Abstract
Current procedures for obtaining and measuring plasma concentrations of HIV protease inhibitors (PIs) are technically challenging. Dried blood spot (DBS) assays offer a way to overcome many of the obstacles. We sought to develop a DBS assay for quantitation of the PI atazanavir (ATV) and to compare this method with a previously validated plasma assay. We prospectively enrolled 48 patients with well-controlled HIV disease who had been on ATV for at least 7 days. ATV was quantified from plasma by use of high-performance liquid chromatography (HPLC). A reversed-phase ultrahigh-performance liquid chromatography (UPLC) assay was utilized for DBS samples. The concentrations of ATV quantified in a DBS matrix showed very strong agreement with those measured in plasma (r2 = 0.988). The mean difference in ATV concentration between the two methods was −10.8% (95% confidence interval [95% CI], −7.65% to −13.95%), indicating that the DBS method has a slight negative bias. A majority (97.8%) of the differences in concentration between the two assays fell within ±2 standard deviations. ATV concentrations were lower in subjects who had detectable HIV RNA in plasma (mean, 543 ng/ml) than in those with HIV RNA of <50 copies/ml (mean, 1,582 ng/ml) (P = 0.03, Wilcoxon rank-sum test). In conclusion, our study demonstrated that ATV quantitation in a DBS matrix is feasible and accurate. DBS use offers a convenient alternative for measuring plasma concentrations of ATV and may have utility in monitoring of drug concentrations in clinical practice and in future studies.
Presently, approximately 33 million persons are living with human immunodeficiency virus (HIV) disease (35). Treatment options for HIV disease have expanded over the last 15 years, particularly with the introduction of protease inhibitors (PIs) as a component of combination antiretroviral therapy (ART). Use of these agents has been associated with significant decreases in morbidity and mortality (13, 16, 29). Despite the efficacy of PIs, a substantial number of patients still experience virological failure (23). PIs show significant interindividual pharmacokinetic variability for identical dosing regimens (7, 14, 31, 33). High PI concentrations have been associated with toxicity, while subtherapeutic concentrations have been associated with virologic failure (2, 3, 9, 11, 12, 28, 30, 31, 32). These findings have led to interest in the use of therapeutic drug monitoring (TDM), which individualizes therapy to maximize outcomes and minimize toxicity (1, 7, 10). Currently, the literature does not support and guidelines do not recommend routine use of TDM in HIV-infected adults (8, 21).
Atazanavir (ATV) is an azapeptide PI approved for use in both treatment-naïve and treatment-experienced patients (18). It has the advantage of being dosed once a day and can be used with or without ritonavir (RTV), although coadministration of RTV is preferred (8). The current techniques for quantitation of ATV (as well as all other PIs) are plasma- or serum-based analytical procedures. These procedures require specific processing of samples and specialized equipment. Measurement of plasma concentration requires the drawing of venous blood followed by immediate processing to obtain plasma and freezing of the sample. Specialized equipment used to measure drug concentrations is expensive, and frozen samples typically are shipped to a centralized lab. These issues limit the ability to collect samples for quantitation of PI concentrations in both high- and low-resource areas.
Dried blood spot (DBS) assays have been available for decades in neonatal screening for inborn errors in metabolism (15). The advantages of DBS techniques include the ease of sample acquisition and transport and the ability to obtain samples in varied settings. Similarly, a DBS matrix for measurement of drug concentrations offers advantages over the conventional plasma matrix. The objectives of this work were to develop a DBS assay for quantitation of ATV concentrations and to compare this method with a validated, externally quality-controlled high-performance liquid chromatography (HPLC) method for ATV quantitation in plasma in patients on chronic, stable doses of an ATV-containing antiretroviral (ARV) regimen.
MATERIALS AND METHODS
Patients.
Patients were recruited from the HIV Clinic of the University of Nebraska Medical Center from January to March 2009. Entry criteria included HIV infection, age greater than 19 years, receipt of ATV (with or without RTV) for at least 7 days prior, and HIV RNA of <50 copies/ml for the last 90 days. Persons who had any intercurrent illness that might interfere with the interpretation of the study were excluded. Demographic information and complete medication lists were obtained. Race and ethnicity data were self-reported. Patients were queried regarding the timing of their last 2 doses of ATV and the number of missed doses in the last 7 days. The study was approved by the University of Nebraska Medical Center Institutional Review Board, and each participant gave informed consent.
Samples.
