comparisons of KH16 versus KH16 (pKHS6), KH16 versus KH16 (pMEK21), and KK21 versus KK21 (pMEK21) is indicated by an asterisk (*).
KatE is the major detoxifier of exogenous hydrogen peroxide in B. abortus 2308.
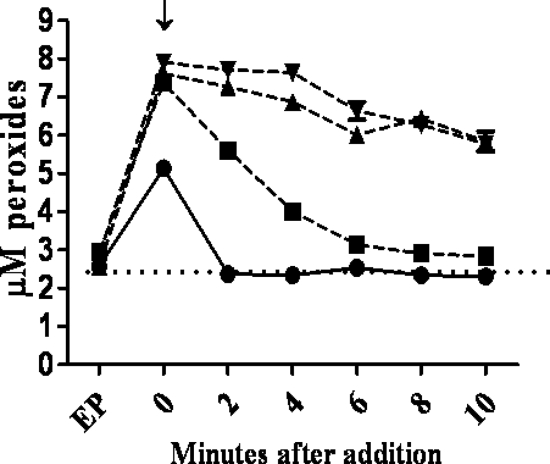
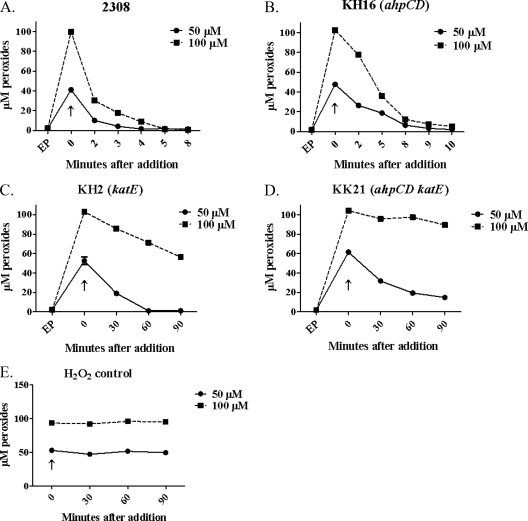
While AhpC appears to be the major detoxifier of endogenous H2O2 in B. abortus 2308, the results presented in Fig. 4 indicate that KatE is the major detoxifier of exogenous H2O2 in this strain. Even at levels of exogenous H2O2 as low as 5 μM, the B. abortus katE and ahpCD katE mutants exhibit a marked defect in their capacity to degrade exogenous H2O2 compared to their parental strains (Fig. 4), and these defects are much more dramatic when these strains are exposed to 50 and 100 μM H2O2 (Fig. 5). The role of KatE in the degradation of exogenous H2O2 is further reflected in the differences in the sensitivities to H2O2 exhibited by the B. abortus ahpC and katE mutants and the ahpCD katE double mutant in a disk sensitivity assay (Table 3), where the strains lacking KatE display a much more pronounced phenotype than the ahpCD mutant.
FIG. 4.
KatE is the predominant detoxifier of 5 μM exogenous H2O2 in B. abortus 2308. Shown are the levels of peroxides measured using the xylenol orange/ferrous iron assay in B. abortus 2308 (•), KH16 (2308 ahpCD) (▪), KH2 (2308 katE) (▴), and KK21 (2308 ahpCD katE) (▾) cell suspensions at selected times after the addition of 5 μM H2O2. The data presented here are representative of multiple (≥3) experiments performed from which equivalent results were obtained. “EP” denotes the levels of endogenous peroxides detected prior to the addition of the H2O2; “↓” denotes the levels of endogenous intracellular peroxides detected immediately after the addition of the H2O2.
FIG. 5.
KatE is the predominant detoxifier of 50 and 100 μM exogenous H2O2 in B. abortus 2308. Shown are the levels of peroxides present in B. abortus 2308 (A), KH16 (B), KH2 (C), and KK21 (D) cell suspensions at selected times following the addition of 50 μM (solid lines) and 100 μM (dashed lines) H2O2. Panel E shows levels of H2O2 detected in cell-free test medium at selected time points following the addition of 50 μM (solid lines) and 100 μM (dashed lines) H2O2. The data presented here are representative of multiple (≥3) experiments performed from which equivalent results were obtained. “EP” denotes the levels of endogenous peroxides detected prior to the addition of the H2O2; “↑” denotes the levels of intracellular peroxides detected immediately after the addition of the H2O2. Note that the time points after addition of the H2O2 differ for some of the panels in this figure.
TABLE 3.
Sensitivity of B. abortus 2308, KH16 (2308 ahpCD), KH2 (2308 katE), and KK21 (2308 ahpCD katE) to H2O2
| Strain | Zone of inhibition (mm)a |
|---|---|
| 2308 | 24 ± 0.58 |
| KH16 | 27 ± 0.58* |
| KH2 | 41 ± 1.5** |
| KK21 | 41 ± 0.0** |
Zone of inhibition around disks containing 10 μl of a 30% solution of H2O2. *, P ≤ 0.05; **, P ≤ 0.005 for comparisons of 2308 versus KH16, KH2, or KK21.
The B. abortus ahpCD mutant KH16 displays increased sensitivity to peroxynitrite.
