Abstract
Agrobacterium VirB2 pilin is required for assembly of the VirB/VirD4 type IV secretion system (T4SS). The propilin is processed by signal sequence cleavage and covalent linkage of the N and C termini, and the cyclized pilin integrates into the inner membrane (IM) as a pool for assembly of the secretion channel and T pilus. Here, by use of the substituted cysteine accessibility method (SCAM), we defined the VirB2 IM topology and then identified distinct contributions of the T4SS ATPase subunits to the pilin structural organization. Labeling patterns of Cys-substituted pilins exposed to the membrane-impermeative, thiol-reactive reagent 3-(N-maleimidopropionyl)biocytin (MPB) supported a topology model in which two hydrophobic stretches comprise transmembrane domains, an intervening hydrophilic loop (residues 90 to 94) is cytoplasmic, and the hydrophilic N and C termini joined at residues 48 and 121 form a periplasmic loop. Interestingly, the VirB4 ATPase, but not a Walker A nucleoside triphosphate (NTP) binding motif mutant, induced (i) MPB labeling of Cys94, a residue that in the absence of the ATPase is located in the cytoplasmic loop, and (ii) release of pilin from the IM upon osmotic shock. These findings, coupled with evidence for VirB2-VirB4 complex formation by coimmunoprecipitation, support a model in which VirB4 functions as a dislocation motor to extract pilins from the IM during T4SS biogenesis. The VirB11 ATPase functioned together with VirB4 to induce a structural change in the pilin that was detectable by MPB labeling, suggestive of a role for VirB11 as a modulator of VirB4 dislocase activity.
The Agrobacterium tumefaciens VirB/VirD4 type IV secretion system (T4SS) delivers effector proteins and DNA to plant cells during infection (1, 14). The 11 VirB proteins and VirD4 substrate receptor mediate assembly of the envelope-spanning translocation channel, whereas the VirB proteins independently of VirD4 are required for polymerization of the extracellular T pilus (6, 32, 46). These T4SS subunits include the three ATPases VirD4, VirB4, and VirB11; a trans-envelope core complex comprised of VirB7, VirB9, and VirB10; subunits involved in assembly or spatial positioning of the core complex (VirB1, VirB6, and VirB8); and other structural components (VirB2 pilin, VirB3, and pilus-associated VirB5) (1, 14, 43, 48, 55, 70). The VirB/VirD4 subunits are conserved among many Gram-negative bacterial T4SSs, and recent structures of homologs of VirD4, VirB5, VirB8, VirB10, and VirB11 and a VirB7/VirB9/VirB10 machine subassembly are supplying exciting new information about T4SS machine architectures (11, 28, 29).
The pilin subunit VirB2 is a component of both the secretion channel and T pilus (39, 47, 48). Its role in substrate transfer was established with a modified chromatin immunoprecipitation (ChIP) assay termed transfer DNA (T-DNA) immunoprecipitation (TrIP), wherein the pilin (but not the T pilus) was shown to form formaldehyde-cross-linkable contacts with the translocating T-DNA substrate (10). TrIP studies with virB mutant strains also supplied evidence that VirB2 occupies a distal portion of the translocation channel near or at the outer membrane (OM) (10). Complementary genetic studies identified mutations in several VirB subunits, including VirB6, VirB9, VirB10, and VirB11, that selectively block T pilus production without affecting substrate transfer (39, 40, 41, 62). These Tra+ Pil− “uncoupling” mutations do not bypass the requirement for VirB2 production for substrate transfer, as the further deletion of virB2 from the Tra+ Pil− mutant strains renders these strains transfer defective (39, 41, 62). Therefore, VirB2 pilin, but not an intact T pilus, is required for passage of substrates to target cells.
The pathways culminating in the integration of VirB2 into the two terminal organelles, the secretion channel and T pilus, are fundamentally poorly understood. The early VirB protein-independent reactions involve insertion of the 12.3-kDa propilin into the inner membrane (IM); cleavage of a long, 47-residue signal sequence, presumably by LepB signal peptidase; and covalent joining of the N-terminal Gln48 and C-terminal Ser121 to form the mature, cyclic pilin (24). This unusual head-to-tail cyclization reaction was also shown for the VirB2 homolog, TrbC (24/51% sequence identity/similarity) of plasmid RP4 (24, 34, 44). Other VirB2 homologs, such as F plasmid TraA (19/47% identity/similarity) (67), remain linear although their N termini are modified by N acetylation (54).
Prevailing models suggest that mature forms of conjugative pilins accumulate in the IM as pools for use in assembly of the channel/pilus upon receipt of an unknown morphogenetic signal(s). The IM-integrated VirB2, TraAF, and TrbCRP4 pilins likely adopt similar topologies, as deduced from similar predicted secondary structures and results of reporter fusion studies with periplasmically active alkaline phosphatase (PhoA) (5, 22, 56). Two hydrophobic domains are thought to orient across the IM so that a small, intervening hydrophilic loop is cytoplasmic and the hydrophilic N and C termini are periplasmic. Detailed studies confirming this overall topology are lacking, and limited information exists regarding the nature of pilin interactions with other T4SS subunits (36, 51). Furthermore, little is known about the mechanism or energetic requirements for dislocation of membrane-integrated forms of conjugative pilins during machine morphogenesis.
In A. tumefaciens, mutations in the Walker A nucleoside triphosphate (NTP) binding site motifs of the VirB4 and VirB11 ATPases render cells defective for substrate transfer and pilus production, indicating that NTP energy consumption by both ATPases is essential for assembly of the two terminal organelles (6, 7, 58, 62, 68). VirB4-like subunits are signatures of all T4SSs described to date, whereas VirB11-like proteins are common but not ubiquitous among the T4SSs (1). Some T4SSs, such as the conjugation machines encoded by Escherichia coli F-like plasmids, lack VirB11 homologs, and yet their conjugative pili extend and retract dynamically by a mechanism(s) dependent on VirB4 homologs (18, 65). On the basis of these observations, it is reasonable to propose that the VirB4-like subunits catalyze early reactions associated with assembly of conjugative pili.
Here, we used the scanning cysteine accessibility method (SCAM) (9) to define the IM topology of cyclized VirB2. We then assayed for contributions of VirB subunits to the pilin structural organization. We present biochemical evidence for VirB4-mediated dislocation of VirB2 pilin from the membrane and also for a contribution by VirB11 in modulating pilin tertiary or quaternary structure. We discuss our findings in the context of recent advances in our understanding of T4SS machine assembly and architecture.
MATERIALS AND METHODS
Bacterial strains and induction conditions.
A348 carries the octopine-type plasmid pTiA6NC (wild type [WT]) (33). PC1000 (ΔvirB operon), PC1004 (ΔvirB4), and PC1211 (ΔvirB2 ΔvirB11) are A348 derivatives (6, 26, 39). Strains JK1002, carrying a nonpolar deletion of virB2 (ΔvirB2), and JK1204, deleted of virB2 and virB4 (ΔvirB2 ΔvirB4), were constructed as follows. The sacB suicide plasmid pJEK03, which carries a nonpolar deletion of virB2 (see below), was introduced by transformation into wild-type A348 and the ΔvirB4 mutant, PC1004. The ΔvirB2 mutation was inserted into the virB2 locus by marker exchange eviction, as described previously (6). Both the deletion mutations and nonpolarity of the deletions in the resulting strains, JK1002 (ΔvirB2) and JK1204 (ΔvirB2 ΔvirB4), were confirmed by immunodetection of VirB proteins synthesized from downstream genes and trans complementation assays, as described previously (6). Conditions for growth of A. tumefaciens and Escherichia coli and for vir gene induction with 100 μM acetosyringone (AS) in induction medium (ABIM) have been described previously (6). Plasmids were maintained in A. tumefaciens and E. coli by addition of carbenicillin (100 μg/ml), kanamycin (50 to 100 μg/ml), tetracycline (5 μg/ml), and gentamicin sulfate (20 to 100 μg/ml).
Protein analysis and immunoblotting.
Proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) or a Tricine-SDS-PAGE system as previously described (59). Proteins were transferred to nitrocellulose membranes, and immunoblots were developed with goat anti-rabbit antibodies conjugated to alkaline phosphatase and histochemical substrates. Alternatively, blots were developed with anti-rabbit antibodies conjugated to horseradish peroxidase, and antibody-antigen interactions were visualized by chemiluminescence (Pierce, Thermo Scientific). Prestained SDS-PAGE molecular size markers were from Bio-Rad.
Plasmid constructions.
The plasmids used in this study are listed in Table 1. Plasmid pJEK01, expressing PvirB-virB2C64S, was constructed as follows. Plasmid pPC927, which carries an NdeI restriction site at the start of virB2 (6), was used as the template to construct the virB2 C64S codon substitution with the mutagenic oligonucleotide listed in Table 1. A 0.5-kb NdeI-XmnI fragment carrying only virB2C64S was introduced downstream of the PvirB promoter, substituting for virB1 of pPC914. Plasmid pJEK1 served as the template for construction of the Cys substitution mutations by Kunkel or QuikChange (Stratagene) mutagenesis as described previously (6). The mutagenic oligonucleotides listed in Table 1 carried the Cys substitution codon and at least 15 bp of 5′ and 3′ flanking complementary sequences. Cys substitution mutations were identified by restriction enzyme digestion and confirmed by sequencing. The resulting plasmids were designated pJKxx, where xx denotes the number of the substituted residue (relative to the N-terminal Met of the propilin). The pJKxx plasmids were introduced into A. tumefaciens strains by ligation to the IncP plasmid pXZ151 (Kanr) or pBBR1MCS (Genr or Kanr), resulting in plasmid pJKBxx or pJKBBxx, respectively. Plasmid pJEK02 was constructed by deleting a 46-bp EcoRV internal fragment in virB2 from plasmid pBB8 (6). Plasmid pJEK03, used to generate nonpolar virB2 deletions on the Ti plasmid, was constructed by introducing a 2.9-kb BamHI-SalI fragment from pJEK02 that encompasses the ΔvirB2 mutation and 5′ and 3′ flanking sequences into the corresponding sites of the sacB-containing plasmid pBB50 (6). Plasmid pKA93 expresses PvirB-virB4 from the broad-host-range vector pBBR1MCS2Gr (3). Plasmid pKA96, expressing PvirB-virB4K439Q, is pBBR1MCS2Gr with a 2.4-kb XbaI/XhoI fragment from pBB11 (6). Plasmid pKA94, expressing PvirB-virB4 and PvirB-virB11, is a cointegrate of plasmids pKA93 and pSR1 joined at their respective XhoI sites (58).
