Abstract
This study was undertaken to characterize the pharmacokinetics and bioavailability of voriconazole in adult lung transplant patients during the early postoperative period, identify factors significantly associated with various pharmacokinetic parameters, and make recommendations for adequate dosing regimens. Thirteen lung transplant patients received two intravenous infusions (6 mg/kg, twice daily [b.i.d.]) immediately posttransplant followed by oral doses (200 mg, b.i.d.) for prophylaxis. Blood samples (9/interval) were collected during one intravenous and one oral dosing interval from each patient. Voriconazole plasma concentrations were measured by high-pressure liquid chromatography (HPLC). NONMEM was used to develop pharmacokinetic models, evaluate covariate relationships, and perform Monte Carlo simulations. There was a good correlation (R2 = 0.98) between the area under the concentration-time curve specific for the dose evaluated (AUC0-∞) and trough concentrations. A two-compartment model adequately described the data. Population estimates of bioavailability, clearance, Vc, and Vp were 45.9%, 3.45 liters/h, 54.7 liters, and 143 liters. Patients with cystic fibrosis (CF) exhibited a significantly lower bioavailability (23.7%, n = 3) than non-CF patients (63.3%, n = 10). Bioavailability increased with postoperative time and reached steady levels in about 1 week. Vp increased with body weight. Bioavailability of voriconazole is substantially lower in lung transplant patients than non-transplant subjects but significantly increases with postoperative time. CF patients exhibit significantly lower bioavailability and exposure of voriconazole and therefore need higher doses. Intravenous administration of voriconazole during the first postoperative day followed by oral doses of 200 mg or 400 mg appeared to be the optimal dosing regimen. However, voriconazole levels should be monitored, and the dose should be individualized based on trough concentrations as a good measure of drug exposure.
Invasive aspergillosis is one of the most severe complications after transplantation (22), with a mortality rate as high as 88.1% (19). Voriconazole (V-Fend), a triazole antifungal agent, has become the treatment of choice for invasive aspergillosis (14, 31). The standard use of a prophylactic voriconazole regimen immediately after transplant in lung transplant patients at our institution offered the opportunity to assess voriconazole pharmacokinetics in this unique patient population.
There is large inter- and intraindividual variability in plasma concentrations of voriconazole regardless of the route of administration or the type of patient population (29). Low voriconazole exposure is associated with poor outcomes in patients with aspergillosis (7, 11, 23, 25-27), while high voriconazole exposure is associated with an increased risk for toxicity, including visual disturbances, elevated transaminase levels, central nervous system disorders (e.g., encephalopathy), and electrolyte disturbances (4, 7, 26, 28). Therefore, it is important to optimize the use and exposure of voriconazole.
The bioavailability of voriconazole after oral administration is 96% in non-transplant populations (24). However, gastrointestinal complications observed after transplant surgery (3, 5, 18, 33) may cause clinically significant lower bioavailability of voriconazole. Therefore, it is important to understand bioavailability of voriconazole in transplant patients.
To date, the bioavailability of voriconazole in transplant patients and the pharmacokinetics of voriconazole in lung transplant patients have not been reported. There is limited information on pharmacokinetics of voriconazole in other transplant populations (13, 15). Population approaches were used previously to investigate the pharmacokinetics of voriconazole in a limited number of studies (13, 17, 20, 21, 32).
The objectives of this prospective single-center observational study of voriconazole were to characterize the pharmacokinetics and bioavailability of voriconazole in lung transplant patients in early postoperative period, to determine the extent of interindividual variability in pharmacokinetics of voriconazole, to identify factors significantly associated with pharmacokinetic parameters, and to make recommendations for adequate dosing regimens.
MATERIALS AND METHODS
Patients.
The protocol was approved by the institutional review board at the University of Pittsburgh. Lung transplant recipients who were started on a voriconazole prophylactic regimen immediately after transplant as part of their standard clinical care and who gave informed consent were enrolled in this prospective study regardless of the reason for transplant. Two intravenous doses were administered first as a 2-hour intravenous infusion (6 mg/kg, twice daily [b.i.d.]) followed by oral doses (200 mg, b.i.d.) for a duration of 3 months posttransplant. The exclusion criteria were as follows: age under 18, coadministration of medications known to influence voriconazole pharmacokinetics, administration of voriconazole to treat an active fungal infection, pretransplant voriconazole administration, and voriconazole dosing regimens other than that associated with fixed oral dosage. Complete dosing history, demographic data, laboratory tests, and current medication use were recorded. All patients received tacrolimus as their primary immunosuppressive agent.
