Abstract
The pharmacokinetics of voriconazole in children receiving 4 mg/kg intravenously (i.v.) demonstrate substantially lower plasma exposures (as defined by area under the concentration-time curve [AUC]) than those in adults receiving the same therapeutic dosage. These differences in pharmacokinetics between children and adults limit accurate prediction of pediatric voriconazole exposure based on adult dosages. We therefore studied the pharmacokinetics and tolerability of higher dosages of an i.v.-to-oral regimen of voriconazole in immunocompromised children aged 2 to <12 years in two dosage cohorts for the prevention of invasive fungal infections. The first cohort received 4 mg/kg i.v. every 12 h (q12h), then 6 mg/kg i.v. q12h, and then 4 mg/kg orally (p.o.) q12h; the second received 6 mg/kg i.v. q12h, then 8 mg/kg i.v. q12h, and then 6 mg/kg p.o. q12h. The mean values for the AUC over the dosing interval (AUCτ) for 4 mg/kg and 6 mg/kg i.v. in cohort 1 were 11,827 and 22,914 ng·h/ml, respectively, whereas the mean AUCτ values for 6 mg/kg and 8 mg/kg i.v. in cohort 2 were 17,249 and 29,776 ng·h/ml, respectively. High interpatient variability was observed. The bioavailability of the oral formulation in children was approximately 65%. The safety profiles were similar in the two cohorts and age groups. The most common treatment-related adverse event was increased gamma glutamyl transpeptidase levels. There was no correlation between adverse events and voriconazole exposure. In summary, voriconazole was tolerated to a similar degree regardless of dosage and age; the mean plasma AUCτ for 8 mg/kg i.v. in children approached that for 4 mg/kg i.v. in adults, thus representing a rationally selected dosage for the pediatric population.
Invasive fungal infections cause severe morbidity and mortality in immunocompromised children, particularly those with hematological malignancies and those undergoing hematopoietic stem cell transplantation (HSCT) (3, 8, 11, 19, 21, 27, 28). Voriconazole is a broad-spectrum antifungal triazole with in vitro and in vivo activity against yeasts and filamentous fungi (2, 5). Voriconazole is approved for adults for primary treatment of invasive aspergillosis, esophageal candidiasis, and candidemia in nonneutropenic patients. It is also approved for the treatment of serious, refractory infections caused by Scedosporium and Fusarium species (16, 25). Voriconazole is more effective than deoxycholate amphotericin B in treatment of invasive aspergillosis in adults, most of whom suffered from invasive pulmonary aspergillosis (9). In addition, voriconazole has been used successfully to treat aspergillosis of the central nervous system and bone (14, 20).
Several case series and single case reports have described the safety and efficacy of voriconazole in pediatric oncology patients and other immunocompromised children with invasive mycoses (10, 24, 26). Voriconazole also has been used to treat Aspergillus airway disease in pediatric patients with cystic fibrosis (10). However, considerably less is known about the pharmacokinetics of this antifungal agent in children than about those in adults.
Current studies indicate that there are major differences between the pharmacokinetics of voriconazole in children and those in adults. The first systematic study of the safety, tolerability, and pharmacokinetics of voriconazole in pediatric patients demonstrated linear plasma pharmacokinetics in patients receiving intravenous (i.v.) voriconazole at dosages of 3 mg/kg every 12 h (q12h) or 4 mg/kg q12h (24). By comparison, voriconazole in adults displays nonlinear Michaelis-Menten plasma pharmacokinetics following similar dosages (17, 18). These differences in pharmacokinetics between children and adults limit accurate prediction of pediatric voriconazole exposure (as defined by area under the concentration-time curve [AUC]) based on adult dosages. Indeed, pharmacokinetic modeling studies demonstrated that the AUC was approximately 3-fold lower in children receiving 4 mg/kg of voriconazole q12h than in adults receiving the same dosage.
Therefore, in order to approximate the plasma exposure achieved in adults, we studied the safety, tolerability, and plasma pharmacokinetics of voriconazole in pediatric patients receiving dosages of 4, 6, and 8 mg/kg i.v. q12h. We also examined the plasma pharmacokinetics of the oral suspension of voriconazole at 4 and 6 mg/kg q12h.
MATERIALS AND METHODS
Study design.
This study was designed and conducted as an open-label, multicenter study of immunocompromised pediatric patients to whom voriconazole was administered for prevention of invasive fungal infections. The study was conducted in two consecutive cohorts, each consisting of a minimum of 18 evaluable patients. The study design, dosages, and cohorts are summarized in Table 1. Each age group included 9 ± 1 evaluable patients aged 2 to <6 years and 9 ± 1 patients aged 6 to <12 years. The first 18 patients were enrolled in cohort 1, in which the following dosing regimen was used: on day 1, a loading dose of 6 mg/kg q12h i.v.; on days 2 to 4, 4 mg/kg q12h i.v.; on days 5 to 8, 6 mg/kg q12h i.v.; and on days 9 to 12, 4 mg/kg twice a day (b.i.d.) orally (p.o.). An interim population pharmacokinetics analysis using data from the first 12 patients in cohort 1 was used to determine which of two possible dosing regimens should be followed for the second cohort of 18 patients.
TABLE 1.
Dosing schedules
| Day(s) | Dose (mg/kg) |
|
|---|---|---|
| Cohort 1 | Cohort 2 | |
| 1 | 6 i.v. | 6 i.v. |
| 2 to 4 | 4 q12h i.v. | 6 q12h i.v. |
| 5 to 8a | 6 q12h i.v. | 8 q12h i.v. |
| 9 to 11 | 4 q12h p.o.c | 6 q12h p.o. |
| 12b | 4 q12h p.o.c | 6 q12h p.o. |
| Up to 30 | To continue on voriconazole if there was a medical need | To continue on voriconazole if there was a medical need |
If the patient was unable to take oral medication, this period could be extended up to day 20. Blood samples were taken on day 4 of this period.
Day 4 of oral dosing. Patients switching to oral suspension on days 10 to 20 had blood samples drawn for pharmacokinetic analysis on days 13 to 23, respectively, such that all of these patients received 4 days of the oral suspension, irrespective of the day of switch.
Oral suspension.