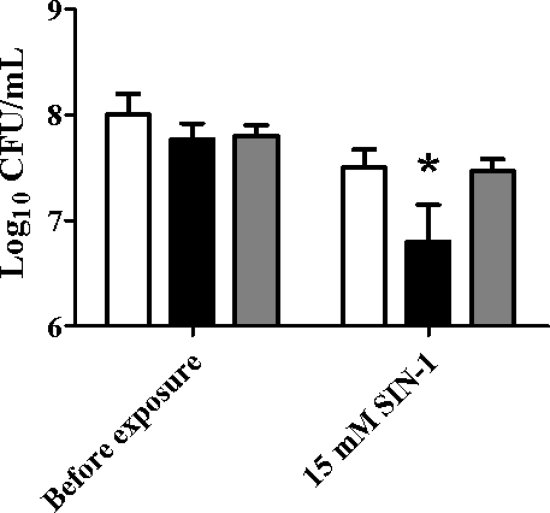
Biochemical studies have shown that in addition to H2O2, AhpC can also detoxify organic peroxides such as tert-butyl hydroperoxide (t-BOOH) (26), cumene hydroperoxide (CHP) (28, 50), and peroxynitrite (ONOO−) in vitro (9), and genetic studies have shown that this peroxiredoxin provides bacterial cells with an important defense against environmental exposure to these compounds (14, 39, 66). The results shown in Fig. 6 suggest that AhpC plays an important role in protecting B. abortus 2308 from exposure to ONOO−. Compared to the parental strain, the ahpC mutant KH16 displays an increased sensitivity to the ONOO− generator SIN-1 in an in vitro assay, and genetic complementation of KH16 with a plasmid-borne wild-type version of the ahpCD locus restores the resistance of the mutant to ONOO− to the same levels as those exhibited by the parent strain. In contrast, the extent to which AhpC contributes to the detoxification of organic peroxides in B. abortus 2308 is unclear. The B. abortus ahpCD mutant KH16 exhibits variable and inconsistent sensitivity to t-BOOH and CHP in standard in vitro assays, and the levels of lipid hydroperoxides present in B. abortus 2308 and KH16 cells following aerobic growth are equivalent (data not shown).
FIG. 6.
The B. abortus ahpCD mutant KH16 exhibits an increased sensitivity to the peroxynitrite generator SIN-1. Viability of B. abortus 2308 (white bars), KH16 (2308 ahpCD) (black bars), and KH16 [pMWV77] (gray bars) before and after a 60-min exposure to SIN-1. The data presented are means and standard deviations for triplicate determinations for each strain in a single experiment. The data presented here are representative of multiple (≥6) experiments performed from which equivalent results and statistical trends were obtained. Statistical significance (P ≤ 0.05) as determined by the Student two-tailed t test for comparison of 2308 versus the other strains is represented by an asterisk (*).
The presence of either AhpC or KatE alone allows B. abortus strains to retain their virulence in the mouse model.
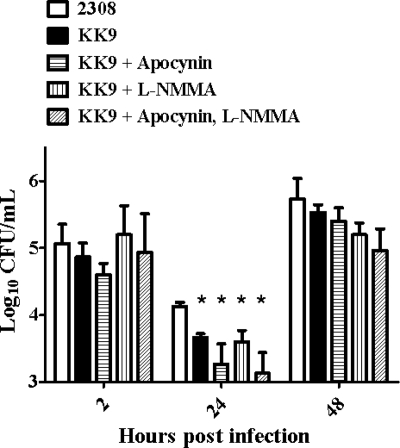
During residence in their mammalian hosts, Brucella strains are exposed to both exogenous ROS produced by the oxidative burst of host phagocytes and endogenous ROS arising as by-products of their own aerobic metabolism (57). To determine to what extent AhpC and KatE protect B. abortus 2308 from H2O2 of exogenous and endogenous origin in the host, the virulence properties of B. abortus 2308 and isogenic ahpCD, katE, and ahpCD katE mutants in cultured murine macrophages and experimentally infected mice were evaluated. Only the B. abortus ahpCD katE mutant KK9 exhibited significant and stable attenuation compared to the parental 2308 strain in cultured murine macrophages (Fig. 7), and this attenuation was consistently observed only when these phagocytes were stimulated with IFN-γ. Notably, the addition of apocynin (a NADPH oxidase inhibitor), L-NMMA (an iNOS inhibitor), or both of these inhibitors in combination to the phagocyte cultures failed to alleviate the attenuation exhibited by the B. abortus ahpCD katE mutant in the IFN-γ-treated macrophages (Fig. 7). These experimental findings suggest that neither AhpC nor KatE is playing a role in protecting B. abortus 2308 from exogenous H2O2 produced as a result of the oxidative burst of these phagocytes. They also suggest that AhpC does not play a prominent role in protecting B. abortus 2308 from exogenous ONOO− generated by the NADPH oxidase and iNOS activity of host macrophages.
FIG. 7.
The B. abortus ahpCD katE mutant KK9 exhibits attenuation in IFN-γ-stimulated cultured murine peritoneal macrophages, and this attenuation is not alleviated by the addition of inhibitors of the oxidative and nitrosative bursts of the host phagocytes. The intracellular survival and replication patterns of B. abortus 2308 and the isogenic ahpCD katE double mutant KK9 in IFN-γ-treated cultured resident peritoneal macrophages from BALB/c mice with or without the addition of the NADPH oxidase inhibitor apocynin and iNOS inhibitor L-NMMA are shown. The data presented are means and standard deviations obtained for each bacterial strain from three separate wells of cultured macrophages at each experimental time point in a single experiment. The data presented here are representative of multiple (≥3) experiments performed from which equivalent results and statistical trends were obtained. Statistical significance (P ≤ 0.05) as determined by the Student two-tailed t test for the comparison of 2308 versus KK9 is represented by an asterisk (*).