At varied and random times after a reported dose of ATV, a single whole-blood sample from each study participant was obtained in EDTA-containing tubes and processed to obtain plasma. Plasma specimens were frozen at −70°C. After sterile cleaning of the skin, single DBS samples were obtained directly from the same patients via lancet puncture. Five 1-cm areas on filter paper (Protein Saver 903 card; Whatman Inc., Piscataway, NJ) were saturated with whole blood, air dried in the horizontal position, and placed in sealed plastic bags for storage. All samples were stored until study enrollment was complete, at which time both DBS and plasma samples were analyzed for ATV concentrations. All measurements of CD4 cell counts and HIV-1 RNA were performed as part of standard patient care. The detection limit for the HIV-1 RNA assay was 50 copies/ml.
Analytical methods.
The analysis of plasma ATV concentrations was performed using a previously developed and validated simultaneous reversed-phase high-performance liquid chromatography (HPLC) assay. The HPLC system included a Waters Alliance 2690 separations module with a Waters 2487 dual-wavelength UV absorbance detector (Waters Corp., Milford, MA). Following the addition of an internal standard (A86093; Abbott Laboratories, North Chicago, IL), a liquid-liquid extraction procedure using methyl tert-butyl ether at a basic pH was carried out to prepare the samples. The chromatographic separation of the compounds and the internal standard was accomplished on a YMC Octyl (C8) 120-Å, 100- by 4.6-mm column with a 3-μm particle size (Waters Corp., Milford, MA). The mobile phase consisted of 54.7% 20 mM acetate buffer, pH 4.9, and 45.3% acetonitrile, with an isocratic flow rate of 1 ml/min. Detection and quantitation of ATV occurred at 212 nm. The assay was linear in the range of 20 ng/ml to 20,000 ng/ml, with a minimum quantifiable limit (LLOQ) of 20 ng/ml when 0.200 ml of human plasma was used. Inter- and intraday accuracy and precision were within ±20% at the LLOQ and ±15% at all other concentrations. This assay has undergone assay validation according to FDA guidelines and is tested twice yearly by participation in an externally administered proficiency program. A series of 5 blinded samples are tested for ATV concentrations, and the results have to be within 20% of the target concentration on 3 of 5 samples in order to pass the round.
Our initial approach to development of a DBS method for ATV was to modify our HPLC plasma assay for the DBS matrix. We tested our standard curve and measured a series of quality controls (QC) and validation samples prepared by spiking whole blood. The QC concentrations were 75, 750, and 7,500 ng/ml, and the validation sample concentrations were 20 (LLOQ), 60, 1,800, and 18,000 ng/ml. The experiment was performed over 5 days, with the standard curve, quality controls, and validation samples spiked daily. The overall HPLC back-calculated standard percent accuracy was ≤5.2% for standards above the LLOQ and 3.0% at the LLOQ. The percent coefficient of variation (CV) was ≤6.3% above the LLOQ and 5.6% at the LLOQ. The overall quality control variability was <5% for both percent CV and percent accuracy. The overall validation sample percent accuracy was ≤6.5% above the LLOQ and 11.3% at the LLOQ. The percent CV was ≤3.7% above the LLOQ and 6.7% at the LLOQ. These assay validation data indicated assay performance characteristics consistent with those of our plasma assay described above. However, a matrix stability issue was revealed with incubation of a series of spotted spiked DBS samples at 4°C, ambient temperature, and 35°C at concentrations of 40, 1,000, and 10,000 ng/ml for intervals of 0, 6, 14, and 30 days. An immediate increase in concentration of the low-concentration sample incubated at 35°C (+25%) was noted, and this increased with time. DBS samples with the lowest ATV concentration were the most sensitive to change. Samples with the middle and high concentrations increased to a lesser but still significant extent at the later time points. The samples at ambient temperature and 4°C increased at a slower but notable rate. We were not able to isolate ATV from the interfering peak and transferred the assay to a reversed-phase ultrahigh-performance liquid chromatography (UPLC) instrument.