A median AUC of approximately 40,000 ng·h/ml was observed in 236 healthy adult volunteers after an i.v. dose of 4 mg/kg q12h, and this was used as a reference value (24). As predefined in the study protocol, if the interim analysis revealed that the observed mean AUC was <40,000 ng·h/ml after the dosage of 6 mg/kg and if there were no safety concerns, then the next 18 patients would be assigned to cohort 2 (on days 1 to 4, 6 mg/kg q12h i.v.; on days 5 to 8, 8 mg/kg q12h i.v.; and on days 9 to 12, 6 mg/kg b.i.d. p.o.). If, however, the mean AUC was ≥40,000 ng·h/ml, or if the AUC was <40,000 ng·h/ml but there were safety concerns, then the next 18 patients would be assigned to cohort 2b (6 mg/kg q12h i.v. on day 1, 5 mg/kg q12h i.v., and then 4 mg/kg b.i.d. p.o. on days 5 to 8, followed by 5 mg/kg b.i.d. p.o. on days 9 to 12). If, on day 8 in cohort 1 or 2 or on day 4 in cohort 2b, the child was unable to take oral treatments, the i.v. period could be extended until day 20. If there was a clinical need, patients were allowed to continue voriconazole treatment until day 30. As summarized in Table 1, the study ultimately enrolled patients into cohort 2.
The study was conducted in accordance with the ethical principals described in the Declaration of Helsinki and received approval by institutional review boards and independent ethics review committees. Investigators obtained written informed consent from each patient's parent or legal guardian. In addition, informed assent also was obtained from the patient when this was possible.
Patients.
Male and female patients aged from 2 to <12 years of age who required antifungal therapy for the prevention of invasive fungal infections and who could, or were likely to, tolerate a transition from i.v. to oral therapy between days 9 and 20 were eligible for enrollment into the study. Patients were expected to develop neutropenia, defined as an absolute neutrophil count of <500 cells/μl lasting more than 10 days, either as a result of chemotherapy for leukemia, lymphoma, or aplastic anemia or in response to preparative treatment for bone marrow or hematopoietic stem cell transplantation. Patients with a history of hypersensitivity or severe intolerance to azole antifungal agents were ineligible, as were those with a creatinine clearance of <30 ml/minute or a history or current evidence of cardiac arrhythmia. While concomitant drug use with some drugs that potentially interact with voriconazole was permitted in accordance with appropriate monitoring and dosage adjustment, patients who were receiving terfenadine, pimozide, quinidine, astemizole, cisapride, omeprazole, or ergot alkaloids and who could not discontinue these drugs at least 24 h before starting the study, or who would need to receive any of these drugs during the study, were excluded. Similarly, those patients who received rifampin, rifabutin, carbamazepine, phenytoin, nevirapine, long-acting barbiturates, or sirolimus in the 14 days prior to the start of the study were excluded. Patients with laboratory safety findings at screening of aspartate aminotransferase (AST), alanine aminotransferase (ALT), or total bilirubin >5 times the upper limit of normal were ineligible. During the study, patients with breakthrough fungal infections, grade III to IV toxicity according to the Common Toxicity Criteria of the National Cancer Institute (http://ctep.cancer.gov/reporting/CTC-3test.html), or any other clinically significant condition were discontinued from the study and were ineligible for dose escalation. Any patient enrolled into the study who subsequently withdrew from participation was considered to be a discontinuation.
Procedures.
This was a 30-day study consisting of a pharmacokinetic period from day 1 to 12 and an optional nonpharmacokinetic period from day 13 to 30. Patients returned to the study center at 23 to 37 days after their last voriconazole dose for a follow-up visit. A physical examination was performed at screening, at the end of therapy (EOT), and at the follow-up visit. The following visual function tests were performed by an ophthalmologist: visual acuity (using Snellen letters or charts), fixation (for patients too young to undergo visual acuity testing), color vision (using the Ishihara test), visual field testing (for patients older than 5 years), and funduscopy (for all patients). Blood and urine samples were collected from each patient before the study and then again every 5 to 7 days or for abnormal laboratory results until normalization. An additional blood sample, or a buccal swab in those with profound neutropenia, was collected to determine CYP2C19 genotype, which was identified in a central laboratory.
Voriconazole administration and formulations.
i.v. voriconazole at a final concentration of 2 mg/ml was administered at an infusion rate of 3 mg/kg/h. Hence, the 4-, 6-, and 8-mg/kg dosages were administered over periods of 80, 120, and 160 min, respectively, every 12 h. Voriconazole powder for oral suspension at a concentration of 40 mg/ml was administered 1 h before or after a meal. Each dosage was provided for a minimum of 4 days.
Pharmacokinetic sampling.
Blood samples for determination of voriconazole concentrations were collected on day 4 of each dosing regimen at the following times: before the dose; after i.v. doses of 4 mg/kg and 6 mg/kg, at 2 min before the end of infusion and 2, 4, 6, 8, and 12 h after the start of the infusion; after the i.v. dose of 8 mg/kg, at 2 min before the end of infusion and 4, 6, 8, and 12 h after the start of the infusion; and after the oral dose, at 0.5, 1, 2, 4, 6, 8, and 12 h of dosing. Samples were centrifuged at 1,500 × g for 10 min at 4°C and submitted to a central laboratory.
Nominal sampling times were used for pharmacokinetic calculations. In this study, there was a total of 857 concentration points collected at steady state. Approximately 97% of these samples were drawn within 10% of the nominal sampling time. The 29 remaining samples (3.4%) from 14 subjects had a deviation of over 10% from the nominal time. Twenty of these 29 samples had a deviation within 25%. These deviations were found to have a minor impact on the calculated AUC.
Pharmacokinetic analysis.
Voriconazole and its major metabolite N-oxide were assayed using a previously validated high-performance liquid chromatography (HPLC) method (22). The lower limit of quantification was 10 ng/ml; concentrations of below 10 ng/ml were set to zero for the analysis of means. The accuracy and precision of the HPLC assay were <10%. Plasma concentrations in each cohort at each nominal time postdose were summarized as the mean, standard deviation, and coefficient of variation. The area under the plasma-concentration time curve over the dosing interval (AUCτ) and the plasma concentration at the end of the i.v. infusion (CEOI) for the i.v. formulation and the AUCτ, maximum observed plasma concentration (Cmax), and time to first occurrence of Cmax (Tmax) for the oral suspension formulation were summarized by dosage, age group, and cohort, and the bioavailability (F) was estimated using noncompartmental analyses for each dosage.
Safety analysis.