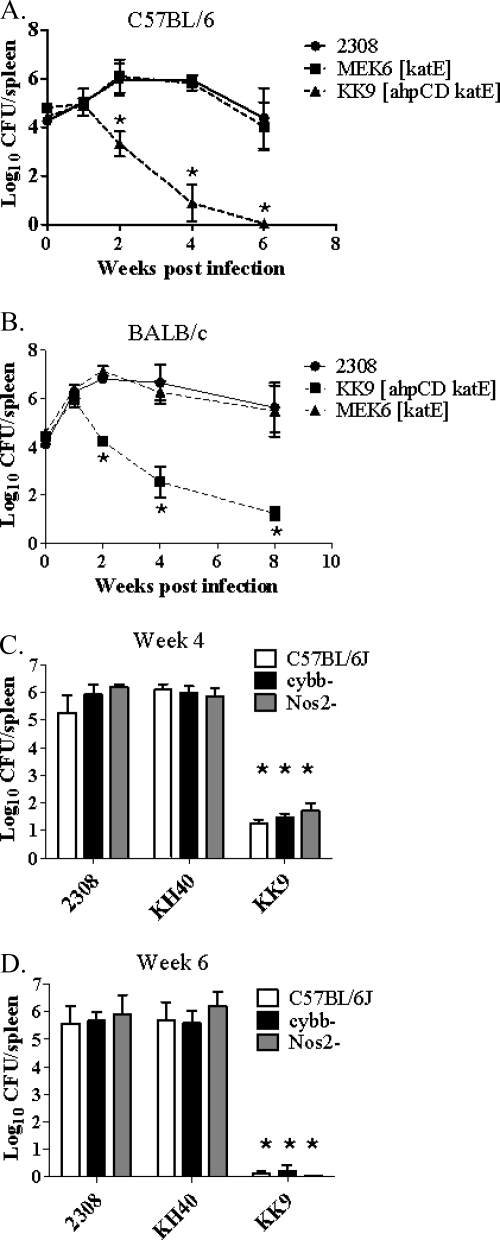
The B. abortus ahpCD katE double mutant KK9 was also the only mutant to display significant attenuation compared to the parental 2308 strain in C57BL/6 (Fig. 8A) or BALB/c (Fig. 8B) mice, and the severe attenuation exhibited by B. abortus KK9 in C57BL/6J mice was not alleviated in congenic NADPH oxidase and iNOS knockout mice (Fig. 8C and D). These data provide further evidence that neither AhpC nor KatE plays a direct role in protecting B. abortus 2308 from the oxidative or nitrosative bursts of host phagocytes. Instead, they support the contention that the buildup of endogenous H2O2 is a biologically relevant environmental stress encountered by B. abortus 2308 during its residence in the murine host (57). Moreover, they demonstrate that the presence of either AhpC or KatE alone is sufficient to alleviate this stress.
FIG. 8.
Spleen colonization profiles of B. abortus 2308, MEK6 (2308 katE), KK9 (2308 ahpCD katE), and KH40 (ahpCD) in C57BL/6(J) mice (A, C, and D), BALB/c mice (B), and NADPH oxidase-deficient (cybb−) and iNOS-deficient (Nos2−) knockout mice in the C57BL/6J background (C and D). The data presented are means and standard deviations for the number of brucellae recovered from the spleens of five mice infected with each strain at each experimental time point in a single experiment. Statistical significance (P ≤ 0.05) as determined by the Student two-tailed t test from comparison of 2308 versus the other strains is represented by an asterisk (*).
DISCUSSION
The experimental findings presented here show that AhpC plays a major role in scavenging H2O2 that is generated as a by-product of respiratory metabolism in B. abortus 2308. This function is similar to that reported for the E. coli AhpC and consistent with the reported biochemical properties of this class of peroxiredoxins in general, which work most efficiently on low levels of H2O2 (61). The capacity of AhpC to scavenge metabolic H2O2 appears to be especially important to B. abortus 2308 for the maintenance of stationary-phase viability when this strain is cultured under nutrient-limited conditions. This function is consistent with the observation that maximum expression of an ahpC-lacZ fusion is observed during stationary phase in B. abortus 2308 (K. Steele, unpublished observations), and AhpC has been proposed to be an important stationary-phase antioxidant (56, 60, 71). The basis for the H2O2-dependent loss of viability of the B. abortus ahpC mutant during stationary phase is not known. But the fact that the ahpC mutant does not exhibit this phenotype during growth in a nutritionally replete medium suggests that certain key biosynthetic enzymes in B. abortus 2308 may be particularly susceptible to H2O2-mediated damage in the absence of AhpC. This phenotype could be masked if the products of the corresponding biosynthetic pathways can be readily obtained from the growth medium. H2O2-mediated damage of the 4Fe-4S clusters in isopropylmalate isomerase, a key enzyme in the leucine biosynthetic pathway, for instance, leads to growth arrest in E. coli katG katE ahpC mutants (29).