TABLE 1.
Strains, plasmids, and oligonucleotide primers for virB2 mutagenesis
| Bacterial strain, plasmid, or mutation | Relevant characteristics or 5′ primer sequencea | Reference or source |
|---|---|---|
| Strains | ||
| E. coli | ||
| DH5α | λφ80d/lacZΔM15 Δ(lacZYA-argF)U169 recA1 endA1 hsdR17(rK− mK+) supE44 thi-1 gyrA relA1 | GIBCO-BRL |
| S17-1 | Tra genes from pRP4 integrated into chromosome | Bio-Rad |
| A. tumefaciens | ||
| A348 | A136 containing pTiA6NC | 33 |
| A348Spcr | A348 with Spcr by spontaneous mutagenesis | 62 |
| PC1000 | A348 deleted of entire virB operon from pTiA6NC | 27 |
| PC1002 | A348 deleted of virB2 from pTIA6NC; polar on virB3 | 6 |
| PC1004 | A348 deleted of virB4 from pTIA6NC | 7 |
| PC1011 | A348 deleted of virB11 from pTIA6NC | 6 |
| PC1211 | A348 deleted of virB2 and virB11 from pTIA6NC | 39 |
| JK1002 | A348 deleted of virB2 from pTiA6NC | This study |
| JK1204 | A348 deleted of virB2 and virB4 from pTiA6NC | This study |
| Plasmids | ||
| Plasmid vectors | ||
| pBSIIKS+.NdeI | Crbr; cloning vector containing NdeI restriction site at the translational start site of lacZ | 6 |
| pXZ151 | Kanr; broad-host-range IncP plasmid containing Plac with downstream polylinker sequence | 75 |
| pBBR1MCS2Kr | Kanr; broad-host-range cloning vector | 45 |
| pBBR1MCS2Gr | Genr; broad-host-range cloning vector | 21 |
| pML122ΔKr | Genr; IncQ RSF1010 derivative | 31 |
| pBB50 | Kanr; ∼3-kb BamHI fragment containing nptII and the Bacillus subtilis sacB gene in pUC7 | 6 |
| virB expression plasmids | ||
| pJEK01 | Crbr; pBIIKS+.NdeI expressing PvirB-virB2C64S | This study |
| pJEK02 | Crbr; pBB8 with a virB2 internal deletion | This study |
| pJEK03 | Crbr Kanr; sacB-containing pBB50 (8) with the ΔvirB2 mutation and flanking sequences from pJK002 | This study |
| pPC914 | Crbr; pBIIKS+.NdeI expressing PvirB-virB1 | 6 |
| pPC927 | Crbr; pBIIKS+ expressing PvirB-virB1, virB2 with NdeI at start site, and virB3 | 6 |
| pKA93 | Genr; pBBR1MCS2Gr expressing PvirB-virB4 | 3 |
| pKA96 | Genr; pBBR1MCS2Gr expressing PvirB-virB4K439Q | This study |
| pKA94 | Genr; pBBR1MCS2Gr expressing PvirB-virB4 and PvirB-virB11 | This study |
| pJKxx | pJEK01 with VirB2 Cys substitutions; xx denotes the position of the codon replacement | This study |
| pJKBxx | Crbr Kanr; pXZ151 ligated to pJKxx plasmids | This study |
| pJKBBxx | Crbr Genr; pBBR1MCS2Gr ligated to pJKxx plasmids | This study |
| pXZB100 | Crbr Kanr; pXZ151 with PvirB-virB11 | 76 |
| pPCB39 | Crbr Kanr; pXZ151 with PvirB-virB11K175Q | 58 |
| Mutation | ||
| C64S | 5′-GGT TAA CAA TAT AAGTAC GTT TAT CC-3′ | |
| G51C | 5′-CAA TCT GCG TGC GGC GGT ACC GAC CCC-3′ | |
| T54C | 5′-CAA TCTGCA GGT GGC GGC TGC GAC CC-3′ | |
| T58C | 5′-GCA CCG ACC CCG CATGCA TGG TTC AC-3′ | |
| I67C | 5′-GCA CGT TTTGCC TTG GTC CGT TC-3′ | |
| F71C | 5′-CTT GGT CCG TGC GGC CAG TCA CTC-3′ | |
| V77C | 5′-CAG TCA CTC GCATGC CTC GGC ATT GT-3′ | |
| I83C | 5′-GGC ATT GTC GCATGC GGG ATC TCC TG-3′ | |
| I85C | 5′-CGC TAT CGG GTGCTC CTG GAT GTT C-3′ | |
| S86C | 5′-GCT ATC GGG ATC TGC TGG ATG TTC GGG-3′ | |
| M88C | 5′-GAT CTC CTG GTGCTT CGG GCG GGC TTC-3′ | |
| L94C | 5′-CGG GCT TCG TGC GGG CTG GTT G-3′ | |
| I104C | 5′-GTC GGC GGC TGC GTT ATC ATG TTT GG-3′ | |
| M107C | 5′-GGC ATT GTT ATC TGC TTT GGG GCG AGC-3′ | |
| S111C | 5′-CAT GTT TGG GGCATGCCT CGG C-3′ | |
| L117C | 5′-CCT CGG CCA AAC GTGCAC TGG CGG-3′ | |
| G119C | 5′-GGC CAA ACG TTA ACT TGC GGT AG-3′ |
SphI restriction sites (GCATGC) are in bold; Cys (TGC) or Ser (AGT) codons are underlined. Antisense oligonucleotides were the reverse complement of the sense primers shown.
T pilus isolation and fractionation.
A. tumefaciens strains were grown to an optical density at 600 nm (OD600) of 0.5 in MG/L medium at 28°C and then induced with acetosyringone (AS) for expression of the vir genes for 6 h at 22°C, as described previously (26). The induced culture (500 μl) was spread on ABIM agar plates, and the plates were incubated for 4 days at 18°C. Cells were then gently scraped off the plates in 1 ml 50 mM KPO4 buffer (pH 5.5) or buffer A (100 mM HEPES [pH 7.5], 250 mM sucrose, 25 mM MgCl2, 0.1 mM KCl) and passed through a 25-gauge needle to collect flagella, pili, and surface proteins. The sheared material was centrifuged at 14,000 × g for 30 min at 4°C, and the supernatant was filtered through a 0.22-μm-pore-size cellulose acetate membrane to remove intact cells and cell debris. The filtered fraction was centrifuged at 100,000 × g for 1 h at 4°C to recover T pili or, alternatively, prepared for viewing in the electron microscope (see below). T pili were either suspended in 50 μl of Laemmli buffer and boiled or suspended in 500 μl KPO4 buffer (pH 5.5) and further fractionated through a 20 to 70% sucrose density gradient as described previously (61). Enriched T pili were used in Cys labeling studies, as described below.
Extracellular VirB2 blot assay.
Surface-exposed VirB2 was detected by colony immunoblotting as described previously (40).
Conjugation assays.
The IncQ plasmid pML122 (Genr) was introduced into various A. tumefaciens donor strains by diparental mating with E. coli strain S17-1(pML122) (42, 62). A. tumefaciens strains carrying pML122 were mated with A348Spcr recipient cells, as described previously (62). Transfer frequencies are reported as transconjugants recovered per donor cell. Experiments were repeated in triplicate, and results of a representative experiment are reported.
Virulence assays.
A. tumefaciens strains were tested for virulence by inoculating wound sites of Kalanchoe daigremontiana leaves. As controls, all leaves were coinoculated with WT A348 and isogenic null mutants as positive and negative controls (6). Relative virulence was assessed by inoculation of serially diluted cultures adjusted to the same OD600 on plant wound sites. Tumor formation was monitored over a period of 6 weeks and scored on a scale of − (avirulent) to +++ (WT virulence).
Chemical labeling of cysteine residues.