Blood sampling and analytical assay.
Serial blood samples (7 ml) were collected within one intravenous and one oral dosing interval from each patient. The sampling time was just prior to (0 h) and at 0.5, 1, 1.5, 2, 4, 6, 8, and 12 h following the second intravenous dose and following administration of a minimum of 5 oral doses (range from the 5th to 37th dose; mean, 15th dose). Blood samples were processed and analyzed for voriconazole plasma concentration using a validated high-pressure liquid chromatography (HPLC) method described previously (15). The assay precision (intraday and interday variability) was 1.3% to 9.0% (0.2 to 9 μg/ml), and the assay bias (deviation from actual value) was 0.7% to 3.1% (0.5 to 9 μg/ml). The linearity range was 0.2 to 9 μg/ml (R2 = 0.9998).
Noncompartmental pharmacokinetic analysis.
The difference between trough concentrations prior to oral dosing (C0) and at 12 h after oral dosing (C12) was tested using a paired two-tailed Student t test to confirm attainment of steady state. Area under the plasma concentration-versus-time curve specific for the dose evaluated (AUC0-∞) was calculated using the trapezoid rule and reverse superposition principle. Time to peak concentration (Tmax) and peak plasma concentrations (Cmax) were directly read off the concentration-versus-time profiles.
Population pharmacokinetic analysis.
A nonlinear mixed-effects pharmacokinetic model (base model) was developed using NONMEM 6.2.0 (GloboMax, Hanover, MD) using the first-order conditional estimation method with interaction. Correlations between pharmacokinetic parameters were always incorporated and estimated. One- and two-compartment models were tested with first- and zero-order elimination and a Michaelis-Menten elimination process, since nonlinear pharmacokinetics of voriconazole has been reported (24). Interindividual variability was described using exponential model Pij = TV(Pj) × eηij, where Pij is the ith individual's estimate of the jth pharmacokinetic parameter, TV(Pj) is the typical value of the jth pharmacokinetic parameter, and ηij is a random variable for the ith individual and the jth pharmacokinetic parameter distributed with a mean of zero and a variance of ωj2. Residual variability was described using combined error model Cobs = Cpred × (1 + ɛ) + ɛ′, where Cobs and Cpred are the observed and predicted concentrations, respectively, and ɛ and ɛ′ are normal random variables with means of zero and variances of σ2 and σ′2, respectively. The adequacy of fitting was examined by plotting predicted versus observed concentrations (goodness of fit), concentrations versus time profiles and weighted residuals versus predicted concentrations.
Covariate relationship exploration.
Association between patient variables and pharmacokinetic parameters were first visually evaluated by plotting empirical Bayes estimates (EBE) against patient variables. Patient variables were then incorporated into the base model one at a time using at least 13 approaches to associate the patient variable with the parameter. A patient variable was considered significant if all the following criteria were met: (i) a decrease in objective function value (OFV) of 6.63 for 1 degree of freedom (P < 0.01), (ii) no significant trend in EBE-versus-patient variables plots, (iii) improved goodness of fit, (iv) reduced interindividual variability, and (v) clinical plausibility for incorporating the patient variable.
Precision of parameter estimation, stability of the covariate models, and normality of the distribution of the parameter estimates were evaluated using bootstrapping (resampling repeated 3,500 times) using Wings for NONMEM (http://wfn.sourceforge.net). Nonparametric statistics (median and 95% confidence interval) of parameter estimates were obtained from bootstrapping.
Monte Carlo simulations.
Voriconazole concentration-versus-time profiles in patients with and without cystic fibrosis (CF) (200 mg, oral, b.i.d.) were simulated using NONMEM to demonstrate that CF patients may exhibit significantly lower exposure of voriconazole than non-CF patients and that CF patients may experience underexposure of voriconazole, with trough concentrations of <1 μg/ml. The simulation procedure is based on drawing random samples for each of the pharmacokinetic parameters from their statistical distributions, reflecting interindividual variability. Every random draw generates a parameter set that characterizes the pharmacokinetics of a “virtual” subject and is subsequently used to generate the concentration-versus-time profile of this virtual subject. A total of 1,500 virtual CF subjects and 1,500 virtual non-CF subjects were simulated using this procedure. This simulation ensemble closely matches the original population statistics. Concentration-versus-time profiles of the virtual populations were summarized and compared by their median and 5% and 95% percentiles (90% prediction interval). The width of the 90% prediction interval reflects the degree of interindividual variability in the original population.