In contrast to what was found for the ahpC mutant, the phenotypes exhibited by the B. abortus katE mutant and ahpCD katE mutant suggest that the sole catalase produced by this bacterium plays a minimal role in detoxifying endogenous H2O2 of metabolic origin during routine aerobic cultivation in vitro. These results are intriguing considering the compensatory roles that AhpC and catalases have been reported to perform in scavenging cytoplasmic H2O2 of metabolic origin in ahpC mutants in other bacteria (15, 61). In many cases, the loss of either ahpC or a catalase gene alone does not produce an aerobic growth defect in bacteria, but the loss of both AhpC and a catalase does. The lack of an observable aerobic growth defect in the B. abortus katE mutant in vitro is also notable because KatG appears to be the major scavenger of endogenous H2O2 in Bradyrhizobium japonicum (46), a close phylogenetic relative of the brucellae. In fact, the observation that the B. abortus ahpCD katE double mutant does not exhibit a detectable defect in growth during routine aerobic cultivation in a rich medium or growth on agar plates suggests that this bacterium produces other antioxidants that are capable of compensating for the loss of AhpC's capacity to detoxify H2O2 of metabolic origin. This proposition is further supported by the observation that introduction of the katE mutation into the B. abortus ahpC mutant did not increase this mutant's loss of stationary-phase viability during aerobic culture in a minimal medium. Furthermore, the B. abortus ahpCD katE mutant can still degrade 50 μM H2O2, suggesting that other antioxidants are present to remove the H2O2. The products of the genes designated BAB1_0941 and BAB1_0504 in the B. abortus 2308 genome sequence would appear to be good candidates for this function. BAB1_0941 is predicted to encode a homolog of the bacterioferritin comigratory protein (Bcp) (30), and BAB1_0504 is predicted to encode an AhpC/TSA (thiol-specific antioxidant)-type peroxiredoxin that has sequence similarity to the PrxV-type peroxiredoxins that protect mammalian mitochondria from H2O2 damage (5). Whether or not the putative peroxiredoxins encoded by BAB1_0941 and/or BAB1_0504 can compensate for loss of AhpC activity in B. abortus 2308 remains to be determined experimentally.
Despite the fact that KatE appears to play a minimal role in protecting B. abortus 2308 from endogenous H2O2 during routine aerobic cultivation in vitro, the studies performed with experimentally infected mice suggest that this enzyme plays a pivotal backup role in protecting this bacterium from the metabolic H2O2 it generates during replication in the host. This proposition is based on two observations. First, although our in vitro studies suggest that the Brucella AhpC has the capacity to degrade H2O2, ONOO−, and possibly organic peroxides (see below), the only described function for monofunctional catalases such as KatE that the authors are aware of is the degradation of H2O2. This strongly suggests that H2O2 toxicity plays a key role in the attenuation exhibited by the B. abortus ahpCD katE mutant. Second, this mutant displays the same level of attenuation in NADPH oxidase-deficient mice that it does in wild-type mice, indicating that AhpC and KatE do not provide protection from exogenous H2O2 produced as a by-product of the oxidative burst of host phagocytes. The fact that the presence of either AhpC or KatE alone allows B. abortus 2308 to maintain persistent infection in mice suggests that brucellae possess functionally redundant systems to protect themselves from the metabolic H2O2 they generate endogenously during replication in the host. This is perhaps to be expected of a bacterium that must deal with exposure to ROS of both endogenous origin as well as those generated by the NADPH oxidase and iNOS activity of host phagocytes (57) during residence in this environment.
The fact that neither AhpC nor KatE appears to play a role in protecting the brucellae from the oxidative burst of macrophages is intriguing, especially considering the role that ROS and IFN-γ have been proposed to play in the brucellacidal activity of these phagocytes (31). Moreover, the temporal nature of the attenuation exhibited by the B. abortus ahpC katE double mutant KK9 in cultured macrophages (e.g., 24 h p.i.) and the observation that attenuation of this mutant was observed only when these phagocytes were stimulated by IFN-γ are what would be predicted for a Brucella mutant with an increased sensitivity to the oxidative burst (31). The apparent lack of correlation between macrophage NADPH oxidase activity and the attenuation exhibited by the B. abortus ahpC katE double mutant is also perplexing given the documented role that Brucella SodC plays in detoxifying O2− produced by host macrophages (22), a process that generates H2O2. One possibility is that the exogenous H2O2 produced as a by-product of the oxidative burst of host macrophages is less of a threat to the brucellae than the primary product of this reaction (e.g., O2−). This would be analogous to the situation observed for Salmonella strains, where mutants lacking SODs that detoxify exogenous O2− (e.g., sodC mutants) are more attenuated in experimental hosts than the corresponding catalase- or AhpC-deficient mutants (17, 25, 68). Clearly, a more comprehensive evaluation of the gene products that protect the brucellae from the respiratory burst of host macrophages is warranted. It will also be important to define the Brucella cellular components that are prone to damage by exogenous and endogenous ROS during replication in host macrophages.
The increased sensitivity of the B. abortus ahpC mutant to the ONOO− generator SIN-1 in in vitro assays suggests that the Brucella AhpC, like its counterparts in Salmonella enterica serovar Typhimurium, Mycobacterium tuberculosis, and Helicobacter pylori, has peroxynitrite reductase activity (9). This enzymatic activity has been proposed to be important as a bacterial defense against ONOO− production by host macrophages (39), but the results obtained in this study with the B. abortus ahpCD mutant KH40 and the ahpCD katE double mutant KK9 in iNOS-deficient mice suggest that AhpC does not play a prominent role in protecting the parental 2308 strain from ONOO− produced by host phagocytes.