Cysteine accessibility experiments were carried out as described previously (41) with minor modifications. Briefly, A. tumefaciens strains producing VirB2 Cys derivatives were induced for vir gene expression by growth at 22°C in ABIM to an A600 of 0.5. Cells from 25-ml cultures were harvested, resuspended in buffer A (100 mM HEPES [pH 7.5 or pH 9.5], 250 mM sucrose, 25 mM MgCl2, 0.1 mM KCl), and distributed into two centrifuge tubes to a final A600 of 12 in 500 μl of buffer A. To one cell suspension, 4-acetamido-40-maleimidylstilbene-2,20-disulfonic acid (AMS) (Molecular Probes) was added to a final concentration of 5 mM, and cells were incubated for 30 min at 25°C. AMS was removed by suspending cells in 10 ml of buffer A and centrifuging. The washed cell pellet was suspended in 500 μl of buffer A. To the AMS-pretreated cells and the second cell suspension, 3-(N-maleimidylpropionyl)biocytin (MPB) (Molecular Probes) was added to a final concentration of 100 μM (from a 20 mM stock freshly dissolved in dimethyl sulfoxide [DMSO]), and the cells were incubated for 5 min at 25°C. The final concentration of DMSO in the reaction mixture did not exceed 0.5% (vol/vol). β-Mercaptoethanol (β-ME) (20 mM final concentration) was added to quench biotinylation, and cells were washed twice in buffer A containing 20 mM β-ME. As controls, sonicated extracts were treated with MPB to monitor Cys accessibility of presumptive cytoplasmic residues. For labeling of T pili, the isolated T pili in 500 μl of buffer A were treated with 100 μM MPB (final concentration) and incubated for 5 min at 25°C. β-ME (40 mM final concentration) was added to quench biotinylation, T pili were pelleted by high-speed centrifugation (100,000 × g), and MPB labeling of Cys-substituted pilin was assessed as described below.
Detection of labeled Cys residues.
MPB-treated cells or T pili were suspended in 200 μl TE (10 mM Tris [pH 7.5], 5 mM EDTA) with 2% (wt/vol) SDS and then vortex mixed vigorously for 30 min at 37°C. Samples were then diluted with 250 μl of buffer B (150 mM Tris [pH 8.0], 0.5 M sucrose, 10 mM EDTA). Lysozyme (1-mg/ml final concentration) was added, and the samples were incubated on ice for 1 h and then vortex mixed for 15 min at 37°C. Triton X-100 (20 μl), EDTA-free protease inhibitor cocktail (30 μl; Pierce Biochemicals) and 13 μl of 1 M MgCl2 stock solution were added, and the samples were vortexed for 10 min at 25°C and then incubated with gentle rocking for 3 h at 4°C. Samples were diluted with 900 μl of buffer B, and cell debris was removed by centrifugation at 14,000 × g for 15 min. Protein A-Sepharose CL-4B (Pharmacia) (30-μl bed volume) was incubated with the supernatant for 60 min at 25°C and centrifuged at 5,000 × g to remove protein A-Sepharose and nonspecifically bound proteins. Anti-VirB2 antibodies coupled to protein A-Sepharose were added to the supernatant and incubated overnight at 4°C. The beads were washed twice in buffer B with 1% Triton X-100 for 10 min, once in buffer B with 0.1% Triton X-100 for 10 min, and once in buffer B for 5 min and then resuspended in 15 μl of 5× Laemmli buffer plus 15 μl buffer B. Samples were boiled for 5 min and centrifuged at 400 × g for 5 min, and the solubilized proteins were subjected to Tricine SDS-PAGE and transferred to nitrocellulose membranes (0.45-μm pore size). Membranes were incubated overnight in blocking buffer (1× phosphate-buffered saline [PBS], 0.1% Tween 20, 5% [wt/vol] bovine serum albumin [BSA]) and then for 4 h in the presence of avidin-horseradish peroxidase (HRP) (Pierce) (2:10,000 dilution of a 2-mg/ml stock solution). Blots were washed three times in buffer C (1× PBS, 0.1% Tween 20, 0.5% BSA), and biotinylated proteins were analyzed by chemiluminescence according to the manufacturer's (Pierce, Thermo Scientific) instructions.
Coimmunoprecipitation.
Coimmunoprecipitation was performed as described previously (42). Briefly, 500 ml of induced A. tumefaciens cultures were harvested, and cells were lysed by French press treatment. Total membranes were recovered by ultracentrifugation, cross-linked with 0.5 mg/ml of dithiobissuccidimidyl propionate (DSP), and solubilized with radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris [pH 7.6], 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS). The solubilized membranes were used as starting material for immunoprecipitation with anti-VirB4, anti-VirB11, or anti-VirB2 antibodies coupled to protein A-Sepharose CL-4B beads (Amersham Biosciences).
Osmotic shock.
Cells were osmotically shocked as described by Chen et al. (13). Briefly, A. tumefaciens strains were induced for vir gene expression by growth in ABIM to an A600 of 0.5. Cells from 65-ml cultures were harvested (final A600 of 18), resuspended in 8 ml (4°C) osmotic shock buffer (20% [wt/vol] sucrose, 33 mM Tris-HCl [pH 8.0], 5 mM CaCl2, and 0.5 mM Na2 EDTA). After a shaking incubation for 10 min, cells were harvested by centrifugation at 8,000 × g for 10 min. The supernatant was removed and the cell pellet resuspended in 1 ml reverse osmosis water (4°C). After shaking incubation for 10 min (4°C), the cell suspension was centrifuged at 8,000 × g for 10 min, and osmotic shock supernatant (shockate fraction) was saved. The osmotic shockate was concentrated by centrifugation at 100,000 × g for 1 h at 4°C or precipitation with 5% trichloroacetic acid (TCA), and the resulting pellet was resuspended in 20 μl of 5× Laemmli buffer. The remaining cell pellet was resuspended in 400 μl 5× Laemmli buffer. Samples were boiled for 5 min and centrifuged at 400 × g for 5 min, and the proteins were analyzed using Tricine-SDS-PAGE or 12.5% SDS-PAGE as described above.
Electron microscopy.
T pili in the ABIM plate supernatant were treated with 1 M MgCl2 (0.1 M final concentration), incubated overnight at 4°C, and harvested by centrifugation at 16,000 × g for 1.5 h at 4°C. The pellet was resuspended in 10 mM Tris-Cl (pH 8.0) and fractionated through a 20 to 70% sucrose density gradient at 99,000 × g for 4.5 h at 4°C. Fractions (400 μl) containing T pili were pooled and centrifuged at 124,000 × g for 1 h at 4°C. The pellet was resuspended in 10 mM Tris-Cl (pH 8.0), and the material was centrifuged through a 1.1- to 1.5-g/ml CsCl step gradient for 18 h at 5°C at 120,000 × g (24). Fractions (250 μl) containing T pili were pooled and centrifuged at 124,000 × g for 1 h at 4°C. The pellet was resuspended in 10 mM Tris-Cl (pH 8.0) and further diluted to 4 ml before centrifugation at 124,000 × g for 1 h at 4°C. The pellet was resuspended in 50 μl of 50 mM HEPES, and a 5-μl sample containing T pili was placed on a 300-mesh carbon-Formvar grid (Ted Pella, Inc.) and stained with 1% uranyl acetate (Ted Pella, Inc.). Pilus samples were examined with a JOEL 1400 electron microscope.
RESULTS
VirB2 Cys mutant phenotypes.
Mutation of Cys64, the only Cys residue in mature VirB2, to Ser results in a slight reduction in virulence when assays are carried out at ambient (25°C) temperature (61). However, at a lower temperature (∼20°C), which was shown to be optimal for VirB/VirD4 T4SS function (4), the Cys64Ser mutant pilin accumulated at wild-type (WT) levels (Fig. 1 A), and the mutant strains exhibited near-wild-type substrate transfer frequencies as monitored by virulence and IncQ plasmid transfer to agrobacterial recipient cells (Fig. 1B). Further, the T pili assembled from the native and Cys64Ser mutant pilins displayed similar fractionation properties in sucrose gradients and appeared morphologically indistinguishable as assessed by uranyl acetate staining and electron microscopy (Fig. 1C and D). These near-WT phenotypes permitted use of Cys-less VirB2 for introduction of single Cys substitution mutations at five- to seven-residue intervals along the length of the mature pilin.
FIG. 1.
Phenotypes of VirB2 Cys substitution mutations. (A) Effects of mutations on total cellular and membrane levels of VirB2. Total cellular material and membrane fractions were subjected to SDS-PAGE, and immunoblots were developed with anti-VirB2 antibodies. Protein samples were loaded on a per-cell-equivalent basis. (B) Upper panel, effects of mutations on T-DNA transfer as monitored by virulence on wounded Kalanchoe leaves (black bars) (−, avirulent; +++, WT virulence) and transfer of the mobilizable IncQ plasmid pML122 to A. tumefaciens recipients (gray bars) (Tc's/Donor, number of transconjugants per donor cell). Lower panel, effects of mutations on T pilus production, as monitored by VirB2 abundance recovered from the shear fraction by ultracentrifugation (top) and colony immunoblots developed with anti-VirB2 antibodies (bottom). (C and D) Pilin fractionation profiles and pilus morphologies produced by ΔvirB2 mutant strains synthesizing native VirB2 (Cys64; WT) or the Cys64Ser (C64S) or Gly119Cys (G119C) mutant protein. (C) Distribution profiles of extracellular pilins in identically prepared 20 to 70% sucrose density gradients, as monitored by immunoblot development with anti-VirB2 antibodies. (D) Morphologies of pili from peak sucrose gradient fractions, as detected by uranyl acetate staining and electron microscopy.
Initial phenotypic studies showed that all 16 Cys-substituted pilins comigrated with native VirB2 in protein gels and that most had little or no effect on pilin function, as monitored by substrate transfer and pilus production (Fig. 1). Two mutations near the N terminus, Gly51Cys and Thr54Cys, exerted destabilizing effects, although both mutant pilins were readily detectable in membrane preparations (Fig. 1A). These mutations as well as three other mutations located near the N-C cyclization junction, Thr58Cys, Leu117Cys, and Gly119Cys, completely blocked substrate transfer (Fig. 1B). Of these, only the Gly51Cys and Leu117Cys mutations also completely blocked T pilus production (Fig. 1B). Strains producing the Gly119Cys mutant pilin accumulated high levels of T pili, and further studies indicated that the pili were morphologically indistinguishable from pili assembled from native VirB2 or the Cys64Ser mutant (Fig. 1C and D). The Gly119Cys mutation therefore selectively blocks substrate transfer without discernible effects on pilus production.