The UPLC system included a Waters Acquity separations module with a tunable UV detector (Waters Corp., Milford, MA). The standard curve for the DBS assay was prepared by spiking 980-μl aliquots of purchased whole blood with 20 μl of ATV stock concentrations prepared in 50% methanol to final concentrations between 20 and 20,000 ng/ml. Using 80 μl per spot, these aliquots were applied to the same type of filter paper as patient samples to produce five spots. Quality control stocks were prepared in 50% methanol from a separate weighing of ATV at three concentrations covering the range of the assay. These were spiked into whole blood in a similar manner as the standards and again applied to the filter paper as five spots. The five dried blood spots, whether standard curve, QC, or patient samples, were punched out using a 5/8-in. tool, combined and transferred to a single culture tube, and reconstituted with type I water produced by a Millipore MilliQ Integral 3 purification system. Following the addition of the internal standard, a liquid-liquid extraction procedure using methyl tert-butyl ether at basic pH was carried out to prepare the samples. The chromatographic separation of the compounds and the internal standard was accomplished using an Acquity UPLC HSS 100- by 2.1-mm column with a 1.8-μm particle size (Waters Corp., Milford, MA). The mobile phase consisted of 41.0% 20 mm sodium acetate buffer, pH 4.9, 23.0% methanol, and 36.0% acetonitrile, with an isocratic flow rate of 0.6 ml/min. Detection of the ATV peak occurred at a wavelength of 212 nm, with a retention time of 3.3 min. The assay was linear in the range of 20 ng/ml to 20,000 ng/ml, with an LLOQ of 20 ng/ml when five 5/8-in. punches were used. These conditions successfully isolated ATV from the interfering peak found with HPLC. A series of six different lots of whole-blood matrix were spiked with low and high QC concentrations to test for potential matrix effects. In contrast with the matrix effects observed with the HPLC method, no apparent effects were noted with the UPLC method.
Statistics.
We planned to obtain 50 paired samples from 50 subjects. This sample size was based upon guidance from the Clinical and Laboratory Standards Institute (CLSI, formerly NCCLS), who recommend 40 patient samples to be run with both the new analytical procedure and the reference method (27). We chose to inflate this number to account for any subject having a concentration of ATV below the limit of quantitation (BLQ).
ATV dose, time of sample collection postdose, and plasma and DBS concentrations were compiled and summary statistics computed. The approach recommended by Bland and Altman was used for comparison of the two methods for quantitation of ATV, as well as linear regression (4). ATV concentrations that were BLQ were assigned a value of 5 ng/ml for method comparison purposes and for investigation of relationships between ATV concentrations and HIV RNA. The Wilcoxon rank-sum test was used to compare ATV concentrations in subjects who had plasma HIV RNA of <50 copies/ml with those in subjects who did not.
RESULTS
Patients.
Forty-eight patients consented to participate in this study. The characteristics of the 48 patients enrolled are summarized in Table 1. Demographics were consistent with the population served at the clinic, with 60% of patients being white and 81% male. Eighty-five percent of patients had HIV RNA levels of <50 copies/ml. Seven patients (15%) had detectable viral loads at the time blood samples were obtained for evaluation of ATV concentrations, despite the requirement for an undetectable viral load for the previous 90 days for entry into the study. The mean CD4+ cell count was 515 cells/mm3. Forty-three patients were taking 300 mg ATV plus 100 mg RTV daily (ATV300/r), and five were taking 400 mg ATV daily (ATV400). All but one individual was taking other ARV medications, with the three most common combinations being tenofovir-emtricitabine (24 patients), tenofovir-abacavir (10 patients), and abacavir-lamivudine (5 patients). All patients taking tenofovir were on RTV-boosted ATV. No patients were taking acid-blocking medications at the time of evaluation, although one patient was on didanosine. Fifteen percent of patients reported missing at least one dose of ATV in the last 7 days.
TABLE 1.
Patient characteristics
| Characteristic | Mean value (range) or no. (%) of subjects |
|---|---|
| Age, yr (mean [range]) | 45 (23-64) |
| Race/ethnicity (no. [%] of subjects) | |
| White | 29 (60) |
| Black | 9 (19) |
| Hispanic | 10 (21) |
| Gender, male (no. [%] of subjects) | 39 (81) |
| CD4+ lymphocyte count/mm3 (no. [%] of subjects)a | |
| <200 | 5 (10) |
| 201-350 | 8 (17) |
| ≥351 | 35 (73) |
| HIV RNA level (no. [%] of subjects) | |
| <50 copies/ml | 41 (85) |
| ≥50 copies/ml | 7 (15) |
| ≥1 missed dose of medication in last 5 days (no. | |
| [%] of subjects) | 6 (13) |
The mean CD4+ lymphocyte count/mm3 for all subjects was 515, with a range of 79 to 1,312.
Atazanavir concentrations.
ATV concentrations measured in plasma (by HPLC) are shown in Table 2. Plasma concentrations of ATV exhibited significant variability, with patients on ATV400 (n = 5) having a mean concentration of 465 ng/ml (range, <20 to 787 ng/ml) and those on ATV300/r (n = 43) having a mean concentration of 1,629 ng/ml (range, <20 to 7,223 ng/ml). Three persons had undetectable ATV concentrations, but only one of these three patients reported noncompliance in the last 7 days and then only admitted to missing a single dose of ATV.