All adverse events (AEs) were recorded, regardless of treatment group or suspected causal relationship to the study drug. Serious adverse events (SAEs) included any event identified as serious according to predefined criteria or any adverse experience that was considered serious by the investigator or industrial sponsor. As this study devoted considerable attention to characterizing visual AEs in detail, the events are described in this report as all-causality nonvisual AEs and as all-causality visual AEs.
All deaths were reported immediately, regardless of elapsed time between last time of dose of study drug and death. Blood samples were obtained at screening; at days 4, 8, and 12; and at the 1-month follow-up visit for laboratory safety tests.
Statistical analysis.
Data are expressed as mean ± standard deviation. Comparisons of proportions were performed by Fisher's exact test. A P value of <0.05 was considered to be significant. Analysis of the relationship between AUCs and AEs are descriptive and are presented for generation of hypotheses for further study of the association between voriconazole exposure and toxicity.
RESULTS
Baseline characteristics.
Among 49 patients who were screened, 48 (29 males and 19 females) from 12 centers in three countries received voriconazole in two dosage cohorts of 24 patients each. The demographic characteristics of patients in both cohorts were similar at baseline and are summarized in Table 2. There were 18 patients in each dosage cohort who completed all three dosing regimens. The total durations of exposure to voriconazole following i.v. and oral dosing phases were similar in the two cohorts, with median durations of 19 days (range, 3 to 26 days) in cohort 1 and 17 days (1 to 30 days) in cohort 2. Durations of exposure at the highest i.v. dosage, which coincided with the second dosing phase in cohorts 1 (6 mg/kg) and 2 (8 mg/kg), were also similar, with median values of 10 days (2 to 16 days) and 9 days (4 to 17 days), respectively. Patients in cohort 1 had a median nonpharmacokinetic dosing period of 8 days (1 to 16 days), compared with 7 days (1 to 18 days) in cohort 2. The pattern of concomitant drugs, including antibacterial agents, antiviral agents, cytotoxic chemotherapy, corticosteroids, cyclosporine, and antiemetics, was consistent with that expected for patients with immunosuppression and malignancies and was similar for the two dosage cohorts. In cohort 1, 16/24 (67%), 9/23 (39%), and 3/22 (14%) of patients received concomitant cytotoxic agents during the three voriconazole dosing periods, respectively. Similarly, in cohort 2, 11/24 (44%), 7/22 (32%), and 2/20 (10%) patients received concomitant cytotoxic drugs during the three voriconazole dosing periods, respectively.
TABLE 2.
Baseline demographic data
| Characteristic | Value |
|
|---|---|---|
| Cohort 1 (n = 24) | Cohort 2 (n = 24) | |
| Gender, no. | ||
| Male | 18 | 11 |
| Female | 6 | 13 |
| Race, no. | ||
| White | 16 | 15 |
| Black | 1 | 4 |
| Asian | 3 | 1 |
| Other | 4 | 4 |
| Mean age, yrs (SD) [n] | ||
| 2-<6 | 3.7 (1.2) [12] | 2.8 (1.1) [12] |
| 6-<12 | 8.7 (1.9) [12] | 8.1 (1.4) [12] |
| All | 6.2 | 5.4 |
| Mean wt, kg (range) | ||
| 2-<6 yr | 18.1 (13.0-23.2) | 15.1 (10.8-20.0) |
| 6-<12 yr | 30.7 (19.0-54.9) | 26.6 (16.6-37.6) |
| All | 24.3 (13.0-54.9) | 20.8 (10.8-37.6) |
| CYP2C19 genotype,a no. (all, 2-<6 yr, 6-<12 yr) | ||
| EM | 15, 7, 8 | 21, 9, 12 |
| HEM | 8, 5, 3 | 2, 2, 0 |
| PM | 0, 0, 0 | 1, 1, 0 |
| Unknown | 1, 0, 1 | 0, 0, 0 |
| Primary underlying condition, no. | ||
| Leukemia | 11 | 5 |
| Aplastic anemia | 0 | 1 |
| HSCTb | 13 | 18 |
EM, homozygous extensive metabolizer; HEM, heterozygous extensive metabolizer; PM, homozygous poor metabolizer.
Preparative regimen for bone marrow or hematopoietic stem cell transplantation.
Pharmacokinetic analysis.
Because the predefined reference median AUC value of 40,000 ng·h/ml after 6 mg/kg q12h i.v. was not achieved in cohort 1 and there were no concerns of safety, the regimen planned for cohort 2 was followed. Mean pharmacokinetic findings following i.v. and oral administration of voriconazole in patients from both age groups after each dosing regimen are shown in Table 3. There was high interpatient variability in the pharmacokinetic parameters observed following either i.v. or oral administration of voriconazole in both cohorts. No significant differences in pharmacokinetic parameters were observed between the younger (2 to <6 years) and older (6 to <12 years) age groups.
TABLE 3.
Pharmacokinetic parameters following i.v. and oral administration of voriconazole in patients in different age groups
| Patient age (yr) | Parameter (unit) | Value (n) [coefficient of variation, %] |
|||||
|---|---|---|---|---|---|---|---|
| Cohort 1 |
Cohort 2 |
||||||
| 4 mg/kg i.v. | 6 mg/kg i.v. | 4 mg/kg p.o. | 6 mg/kg i.v. | 8 mg/kg i.v. | 6 mg/kg p.o. | ||
| 2-<6 | AUCτ (ng·h/ml)a | 11,722 (12) [76] | 21,931 (11) [125] | 3,788 (10) [78] | 18,216 (10) [87] | 25,566 (10) [81] | 6,959 (9) [104] |
| Cmax (ng/ml)a | 3,352 (12) [71] | 4,690 (11) [111] | 956 (12) [85] | 4,609 (10) [93] | 4,804 (10) [83] | 1,433 (10) [66] | |
| Tmax (h)b | 1.36 (12) [15] | 1.97 (11) [0] | 1.50 (12) [144] | 1.97 (10) [0] | 2.63 (10) [0] | 1.00 (10) [58] | |
| F (%)b | —c | — | 43.6 (10) [88] | — | — | 63.4 (8) [88] | |
| 6-<12 | AUCτ (ng·h/ml) | 11,954 (10) [78] | 24,047 (10) [129] | 7,346 (9) [60] | 16,234 (9) [66] | 34,681 (10) [81] | 10,076 (9) [56] |
| Cmax (ng/ml) | 3,067 (11) [64] | 4,009 (10) [88] | 1,555 (9) [54] | 3,986 (10) [67] | 6,924 (10) [123] | 2,213 (9) [49] | |
| Tmax (h) | 1.36 (11) [16] | 1.97 (10) [0] | 1.33 (9) [82] | 2.17 (10) [30] | 3.04 (10) [22] | 1.72 (9) [98] | |
| F (%) | — | — | 90.9 (9) [86] | — | — | 66.7 (7) [53] | |
| 2-<12 (all patients) | AUCτ (ng·h/ml) | 11,827 (22) [75] | 22,914 (21) [125] | 5,184 (19) [71] | 17,249 (19) [80] | 29,776 (20) [82] | 8,373 (18) [80] |
| Cmax (ng/ml) | 3,212 (23) [67] | 4,353 (21) [103] | 1,178 (21) [70] | 4,286 (20) [85] | 5,767 (20) [121] | 1,761 (19) [57] | |
| Tmax (h) | 1.36 (23) [15] | 1.97 (21) [0] | 1.43 (21) [122] | 2.07 (20) [22] | 2.84 (20) [18] | 1.34 (19) [93] | |
| F (%) | — | — | 66.0 (19) [97] | — | — | 65.1 (15) [70] | |
Geometric mean.