In order to illustrate voriconazole exposure in different clinical scenarios and thus make clinical recommendations for adequate dosing regimens, voriconazole concentration-versus-time profiles were simulated for five hypothetical dosing regimens (b.i.d.): oral administration only (200, 400, and 600 mg) or combined administration of two doses of a 2-hour intravenous infusion (6 mg/kg) followed by oral administration (200 mg and 400 mg). A total of 1,500 virtual subjects were simulated for each regimen using the same procedure mentioned above. In addition, simulation of individual profiles was also performed using a fixed dose of 200 mg or a body weight-adjusted dose of 3 mg/kg and compared with each other, in order to evaluate whether the variability among the pharmacokinetic profiles was reduced by using a body weight-adjusted dose compared to a fixed dose.
RESULTS
Patients.
A total of 13 patients were enrolled in this study. Table 1 summarizes the characteristics of the patients, including the primary diagnosis, age, body weight, race, gender, days posttransplant on the day of the oral study, and laboratory biochemical profiles prior to transplant, immediately after transplant, and on the day of the oral study. One patient did not complete the oral study.
TABLE 1.
Characteristics of patients
| Parameter | Resulta |
|---|---|
| No. of patients with: | |
| Cystic fibrosis | 3 |
| Emphysema | 5 |
| Idiopathic pulmonary fibrosis | 4 |
| Scleroderma | 1 |
| Patient age (yr) | 50.9 ± 16.1 (19-70) |
| Wt (kg) | 68.0 ± 15.2 (46-91) |
| Ideal body wt (kg) | 59.6 ± 8.2 (45.5-75.3) |
| Race (Caucasian/other) | 12/0 |
| Gender (male/female) | 7/6 |
| Days posttransplant on day of oral study | 8.5 ± 4.4 (3-19) |
| Alkaline phosphatase (U/liter)b | 82.4 ± 31.8 (54-169) |
| Alanine aminotransferase (U/liter)b | 30.3 ± 8.3 (22-52) |
| Aaspartate aminotransferase (U/liter)b | 28.1 ± 14.5 (20-75) |
| Gamma-glutamyl transpeptidase (U/liter)b | 35.1 ± 19.0 (15-71) |
| Serum creatinine (mg/dl)c | 0.78 ± 0.16 (0.5-1)/0.85 ± 0.22 (0.5-1.1) |
| Creatinine clearance (ml/min)c | 85.6 ± 36.9 (55.6-177.8)/85.7 ± 40.4 (40.5-177.8) |
Values are means ± standard deviations (range) unless otherwise specified.
Value measured before the transplantation.
Shown in the format measurement within 1 day after the transplantation/measurements on the day of the oral study.
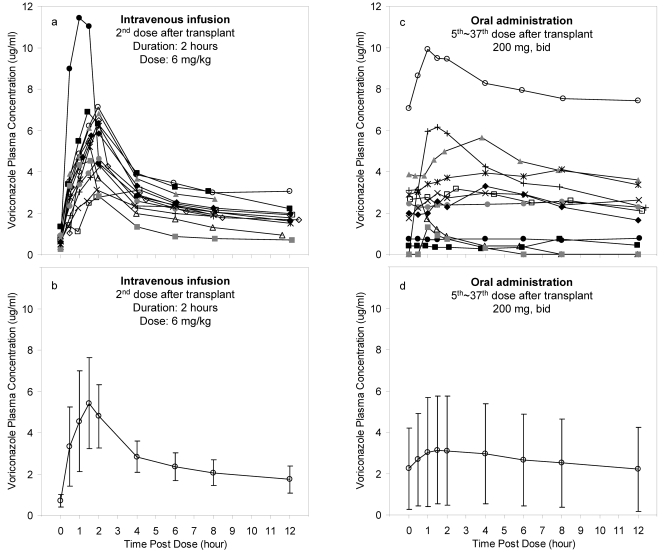
There was a wide variation in voriconazole plasma concentrations (Fig. 1). Most voriconazole plasma concentrations (72.5%) were maintained within 1 to 6 μg/ml, while 17.9% and 9.7% of voriconazole plasma concentrations were below 1 μg/ml or above 6 μg/ml, respectively.