TABLE 2.
ATV plasma concentrations
| ATV dose (mg) QDa | No. of patients | Avg time postdose (h) of sample collection | ATV plasma concn (ng/ml) |
No. of patients with ATV concn BLQ | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | Median | SD | Minimumb | Maximum | ||||
| 400 | 5 | 13.06 | 465 | 366 | 215 | 342 | 787 | 1 |
| 300 | 43 | 16.60 | 1,629 | 1,137 | 1,442 | 196 | 7,223 | 2 |
QD, once daily.
Minimum excludes participants with levels BLQ.
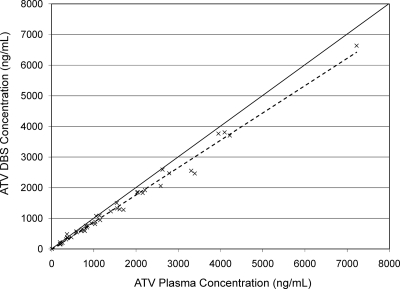
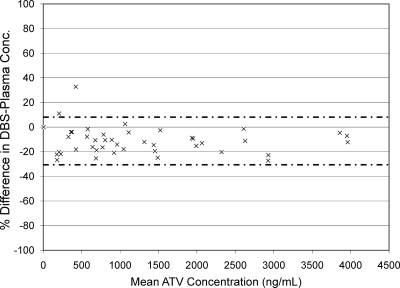
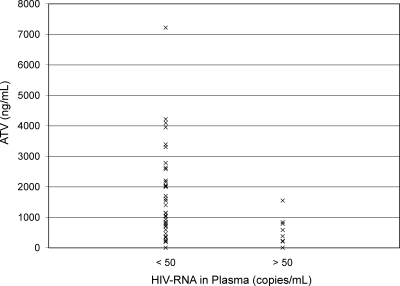
Figure 1 shows the relationship between ATV concentrations quantified in plasma and from DBSs (r2 = 0.988). Figure 2 shows a Bland-Altman plot of the percent difference in ATV concentration (DBS concentration-plasma concentration) between the two methods versus the mean ATV concentration of the two methods. In this analysis, the mean percent difference in ATV concentration between the two methods was −10.8% (95% confidence interval [95% CI], −7.65% to −13.95%), indicating that the DBS method has a slight negative bias. The majority (97.8%) of the differences in concentration between the two assays fell within ±2 standard deviations. Figure 3 presents ATV plasma concentrations in subjects who had HIV RNA of <50 copies/ml versus those in subjects who did not. ATV concentrations were lower in subjects who had detectable HIV RNA in plasma than in those with HIV RNA of <50 copies/ml (P = 0.03, Wilcoxon rank-sum test). The average ATV concentration in subjects with HIV RNA of <50 copies/ml was 1,582 ng/ml (interquartile range, 593 ng/ml to 2,156 ng/ml), compared with an average of 543 ng/ml (interquartile range, 219 ng/ml to 713 ng/ml) in subjects with HIV RNA of >50 copies/ml. All patients with HIV RNA of >50 copies/ml were on RTV-boosted regimens. The average times after the last dose of ATV that samples were collected were 16.3 h in those with HIV RNA of <50 copies/ml and 15.6 h in those with detectable HIV RNA.
FIG. 1.
Correlation of ATV concentrations as measured in plasma and from DBSs. The solid line is the line of identity. The dashed line is the line of best fit (y = 0.89x − 18.3; r2 = 0.988).
FIG. 2.
Percent difference in atazanavir concentrations versus mean atazanavir concentrations. The difference is the DBS concentration minus the plasma concentration. The mean atazanavir concentration is the mean of the values measured in plasma and from DBSs. The mean difference is −10.8%. The majority (97.8%) of the differences are contained within ±2 standard deviations of the mean difference, as bracketed with the dashed lines. For illustration purposes only, one concentration, >7,000 ng/ml, is not shown.
FIG. 3.
ATV concentrations by HIV RNA level. Atazanavir concentrations in plasma versus the levels of HIV RNA in plasma at the time the atazanavir concentrations were obtained.