Arithmetic mean.
—, not calculated.
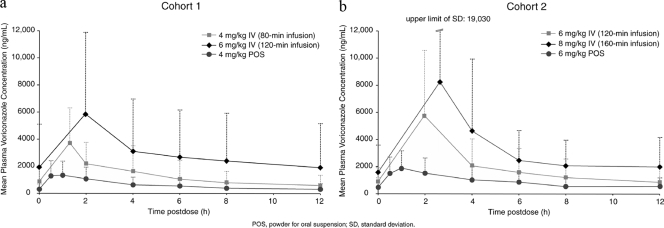
Plasma voriconazole concentrations increased in a dose-dependent manner with i.v. or oral administration. The approximately 3-fold increase in median AUCτ of i.v. voriconazole from 4 mg/kg to 8 mg/kg suggests nonlinear saturability. As expected, in all dosing periods, the mean pharmacokinetic parameters were higher in cohort 2 than in cohort 1 (Table 3 and Fig. 1). Within both cohorts, the median AUCτ values of i.v. voriconazole were similar in patients aged 2 to 5 years and patients aged 6 to 11 years, with exception of the 8-mg/kg i.v. dosage in cohort 2, where older patients demonstrated higher levels of exposure.
FIG. 1.
Mean plasma voriconazole concentrations in cohort 1 (a) and cohort 2 (b).
After oral administration of voriconazole, peak concentrations were observed between 1 and 3 h and between 30 min and 1 h after i.v. administration of voriconazole parenteral solution (Fig. 1). After oral administration of voriconazole, children aged 2 to <6 years tended to have lower peak concentrations and AUCs than those aged 6 to <12 years. The oral bioavailability of approximately 65% was similar after administration of 4-mg/kg and 6-mg/kg suspensions.
Safety evaluation. (i) All-causality nonvisual adverse events.
Table 4 describes all-causality nonvisual AEs observed in at least three patients in either cohort. There was no consistent pattern between the dosing regimen and the frequency of all-causality AEs. In addition, an analysis by voriconazole exposure (<40,000 ng·h/ml and ≥40,000 ng·h/ml) showed no consistent association between exposure and the frequency of all-causality AEs. The most commonly reported all-causality nonvisual AEs were mucositis, fever, rash, hypertension, and pruritus. Most AEs were mild to moderate, and the AE profiles were similar in the two cohorts, with the exceptions that mucositis and pruritus were more common in cohort 1 than cohort 2 and rash, edema, and epistaxis were more common in cohort 2 than cohort 1. Skin-related events (rash and pruritus) were seen in 13 patients in cohort 1 and 12 in cohort 2.
TABLE 4.
All-causality nonvisual adverse events reported for at least three patients in either cohort 1 or cohort 2
| Adverse event | No.a in cohort: |
|
|---|---|---|
| 1 (n = 24) | 2 (n = 24) | |
| Mucositis | 14 | 7 |
| Fever | 8 | 7 |
| Rash | 7 | 10 |
| Hypertension | 6 | 9 |
| Pruritus | 6 | 2 |
| Vomiting | 5 | 6 |
| Diarrhea | 5 | 4 |
| Nausea | 5 | 1 |
| Headache | 4 | 3 |
| Hyperbilirubinemia | 4 | 4 |
| Rhinitis | 3 | 2 |
| Alopecia | 3 | 1 |
| Hypomagnesaemia | 3 | 2 |
| Injection site complications | 3 | 0 |
| Edema | 2 | 5 |
| Increased cough | 2 | 3 |
| Epistaxis | 1 | 4 |
At least one all-causality adverse occurred in 24 of 24 patients in cohort 1 and in 23 of 24 patients in cohort 2.
(ii) All-causality visual adverse events and function.
As described in Table 5, seven (29%) of 24 patients in cohort 1 and two (8%) of 24 patients in cohort 2 experienced an all-causality visual AE. In both cohorts, patients <6 years old reported fewer visual AEs than patients aged 6 to ≤12 years old. The frequencies of visual AEs did not increase with increasing voriconazole exposure, as most events were reported at an AUC value of <40,000 ng·h/ml. A single patient in each cohort complained of a treatment-related visual AE. The first such patient, a 2-year-old male in cohort 1, had moderate photophobia, which was attributed to voriconazole and which lasted several days after initiation of the second i.v. treatment. The second patient, a 9-year-old female in cohort 2, experienced intermittent blurred vision, which also was attributed to voriconazole and which lasted for several days after each dosing phase. However, this patient also had a pineoblastoma and optic neuropathy at screening and a history of a left lateral visual field defect.
TABLE 5.