VirB2 inner membrane topology.
VirB2 possesses two stretches of hydrophobic residues and two hydrophilic regions; here, we will adopt a nomenclature devised for the VirB2 homolog TraAF in referring to these domains (Fig. 2 A) (56). To define the IM topology of the cyclized pilin and assay for possible effects of VirB proteins on pilin structure, we treated cells producing the Cys-substituted pilins with the biotinylated maleimide probe MPB. MPB (542 Da) transits the outer membrane (OM) through porins but only inefficiently crosses the IM (9, 49, 50). MPB therefore labels periplasmic Cys residues but not those buried in structural folds or membranes or residues located in the cytoplasm. Because the early pilin processing and membrane integration reactions proceed independently of VirB proteins (47), we first developed a topology model for the membrane-integrated pilin in a ΔvirB operon mutant background.
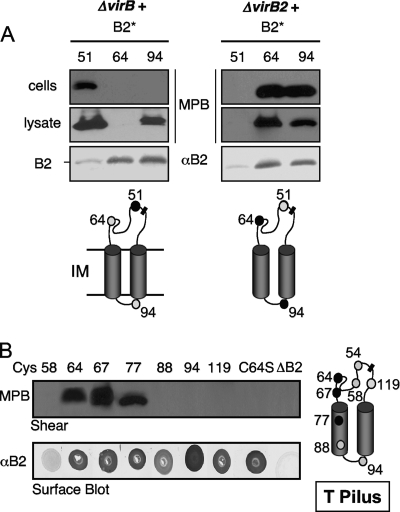
FIG. 2.
MPB accessibility of Cys substitutions. (A) Predicted domain architecture of VirB2. Numbers correspond to the N and C termini of mature pilin and a compilation of hydrophobic domain (gray cylinders) boundaries as predicted by computer hydropathy programs: TMHMM (boundaries predicted: TM1 66 and 88, TM2 92 and 114), Mobyle (70 and 90, 93 and 113), Phobius (65 and 87, 94 and 113), SOUSI (61 and 83, 91 and 113), and HMMTOP (63 and 85, 92 and 114). Domain numbering (I to IV) follows the nomenclature devised for the VirB2 homolog, TraA, of the F plasmid (56). (B) ΔvirB operon strains producing pilins with Cys substitutions at the residues indicated were treated with thiol-reactive MPB without and with pretreatment with AMS. Cys-substituted pilins were isolated by immunoprecipitation and analyzed for MPB labeling. Immunoprecipitates also were analyzed for VirB2 protein abundance by blot development with anti-VirB2 antibodies (αB2). (C) MPB labeling profiles obtained by treatment of intact and lysed cells (by sonication) at neutral and alkaline pH. (D) A topology model for membrane-integrated VirB2 pilin. Thick black-outlined circles with gray centers, Cys substitution mutations at these positions were labeled by MPB treatment of intact cells; thick black-outlined circles with white centers, substitutions at these positions were not labeled under any condition; gray circles with gray outlines, substitutions labeled upon sonication (Cys94) or MPB treatment at alkaline pH (Cys64). Peri, periplasm; IM, inner membrane; Cyto, cytoplasm. Bar, N-C cyclization junction.
As shown in Fig. 2B, Cys residues distributed throughout the hydrophilic domain I formed by head-to-tail cyclization were labeled upon MPB treatment of intact cells. MPB labeling was blocked by pretreatment with the membrane-impermeative, nonbiotinylated maleimide derivative AMS, consistent with a periplasmic location for this hydrophilic loop (8, 9). Interestingly, native Cys64 was not MPB labeled in the ΔvirB operon mutant background, even though flanking Cys replacements at positions 54, 58, 67, and 71 were labeled (Fig. 2B). Previous studies have shown that Cys residues near a membrane interface or in a structural fold that are inaccessible to thiol-reactive reagents at pH 7.5 can be rendered accessible when labeling is carried out at alkaline pH of 9 or higher (8). At pH 9.5, Cys64 was MPB labeled (Fig. 2C), suggesting that this residue is buried in a structural fold in the periplasm when the pilin is produced in the absence of other VirB proteins. Cys residues located within both hydrophobic domains II and IV or in the intervening hydrophilic loop (Cys94) were not labeled under neutral or alkaline reaction conditions (Fig. 2B and C). However, Cys94 was labeled upon treatment of sonicated cell extracts (Fig. 2C), indicating that this residue is located in the cytoplasm. As expected, substitutions in the hydrophobic domains were not labeled upon treatment of sonicated cell extracts (Fig. 2C).
Overall, the observed MPB labeling patterns support a topology model for the membrane-integrated pilin as depicted in Fig. 2D. This topology model is compatible with predictions that the signal sequence cleavage and N-C cyclization reactions occur in the periplasm (24, 25). A cytoplasmic location for the positively charged (GR+ASL) central loop is also in accordance with the positive-inside rule (71).
Disposition of VirB2 in VirB-producing cells and T pili.
Next, we asked whether the Cys-substituted pilins exhibited differences in MPB labeling when synthesized in the presence of other VirB proteins. Overall, we detected three differences in MPB labeling patterns of Cys-substituted pilins produced in the absence versus presence other VirB proteins (Fig. 3 A). Two residues in hydrophilic domain I, Cys51 and Cys64, displayed opposite labeling patterns: Cys51 was labeled only in a strain lacking other VirB proteins, whereas Cys64 was labeled only in the VirB-producing strain. Most intriguingly, however, Cys94, located in the hydrophilic domain III, was labeled only in the VirB-producing strain. Previously, using the same experimental conditions, we showed that Cys replacements in the N-terminal cytoplasmic domain of the bitopic protein VirB10 (40) as well as cytoplasmic loops of polytopic VirB6 (41) were not labeled upon MPB treatment of whole cells but were labeled upon treatment of sonicated cell extracts. Here, we further confirmed that none of the four Cys residues of native VirB11, a peripheral membrane ATPase (58), was accessible to MPB upon treatment of cells producing the VirB2 Cys94 variant (data not shown). These findings indicate that MPB labeling of Cys94 in a functionally WT strain is not due to cell lysis or MPB diffusion across the IM.
FIG. 3.
(A) Comparison of MPB labeling patterns of Cys-substituted pilins synthesized in the absence (ΔvirB) or presence (ΔvirB2) of other VirB proteins. Pilins (B2*) carried Cys substitutions at the residues indicated at the tops of the blots. Intact cells and cell lysates were MPB treated and analyzed as described in the text. VirB2 protein abundance in immunoprecipitates was assessed by immunoblot analysis with anti-VirB2 antibodies (αB2). Schematic diagrams of MPB labeling patterns are presented below the blots for each strain. Thin lines, hydrophilic loops; gray cylinders, hydrophobic domains; bar, cyclization junction; black-filled circles, MPB labeled upon treatment of intact cells; gray-filled circles, no MPB labeling. IM, inner membrane. Data are presented only for Cys replacements displaying differences in labeling patterns between the two genetic backgrounds. (B) MPB labeling patterns of pilins incorporated into the T pilus. Corresponding colony immunoblots developed with anti-VirB2 antibodies are shown for each mutant strain. The schematic at the right shows MPB accessibility of Cys residues in the isolated T pilus. Black and gray circles, accessible and inaccessible residues, respectively.
We were also interested in comparing MPB labeling profiles of pilins associated with the cell versus the T pilus. In contrast to the profiles obtained for cell-associated pilins, only three residues were labeled upon treatment of isolated T pili, namely, Cys64 and Cys67 in the hydrophilic N-C loop domain I and Cys77 within hydrophobic domain II (Fig. 3B). Cys residues at positions 54 and 58 in domain I did not label, even though these strains produced some pili (Fig. 2B); Cys51 and Cys71 substitutions completely blocked pilus production, preventing a test for surface accessibility in the T pilus. However, another domain I substitution, Cys119, was not labeled in isolated T pili, nor was Cys94 in the central hydrophilic loop (Fig. 3B). A region of the hydrophilic N-C loop including residues 64 to 71 and part of hydrophobic domain II (residues 72 to 77) thus appears to form a surface patch in the T pilus. The remainder of hydrophobic domain II, all of hydrophobic domain IV, and the small central hydrophilic domain III are MPB inaccessible and likely buried in the T pilus.
VirB4 and VirB11 ATPases influence the disposition of membrane pilin.
We next wanted to identify the VirB proteins(s) responsible for mediating the observed differences in MPB labeling of cell-associated pilins when synthesized in VirB-lacking versus VirB-producing cells. We reasoned that the simplest explanation for MPB accessibility of Cys94 is that one or more VirB/VirD4 T4SS subunits catalyze a structural reorganization of pilin so that its cytoplasmic loop is exposed in the periplasm. In this T4SS, the VirB4 and VirB11 ATPases are required for assembly of the T pilus, and both VirB ATPases plus the VirD4 ATPase (the substrate receptor) are required for substrate translocation (16). To test a hypothesis that one or more of the ATPases catalyze a topological reorganization of pilin, we developed MPB labeling profiles for the Cys-substituted pilins synthesized in strains lacking or producing these T4SS ATPases.