DISCUSSION
In this report, we describe the development of a DBS assay to determine ATV concentrations. The concentrations of ATV quantified in a DBS matrix measured by UPLC showed very strong agreement with those measured in plasma by HPLC. The mean difference of −10.8%, with a 95% confidence interval of −7.65% to −13.95%, indicate a negative bias of the DBS method. This bias is sufficiently small to provide high confidence in an analytical result measured via the DBS method and is suitable for clinical use. The minimal effective concentration of ATV needed to prevent HIV viral replication is estimated to be 150 ng/ml (31). Trough ATV concentrations average approximately 800 ng/ml with the ATV300/r regimen. Thus, the magnitude of separation among the usual trough, the threshold trough, and the 20 ng/ml LLOQ of the assay is wide enough that a −10% bias would not lead to a different clinical interpretation. Use of this assay in formal pharmacokinetic studies would simplify sample acquisition, and a correction factor of 10% to better correlate with concentrations measured in plasma could easily be integrated. The source of the bias in the DBS method is not known; potential sources of variation have been discussed in a recent review paper (24).
To our knowledge, only two prior studies describing the quantification of antiretroviral drug concentrations using a DBS assay have been published (20, 34). Koal and colleagues used simultaneous liquid chromatography-tandem mass spectrometry to evaluate plasma and DBS samples for lopinavir, ATV, RTV, saquinavir, and efavirenz concentrations in 70 patients. A summary correlation curve of all five ARV drugs tested showed a negative bias of approximately 15% and a high correlation with plasma concentrations (r2 = 0.9772). The use of UPLC in the present method allowed the resolution of the ATV peak from endogenous interfering peaks and reduced analysis time and solvent usage. Our UPLC-DBS assay using five 5/8-in. punches achieved the same lower limit of quantitation of 20 ng/ml as the plasma HPLC assay, and this has been shown to be sufficient for pharmacokinetic studies in adults and children (19, 33). Finally, UPLC instrumentation has a considerably lower acquisition cost than a triple quadruple mass spectrometer, making it more readily available.
In the subjects who participated in this study, ATV concentrations were lower in those who had detectable levels of HIV RNA in plasma than in those who had undetectable HIV RNA. The association between the lack of viral suppression and lower ATV concentrations, as found in our study, has been observed by others. Alexander and colleagues evaluated untimed plasma samples for ARV concentrations in 122 patients initiating ART and found a single “low” concentration within the first 6 weeks to be associated with more-rapid immunologic failure and failure to achieve virologic success in the first year (2). In an evaluation of 210 patients with HIV infection, Oette and colleagues noted that both adequate unscheduled drug concentrations and adequate trough concentrations of ARVs were associated with virologic success at 12 weeks (28). Low ARV concentrations are often surrogate markers of poor medication adherence (33). In contrast, patients in our trial with nonsuppressed HIV RNA had not reported any lack of adherence in the last 7 days. Interestingly, all six patients who admitted to nonadherence had completely suppressed HIV RNA levels, despite one patient having completely undetectable ATV concentrations. ATV concentrations were generally lower in those who reported poor adherence.
There has been recent interest in the use of DBS assays for diagnosis and monitoring of patients with HIV infection, particularly in low-income countries (25). DBSs were first used to detect HIV infection in postnatal serosurveillance studies (17). Their use with HIV has expanded to include nucleic acid detection, viral load determination, and drug resistance genotyping (5, 26, 36). The DBS assay offers a number of advantages over conventional methods, which involve venous puncture followed by rapid plasmid extraction and freezing for transport to a reference laboratory for further analysis. DBSs are obtained without venous puncture or the use of trained phlebotomists, and they do not require on-site processing, such as rapid centrifugation or a robust cold chain. DBS methodology also minimizes the risk of potential occupational exposure to infected blood products, and after sample acquisition, DBS samples can be stored at room temperature for at least 7 days and transported via traditional postal means to reference laboratories (34). The collection and processing of blood samples for ARV concentrations in the setting of routine patient care are challenging regardless of the geography. DBS samples allow a bypass of this difficulty, as they may even be obtained directly by patients and submitted via postal system for analysis at distant sites. A report describing blood samples self-obtained by patients and spotted onto filter paper every 2 weeks to monitor the antiepileptic agent lamotrigine during pregnancy is an illustration of the utility of a DBS assay (6).
The utility of DBS samples may enhance the ability to evaluate ARV pharmacokinetics at sites distant to reference laboratories, allowing for TDM at any site. While TDM is not recommended for routine use, it may be useful in managing significant drug-drug and drug-food interactions, identifying nonadherence, evaluating patients with poor virologic response despite good adherence, adjusting ARV dosing in patients with organ dysfunction, identifying patients with adverse effects due to increased drug concentrations, maximizing therapeutic drug levels in treatment-experienced patients with resistant virus and reduced susceptibility to ARVs, and dosing guidance in children and pregnant women (8, 22). Challenges for the implementation of TDM include the confounding of nonadherence, the paucity of controlled trials demonstrating the benefit of TDM, and data identifying the pharmacokinetic parameter and concentration threshold that best predicts ARV response (1, 14).