In addition to their ability to detoxify H2O2 and ONOO−, bacterial AhpC proteins have also been shown to be able to degrade organic peroxides. Indeed, the name alkyl hydroperoxide reductase reflects the fact that degradation of organic peroxides was the first property identified for many members of this class of bacterial enzymes (28). Thus, it is notable that no conclusive evidence was obtained from the studies described in this report supporting a role for AhpC in the detoxification of organic peroxides in B. abortus 2308. One possible explanation for these findings is that this bacterial strain also possesses the organic peroxide scavenger Ohr (40). Phenotypic analysis of an ohr mutant indicates a role for Ohr in the detoxification of the organic peroxides tert-butyl hydroperoxide and cumene hydroperoxide in B. abortus 2308 (J. Baumgartner, unpublished). Consequently, further phenotypic analysis of ahpC and ohr mutants and ahpC ohr double mutants will be needed to determine whether or not AhpC can detoxify organic peroxides in B. abortus 2308.
In summary, the results presented in this report indicate that AhpC and KatE play distinct but complementary roles in protecting B. abortus 2308 from exposure to H2O2. In order to better understand the contributions of these antioxidants to the physiology and intracellular lifestyle of this bacterium, it will be important in future studies to examine the coordinate regulation of ahpCD and katE during different stages of growth and in response to oxidative stress. It will also be important to determine how Brucella KatE is exported to the periplasm.
Acknowledgments
We thank Eric Anderson, Jennifer Gaines, Michael Kovach, Clayton Caswell, and Daniel Martin for their technical and intellectual contributions to this work.
These studies were funded by a grant from the National Institute of Allergy and Infectious Diseases (AI48499) to R.M.R.
Footnotes
Published ahead of print on 30 July 2010.
REFERENCES
- 1.Alton, G. G., L. M. Jones, R. D. Angus, and J. M. Verger. 1988. Techniques for the brucellosis laboratory. Institut National de la Recherche Agronomique, Paris, France.
- 2.Ariza, J., M. Bosilkovski, A. Cascio, J. D. Colmenero, M. J. Corbel, M. E. Falagas, Z. A. Memish, M. R. H. Roushan, E. Rubinstein, N. V. Sipsas, J. Solera, E. J. Young, and G. Pappas. 2007. Perspectives for the treatment of brucellosis in the 21st century: the Ioannina recommendations. PLoS Med. 4:1872-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. D. Struhl. 2000. Current protocols in molecular biology. John Wiley & Sons, New York, NY.
- 4.Baillon, M.-L. A., A. H. M. van Vliet, J. M. Ketley, C. Constantinidou, and C. W. Penn. 1999. An iron-regulated alkyl hydroperoxide reductase (AhpC) confers aerotolerance and oxidative stress resistance to the microaerophilic pathogen Campylobacter jejuni. J. Bacteriol. 181:4798-4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banmeyer, I., C. Marchand, A. Clippe, and B. Knoops. 2005. Human mitochondrial peroxiredoxin 5 protects from mitochondrial DNA damages induced by hydrogen peroxide. FEBS Lett. 579:2327-2333. [DOI] [PubMed] [Google Scholar]
- 6.Beck, B. L., L. B. Tabatabai, and J. E. Mayfield. 1990. A protein isolated from Brucella abortus is a Cu-Zn superoxide dismutase. Biochemistry 29:372-376. [DOI] [PubMed] [Google Scholar]
- 7.Bowe, F., and F. Heffron. 1994. Isolation of Salmonella mutants defective for intracellular survival. Methods Enzymol. 236:509-526. [DOI] [PubMed] [Google Scholar]
- 8.Brenot, A., K. Y. King, and M. G. Caparon. 2005. The PerR regulon in peroxide resistance and virulence of Streptococcus pyogenes. Mol. Microbiol. 55:221-234. [DOI] [PubMed] [Google Scholar]
- 9.Bryk, R., P. Griffin, and C. Nathan. 2000. Peroxynitrite reductase activity of bacterial peroxiredoxins. Nature 407:211-215. [DOI] [PubMed] [Google Scholar]
- 10.Bryk, R., C. D. Lima, H. Erdjument-Bromage, P. Tempst, and C. Nathan. 2002. Metabolic enzymes of mycobacteria linked to antioxidant defense by a thioredoxin-like protein. Science 295:1073-1077. [DOI] [PubMed] [Google Scholar]
- 11.Bsat, N., L. Chen, and J. D. Helmann. 1996. Mutation of the Bacillus subtilis alkyl hydroperoxide reductase (ahpCF) operon reveals compensatory interactions among hydrogen peroxide stress genes. J. Bacteriol. 178:6579-6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chauhan, R., and S. C. Mande. 2002. Site-directed mutagenesis reveals a novel catalytic mechanism of Mycobacterium tuberculosis alkylhydroperoxidase C. Biochem. J. 367:255-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chelikani, P., I. Fita, and P. C. Loewen. 2004. Diversity of structures and properties among catalases. Cell. Mol. Life Sci. 61:192-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen, L., Q. W. Xie, and C. Nathan. 1998. Alkyl hydroperoxide reductase subunit C (AhpC) protects bacterial and human cells against reactive nitrogen intermediates. Mol. Cell 1:795-805. [DOI] [PubMed] [Google Scholar]