All-causality visual adverse events and their attribution assigned by investigator
| Cohort | Age (yr) | Gender | Visual adverse event(s) | Investigator-assigned attribution(s) |
|---|---|---|---|---|
| 1 | 2 | Male | Mild dry eyes, moderate photophobia | Concomitant chemotherapy, voriconazole |
| 4 | Male | Mild dry eyes, moderate eye hemorrhage | Total body irradiation, thrombocytopenia | |
| 5 | Male | Mild papilledema | Concomitant retinoid treatment | |
| 9 | Male | Moderate conjunctivitis | Bone marrow transplant conditioning treatment | |
| 9 | Male | Mild conjunctivitis | Air conditioning system | |
| 11 | Female | Moderate ocular hemorrhage, moderate eye pain, moderate eye pain | Thrombocytopenia, periorbital swelling, eyelash caught in eye | |
| 11 | Male | Moderate papilledema | Concomitant cyclosporine treatment | |
| 2 | 9 | Female | Mild worsening of blurred vision | Worsening of preexisting condition (considered not to be related to study drug and resolved spontaneously) |
| 9 | Female | Moderate blurred vision | Voriconazole |
Among the 17 patients who had visual acuity data at baseline and at the end of therapy or follow-up visit, one patient from cohort 1 and three patients from cohort 2 experienced deterioration. In the opinion of the ophthalmologist, visual acuity deterioration was related to “tiredness” (lack of cooperation with the exam) or underlying illness in two of the patients, while no relevant comments were provided for the two remaining patients. All fixation tests were normal. There was only one patient, the aforementioned 9-year-old female with pineoblastoma in cohort 2, whose normal baseline color vision test became abnormal, in the left eye, at the 1-month follow-up visit. Eight patients, six in cohort 1 and two in cohort 2, with a normal baseline funduscopy test result returned an abnormal result after receiving the study drug, but in each case the abnormality was attributed to underlying conditions. Compared with baseline, no patients had changes in their color vision tests at EOT or at the 1-month follow-up visit.
(iii) Serious adverse events.
Eight patients experienced a serious AE during treatment with voriconazole, none of which were assessed by the investigators as treatment related. Ten patients experienced a posttherapy serious AE within 30 days of the last dose of study drug. The minimum AUC value of 875 ng·h/ml in the <40,000 ng·h/ml category and the maximum AUC value of 178,000 ng·h/ml in the ≥40,000 ng·h/ml category both occurred in patients treated in cohort 1. The maximum AUC value was observed in a 7-year-old male patient who received chemotherapy in preparation for HSCT. While the patient experienced a serious AE of worsening pericardial effusion, this was thought to be related to the immunosuppressive regimen. This patient completed the study.
(iv) Treatment-related adverse events and study discontinuations.
The most common drug-related AEs were increased cyclosporine concentrations and increased gamma glutamyl transpeptidase (GGTP) levels; visual disturbances were uncommon. Four patients (16.6%) from cohort 1 and nine patients (37.5%) from cohort 2 experienced a treatment-related AE (P = 0.19). Of these, two in cohort 1 and five in cohort 2 had hepatic-related events. Eleven patients had mild to moderate treatment-related AEs, while two in cohort 2 experienced severe treatment-related AEs. One, a 5-year-old male, had a serum GGTP concentration increase after 8-mg/kg i.v. dosing. The GGTP values decreased after completion of voriconazole, but at the 1-month follow-up visit they were still abnormal. The second, a 9-year-old girl, experienced severe pruritus on day 4 when she received an i.v. dose of 6 mg/kg. Despite this AE, her dose was escalated to 8 mg/kg according to the study protocol, and the event resolved on day 7.
A single patient from each cohort discontinued study drug due to treatment-related AEs. In cohort 1, a 2-year-old male with normal serum hepatic enzymes and bilirubin at baseline discontinued study drug after 4 days of 4-mg/kg oral suspension due to a voriconazole-related increase of total bilirubin, AST, ALT, and GGTP. At the 1-month follow-up visit, all had decreased to slightly above normal values. In cohort 2, a 2-year-old male with elevated ALT and GGTP values at baseline discontinued the study drug on day 15 of treatment after 5 days of 6-mg/kg oral suspension, due to voriconazole-related hepatic transaminase elevation. At follow-up on day 46, the ALT value had returned to baseline, while the GGTP value was below that recorded at baseline. Three more patients from cohort 1 and five patients from cohort 2 discontinued for reasons not related to the study drug. Among these eight patients, five patients withdrew consent; one patient discontinued on day 12 because of tonic seizure, right hemiparesis, and lethargy thought to be related to cyclosporine therapy; one patient with acute myelogenous leukemia and septic shock discontinued after 14 days of 6-mg/kg i.v. voriconazole due to hyperbilirubinemia that was considered to be related to the underlying illness; and the last patient discontinued during the nonpharmacokinetic period because her neutrophil count improved sufficiently to warrant discontinuation of voriconazole therapy.
DISCUSSION
Initial pharmacokinetic studies in children demonstrated that the standard adult dosage of 4 mg/kg q12 h of i.v. voriconazole resulted in approximately 3-fold-lower plasma exposures in pediatric patients than in adults. Thus, a subsequent pharmacokinetic study of higher dosages was critically needed in order to understand the dosage of i.v. voriconazole in pediatric patients that would approach the median adult plasma exposure associated with the 4-mg/kg dosage that was effective in treatment of invasive aspergillosis (9). This multicenter study of the safety, tolerability, and plasma pharmacokinetics of the parenteral formulation of voriconazole in immunocompromised pediatric patients is to our knowledge the first systematic investigation to study dosages of >4 mg/kg of i.v. voriconazole in children. This report also describes the first assessment of the plasma pharmacokinetics of the pediatric oral suspension of voriconazole.
The elimination of voriconazole in children was previously found to be linear over the dosage range of 3 mg/kg q12h i.v. and 4 mg/kg q12h i.v. (24). This current study was designed to determine the plasma pharmacokinetics, safety, and tolerability of i.v. voriconazole in dosages from 4 mg/kg q12h to 8 mg/kg q12h, as well as to understand the pharmacokinetic properties of the pediatric oral suspension. As previous studies of voriconazole have demonstrated marked interindividual variation in plasma pharmacokinetic parameters but comparatively minimal intraindividual variation, dosage escalation was conducted within patients in this current study. Intrapatient dosage escalation also permits the patient to serve as his or her own control for the variables of body weight and CYP2C19 genotype. This approach permits a more reliable analysis of differences of plasma pharmacokinetics across the dosages 4 to 8 mg/kg q12h i.v. The same study design also allowed patients to serve as their own controls for analysis of bioavailability of the oral formulation.