In conclusion, our study demonstrates that ATV quantitation in a DBS matrix is both feasible and accurate and may have utility in both clinical practice and pharmacokinetic studies. Further evaluation of this technology for other antiretroviral agents is warranted.
Acknowledgments
This study was supported by a grant from the Bristol-Myers Squibb Virology Fellows Research Training Program (T.V.S.) and grants U0I-AI68632 and U01-AI68630 from the National Institute of Allergy and Infectious Disease (C.V.F.).
We are very grateful to Frances Van Meter, study coordinator; Sebrina Taylor, laboratory technician; and Deanna Hansen, administrative assistant.
Footnotes
Published ahead of print on 26 July 2010.
REFERENCES
- 1.Aarnoutse, R. E., J. M. Schapiro, C. A. Boucher, Y. A. Hekster, and D. M. Burger. 2003. Therapeutic drug monitoring: an aid to optimizing response to antiretroviral drugs? Drugs 63:741-753. [DOI] [PubMed] [Google Scholar]
- 2.Alexander, C. S., J. J. Asselin, L. S. L. Ting, J. S. Montaner, R. S. Hogg, B. Yip, M. B. O'Shaughnessy, and P. R. Harrigan. 2003. Antiretroviral concentrations in untimed plasma samples predicts therapy outcomes in populations with advanced disease. J. Infect. Dis. 188:541-548. [DOI] [PubMed] [Google Scholar]
- 3.Baxter, J. D., T. C. Merigan, D. N. Wentworth, J. D. Neaton, M. L. Hoover, R. M. Hoetelmans, S. C. Piscitelli, W. H. Verbiest, D. L. Mayers, and the CPCRA 046 Study Team for the Terry Beirn Community Programs for Clinical Research on AIDS. 2002. Both baseline HIV-1 drug resistance and antiretroviral drug levels are associated with short-term virologic responses to salvage therapy. AIDS 16:1131-1138. [DOI] [PubMed] [Google Scholar]
- 4.Bland, J. M., and D. G. Altman. 1986. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 327:307-310. [PubMed] [Google Scholar]
- 5.Buckton, A. J., S. L. Bisset, R. E. Myers, S. Beddows, S. Edwards, P. A. Cane, and D. Pillay. 2008. Development and optimization of an internally controlled dried blood spot assay for surveillance of human immunodeficiency virus type-1 drug resistance. J. Antimicrob. Chemother. 62:1191-1198. [DOI] [PubMed] [Google Scholar]
- 6.De Haan, G. J., P. Edelbroek, M. Engelsman, D. Lindhout, M. Devile-Notschaele, and P. Augustijn. 2004. Gestation-induced changes in lamotrigine pharmacokinetics: a monotherapy study. Neurology 63:571-573. [DOI] [PubMed] [Google Scholar]
- 7.de Maat, M. M., A. D. Huitema, J. W. Mulder, P. D. Meenhorst, E. C. van Gorp, A. T. Mairuhu, and J. H. Beijnen. 2003. Subtherapeutic antiretroviral plasma concentrations in routine clinical outpatient HIV care. Ther. Drug Monit. 25:367-373. [DOI] [PubMed] [Google Scholar]
- 8.Department of Health and Human Services. 26 January 2010, accession date. Panel on antiretroviral guidelines for adults and adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents: December 1, 2009, p. 1-161. http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf.