We did not detect any effects of the VirD4 substrate receptor on MPB labeling patterns (J. E. Kerr, unpublished data). Intriguingly, however, in VirB4-producing strains, we detected MPB labeling of Cys94. As shown in Fig. 4, the Cys94-substituted pilin was labeled in a ΔvirB4 mutant complemented with native VirB4 in trans but not in the ΔvirB4 parental strain or a ΔvirB4 strain engineered to synthesize a Walker A NTP binding motif mutant (K439Q) (Fig. 4A). ΔvirB4 strains producing either native VirB4 or the K439Q mutant accumulate these proteins at comparable levels and display WT Tra+ Pil+ and Tra− Pil− null phenotypes, respectively (7, 20). Also of interest, the Cys94-substituted pilin was MPB labeled when coproduced with VirB4 in a ΔvirB operon strain but not when synthesized in ΔvirB operon strains lacking VirB4 or producing the K439Q Walker A mutant (Fig. 4B). In all VirB4-producing strains, other Cys substitutions located in hydrophilic domain I were labeled, arguing against the notion that VirB4 induces a topological inversion of the pilin in the IM (Fig. 4B and data not shown).
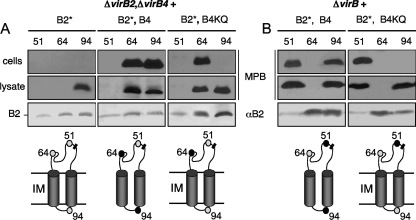
FIG. 4.
Effects of VirB4 ATPase on MPB labeling patterns of pilins. (A) Cys-substituted pilins synthesized in strain JK1204 deleted of native virB2 and virB4 (nonpolar ΔvirB2, ΔvirB4). (B) Cys-substituted pilins produced in strain PC1000 (ΔvirB operon mutant). JK1204 and PC1000 were engineered to synthesize mutant pilins (B2*) with Cys substitutions at the residues indicated at the tops of the blots alone or with native VirB4 (B4) or a Walker A mutant (KQ) derivative. Panels showing MPB labeling patterns, VirB2* abundance in the immunoprecipitates, and schematic representations of the labeling patterns are as described in the Fig. 3A legend. Data are presented only for Cys replacements displaying differences in labeling patterns among the strains analyzed.
VirB4 was also required for labeling of Cys64 (Fig. 4A). However, in this case, (i) both the native and Walker A mutant forms of VirB4 mediated Cys64 labeling, and (ii) VirB4 exerted its effects on Cys64 labeling only in the presence of one or more other VirB proteins (Fig. 4A and B; see below). Finally, VirB4 synthesis did not affect the Cys51 labeling pattern (Fig. 4A and B).
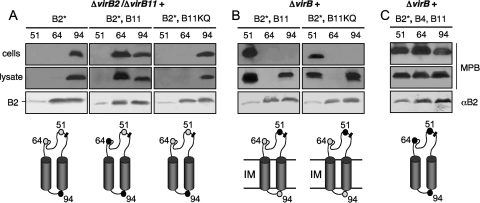
We identified one VirB11-dependent change in MPB labeling (Fig. 5 A and B). Periplasmic Cys64 was labeled in a ΔvirB11 mutant complemented with virB11 in trans but not in the isogenic ΔvirB11 strains lacking VirB11 or producing a VirB11 Walker A mutant (K175Q) (58). Cys64 was labeled only in a strain producing VirB11 and other VirB proteins, suggesting that VirB11 coordinates with at least one other VirB protein to induce a structural change in the periplasmic domain of VirB2 (see below).
FIG. 5.
Effects of VirB11 ATPase synthesis on MPB labeling patterns of pilins. (A) Cys-substituted pilins synthesized in strain PC1211 (nonpolar ΔvirB2, ΔvirB11). (B and C) Cys-substituted pilins produced in strain PC1000 (ΔvirB operon mutant). PC1211 and PC1000 were engineered to synthesize mutant pilins (B2*) with Cys substitutions at the residues indicated at the tops of the blots alone or together with VirB11 (B11), a VirB11 Walker A mutant (KQ) derivative, or both native VirB11 and VirB4. Panels showing MPB labeling patterns, VirB2* abundance in the immunoprecipitates, and schematic representations of the labeling patterns are as described in the Fig. 3 legend. Data are presented only for Cys replacements displaying differences in labeling patterns among the strains analyzed.
VirB4 and VirB11 coordinate structural changes of membrane-integrated pilin.
In the studies described above, experiments were designed to test for effects of each VirB ATPase on the pilin structural organization. Next, we asked whether VirB4 and VirB11 function together to catalyze structural changes independently of other VirB proteins (Fig. 5C). As expected from the results described above (Fig. 4B), Cys94 was MPB labeled in a strain coproducing the mutant pilin and both ATPases. Strikingly, however, Cys64 also was labeled, showing that synthesis of VirB4 and VirB11 is necessary and sufficient for labeling of this periplasmic residue. Cys51 was also labeled in this strain (Fig. 5C), but this residue is also labeled even in the complete absence of other VirB proteins (Fig. 3A).
Taken together, the results of our MPB labeling studies identified an individual effect of VirB4 and a combined effect of VirB4 and VirB11 on the structural organization of VirB2 (Fig. 6 A). Synthesis of native VirB4 sufficed among the VirB proteins for labeling of Cys94, cosynthesis of VirB4 and VirB11 sufficed for labeling of Cys64, and Cys51 was labeled only in the absence of one or more other VirB proteins. Formulated as steps in a T4SS biogenesis pathway, we propose a working model in which (i) VirB4 catalyzes dislocation of VirB2 from the IM (rendering Cys94 accessibility), ii) VirB11 coordinates with VirB4 (most likely prior to pilin dislocation) to induce a structural change in the periplasmic domain of the pilin (rendering Cys64 accessibility), and (iii) VirB4 and VirB11 coordinate with other VirB proteins to mediate pilin binding with a VirB partner protein(s) (rendering Cys51 inaccessibility) required for further channel and T pilus assembly (Fig. 6A).
FIG. 6.
(A) Schematic summarizing effects of VirB4, VirB11, and other VirB subunits on VirB2 MPB labeling patterns. (B) VirB protein complexes isolated from detergent-solubilized membrane extracts by immunoprecipitation (IP) with anti-VirB2 or anti-VirB4 antiserum. Strains: ΔvirB4, PC1004; ΔvirB2, JK1002; ΔvirB operon, PC1000; ΔvirB11, PC1011 (engineered to synthesize the VirB proteins indicated in addition to those produced from the Ti plasmid). B2, VirB2; B4, VirB4; B4KQ, VirB4K439Q; B11, VirB11. Anti-VirB2 (αB2) and anti-VirB4 (αB4) antibodies were used for immunoprecipitation (IP) (left) and development of immunoblots (Blot Dev) (right).
Formation of a precipitable VirB2-VirB4 complex.
An assumption of this model is that VirB4 interacts with VirB2 pilin. As shown in Fig. 6B, we found that the anti-VirB2 and anti-VirB4 antibodies each coprecipitated VirB2 and VirB4 regardless of the synthesis of other VirB subunits. The antibodies also coprecipitated a presumptive complex of VirB2 and the VirB4 Walker A mutant protein independently of other VirB subunits. Neither the anti-VirB2 nor anti-VirB4 antibodies precipitated VirB4 or VirB2 proteins nonspecifically. These findings suggest that VirB4 exerts its effect on VirB2 through a direct protein-protein contact. In contrast, the anti-VirB2 antibodies did not precipitate detectable levels of VirB11, indicating that VirB11 might exert its effect on VirB2 indirectly through transient or weak-affinity interactions with VirB4.
VirB4-mediated release of VirB2 upon osmotic shock.
To complement the Cys accessibility studies described above, we assayed for VirB4-mediated release of VirB2 upon osmotic shock treatment of cells. VirB2 accumulated at appreciably higher levels in material released by osmotic shock (shockate) of WT strain A348 and a ΔvirB4 mutant complemented with virB4 in trans than in material released from the isogenic ΔvirB4 strains lacking VirB4 or producing the VirB4 K439Q Walker A mutant (Fig. 7). VirB2 also accumulated at appreciably higher levels in the shockate of a ΔvirB operon mutant coproducing only VirB4 and VirB2 among the VirB proteins than in those of isogenic strains lacking VirB4 or producing the VirB4 K439Q Walker A mutant. VirB2 also accumulated at low levels in the shockate fraction of cells producing only VirB11, providing additional evidence that VirB11 alone does not mediate release of pilin from the IM.
FIG. 7.
VirB4-mediated release of VirB2 upon osmotic shock treatment, showing the presence of proteins indicated at the right in material released by osmotic shock (OS) or in the cell pellet (CP) obtained from centrifugation of shocked cells. (A) Detection of VirB2, periplasmic ChvE, and cytoplasmic VirE2. (B) Detection of outer membrane protein VirB7 and inner membrane proteins VirB10 and VirB11. Strains: ΔvirB2 ΔvirB4, JK1204; ΔvirB, PC1000; WT, A348 (engineered to synthesize the VirB proteins listed in addition to those produced from the Ti plasmid). B2, VirB2; B4, VirB4; B4KQ, VirB4K439Q; B11, VirB11; B11KQ, VirB11K175Q.
To evaluate whether cell lysis accompanied osmotic shock treatment or whether VirB4 synthesis disrupted integrity of the IM or induced release of IM vesicles, shockate fractions were examined for the presence of cytoplasmic (VirE2) and periplasmic (ChvE) markers and of IM (VirB10 and VirB11) and OM (VirB7 and porins) markers. As shown in Fig. 7A and B, a small amount of the VirE2 secretion substrate was detectable in shockates independently of the VirB/VirD4 T4SS, as previously reported (12, 17); even so, levels of released VirE2 were unaffected by VirB4 synthesis. Periplasmic ChvE accumulated at abundant levels in the shockates, and levels were also unaffected by VirB4 synthesis. The IM markers, VirB10 and VirB11 (40, 58), were detectable at very low levels in the shockate fractions and abundantly in the cell pellets separated from the shockate fractions by centrifugation. Finally, OM VirB7 was detected at comparable levels in shockates from all VirB7-producing strains. We lack antibodies to A. tumefaciens porins; however, prominent protein species characteristic of the ∼30- to 40-kDa OM porins were also visualized in the shockate fractions by SDS-PAGE and Coomassie blue staining (data not shown). The results of these experiments suggest that osmotic shock treatment did not induce massive cell lysis and, furthermore, that VirB4 synthesis did not disrupt membrane integrity or induce vesicle formation.