The clearance of voriconazole varies depending upon the allelic polymorphisms of CYP2C19. Single-nucleotide polymorphisms in the gene encoding the protein of CYP2C19 result in two phenotypes: poor metabolizers (PMs) and extensive metabolizers (7, 12). Extensive metabolizers are further classified into homozygous (EM) and heterozygous (HEM) populations. Approximately 15 to 20% of the Asian population is comprised of PMs, while only 3 to 5% of the Caucasian and African human population display this phenotype. While the CYP2C19 genotype is the most important determinant of voriconazole clearance and a major factor in interpatient variability, this genotypic classification does not adequately account for the differences observed in drug exposure and clearance between pediatric and adult patients. The greater clearance of voriconazole in pediatric patients may be better explained by the increased ratio of hepatic to total body mass in children or perhaps by age-related differences in expression of CYP2C19 enzymes.
The study reported here demonstrates that over the range of 4 mg/kg q12h i.v. and 8 mg/kg q12h i.v., the elimination of voriconazole in children is nonlinear. These findings compare with those of adults, where the elimination of voriconazole follows Michaelis-Menten-type saturation plasma pharmacokinetics over the dosage range of 3 and 4 mg/kg. As patients in this study demonstrated wide intersubject variation and may not have achieved steady state in all cases, calculation of Km and Vmax for characterization of the nonlinear saturation pharmacokinetic properties of voriconazole in this population would not have been accurate.
By noncompartmental analysis, the mean AUC achieved in adults following the currently recommended dosage of 4 mg/kg was approximated in children receiving 8 mg/kg i.v. Within both cohorts, the pharmacokinetic parameters of voriconazole after i.v. dosing were similar in patients aged 2 to 5 years and in patients aged 6 to 11 years; however, the older patients receiving 8 mg/kg in cohort 2 demonstrated somewhat higher levels of plasma exposures, perhaps related to diminished clearance in the older population. Nevertheless, these pharmacokinetic observations are consistent with those of the earlier pediatric study that examined the pharmacokinetics of i.v. voriconazole administered at 3 mg/kg b.i.d. and 4 mg/kg b.i.d.; that study demonstrated no significant differences in Cmax or AUC between the 2- to 5-year and the 6- to 11-year age groups. After oral dosing, older patients in the current study also had higher median AUCs and Cmaxs than did younger patients in both cohorts.
Voriconazole in adults is well absorbed, with bioavailability reaching 96% (17). In this study, however, the bioavailability was only approximately 66%, and there were no differences between younger and older patients. The reasons for lower bioavailability in children compared with adults are incompletely characterized but may be related to developmental differences in the activity of intestinal drug-metabolizing enzymes and efflux transporters.
The current study was conducted in immunocompromised children in whom there was a high risk of developing invasive fungal infection, which was predominantly associated with treatment of the underlying neoplastic diseases with cytotoxic chemotherapy or hematopoietic stem cell transplantation. The number and type of all-causality AEs were typical for this population; the AE profile was consistent with their underlying conditions and concomitant treatments and was similar to that seen in neutropenic adults. The types of AEs observed in this study were similar to those in the earlier pediatric and adult studies of voriconazole, where most events occurred in three categories: hepatic, cutaneous, and visual.
Abnormalities in hepatic transaminases are known side effects of voriconazole and have been observed in 12 to 20% of patients in previous studies (23). When all-causality liver events were analyzed, there were no differences between cohorts, nor were they different from those observed in the previous pediatric pharmacokinetic study (24). Moreover, when liver-associated laboratory abnormalities, including increased concentrations of serum hepatic transaminases and bilirubin, are analyzed, their occurrence was the same in both dosage cohorts and the frequency similar to that seen in immunocompromised adults treated with voriconazole (9, 14, 17, 26).
Although there were more patients with treatment-related AEs in cohort 2 than in cohort 1 (9 versus 4), the number of AEs was only slightly different (14 versus 11, respectively). These differences were primarily associated with AEs related to the liver (increased hepatic enzymes, hyperbilirubinemia, and jaundice; 8 versus 5 events, respectively) and to the skin (pruritis and rash; 2 versus 0 events, respectively). When the distribution of AEs was analyzed as a function of plasma exposure of voriconazole (<40,000 ng·h/ml versus ≥40,000 ng·h/ml), the distribution of hepatic AEs was not different. This finding is consistent with a previous analysis of 1,053 adults who had received standard dosages of voriconazole and who demonstrated only a weak correlation between voriconazole plasma concentrations and AST, alkaline phosphatase, or bilirubin but not ALT abnormalities (23).
With the exception of cases of pruritus and rash in two patients in cohort 2 that were attributed by the investigator to voriconazole, the other cutaneous-related events were related to concomitant therapies, other illness, or to graft-versus-host disease. The relatively high frequency of cutaneous-related AEs in this study population, where approximately one-third of patients were suffering from graft-versus-host disease and receiving multiple concomitant medications, is expected. Rash is a known adverse reaction to voriconazole. The patterns of cutaneous reactions to voriconazole include a solar hypersensitivity that is well described in an ambulatory population (4) but seldom observed among inpatients (26). Consistent with these earlier observations, only 2 of 24 cutaneous AEs in this study were associated primarily with voriconazole treatment.
In order to thoroughly determine the possible ocular effects of voriconazole in pediatric patients, this study included a robust range of visual function tests. Due to the young age of the patients and the severity of disease in this population, there were difficulties in completing all of the visual function tests, particularly the visual field, visual acuity, and fixation tests. Hence, an insufficient number of patients underwent the visual field test to produce meaningful results. In addition, full visual acuity data, including baseline and EOT or 1-month follow-up tests, were available for only 17/48 patients, and full fixation test results were collected for 26/48 patients. However, based on the wide range of visual tests performed in this study, there was no evidence that voriconazole treatment affected the developing eye.
In this study, there were seven patients (29%) in cohort 1 and two (8%) in cohort 2 with all-causality visual AEs but only two (4%) of 48 patients (one patient in each cohort) with treatment-related visual AEs. While voriconazole may have contributed to a decline in visual acuity, other concomitantly administered agents, such as opiates for pain control, and lack of cooperation in this pediatric oncology population also may have contributed to impaired visual acuity. Supporting this possibility was the lack of a relationship between increasing voriconazole exposure and development of visual AEs. Of those patients whose visual function tests changed from baseline during the study, most changes could be attributed to underlying conditions. These findings are consistent with the previous pediatric pharmacokinetic study in which 3 (11%) of 28 patients experienced five voriconazole-related visual disturbances (eye pain, itchy eyes, photophobia, blurred vision, and strabismus) (24). Although the frequencies of visual AEs in this study and in adults are similar, there were no well-documented episodes of photopsia in the children studied here. By comparison, photopsia constitutes the majority of visual AEs in adults. This difference may be the result of underreporting of photopsia by the children in this study. Nonetheless, all visual events in this study and in the previous prospective trial were transient and resolved without intervention.