FIG. 1.
Plasma concentration-versus-time profiles of voriconazole. (a) Individual plasma concentration-versus-time profiles of voriconazole collected during an intravenous infusion dosing interval. (b) Mean plasma concentration-versus-time profiles of voriconazole with standard deviations (error bars) collected during an intravenous infusion dosing interval. (c) Individual plasma concentration-versus-time profiles of voriconazole collected during an oral dosing interval (one patient did not complete oral study). (d) Mean plasma concentration-versus-time profiles of voriconazole with standard deviations (error bars) collected during an oral dosing interval.
Noncompartmental analysis.
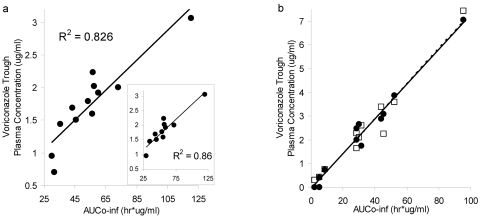
Trough concentrations (C0 and C12) were not significantly different (P = 0.82), and the difference between the trough concentrations (C12 − C0)/C12 averaged −2.7%, indicating that steady state had been reached in most of the patients at the time of the oral study. Figure 2 illustrates a good correlation between voriconazole trough plasma concentrations and the corresponding AUC0-∞ for both intravenous infusion (non-steady state, R2 = 0.86) and oral dose (steady state, R2 = 0.98). Tmax (mean ± standard deviation [SD]) for oral dose was 1.9 ± 1.3 h. Cmax (mean ± SD) for intravenous infusion and oral dose was 5.9 ± 2.2 μg/ml and 3.6 ± 2.6 μg/ml, respectively.
FIG. 2.
Correlation between AUC0-∞ and voriconazole trough plasma concentrations. (a) R2 = 0.83 when AUC0-∞ and trough concentrations (C12) were correlated during an intravenous infusion dosing interval (non-steady state). (Inset) R2 = 0.86 when a potential outlier is omitted. Two patients had very similar C12 and AUC and therefore cannot be visually separated in the figure. (b) R2 = 0.98 (dashed line) and R2 = 0.96 (solid line) when AUC0-∞ was correlated with trough concentrations (C0 [•] and C12 [□], respectively) during an oral dosing interval (steady state; one patient did not complete oral study).
Population pharmacokinetic analysis.
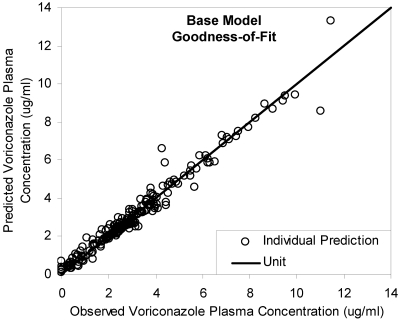
A two-compartment model with first-order absorption and elimination adequately described the data. The population estimates (interindividual) of bioavailability, clearance, volume of distribution of the central compartment (Vc) and peripheral compartment (Vp), intercompartment clearance (Q), and absorption rate constant (ka) were 45.9% (82.9%), 3.45 liters/h (107%), 54.7 liters (78.4%), 143 liters (88.3%), 22.6 liters/h (50.1%) and 0.591 h−1 (115.2%). The proportional and additive residual variability was 0.31 and 0.49 μg/ml, respectively. Individual predictions agreed well with observations (Fig. 3). Weighted residuals were approximately normally distributed and were mostly within about 2 units of the null ordinate.
FIG. 3.
Goodness-of-fit of base model. Individual predictions agreed well with observations (R2 = 0.96).
Based on the individual estimates obtained from the base model, mean bioavailability (SD) was 23.7% (19.4%, n = 3) and 63.3% (15.2%, n = 10) in CF and non-CF patients, respectively. Bioavailability was significantly lower in CF patients than non-CF patients (P = 0.0032, two-tailed Student's t test).
Covariate relationship exploration.