DISCUSSION
In this study, we developed MPB accessibility profiles for a collection of Cys-substituted pilins with the aim of defining intermediary steps of the VirB/VirD4 T4SS assembly pathway. We supplied evidence for a new role for the VirB4 ATPase in catalyzing extraction of pilin monomers from the IM, and we also advanced an understanding of the pilin structural state when integrated in the IM and polymerized as the extracellular T pilus.
Our MPB accessibility studies showed that VirB4 mediates MPB labeling of Cys94, a substitution that in the absence of the ATPase is located in the cytoplasm (Fig. 2 and 3). VirB4 synthesis does not appear to affect cell growth or IM permeability to MPB, as we were unable to detect labeling of Cys residues in cytoplasmic proteins or domains of polytopic proteins in any VirB4 producing strains. The most likely explanation for Cys94 labeling, therefore, is that VirB4 catalyzes extraction of the pilin into the periplasmic compartment. This proposal was further strengthened by evidence for a precipitable VirB2-VirB4 complex (Fig. 6) and our finding that VirB4 mediated release of VirB2 from the IM upon osmotic shock (Fig. 7). VirB4 was both necessary and sufficient among the VirB subunits for Cys94 labeling and VirB2 release, suggesting that it functions as a pilin dislocase independently of other channel constituents. A Walker A mutation also abolished both activities, suggesting that VirB4 acts catalytically through ATP binding/hydrolysis. Here, we characterized effects of a K439Q Walker A mutation on the VirB2 structural organization, as this mutation was shown previously to abolish VirB/VirD4-mediated substrate transfer and pilus biogenesis (7, 20, 21). Similar charge-altering substitutions of conserved Walker A or B residues of VirB4 or homologs TrbERP4 and TrwKR388 also block protein function, and such mutations correspondingly abolish TrwKR388 ATPase activity in vitro (2). Other Walker A mutations preserving the structure or charge of the ATP binding pocket, e.g., VirB4(K439R) (74) and TrbERP4(G495A, K501A) (57), confer partial-function phenotypes. In one case, this was cited as evidence that VirB4 ATPase activity is not required for pilus biogenesis (74), but a more likely explanation is that the K439R mutant protein retains ATP binding or hydrolysis at levels sufficient for residual protein function.
VirB4 has been difficult to characterize in vitro due to problems of insolubility (57), yet the described biochemical properties of VirB4 or its homologs are reminiscent of other known or proposed membrane dislocases. There is in silico (53) and compelling experimental (2, 23) evidence for oligomerization of VirB4 or VirB4-like TraBpKM101 and TrwKR388 as homohexamers. These proteins also adopt other oligomeric states in vitro, e.g., dimers, but only the hexameric forms are catalytically active (23). A. tumefaciens VirB4 also embeds into or spans the IM (20), and membrane-associated forms of TraBpKM101, TrwKR388, and VirB4-like TraCF were also detected by fractionation (2, 23, 65). Finally, several VirB4-like ATPases form stabilizing interactions with other channel components; A. tumefaciens VirB4 interacts with VirB3 and VirB8 (55, 74), as well as VirB10 and the VirD4 and VirB11 ATPases (3). These physical properties—ATPase activity, homohexamer formation, and association with membrane and membrane proteins—are shared by the FtsH dislocase/protease as well as the GspE ATPases implicated in functioning as pilin dislocases during assembly of the type IV pilus systems (19, 37, 38, 63). The GspE ATPases are related to A. tumefaciens VirB11, and several of these family members have been shown to undergo profound conformational changes upon ATP hydrolysis (35, 63, 64, 73). Although our data indicate that VirB4, not VirB11, functions as a dislocation motor, we did find that both ATPases act synergistically to induce a structural change in the pilin as detected by labeling of periplasmic Cys64 (Fig. 5C). VirB11 might modulate VirB4 dislocase activity through ATP-mediated conformational changes to regulate pilin binding or release or induce a structural state necessary for formation of pilin binding partner interactions in the periplasm. Alternatively, assembly of a VirB4-VirB11 ternary complex might be important for sensing and integrating spatial, temporal, or other signals controlling machine morphogenesis. Our ongoing studies are examining these possibilities to gain a more detailed mechanistic understanding of how VirB4 coordinates with VirB11 to energize extraction of pilin subunits from the membrane.
Besides exploiting the power of SCAM to define the topological orientation of VirB2 in the IM and identify VirB4/VirB11-mediated dynamic changes in the pilin structural state, our phenotypic studies of the Cys-substituted pilins generated new insights regarding the contributions of residues/domains to VirB2 function. Although substitutions in domains II, III, and IV were phenotypically silent, several in domain I disrupted protein function (Fig. 2). As might be expected, some substitutions near the processing/cyclization junction were destabilizing (Cys51 and Cys54) or blocked protein function without discernible effects on stability (Cys117). Similar effects were described earlier for mutations located near the cleavage site but within the signal sequence (47). However, some substitutions near the cyclization junction (Cys54, Cys58, and Cys119) selectively blocked substrate transfer without affecting T pilus biogenesis. These Cys substitutions did not appear to affect early pilin-processing reactions; we detected only the mature pilins in protein gels, and the mutant pilins were also resistant to carboxypeptidase, suggestive of head-to-tail cyclization (J. E. Kerr, unpublished data). In studies of the related pilins TraAF (30, 52) and TrbCRP4 (25), Tra+ Pil− “uncoupling” mutations were also recovered in the hydrophilic domains (30, 52). These findings underscore the general notion that conjugative pilins are configured differently in the mating channel and pilus. They also point to the importance of the hydrophilic regions of pilins specifically for substrate transfer. Our domain I substitutions in VirB2 were MPB labeled (Fig. 2), raising the possibility that these hydrophilic residues are exposed in the interior of the translocation channel; if so, the Cys54, Cys58, and Cys119 substitutions might directly impede substrate translocation through structural effects on channel architecture. Alternatively, the differential labeling of domain I residues Cys51 and Cys64 in strains lacking versus producing other VirB proteins (Fig. 3) is consistent with the notion that the Tra− Pil+ “uncoupling” mutations might selectively disrupt formation of pilin contacts with another VirB subunit(s) required for assembly of the translocation channel but dispensable for T pilus production.
Our MPB labeling studies also supplied new information relating to the packing geometry of VirB2 in the T pilus. Hydrophobic domains II and IV are probably buried in packing interfaces between adjacent pilins. The central hydrophilic loop (domain III), marked by Cys94, as well as the N-C cyclization junction site of domain I, marked by Cys54, Cys58, and Cys119, also appear to be buried in the interior of the T pilus. If the T pilus has a central lumen analogous to that detected for the F pilus (69, 72), these hydrophilic regions might line the pilus lumen. Interestingly, whereas the central domain III of TraAF is also buried in the F pilus, its C terminus is surface displayed and can even accommodate epitope tags that are accessible to antibody (60). We were unable to generate functional forms of VirB2 bearing epitope tags near the N-C junction, preventing conclusions regarding surface display of this region of VirB2 (J. E. Kerr, unpublished data). However, portions of hydrophilic domain I, as shown by labeling of Cys64 and Cys67, and of hydrophobic domain II, as shown by labeling of Cys77, are surface displayed (Fig. 3B). Residues 64 to 69 of VirB2 correspond to residues 17 to 22 of TraAF, a region also found to be exposed laterally on the pilus by bacteriophage binding and antibody labeling of immunodominant epitopes (30, 52, 60). The surface display of a stretch of hydrophobicity of VirB2 (marked by Cys77 labeling) is intriguing in view of observations that, in contrast to F pili, which dynamically extend and retract (18), T pili and related pili are sloughed or broken from the cell surface. The released fibers are thought to promote nonspecific cell-cell aggregation as a prerequisite to mating junction formation (66). A VirB2 packing geometry resulting in patches of surface hydrophobicity might account for the tendency of T pili to bundle and induce cellular aggregation.
Although a detailed understanding of VirB2 structural organization in the secretion channel and T pilus will require further investigation, recent advances in knowledge of the structure of the envelope-spanning “core” complex from the pKM101 conjugation system have shed light on the overall T4SS architecture. This “core” complex is composed of 14 copies each of TraN, TraO, and TraF, which are homologs of A. tumefaciens VirB7, VirB9, and VirB10, respectively (11, 29). The core is configured as a large, ring-shaped complex of ∼185 Å in length and width, whose interior chamber ranges from ∼8 nm at the widest point to 5 nm at the IM and 3 nm at the OM (11, 29). The chamber is sufficient in size to accommodate the translocation channel and, possibly, the T pilus (11, 15, 29). In the context of our present findings, we suggest that VirB4 and probably also VirB11 spatially position near or at the base of the core complex where the two ATPases couple pilin extraction to translocation into the core chamber. This fits with earlier evidence for complex formation between VirB10 and the VirB4/VirB11/VirD4 ATPases (3) and with more recently described interaction networks among VirB3, VirB4, VirB5, and VirB8 that have been postulated to be important for T pilus biogenesis (55, 74). Conceivably, these VirB proteins assemble as a substructure within the core chamber, and the membrane-extracted pilin monomers interact dynamically or stably with this substructure to complete channel or pilus morphogenesis.