- 15.Cosgrove, K., G. Coutts, I.-M. Jonsson, A. Tarkowski, J. F. Kokai-Kun, J. J. Mond, and S. J. Foster. 2007. Catalase (KatA) and alkyl hydroperoxide reductase (AhpC) have compensatory roles in peroxide stress resistance and are required for survival, persistence, and nasal colonization in Staphylococcus aureus. J. Bacteriol. 189:1025-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Croxen, M. A., P. B. Ernst, and P. S. Hoffman. 2007. Antisense RNA modulation of alkyl hydroperoxide reductase levels in Helicobacter pylori correlates with organic peroxide toxicity but not infectivity. J. Bacteriol. 189:3359-3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Groote, M. A., U. A. Ochsner, M. U. Shiloh, C. Nathan, J. M. McCord, M. C. Dinauer, S. J. Libby, A. Vazquez-Torres, Y. Xu, and F. C. Fang. 1997. Periplasmic superoxide dismutase protects Salmonella from products of the phagocyte NADPH-oxidase and nitric oxide synthase. Proc. Natl. Acad. Sci. U. S. A. 94:13997-14001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding, A. H., C. F. Nathan, and D. J. Stuehr. 1988. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages: comparison of activating cytokines and evidence for independent production. J. Immunol. 141:2407-2412. [PubMed] [Google Scholar]
- 19.Elzer, P. H., R. W. Phillips, M. E. Kovach, K. M. Peterson, and R. M. Roop II. 1994. Characterization and genetic complementation of a Brucella abortus high-temperature-requirement A (htrA) deletion mutant. Infect. Immun. 62:4135-4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fridovich, I. 1995. Superoxide radical and superoxide dismutases. Annu. Rev. Biochem. 64:97-112. [DOI] [PubMed] [Google Scholar]
- 21.Gee, J. M., M. E. Kovach, V. K. Grippe, S. Hagius, J. V. Walker, P. H. Elzer, and R. M. Roop II. 2004. Role of catalase in the virulence of Brucella melitensis in pregnant goats. Vet. Microbiol. 102:111-115. [DOI] [PubMed] [Google Scholar]
- 22.Gee, J. M., M. W. Valderas, M. E. Kovach, V. K. Grippe, G. T. Robertson, W.-L. Ng, J. M. Richardson, M. E. Winkler, and R. M. Roop II. 2005. The Brucella abortus Cu,Zn superoxide dismutase is required for optimal resistance to oxidative killing by murine macrophages and wild-type virulence in experimentally infected mice. Infect. Immun. 73:2873-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerhardt, P. 1958. The nutrition of brucellae. Microbiol. Rev. 22:81-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hassan, H. M., and I. Fridovich. 1979. Paraquat and Escherichia coli: mechanism of production of extracellular superoxide radical. J. Biol. Chem. 254:10846-10852. [PubMed] [Google Scholar]
- 25.Hébrard, M., J. P. M. Viala, S. Méresse, F. Barras, and L. Aussel. 2009. Redundant hydrogen peroxide scavengers contribute to Salmonella virulence and oxidative stress resistance. J. Bacteriol. 191:4605-4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hillas, P. J., F. S. del Alba, J. Oyarzabal, A. Wilks, and P. R. Ortiz De Montellano. 2000. The AhpC and AhpD antioxidant defense system of Mycobacterium tuberculosis. J. Biol. Chem. 275:18801-18809. [DOI] [PubMed] [Google Scholar]
- 27.Imlay, J. A. 2008. Cellular defenses against superoxide and hydrogen peroxide. Annu. Rev. Biochem. 77:755-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacobson, F. S., R. W. Morgan, M. F. Christman, and B. N. Ames. 1989. An alkyl hydroperoxide reductase from Salmonella typhimurium involved in the defense of DNA against oxidative damage. Purification and properties. J. Biol. Chem. 264:1488-1496. [PubMed] [Google Scholar]
- 29.Jang, S., and J. A. Imlay. 2007. Micromolar intracellular hydrogen peroxide disrupts metabolism by damaging iron-sulfur enzymes. J. Biol. Chem. 282:929-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeong, W., M.-K. Cha, and I. H. Kim. 2000. Thioredoxin-dependent hydroperoxidase activity of bacterioferritin comigratory protein (BCP) as a new member of the thiol-specific antioxidant protein (TSA)/alkyl hydroperoxide peroxidase (AhpC) family. J. Biol. Chem. 275:2924-2930. [DOI] [PubMed] [Google Scholar]
- 31.Jiang, X., B. Leonard, R. Benson, and C. L. Baldwin. 1993. Macrophage control of Brucella abortus: role of reactive oxygen intermediates and nitric oxide. Cell. Immunol. 151:309-319. [DOI] [PubMed] [Google Scholar]
- 32.Johnson, N. A., Y. Liu, and H. M. Fletcher. 2004. Alkyl hydroperoxide peroxidase subunit C (ahpC) protects against organic peroxides but does not affect the virulence of Porphyromonas gingivalis W83. Oral Microbiol. Immunol. 19:233-239. [DOI] [PubMed] [Google Scholar]
- 33.Kim, J.-A., Z. Sha, and J. E. Mayfield. 2000. Regulation of Brucella abortus catalase. Infect. Immun. 68:3681-3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Köhler, S., S. Michaux-Charachon, F. Porte, M. Ramuz, and J.-P. Liautard. 2003. What is the nature of the replicative niche of a stealthy bug named Brucella? Trends Microbiol. 11:215-219. [DOI] [PubMed] [Google Scholar]
- 35.Kovach, M. E., P. H. Elzer, D. S. Hill, R. M. Roop II, and K. M. Peterson. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic resistance cassettes. Gene 166:175-176. [DOI] [PubMed] [Google Scholar]
- 36.La Carbona, S., N. Sauvageot, J.-C. Giard, A. Benachour, B. Posteraro, Y. Auffray, M. Sanguinetti, and A. Hartke. 2007. Comparative study of the physiological roles of three peroxidases (NADH peroxidase, alkyl hydroperoxide reductase and thiol peroxidase) in oxidative stress response, survival inside macrophages and virulence of Enterococcus faecalis. Mol. Microbiol. 66:1148-1163. [DOI] [PubMed] [Google Scholar]
- 37.LeBlanc, J. J., R. J. Davidson, and P. S. Hoffman. 2006. Compensatory functions of two alkyl hydroperoxide reductases in the oxidative defense system of Legionella pneumophila. J. Bacteriol. 188:6235-6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loewen, P. C. 1997. Bacterial catalases, p. 273-308. In J. G. Scandalios (ed.), Oxidative stress and the molecular biology of antioxidant defenses. Cold Spring Harbor Laboratory Press, Plainview, New York, NY.