Among all the 12 patient variables tested (the primary diagnosis, age, body weight, race, gender, postoperative time on the day of the oral study, alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase, gamma-glutamyl transpeptidase, serum creatinine, and creatinine clearance), three were found to be significantly associated with pharmacokinetic parameters in lung transplant patients in this study: cystic fibrosis, postoperative time, and body weight.
Model 1: cystic fibrosis (CF).
The most important patient variable associated with bioavailability was CF. OFV decreased by 11.65 from −47.55 (base model) to −59.20 when CF was incorporated in bioavailability, indicating substantial model improvement (P = 0.0006). Interindividual variability in bioavailability decreased by 30.7% from 82.9% (base model) to 57.5%, while interindividual variability in other pharmacokinetic parameters did not change significantly.
The association between CF and bioavailability (F) was best described using the equation F = FCF + F′ × NCF (model 1), where FCF denotes bioavailability of CF patients, F′ denotes the difference in bioavailability between CF and non-CF patients, and NCF is 1 for non-CF patients and 0 for CF patients. Population estimates of FCF and F′ (95% confidence interval) were 10.7% (1.1% ∼ 23.4%) and 72% (35.3% ∼ 97.2%), respectively. Based on the model, bioavailability of voriconazole was significantly lower in CF patients (10.7%) than non-CF patients (82.7%), by 87%.
Model 2: postoperative time (POT).
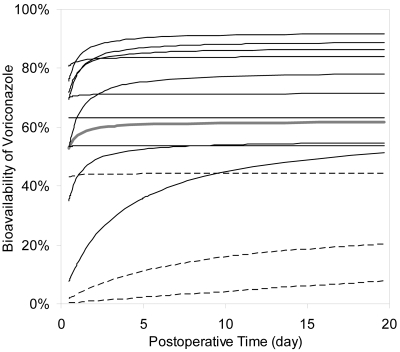
Another important factor associated with bioavailability was POT. OFV decreased by 10.94 from −47.55 (base model) to −58.49 when POT was incorporated in bioavailability, indicating substantial model improvement (P = 0.0009). The association between POT and bioavailability (F) was best described using the equation F = (Fmax × POT)/(POT + Fc) (model 2), where Fmax denotes the maximal bioavailability that can be reached in the patients in this study, and Fc is a constant. Interindividual variability was incorporated both in Fmax and Fc and estimated. Population estimates (95% confidence interval) of Fmax and Fc were 61.9% (43.5% ∼ 72.8%) and 1.97 h (0.04 ∼ 4.2), respectively. The interindividual variability of Fmax and Fc was 61.5% and 217.3%, respectively. Even the maximal bioavailability (61.9%) in lung transplant patient population was still much lower than that in non-transplant subjects (96%). The low value of Fc indicates that bioavailability would increase rapidly with POT.
According to the equation and individual parameter estimates obtained from model 2, bioavailability of voriconazole significantly and rapidly increased with POT in most of the patients and eventually reached maximal levels within 1 week posttransplant (Fig. 4). Figure 4 also shows that bioavailability was significantly lower in CF patients than non-CF patients. The large variability demonstrated in Fig. 4 is consistent with the large interindividual variability in Fmax and Fc.
FIG. 4.
Change of bioavailability of voriconazole over postoperative time (POT) in patients with and without cystic fibrosis (CF). Individual parameter estimates of bioavailability obtained from model 2 were plotted against postoperative time. Bioavailability significantly, rapidly increased with POT in most of the patients, and eventually reached the maximal level within 1 week after transplant. Bioavailability was significantly lower in CF patients (dashed line) than non-CF patients (solid line). Solid gray line, population estimates from model 2.
Model 3: body weight (WT).
Vp significantly increased with WT. OFV decreased by 7.29 from −47.55 (base model) to −54.84 when WT was incorporated in Vp, indicating substantial model improvement (P = 0.0069). Interindividual variability in bioavailability decreased by 31.9% from 88.3% (base model) to 61.2%, while interindividual variability in other pharmacokinetic parameters did not change significantly. The association between Vp and WT was best described using the equation Vp = TV(Vp) × (WT/WT̄)a (model 3), where TV(Vp) denotes the typical value of Vp in the patients in this study, i.e., the Vp in a patient with average body weight (68 kg), and a is a constant to be estimated. Population estimates of TV(Vp) and a were 148 liters (95% confidence interval, 84 ∼ 223 liters) and 3.56 (95% confidence interval, 0.43 ∼ 6.33), respectively.