In sum, we have presented evidence for a new function for A. tumefaciens VirB4 as a pilin dislocase. Given that VirB4-like subunits are signatures of all bacterial and archaeal T4SSs (1), it is tempting to propose a universal mechanism in which VirB4-like subunits catalyze the extraction of pilins or other hydrophobic proteins for use in assembly of the extramembranous portions of the translocation channel.
Acknowledgments
We thank William Dowhan and Mikhail Bogdanov for discussions on SCAM and members of our laboratory for helpful comments.
This study was supported by NIH grant GM48746 and Molecular Basis of Infectious Diseases (MBID) training grant 1 T32 AI55449.
Footnotes
Published ahead of print on 23 July 2010.
REFERENCES
- 1.Alvarez-Martinez, C. E., and P. J. Christie. 2009. Biological diversity of prokaryotic type IV secretion systems. Microbiol. Mol. Biol. Rev. 73:775-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arechaga, I., A. Pena, S. Zunzunegui, M. del Carmen Fernandez-Alonso, G. Rivas, and F. de la Cruz. 2008. ATPase activity and oligomeric state of TrwK, the VirB4 homologue of the plasmid R388 type IV secretion system. J. Bacteriol. 190:5472-5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atmakuri, K., E. Cascales, and P. J. Christie. 2004. Energetic components VirD4, VirB11 and VirB4 mediate early DNA transfer reactions required for bacterial type IV secretion. Mol. Microbiol. 54:1199-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baron, C., N. Domke, M. Beinhofer, and S. Hapfelmeier. 2001. Elevated temperature differentially affects virulence, VirB protein accumulation, and T pilus formation in different Agrobacterium tumefaciens and Agrobacterium vitis strains. J. Bacteriol. 183:6852-6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beijersbergen, A., S. J. Smith, and P. J. Hooykaas. 1994. Localization and topology of VirB proteins of Agrobacterium tumefaciens. Plasmid 32:212-218. [DOI] [PubMed] [Google Scholar]
- 6.Berger, B. R., and P. J. Christie. 1994. Genetic complementation analysis of the Agrobacterium tumefaciens virB operon: virB2 through virB11 are essential virulence genes. J. Bacteriol. 176:3646-3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berger, B. R., and P. J. Christie. 1993. The Agrobacterium tumefaciens virB4 gene product is an essential virulence protein requiring an intact nucleoside triphosphate-binding domain. J. Bacteriol. 175:1723-1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bogdanov, M., J. Xie, P. Heacock, and W. Dowhan. 2008. To flip or not to flip: lipid-protein charge interactions are a determinant of final membrane protein topology. J. Cell Biol. 182:925-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bogdanov, M., W. Zhang, J. Xie, and W. Dowhan. 2005. Transmembrane protein topology mapping by the substituted cysteine accessibility method (SCAM(TM)): application to lipid-specific membrane protein topogenesis. Methods 36:148-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cascales, E., and P. J. Christie. 2004. Definition of a bacterial type IV secretion pathway for a DNA substrate. Science 304:1170-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chandran, V., R. Fronzes, S. Duquerroy, N. Cronin, J. Navaza, and G. Waksman. 2009. Structure of the outer membrane complex of a type IV secretion system. Nature 462:1011-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen, L., C. M. Li, and E. W. Nester. 2000. Transferred DNA (T-DNA)-associated proteins of Agrobacterium tumefaciens are exported independently of virB. Proc. Natl. Acad. Sci. U. S. A. 97:7545-7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen, Y.-C., L.-A. Chen, S.-J. Chen, M.-C. Chang, and T.-L. Chen. 2004. A modified osmotic shock for periplasmic release of a recombinant creatinase from Escherichia coli. Biochem. Eng. J. 19:211-215. [Google Scholar]
- 14.Christie, P. J. 2004. Bacterial type IV secretion: the Agrobacterium VirB/D4 and related conjugation systems. Biochim. Biophys. Acta 1694:219-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Christie, P. J. 2009. Structural biology: translocation chamber's secrets. Nature 462:992-994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christie, P. J., K. Atmakuri, V. Krishnamoorthy, S. Jakubowski, and E. Cascales. 2005. Biogenesis, architecture, and function of bacterial type IV secretion systems. Annu. Rev. Microbiol. 59:451-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christie, P. J., J. E. Ward, S. C. Winans, and E. W. Nester. 1988. The Agrobacterium tumefaciens virE2 gene product is a single-stranded-DNA-binding protein that associates with T-DNA. J. Bacteriol. 170:2659-2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clarke, M., L. Maddera, R. L. Harris, and P. M. Silverman. 2008. F-pili dynamics by live-cell imaging. Proc. Natl. Acad. Sci. U. S. A. 105:17978-17981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Craig, L., and J. Li. 2008. Type IV pili: paradoxes in form and function. Curr. Opin. Struct. Biol. 18:267-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dang, T. A., and P. J. Christie. 1997. The VirB4 ATPase of Agrobacterium tumefaciens is a cytoplasmic membrane protein exposed at the periplasmic surface. J. Bacteriol. 179:453-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dang, T. A., X. R. Zhou, B. Graf, and P. J. Christie. 1999. Dimerization of the Agrobacterium tumefaciens VirB4 ATPase and the effect of ATP-binding cassette mutations on the assembly and function of the T-DNA transporter. Mol. Microbiol. 32:1239-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Das, A., and Y. H. Xie. 1998. Construction of transposon Tn3phoA: its application in defining the membrane topology of the Agrobacterium tumefaciens DNA transfer proteins. Mol. Microbiol. 27:405-414. [DOI] [PubMed] [Google Scholar]
- 23.Durand, E., C. Oomen, and G. Waksman. 2010. Biochemical dissection of the ATPase TraB, the VirB4 homologue of the Escherichia coli pKM101 conjugation machinery. J. Bacteriol. 192:2315-2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eisenbrandt, R., M. Kalkum, E. M. Lai, R. Lurz, C. I. Kado, and E. Lanka. 1999. Conjugative pili of IncP plasmids, and the Ti plasmid T pilus are composed of cyclic subunits. J. Biol. Chem. 274:22548-22555. [DOI] [PubMed] [Google Scholar]
- 25.Eisenbrandt, R., M. Kalkum, R. Lurz, and E. Lanka. 2000. Maturation of IncP pilin precursors resembles the catalytic dyad-like mechanism of leader peptidases. J. Bacteriol. 182:6751-6761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fernandez, D., T. A. Dang, G. M. Spudich, X. R. Zhou, B. R. Berger, and P. J. Christie. 1996. The Agrobacterium tumefaciens virB7 gene product, a proposed component of the T-complex transport apparatus, is a membrane-associated lipoprotein exposed at the periplasmic surface. J. Bacteriol. 178:3156-3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernandez, D., G. M. Spudich, X. R. Zhou, and P. J. Christie. 1996. The Agrobacterium tumefaciens VirB7 lipoprotein is required for stabilization of VirB proteins during assembly of the T-complex transport apparatus. J. Bacteriol. 178:3168-3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fronzes, R., P. J. Christie, and G. Waksman. 2009. The structural biology of type IV secretion systems. Nat. Rev. Microbiol. 7:703-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fronzes, R., E. Schafer, L. Wang, H. R. Saibil, E. V. Orlova, and G. Waksman. 2009. Structure of a type IV secretion system core complex. Science 323:266-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frost, L. S., and W. Paranchych. 1988. DNA sequence analysis of point mutations in traA, the F pilin gene, reveal two domains involved in F-specific bacteriophage attachment. Mol. Gen. Genet. 213:134-139. [DOI] [PubMed] [Google Scholar]
- 31.Fullner, K. J. 1998. Role of Agrobacterium virB genes in transfer of T complexes and RSF1010. J. Bacteriol. 180:430-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fullner, K. J., J. C. Lara, and E. W. Nester. 1996. Pilus assembly by Agrobacterium T-DNA transfer genes. Science 273:1107-1109. [DOI] [PubMed] [Google Scholar]
- 33.Garfinkel, D. J., R. B. Simpson, L. W. Ream, F. F. White, M. P. Gordon, and E. W. Nester. 1981. Genetic analysis of crown gall: fine structure map of the T-DNA by site-directed mutagenesis. Cell 27:143-153. [DOI] [PubMed] [Google Scholar]
- 34.Haase, J., and E. Lanka. 1997. A specific protease encoded by the conjugative DNA transfer systems of IncP and Ti plasmids is essential for pilus synthesis. J. Bacteriol. 179:5728-5735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hare, S., R. Bayliss, C. Baron, and G. Waksman. 2006. A large domain swap in the VirB11 ATPase of Brucella suis leaves the hexameric assembly intact. J. Mol. Biol. 360:56-66. [DOI] [PubMed] [Google Scholar]
- 36.Harris, R. L., K. A. Sholl, M. N. Conrad, M. E. Dresser, and P. M. Silverman. 1999. Interaction between the F plasmid TraA (F-pilin) and TraQ proteins. Mol. Microbiol. 34:780-791. [DOI] [PubMed] [Google Scholar]
- 37.Hazes, B., and L. Frost. 2008. Towards a systems biology approach to study type II/IV secretion systems. Biochim. Biophys. Acta 1778:1839-1850. [DOI] [PubMed] [Google Scholar]