- 39.Master, S. S., B. Springer, P. Sander, E. C. Boettger, V. Deretic, and G. S. Timmins. 2002. Oxidative stress response genes in Mycobacterium tuberculosis: role of ahpC in resistance to peroxynitrite and stage-specific survival in macrophages. Microbiology 148:3139-3144. [DOI] [PubMed] [Google Scholar]
- 40.Mongkolsuk, S., W. Praituan, S. Loprasert, M. Fuangthong, and S. Chamnongpol. 1998. Identification and characterization of a new organic hydroperoxide resistance (ohr) gene with a novel pattern of oxidative stress regulation from Xanthomonas campestris pv. phaseoli. J. Bacteriol. 180:2636-2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mongkolsuk, S., W. Whangsuk, P. Vattanaviboon, S. Loprasert, and M. Fuangthong. 2000. A Xanthomonas alkyl hydroperoxide reductase subunit C (ahpC) mutant showed an altered peroxide stress response and complex regulation of the compensatory response of peroxide detoxification enzymes. J. Bacteriol. 182:6845-6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muijsers, R. B. R., E. van den Worm, G. Folkerts, C. J. Beukelman, A. S. Koster, D. S. Postma, and F. P. Nijkamp. 2000. Apocynin inhibits peroxynitrite formation by murine macrophages. Br. J. Pharmacol. 130:932-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nicoletti, P. 1989. Relationship between animal and human brucellosis, p. 41-51. In E. J. Young and M. J. Corbel (ed.), Brucellosis: clinical and laboratory aspects. CDC Press, Boca Raton, FL.
- 44.Olczak, A. A., J. W. Olson, and R. J. Maier. 2002. Oxidative-stress resistance mutants of Helicobacter pylori. J. Bacteriol. 184:3186-3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Olczak, A. A., R. W. Seyler, Jr., J. W. Olson, and R. J. Maier. 2003. Association of Helicobacter pylori antioxidant activities with host colonization proficiency. Infect. Immun. 71:580-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Panek, H. R., and M. R. O'Brian. 2004. KatG is the primary detoxifier of hydrogen peroxide produced by aerobic metabolism in Bradyrhizobium japonicum. J. Bacteriol. 186:7874-7880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pappas, G., P. Papadimitriou, N. Akritidis, L. Christou, and E. V. Tsianos. 2006. The new global map of human brucellosis. Lancet Infect. Dis. 6:91-99. [DOI] [PubMed] [Google Scholar]
- 48.Parsonage, D., P. A. Karplus, and L. B. Poole. 2008. Substrate specificity and redox potential of AhpC, a bacterial peroxiredoxin. Proc. Natl. Acad. Sci. U. S. A. 105:8209-8214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Poole, L. B. 1996. Flavin-dependent alkyl hydroperoxide reductase from Salmonella typhimurium. 2. Cystine disulfides involved in catalysis of peroxide reduction. Biochemistry 35:65-75. [DOI] [PubMed] [Google Scholar]
- 50.Poole, L. B., and H. R. Ellis. 1996. Flavin-dependent alkyl hydroperoxide reductase from Salmonella typhimurium. 1. Purification and enzymatic activities of overexpressed AhpF and AhpC proteins. Biochemistry 35:56-64. [DOI] [PubMed] [Google Scholar]
- 51.Rankin, S., Z. Li, and R. R. Isberg. 2002. Macrophage-induced genes of Legionella pneumophila: protection from reactive intermediates and solute imbalance during intracellular growth. Infect. Immun. 70:3637-3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rest, R. F., and D. C. Robertson. 1975. Characterization of the electron transport system in Brucella abortus. J. Bacteriol. 122:139-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Robertson, G. T., and R. M. Roop II. 1999. The Brucella abortus host factor I (HF-I) protein contributes to stress resistance during stationary phase and is a major determinant of virulence in mice. Mol. Microbiol. 34:690-700. [DOI] [PubMed] [Google Scholar]
- 54.Robertson, G. T., M. E. Kovach, C. A. Allen, T. A. Ficht, and R. M. Roop II. 2000. The Brucella abortus Lon functions as a generalized stress response protease and is required for wild-type virulence in BALB/c mice. Mol. Microbiol. 35:577-588. [DOI] [PubMed] [Google Scholar]