Monte Carlo simulations.
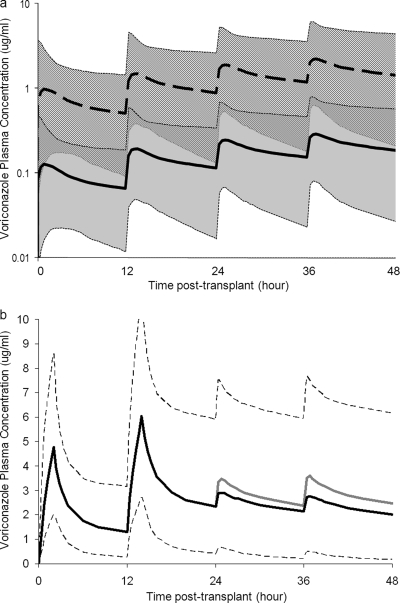
The statistical distribution of pharmacokinetic parameters and interindividual variability obtained from model 1 (see above) was used to simulate CF and non-CF virtual subjects (Fig. 5a). Median voriconazole plasma concentration and median AUC were 6.7 times higher in non-CF patients than CF patients. Furthermore, the 90% prediction interval for CF patients did not include the median concentration-versus-time profiles of non-CF patients, and vice versa. This indicates significantly lower exposure of voriconazole in CF patients than non-CF patients.
FIG. 5.
Monte Carlo simulation. (a) Simulated voriconazole concentration-versus-time profiles during the first 2 days posttransplant in lung transplant patients with and without cystic fibrosis (CF). The median simulated voriconazole concentration in CF patients (solid line) and non-CF patients (dashed line) with 90% prediction intervals of CF patients (gray shading) and non-CF patients (hatching) is displayed. Extension of the profiles beyond 2 days posttransplant is not shown. (b) Simulated voriconazole concentration-versus-time profiles (extended until steady state was reached) in lung transplant patients receiving two doses of 2-h intravenous infusion (6 mg/kg) followed by oral doses (b.i.d.). The medians of simulated voriconazole concentration with intravenous infusion followed by oral dose of 200 mg (black line) and 400 mg (gray line) are compared. Only the 90% prediction interval for intravenous infusion followed by oral dose of 200 mg (dashed line) is displayed.
The 90% prediction interval of the entire concentration-versus-time profiles (including peak levels) in CF patients remained below 1 μg/ml for the first 3 days posttransplant. The 90% prediction interval of trough concentration in CF patients remained below 1 μg/ml for the first 4 days posttransplant. Median concentration-versus-time profiles in CF patients remained below 0.5 μg/ml for the duration of the study. This indicates underexposure of voriconazole in CF patients, with trough concentrations of <1 μg/ml in 90% of the patients during the first 4 days posttransplant. In addition, the large interindividual variability is confirmed by wide 90% prediction intervals.
Statistical distributions of pharmacokinetic parameters and interindividual variability obtained from model 2 (see above) were used to simulate different dosing regimens. Median trough concentrations stayed above 1 μg/ml after the first loading dose and were maintained between 2 and 3 μg/ml at steady state when patients received two 2-hour intravenous infusions followed by oral doses (Fig. 5b). In contrast, simulation with mere oral administration (b.i.d.) at 200 mg, 400 mg, and 600 mg resulted in median trough concentrations below 1 μg/ml for the first 3.5 days, 1.5 days, and 1 day posttransplant, respectively. In addition, simulated individual profiles using a fixed dose of 200 mg or a body weight-adjusted dose of 3 mg/kg were compared with each other, and the variability among the pharmacokinetic profiles was not reduced by using a body weight-adjusted dose compared to a fixed dose, which confirmed the adequacy of fixed oral dosing regimens.
DISCUSSION
This is the first evaluation of bioavailability of voriconazole in transplant patients and the first pharmacokinetic study of voriconazole in lung transplant patients.
Prospective intense sampling (nine samples per dosing interval) in the early posttransplant period in a small group of relatively homogenous patients (n = 13) was used in this study to provide accurate and precise parameter estimation. Oral pharmacokinetic profiles of voriconazole are characterized by an early and sharp increase of voriconazole concentration, with the peak concentration being reached around 2 h after dosing. This observation is consistent with rapid absorption of voriconazole and is similar to what has been observed in non-transplant patients (24). Despite the relative homogeneity of the population studied, a large interindividual variability in voriconazole pharmacokinetics was demonstrated. This is consistent with what was previously reported (15, 29). Nonlinear pharmacokinetics was not observed in this study (a Michaelis-Menten elimination process did not improve the fit).