- 38.Ito, K., and Y. Akiyama. 2005. Cellular functions, mechanism of action, and regulation of FtsH protease. Annu. Rev. Microbiol. 59:211-231. [DOI] [PubMed] [Google Scholar]
- 39.Jakubowski, S. J., E. Cascales, V. Krishnamoorthy, and P. J. Christie. 2005. Agrobacterium tumefaciens VirB9, an outer-membrane-associated component of a type IV secretion system, regulates substrate selection and T pilus biogenesis. J. Bacteriol. 187:3486-3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jakubowski, S. J., J. E. Kerr, I. Garza, V. Krishnamoorthy, R. Bayliss, G. Waksman, and P. J. Christie. 2009. Agrobacterium VirB10 domain requirements for type IV secretion and T pilus biogenesis. Mol. Microbiol. 71:779-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jakubowski, S. J., V. Krishnamoorthy, E. Cascales, and P. J. Christie. 2004. Agrobacterium tumefaciens VirB6 domains direct the ordered export of a DNA substrate through a type IV secretion system. J. Mol. Biol. 341:961-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jakubowski, S. J., V. Krishnamoorthy, and P. J. Christie. 2003. Agrobacterium tumefaciens VirB6 protein participates in formation of VirB7 and VirB9 complexes required for type IV secretion. J. Bacteriol. 185:2867-2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Judd, P. K., R. B. Kumar, and A. Das. 2005. Spatial location and requirements for the assembly of the Agrobacterium tumefaciens type IV secretion apparatus. Proc. Natl. Acad. Sci. U. S. A. 102:11498-11503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kalkum, M., R. Eisenbrandt, R. Lurz, and E. Lanka. 2002. Tying rings for sex. Trends Microbiol. 10:382-387. [DOI] [PubMed] [Google Scholar]
- 45.Kovach, M. E., R. W. Phillips, P. H. Elzer, R. M. Roop II, and K. M. Peterson. 1994. pBBR1MCS: a broad-host-range cloning vector. Biotechniques 16:800-802. [PubMed] [Google Scholar]
- 46.Lai, E. M., O. Chesnokova, L. M. Banta, and C. I. Kado. 2000. Genetic and environmental factors affecting T pilin export and T pilus biogenesis in relation to flagellation of Agrobacterium tumefaciens. J. Bacteriol. 182:3705-3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lai, E. M., R. Eisenbrandt, M. Kalkum, E. Lanka, and C. I. Kado. 2002. Biogenesis of T pili in Agrobacterium tumefaciens requires precise VirB2 propilin cleavage and cyclization. J. Bacteriol. 184:327-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lai, E. M., and C. I. Kado. 1998. Processed VirB2 is the major subunit of the promiscuous pilus of Agrobacterium tumefaciens. J. Bacteriol. 180:2711-2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Long, J. C., S. Wang, and S. B. Vik. 1998. Membrane topology of subunit a of the F1F0 ATP synthase as determined by labeling of unique cysteine residues. J. Biol. Chem. 273:16235-16240. [DOI] [PubMed] [Google Scholar]
- 50.Loo, T. W., and D. M. Clarke. 1995. Membrane topology of a cysteine-less mutant of human P-glycoprotein. J. Biol. Chem. 270:843-848. [DOI] [PubMed] [Google Scholar]
- 51.Majdalani, N., and K. Ippen-Ihler. 1996. Membrane insertion of the F-pilin subunit is Sec independent but requires leader peptidase B and the proton motive force. J. Bacteriol. 178:3742-3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Manchak, J., K. G. Anthony, and L. S. Frost. 2002. Mutational analysis of F-pilin reveals domains for pilus assembly, phage infection and DNA transfer. Mol. Microbiol. 43:195-205. [DOI] [PubMed] [Google Scholar]
- 53.Middleton, R., K. Sjolander, N. Krishamurthy, J. Foley, and P. Zambryski. 2005. Predicted hexameric structure of the Agrobacterium VirB4 C-terminus suggests VirB4 acts as a docking site during type IV secretion. Proc. Natl. Acad. Sci. U. S. A. 102:1685-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moore, D., C. M. Hamilton, K. Maneewannakul, Y. Mintz, L. S. Frost, and K. Ippen-Ihler. 1993. The Escherichia coli K-12 F plasmid gene traX is required for acetylation of F pilin. J. Bacteriol. 175:1375-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mossey, P., A. Hudacek, and A. Das. 2010. Agrobacterium tumefaciens type IV secretion protein VirB3 is an inner membrane protein and requires VirB4, VirB7 and VirB8 for stabilization. J. Bacteriol. 192:2830-2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paiva, W. D., T. Grossman, and P. M. Silverman. 1992. Characterization of F-pilin as an inner membrane component of Escherichia coli K12. J. Biol. Chem. 267:26191-26197. [PubMed] [Google Scholar]
- 57.Rabel, C., A. M. Grahn, R. Lurz, and E. Lanka. 2003. The VirB4 family of proposed traffic nucleoside triphosphatases: common motifs in plasmid RP4 TrbE are essential for conjugation and phage adsorption. J. Bacteriol. 185:1045-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rashkova, S., G. M. Spudich, and P. J. Christie. 1997. Characterization of membrane and protein interaction determinants of the Agrobacterium tumefaciens VirB11 ATPase. J. Bacteriol. 179:583-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rashkova, S., X.-R. Zhou, and P. J. Christie. 2000. Self-assembly of the Agrobacterium tumefaciens VirB11 traffic ATPase. J. Bacteriol. 182:4137-4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rondot, S., K. G. Anthony, S. Dubel, N. Ida, S. Wiemann, K. Beyreuther, L. S. Frost, M. Little, and F. Breitling. 1998. Epitopes fused to F-pilin are incorporated into functional recombinant pili. J. Mol. Biol. 279:589-603. [DOI] [PubMed] [Google Scholar]
- 61.Sagulenko, V., E. Sagulenko, S. Jakubowski, E. Spudich, and P. J. Christie. 2001. VirB7 lipoprotein is exocellular and associates with the Agrobacterium tumefaciens T pilus. J. Bacteriol. 183:3642-3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sagulenko, Y., V. Sagulenko, J. Chen, and P. J. Christie. 2001. Role of Agrobacterium VirB11 ATPase in T pilus assembly and substrate selection. J. Bacteriol. 183:5813-5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Savvides, S. N. 2007. Secretion superfamily ATPases swing big. Structure 15:255-257. [DOI] [PubMed] [Google Scholar]
- 64.Savvides, S. N., H. J. Yeo, M. R. Beck, F. Blaesing, R. Lurz, E. Lanka, R. Buhrdorf, W. Fischer, R. Haas, and G. Waksman. 2003. VirB11 ATPases are dynamic hexameric assemblies: new insights into bacterial type IV secretion. EMBO J. 22:1969-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schandel, K. A., M. M. Muller, and R. E. Webster. 1992. Localization of TraC, a protein involved in assembly of the F conjugative pilus. J. Bacteriol. 174:3800-3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schroder, G., and E. Lanka. 2005. The mating pair formation system of conjugative plasmids—a versatile secretion machinery for transfer of proteins and DNA. Plasmid 54:1-25. [DOI] [PubMed] [Google Scholar]
- 67.Shirasu, K., and C. I. Kado. 1993. Membrane location of the Ti plasmid VirB proteins involved in the biosynthesis of a pilin-like conjugative structure on Agrobacterium tumefaciens. FEMS Microbiol. Lett. 111:287-294. [DOI] [PubMed] [Google Scholar]
- 68.Shirasu, K., N. Z. Koukolikova, B. Hohn, and C. I. Kado. 1994. An inner-membrane-associated virulence protein essential for T-DNA transfer from Agrobacterium tumefaciens to plants exhibits ATPase activity and similarities to conjugative transfer genes. Mol. Microbiol. 11:581-588. [DOI] [PubMed] [Google Scholar]
- 69.Silverman, P. M. 1997. Towards a structural biology of bacterial conjugation. Mol. Microbiol. 23:423-429. [DOI] [PubMed] [Google Scholar]
- 70.Sivanesan, D., M. A. Hancock, A. M. V. Giraldo, and C. Baron. 2010. Quantitative analysis of VirB8-VirB9-VirB10 interactions provides a dynamic model of type IV secretion system core complex assembly. Biochemistry 49:4483-4493. [DOI] [PubMed] [Google Scholar]
- 71.von Heijne, G., and Y. Gavel. 1988. Topogenic signals in integral membrane proteins. Eur. J. Biochem. 174:671-678. [DOI] [PubMed] [Google Scholar]
- 72.Wang, Y. A., X. Yu, P. M. Silverman, R. L. Harris, and E. H. Egelman. 2009. The structure of F-pili. J. Mol. Biol. 385:22-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yeo, H. J., S. N. Savvides, A. B. Herr, E. Lanka, and G. Waksman. 2000. Crystal structure of the hexameric traffic ATPase of the Helicobacter pylori type IV secretion system. Mol. Cell 6:1461-1472. [DOI] [PubMed] [Google Scholar]
- 74.Yuan, Q., A. Carle, C. Gao, D. Sivanesan, K. A. Aly, C. Hoppner, L. Krall, N. Domke, and C. Baron. 2005. Identification of the VirB4-VirB8-VirB5-VirB2 pilus assembly sequence of type IV secretion systems. J. Biol. Chem. 280:26349-26359. [DOI] [PubMed] [Google Scholar]
- 75.Zhou, X.-R., and P. J. Christie. 1999. Mutagenesis of Agrobacterium VirE2 single-stranded DNA-binding protein identifies regions required for self-association and interaction with VirE1 and a permissive site for hybrid protein construction. J. Bacteriol. 181:4342-4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou, X.-R., and P. J. Christie. 1997. Suppression of mutant phenotypes of the Agrobacterium tumefaciens VirB11 ATPase by overproduction of VirB proteins. J. Bacteriol. 179:5835-5842. [DOI] [PMC free article] [PubMed] [Google Scholar]