- 9.Durant, J., P. Clevenbergh, R. Garraffo, P. Halfon, S. Icard, P. Del Giudice, N. Montagne, J. M. Schapiro, and P. Dellamonica. 2000. Importance of protease inhibitor plasma levels in HIV-infected patients treated with genotypic-guided therapy: pharmacological data from the Viradapt Study. AIDS 14:1333-1339. [DOI] [PubMed] [Google Scholar]
- 10.Fletcher, C. V., P. L. Anderson, T. N. Kakuda, T. W. Schacker, K. Henry, C. R. Gross, and R. C. Brundage. 2002. Concentration-controlled compared with conventional antiretroviral therapy for HIV infection. AIDS 16:551-560. [DOI] [PubMed] [Google Scholar]
- 11.Gandhi, M., N. Ameli, P. Bacchetti, S. J. Gange, K. Anastos, A. Levine, C. L. Hyman, M. Cohen, M. Young, Y. Huang, R. M. Greenblatt, and the Women's Interagency HIV Study (WIHS). 2009. Protease inhibitor levels in hair strongly predict virologic response to treatment. AIDS 23:471-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gatti, G., A. Di Biagio, R. Casazza, C. De Pascalis, M. Bassetti, M. Cruciani, S. Vella, and D. Bassetti. 1999. The relationship between ritonavir plasma levels and side effects: implications for therapeutic drug monitoring. AIDS 13:2083-2089. [DOI] [PubMed] [Google Scholar]
- 13.Gortmaker, S. L., M. Hughes, J. Cervia, M. Brady, G. M. Johnson, G. R. Seage III, L. Y. Song, W. M. Dankner, J. M. Oleske, and the Pediatric AIDS Clinical Trials Group Protocol 219 Team. 2001. Effect of combination therapy including protease inhibitors on mortality among children and adolescents infected with HIV-1. N. Engl. J. Med. 345:1522-1528. [DOI] [PubMed] [Google Scholar]
- 14.Guiard-Schmid, J. B., J. M. Poirier, J. L. Meynard, P. Bonnard, A. H. Gbadoe, C. Amiel, F. Calligaris, B. Abraham, G. Pialoux, P. M. Girard, P. Jaillon, and W. Rozenbaum. 2003. High variability of plasma drug concentrations in dual protease inhibitor regimens. Antimicrob. Agents Chemother. 47:986-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guthrie, R., and A. Susi. 1963. A simple phenylalanine method for detecting phenylketonuria in large populations of newborn infants. Pediatrics 32:338-343. [PubMed] [Google Scholar]
- 16.Hammer, S. M., K. E. Squires, M. D. Hughes, J. M. Grimes, L. M. Demeter, J. S. Currier, J. J. Eron, Jr., J. E. Feinberg, H. H. Balfour, Jr., L. R. Deyton, J. A. Chodakewitz, and M. A. Fischl. 1997. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. N. Engl. J. Med. 337:725-733. [DOI] [PubMed] [Google Scholar]
- 17.Hannon, W. H., D. S. Lewis, W. K. Jones, and M. K. Powell. 1989. A quality assurance program for human immunodeficiency virus seropositivity screening of dried-blood spot specimens. Infect. Control Hosp. Epidemiol. 10:8-13. [DOI] [PubMed] [Google Scholar]
- 18.Havlir, D. V., and S. D. Marro. 2004. Atazanavir: new option for treatment of HIV infection. Clin. Infect. Dis. 38:1599-1604. [DOI] [PubMed] [Google Scholar]
- 19.Kiser, J. J., C. V. Fletcher, P. M. Flynn, C. K. Cunningham, C. M. Wilson, B. G. Kapogiannis, H. Major-Wilson, R. M. Viani, N. X. Liu, L. R. Muenz, D. R. Harris, P. L. Havens, and the Adolescent Trials Network for HIV/AIDS Interventions. 2008. Pharmacokinetics of antiretroviral regimens containing tenofovir disoproxil fumarate and atazanavir-ritonavir in young adults with HIV infection. Antimicrob. Agents Chemother. 52:631-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koal, T., H. Burhenne, R. Römling, M. Svoboda, K. Resch, and V. Kaever. 2005. Quantification of antiretroviral drugs in dried blood spot samples by means of liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 19:2995-3001. [DOI] [PubMed] [Google Scholar]
- 21.Kredo, T., J. S. Van derWalt, N. Siegfried, and K. Cohen. 2009. Therapeutic drug monitoring of antiretrovirals for people with HIV. Cochrane Database of Systematic Reviews, issue 3, article no. CD007268. doi: 10.1002/14651858.CD007268.pub2. [DOI] [PubMed]
- 22.La Porte, C. J. L., D. Black, T. Blaschke, C. A. B. Boucher, C. V. Fletcher, C. Flexner, J. G. Gerber, A. D. M. Kashuba, J. Schapiro, and D. M. Burger. 2006. Updated guideline to perform therapeutic drug monitoring for antiretroviral agents. Rev. Antivir. Ther. 3:4-14. [Google Scholar]
- 23.Ledergerber, B., M. Egger, M. Opravil, A. Telenti, B. Hirschel, M. Battegay, P. Vernazza, P. Sudre, M. Flepp, H. Furrer, P. Francioli, and R. Weber. 1999. Clinical progression and virological failure on highly active antiretroviral therapy in HIV-1 patients: a prospective cohort study. Lancet 353:863-868. [DOI] [PubMed] [Google Scholar]
- 24.Li, W., and F. L. S. Tse. 2010. Dried blood spot sampling in combination with LC-MS/MS for quantitative analysis of small molecules. Biomed. Chromatogr. 24:49-65. [DOI] [PubMed] [Google Scholar]
- 25.Lofgren, S. M., A. B. Morrissey, C. C. Chevallier, A. I. Malabeja, S. Edmonds, B. Amos, D. J. Sifuna, L. von Seidlein, W. Schimana, W. S. Stevens, J. A. Bartlett, and J. A. Crump. 2009. Evaluation of a dried blood spot HIV-1 RNA program for early infant diagnosis and viral load monitoring at rural and remote healthcare facilities. AIDS 23:2459-2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mbida, A. D., S. Sosso, P. Flori, H. Saoudin, P. Lawrence, M. Monny-Lobe, Y. Oyono, E. Ndzi, G. Cappelli, F. Lucht, B. Pozzetto, O. O. Oukem-Boyer, and T. Bourlet. 2009. Measure of viral load by using the Abbott real-time HIV-1 assay on dried blood and plasma spot specimens collected in 2 rural dispensaries in Cameroon. J. Acquir. Immune Defic. Syndr. 52:9-16. [DOI] [PubMed] [Google Scholar]
- 27.NCCLS. 2002. Method comparison and bias estimation using patient samples; approved guideline, 2nd ed. NCCLS document EP9-A2. NCCLS, Wayne, PA.