The large interindividual variability in voriconazole exposure has given rise to concerns about voriconazole dose management in transplant patients, especially when it results in underexposure. Unpublished results (>3,500 samples) from our research group showed that nearly 15.2% of transplant patients on recommended doses have undetectable trough concentrations, and nearly 45% of the patients have trough concentrations of <1 μg/ml. Drug underexposure may be caused by decreased absorption or increased elimination. Elimination of voriconazole is determined by liver function and cytochrome P450 polymorphism. Therefore, elimination is unlikely to increase in transplant patients. Therefore, we hypothesized that decreased bioavailability is responsible for underexposure of voriconazole in transplant patients. In this study, bioavailability of voriconazole was substantially lower during the early postoperative period in lung transplant patients (45.9%) than in non-transplant subjects (96%), likely due to gastrointestinal complications observed after transplant surgery (3, 5, 18, 33).
Furthermore, we demonstrated that bioavailability of voriconazole was significantly lower in CF patients than non-CF patients, by 87%. It is typical that the mean bioavailability calculated using individual estimates of bioavailability obtained from the base model (23.7% for CF patients and 63.3% for non-CF patients) were different from the population estimates in model 2 (10.7% for CF patients and 82.7% for non-CF patients). Unlike the mean, the population estimate is the posterior mode of the marginal likelihood distribution for that parameter value versus the objective function (i.e., the maximum likelihood point in the distribution). If the distributions are not strictly normal (log normal is enough to skew this), the mean will not equal the mode.
The low voriconazole exposure observed in patients with CF in this study agrees with the observations reported by Berge et al. (2) that voriconazole plasma concentrations were <0.5 μg/ml in over 30% of CF lung transplant patients and <1.5 μg/ml in nearly 70% of the patients. However, those authors did not perform a pharmacokinetic analysis to determine the cause of underexposure, since only trough and peak concentrations from therapeutic drug monitoring were obtained. Population pharmacokinetic analysis and Monte Carlo simulation in our study demonstrated that the reduced bioavailability in CF patients is the potential cause of underexposure.
CF is well known to cause malabsorption and reduced bioavailability of several highly lipophilic compounds, such as vitamins A, D, E, and K (9), cyclosporine (30), and ibuprofen (12). Due to its high lipophilicity and low water solubility, absorption of voriconazole is associated with digestion of fat and the subsequent formation of micelles. However, this process is severely impaired in CF patients for many reasons, including (i) pancreatic insufficiency, leading to decreased secretion of pancreatic enzymes (lipase), (ii) reduced activity of lipase due to low duodenal pH caused by decreased secretion of pancreatic bicarbonate (16) and gastric acid hypersecretion (6), (iii) precipitation of bile salts at low duodenal pH, leading to low duodenal bile salt concentration and a diminished bile salt pool (precipitated bile salts are not reabsorbed) (10), and (iv) intestinal mucosal dysfunction, alterations in the intestinal mucus layer, and accelerated intestinal transit time (8, 30).
It is important to identify factors that significantly contribute to the large inter- and intraindividual variability of voriconazole in this population by exploring associations between patient variables and pharmacokinetic parameters. The 12 patient variables tested in this study covered a wide range of values within each of the categories tested. Bioavailability increased rapidly over POT and reached maximal levels within 1 week in most of the patients (Fig. 4), probably because of improved gastrointestinal function over POT. The values of CL/F and Vd/F of voriconazole have been reported to rapidly and dramatically decrease with POT in liver transplant patients (13). The authors of that study proposed increased bioavailability with POT as the primary reason, which is partly supported by this study.
A final model was also built using a standard forward addition and reverse removal approach. However, despite the statistically significant improvement of the final model and the covariate models (models 1, 2, and 3) compared to the base model, visual inspection of the goodness-of-fit plots of the final model and covariate models showed a corrected bias of population predictions only at low concentrations. This suggested that the patient variables tested and selected in this study (CF, POT, and body weight) explain only part of the variability in the pharmacokinetics of voriconazole in lung transplant patients, while some other variables that were not collected in this study are still needed to account for the remaining variability. Future studies should collect more variables and further explore factors that are significantly associated with pharmacokinetics of voriconazole in lung transplant patients.