- 55.Rocha, E. R., and C. J. Smith. 1999. Role of the alkyl hydroperoxide reductase (ahpCF) gene in oxidative stress defense of the obligate anaerobe Bacteroides fragilis. J. Bacteriol. 181:5701-5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roop, R. M., II, J. M. Gee, G. T. Robertson, J. M. Richardson, W.-L. Ng, and M. E. Winkler. 2003. Brucella stationary-phase gene expression and virulence. Annu. Rev. Microbiol. 57:57-76. [DOI] [PubMed] [Google Scholar]
- 57.Roop, R. M., II, J. M. Gaines, E. S. Anderson, C. C. Caswell, and D. W. Martin. 2009. Survival of the fittest: how Brucella strains adapt to their intracellular niche in the host. Med. Microbiol. Immunol. 198:221-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 59.Sangari, F. J., and J. Agüero. 1996. Molecular basis of Brucella pathogenicity: an update. Microbiologia 12:207-218. [PubMed] [Google Scholar]
- 60.Schurig-Briccio, L. A., R. N. Farías, L. Rodríquez-Montelongo, R. R. Rintoul, and V. A. Rapisarda. 2009. Protection against oxidative stress in Escherichia coli stationary phase by a phosphate concentration-dependent genes expression. Arch. Biochem. Biophys. 483:106-110. [DOI] [PubMed] [Google Scholar]
- 61.Seaver, L. C., and J. A. Imlay. 2001. Alkyl hydroperoxide reductase is the primary scavenger of endogenous hydrogen peroxide in Escherichia coli. J. Bacteriol. 183:7173-7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Seaver, L. C., and J. A. Imlay. 2001. Hydrogen peroxide fluxes and compartmentalization inside growing Escherichia coli. J. Bacteriol. 183:7182-7189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sha, Z., T. J. Stabel, and J. E. Mayfield. 1994. Brucella abortus catalase is a periplasmic protein lacking a standard signal sequence. J. Bacteriol. 176:7375-7377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Springer, B., S. Master, P. Sander, T. Zahrt, M. McFalone, J. Song, K. G. Papavinasasundaram, M. J. Colston, E. Boettger, and V. Deretic. 2001. Silencing of oxidative stress response in Mycobacterium tuberculosis: expression patterns of ahpC in virulent and avirulent strains and effect of ahpC inactivation. Infect. Immun. 69:5967-5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sriranganathan, N., S. M. Boyle, G. Schurig, and H. Misra. 1991. Superoxide dismutases of virulent and avirulent strains of Brucella abortus. Vet. Microbiol. 26:359-366. [DOI] [PubMed] [Google Scholar]
- 66.Storz, G., F. S. Jacobson, L. A. Tartaglia, R. W. Morgan, L. A. Silveira, and B. N. Ames. 1989. An alkyl hydroperoxide reductase induced by oxidative stress in Salmonella typhimurium and Escherichia coli: genetic characterization and cloning of ahp. J. Bacteriol. 171:2049-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Switala, J., and P. C. Loewen. 2002. Diversity of properties among catalases. Arch. Biochem. Biophys. 401:145-154. [DOI] [PubMed] [Google Scholar]
- 68.Taylor, P. D., C. J. Inchley, and M. P. Gallagher. 1998. The Salmonella typhimurium AhpC polypeptide is not essential for virulence in BALB/c mice but is recognized as an antigen during infection. Infect. Immun. 66:3208-3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Valderas, M. W., R. B. Alcantara, J. E. Baumgartner, B. H. Bellaire, G. T. Robertson, W.-L. Ng, J. M. Richardson, M. E. Winkler, and R. M. Roop II. 2005. Role of HdeA in acid resistance and virulence in Brucella abortus 2308. Vet. Microbiol. 107:307-312. [DOI] [PubMed] [Google Scholar]
- 70.Valderas, M. W., and R. M. Roop II. 2006. Brucella and bioterrorism, p. 139-153. In B. Anderson, H. Friedman, and M. Bendinelli (ed.), Microorganisms and bioterrorism. Springer, New York, NY.
- 71.Wasim, M., A. N. Bible, Z. Xie, and G. Alexandre. 2009. Alkyl hydroperoxide reductase has a role in oxidative stress resistance and in modulating changes in cell-surface properties in Azospirillum brasilense Sp245. Microbiology 155:1192-1202. [DOI] [PubMed] [Google Scholar]
- 72.Wilson, T., G. W. de Lisle, J. A. Marcinkeviciene, J. S. Blanchard, and D. M. Collins. 1998. Antisense RNA to ahpC, an oxidative stress defence gene involved in isoniazid resistance, indicates that AhpC of Mycobacterium bovis has virulence properties. Microbiology 144:2687-2695. [DOI] [PubMed] [Google Scholar]
- 73.Wolff, S. P. 1994. Ferrous ion oxidation in the presence of ferric ion indicator xylenol orange for measurement of hydroperoxides. Methods Enzymol. 233:289-303. [Google Scholar]