- 28.Oette, M., A. Kroidl, K. Gobels, A. Stabbert, M. Menge, A. Sagir, D. Kuschak, T. O'Hanley, J. G. Bode, and D. Haussinger. 2006. Predictors of short-term success of antiretroviral therapy in HIV infection. J. Antimicrob. Chemother. 58:147-153. [DOI] [PubMed] [Google Scholar]
- 29.Palella, F. J., K. M. Delaney, A. C. Moorman, M. O. Loveless, J. Fuhrer, G. A. Satten, D. J. Aschman, and S. D. Holmberg. 1998. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N. Engl. J. Med. 338:853-860. [DOI] [PubMed] [Google Scholar]
- 30.Powderly, W. G., M. S. Saag, S. Chapman, G. Yu, B. Quart, and N. J. Clendeninn. 1999. Predictors of optimal virological response to potent antiretroviral therapy. AIDS 13:1873-1880. [DOI] [PubMed] [Google Scholar]
- 31.Ray, J. E., D. Marriott, M. T. Bloch, and A. J. McLachlan. 2005. Therapeutic drug monitoring of atazanavir: surveillance of pharmacotherapy in the clinic. Br. J. Clin. Pharmacol. 60:291-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Solas, C., S. Basso, L. Poizot-Martin, I. Ravaux, H. Gallais, J. A. Gastaut, A. Durand, and B. Lacarelle. 2002. High indinavir Cmin is associated with higher toxicity in patients on indinavir-ritonavir 800/100mg twice-daily regimen. J. Acquir. Immune Defic. Syndr. 29:374-377. [DOI] [PubMed] [Google Scholar]
- 33.Swindells, S., A. G. DiRienzo, T. Wilkin, C. V. Fletcher, D. M. Margolis, G. D. Thal, C. Godfrey, B. Bastow, M. G. Ray, H. Wang, R. W. Coombs, J. McKinnon, J. W. Mellors, and the AIDS Clinical Trials Group 5201 Study Team. 2006. Regimen simplification to atazanavir-ritonavir alone as maintenance antiretroviral therapy after sustained virologic suppression. JAMA 296:806-814. [DOI] [PubMed] [Google Scholar]
- 34.ter Heine, R., H. Rosing, E. C. M. van Gorp, J. W. Mulder, W. A. van der Steeg, J. H. Beijnen, and A. D. R. Huitema. 2008. Quantification of protease inhibitors and non-nucleoside reverse transcriptase inhibitors in dried blood spots by liquid chromatography-triple quadruple mass spectrometry. J. Chromatogr. B 867:205-212. [DOI] [PubMed] [Google Scholar]
- 35.World Health Organization. 2009. AIDS epidemic update. http://data.unaids.org/pub/Report/2009/JC1700_Epi_Update_2009_en.pdf.
- 36.Zhang, Q., L. Wang, Y. Jiang, L. Fang, P. Pan, S. Gong, J. Yao, Y. W. Tang, S. H. Vermund, and Y. Jia. 2008. Early infant human immunodeficiency virus type 1 detection suitable for resource-limited setting with multiple circulating subtypes by use of nested three-monoplex DNA PCR and dried blood spots. J. Clin. Microbiol. 46:721-726. [DOI] [PMC free article] [PubMed] [Google Scholar]