Assessing attribution of ophthalmic, functional, and funduscopic abnormalities in immunocompromised children is challenging. While photopsia and visual hallucinations are well described in adults (26, 29), these complaints are not as commonly reported in children. This may be related in part to difficulty in verbally describing these events in a younger population. For example, symptoms of photophobia may overlap with those of photopsia. Yet another challenge is the ability to discern common ophthalmic problems, such as conjunctivitis, and nonspecific funduscopic changes, such as papilledema and retinal hemorrhages, which may occur during antineoplastic therapy or HSCT, from potential study drug effects. The fact that there have been no consistent patterns of ophthalmic, funduscopic, or visual defects observed in adults over time, other than photopsia and visual hallucinations, provides some cautious guide to interpreting attribution in children. The mechanism of photopsia appears to be a transient neurochemical effect, while the mechanism of visual hallucinations appears to be directly related to serum concentrations (29). Recognizing the challenges of assessing attribution and considering the wide range of visual tests performed in this study, there appears to be no evidence that voriconazole treatment affected the developing eye.
There is increasing recognition of the importance of distinguishing the pharmacokinetic characteristics of compounds in children and adults (6). As with other pharmacokinetic studies of voriconazole in adults and children, this study showed high levels of interpatient variability in pharmacokinetic parameters (6, 17, 18, 24). Due to this high interpatient variability and nonlinear pharmacokinetic profile, noncompartmental analysis may not be appropriate for dosing recommendations for voriconazole. Therefore, the pharmacokinetic data from this study and two other pediatric pharmacokinetic studies were investigated further in a population-based pharmacokinetic analysis. Based on an overall assessment of these aggregate data, a population-based pharmacokinetic analysis, and the risk-benefit analysis of unpredictably elevated circulating concentrations, the European Agency for the Evaluation of Medicines (EMEA) advocated the dosage of 7 mg/kg q12h i.v. voriconazole for life-threatening infections for children aged 2 to 12 years.
The fact that this study and the earlier study of lower dosages of 3 and 4 mg/kg (24) enrolled predominantly Caucasian children is a limitation that warrants caution in dosage of children of Asian ancestry. Both the descriptive pharmacokinetics reported in the present study and their model-based analysis (13) are based upon a mostly non-Asian patient population. As children with Asian backgrounds may display a poor metabolizer phenotype, careful therapeutic drug monitoring may be especially important in this population in order to avoid potentially toxic serum concentrations.
Underscoring the need for accurate dosing of voriconazole in immunocompromised children, Neeley and colleagues identified a pharmacodynamic association between a voriconazole trough of >1,000 ng/ml and survival (15). The fact that the pharmacokinetics of voriconazole in children is characterized by wide interpatient variation (13, 15, 24) warrants the use of therapeutic drug monitoring in patients with invasive aspergillosis and other life-threatening invasive fungal infections in order to attain therapeutic levels while avoiding toxicity (1).
In summary, this report describes the plasma pharmacokinetics of higher dosages of voriconazole needed to treat an immunocompromised pediatric patient population with exposures comparable to those of adult patients. In order to attain exposure of voriconazole in plasma comparable to that achieved with the 4-mg/kg i.v. dosage in adults, children aged 2 to 11 years old would need a dosage approaching 8 mg/kg. The study also demonstrates that the oral bioavailability of voriconazole in children is much lower than that in adults, suggesting the need for higher weight-adjusted oral dosages than those used for i.v. treatment. However, due to very high interpatient variability and the nonlinear pharmacokinetic profile of voriconazole, formal dosing recommendations cannot be based solely on noncompartmental analysis. Instead, a population-based pharmacokinetic model should guide such formal dosing recommendations. Nevertheless, when combined with an overall favorable safety profile indicating the absence of dose-dependent toxicity and the current exposure profiles, the current data reported here indicate that a dosage of voriconazole of approximately 8 mg/kg i.v. provides comparable and safe exposure in immunocompromised pediatric patients.
Footnotes
Published ahead of print on 26 July 2010.
REFERENCES
- 1.Andes, D., A. Pascual, and O. Marchetti. 2009. Antifungal therapeutic drug monitoring: established and emerging indications. Antimicrob. Agents Chemother. 53:24-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boucher, H. W., A. H. Groll, C. C. Chiou, and T. J. Walsh. 2004. Newer systemic antifungal agents: pharmacokinetics, safety and efficacy. Drugs 64:1997-2020. [DOI] [PubMed] [Google Scholar]
- 3.Burgos, A., T. E. Zaoutis, C. C. Dvorak, J. A. Hoffman, K. M. Knapp, J. J. Nania, P. Prasad, W. J. Steinbach. 2008. Pediatric invasive aspergillosis: a multicenter retrospective analysis of 139 contemporary cases. Pediatrics 121:e1286-e1294. [DOI] [PubMed] [Google Scholar]
- 4.Denning, D. W., and C. E. Griffiths. 2001. Muco-cutaneous retinoid-effects and facial erythema related to the novel triazole antifungal agent voriconazole. Clin. Exp. Dermatol. 26:648-653. [DOI] [PubMed] [Google Scholar]
- 5.Ghannoum, M. A., and D. M. Kuhn. 2002. Voriconazole—better chances for patients with invasive mycoses. Eur. J. Med. Res. 7:242-256. [PubMed] [Google Scholar]
- 6.Ginsberg, G., D. Hattis, B. Sonawane, A. Russ, P. Banati, M. Kozlak, S. Smolenski, and R. Goble. 2002. Evaluation of child/adult pharmacokinetic differences from a database derived from the therapeutic drug literature. Toxicol. Sci. 66:185-200. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein, J. A., and S. M. F. de Morais. 1994. Biochemistry and molecular biology of the human CYP2C subfamily. Pharmacogenetics 4:285-299. [DOI] [PubMed] [Google Scholar]
- 8.Groll, A. H., M. Kurz, and W. Schneider. 1999. Five-year-survey of invasive aspergillosis in a paediatric cancer centre. Epidemiology, management and long-term survival. Mycoses 42:431-442. [DOI] [PubMed] [Google Scholar]
- 9.Herbrecht, R., D. W. Denning, T. F. Patterson, J. E. Bennett, R. E. Greene, J. W. Oestmann, et al. 2002. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N. Engl. J. Med. 8:347:408-415. [DOI] [PubMed] [Google Scholar]
- 10.Hilliard, T., S. Edwards, R. Buchdahl, J. Francis, M. Rosenthal, I. Balfour-Lynn, A. Bush, and J. Davies. 2005. Voriconazole therapy in children with cystic fibrosis. J. Cystic Fibrosis 4:215-220. [DOI] [PubMed] [Google Scholar]
- 11.Hovi, L., U. M. Saarinen-Pihkala, K. Vettenranta, and H. Saxen. 2000. Invasive fungal infections in pediatric bone marrow transplant recipients: single center experience of 10 years. Bone Marrow Transplant. 26:999-1004. [DOI] [PubMed] [Google Scholar]
- 12.Hyland, R., B. C. Jones, and D. A. Smith. 2003. Identification of the cytochrome P450 enzymes involved in the N-oxidation of voriconazole. Drug Metab. Dispos. 31:540-547. [DOI] [PubMed] [Google Scholar]
- 13.Karlsson, M. O., I. Lutsar, and P. A. Milligan. 2009. Population pharmacokinetic analysis of voriconazole plasma concentration data from pediatric studies. Antimicrob. Agents Chemother. 53:935-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mouas, H., I. Lutsar, B. Dupont, O. Fain, R. Herbrecht, F. X. Lescure, and O. Lortholary. 2005. Voriconazole for invasive bone aspergillosis: a worldwide experience of 20 cases. Voriconazole/Bone Invasive Aspergillosis Study Group. Clin. Infect. Dis. 40:1141-1147. [DOI] [PubMed] [Google Scholar]
- 15.Neely, M., T. Rushing, A. Kovacs, R. Jelliffe, and J. Hoffman. 2010. Voriconazole pharmacokinetics and pharmacodynamics in children. Clin. Infect. Dis. 50:27-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfizer Inc. 2006. Voriconazole package insert. Pfizer Inc., New York, NY.
- 17.Purkins, L., N. Wood, P. Ghahramani, K. Greenhalgh, M. J. Allen, and D. Kleinermans. 2002. Pharmacokinetics and safety of voriconazole following intravenous- to oral-dose escalation regimens. Antimicrob. Agents Chemother. 46:2546-2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Purkins, L., N. Wood, K. Greenhalgh, M. D. Eve, S. D. Oliver, and D. Nichols. 2003. The pharmacokinetics and safety of intravenous voriconazole—a novel wide-spectrum antifungal agent. Br. J. Clin. Pharmacol. 56(Suppl. 1):2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosen, G. P., K. Nielsen, S. Glenn, J. Abelson, J. Deville, and T. B. Moore. 2005. Invasive fungal infections in pediatric oncology patients: 11-year experience at a single institution. J. Pediatr. Hematol. Oncol. 27:135-140. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz, S., M. Ruhnke, P. Ribaud, L. Corey, T. Driscoll, O. A. Cornely, U. Schuler, I. Lutsar, P. Troke, and E. Thiel. 2005. Improved outcome in central nervous system aspergillosis, using voriconazole treatment. Blood 106:2641-2645. [DOI] [PubMed] [Google Scholar]
- 21.Steinbach, W. J., and T. J. Walsh. 2006. Mycoses in pediatric patients. Infect. Dis. Clin. North Am. 20:663-678. [DOI] [PubMed] [Google Scholar]
- 22.Stopher, D. A., and R. Gage. 1997. Determination of a new antifungal agent, voriconazole, by multidimensional high-performance liquid chromatography with direct plasma injection onto a size-exclusion column. J. Chromatogr. B Biomed. Sci. Appl. 691:441-448. [DOI] [PubMed] [Google Scholar]
- 23.Tan, K., N. Brayshaw, K. Tomaszewski, P. Troke, and N. Wood. 2006. Investigation of the potential relationships between plasma voriconazole concentrations and visual adverse events or liver function test abnormalities. J. Clin. Pharmacol. 46:235-243. [DOI] [PubMed] [Google Scholar]
- 24.Walsh, T. J., M. O. Karlsson, T. Driscoll, A. G. Arguedas, P. Adamson, X. Saez-Llorens, A. J. Vora, A. C. Arrieta, J. Blumer, I. Lutsar, P. Milligan, and N. Wood. 2004. Pharmacokinetics and safety of intravenous voriconazole in children after single- or multiple-dose administration. Antimicrob. Agents Chemother. 48:2166-2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walsh, T. J., I. Lutsar, T. Driscoll, B. Dupont, M. Roden, P. Ghahramani, M. Hodges, A. H. Groll, and J. R. Perfect. 2002. Voriconazole in the treatment of aspergillosis, scedosporiosis and other invasive fungal infections in children. Pediatr. Infect. Dis. J. 21:240-248. [DOI] [PubMed] [Google Scholar]
- 26.Walsh, T. J., P. Pappas, D. J. Winston, H. M. Lazarus, F. Petersen, J. Raffalli, S. Yanovich, P. Stiff, R. Greenberg, G. Donowitz, J. Lee, M. Schuster, A. Reboli, J. Wingard, C. Arndt, J. Reinhardt, S. Hadley, R. Finberg, M. Laverdiere, J. Perfect, G. Garber, G. Fioritoni, and E. Anaissie. 2002. Voriconazole compared with liposomal amphotericin B for empirical antifungal therapy in patients with neutropenia and persistent fever. N. Engl. J. Med. 346:225-234. [DOI] [PubMed] [Google Scholar]
- 27.Zaoutis, T. E., S. E. Coffin, J. H. Chu, K. Heydon, H. M. Greves, and T. J. Walsh. 2005. Risk factors for mortality in children with candidemia. Pediatr. Infect. Dis. 24:736-739. [DOI] [PubMed] [Google Scholar]
- 28.Zaoutis, T. E., K. Heydon, J. H. Chu, T. J. Walsh, and W. J. Steinbach. 2006. Epidemiology, outcomes, and costs of invasive aspergillosis in immunocompromised children in the United States, 2000. Pediatrics 117:e711-e716. [DOI] [PubMed] [Google Scholar]
- 29.Zonios, D. I., J. Gea-Banacloche, R. Childs, and J. E. Bennett. 2008. Hallucinations during voriconazole therapy. Clin. Infect. Dis. 47:e7-e10. [DOI] [PMC free article] [PubMed] [Google Scholar]