The large variability in voriconazole exposure following weight-adjusted or fixed doing regimens necessitates individualizing voriconazole dosing to maximize therapeutic efficacy and minimize toxicity in lung transplant patients. This is particularly important because simple efficacy measures for molds to which patient dose can be titrated are not available yet. So far there have been animal model data only for Candida, yielding a predictive pharmacodynamic parameter (AUC/MIC) and a potential target value (1), with no equivalent data for molds. However, there is a simple HPLC/UV assay available to monitor voriconazole levels. Therapeutic monitoring has been proposed (4, 7) and is currently performed at our institution with an intent to keep the trough concentration above 1 μg/ml. However, trough concentrations have never been documented as surrogate markers of voriconazole exposure in lung transplant patients.
The good correlation observed in this study between the voriconazole trough plasma concentrations and the corresponding AUC0-∞ for both intravenous infusion (non-steady state, R2 = 0.86) and oral dose (steady state, R2 = 0.98) (Fig. 2) indicates that trough concentration is a good measure of voriconazole exposure in this population.
These findings are likely to be clinically relevant. Based on Monte Carlo simulations, CF patients are very likely to experience underexposure of voriconazole and therefore need higher doses. Mere oral administration of voriconazole is likely to cause underexposure of voriconazole in lung transplant patients in the early posttransplant period, while intravenous administration during the first postoperative day followed by oral doses is likely to result in appropriate drug exposure. However, therapeutic drug monitoring of voriconazole is still necessary in lung transplant patients due to the large interindividual variability.
In conclusion, a population pharmacokinetic model was developed for voriconazole in lung transplant patients in the early postoperative period. Large interindividual variability in voriconazole pharmacokinetics was demonstrated. Bioavailability of voriconazole is substantially lower in lung transplant patients (45.9%) than non-transplant subjects (96%) but increased significantly with postoperative time, likely due to recovery of gastrointestinal functions. Exposure and bioavailability of voriconazole are significantly lower in CF patients, likely due to impaired absorption of voriconazole caused by physiological changes associated with CF. We recommend intravenous infusion (6 mg/kg) during the first postoperative day followed by oral doses (200 mg or 400 mg) as an adequate dosing regimen in lung transplant patients. Given the large variability in the pharmacokinetics and the good correlation between AUC and trough concentrations, trough concentrations should be used to individualize voriconazole dose.
Acknowledgments
This work was supported by the Clinical Pharmacokinetics Laboratory at University of Pittsburgh School of Pharmacy and by funds received from the Thomas E. Starzl Transplant Institute Young Investigator Award to B. Capitano.
We acknowledge Shimin Zhang for technical assistance.
Footnotes
Published ahead of print on 2 August 2010.
REFERENCES
- 1.Andes, D., K. Marchillo, T. Stamstad, and R. Conklin. 2003. In vivo pharmacokinetics and pharmacodynamics of a new triazole, voriconazole, in a murine candidiasis model. Antimicrob. Agents Chemother. 47:3165-3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berge, M., R. Guillemain, V. Boussaud, M. H. Pham, P. Chevalier, A. Batisse, C. Amrein, E. Dannaoui, M. A. Loriot, A. Lillo-Le Louet, and E. M. Billaud. 2009. Voriconazole pharmacokinetic variability in cystic fibrosis lung transplant patients. Transpl. Infect. Dis. 11:211-219. [DOI] [PubMed] [Google Scholar]
- 3.Bowers, L. D., and D. M. Canafax. 1984. Cyclosporine: experience with therapeutic monitoring. Ther. Drug Monit. 6:142-147. [PubMed] [Google Scholar]
- 4.Boyd, A. E., S. Modi, S. J. Howard, C. B. Moore, B. G. Keevil, and D. W. Denning. 2004. Adverse reactions to voriconazole. Clin. Infect. Dis. 39:1241-1244. [DOI] [PubMed] [Google Scholar]
- 5.Christenson, J. T., M. Schmuziger, J. Maurice, F. Simonet, and V. Velebit. 1994. Postoperative visceral hypotension the common cause for gastrointestinal complications after cardiac surgery. Thorac. Cardiovasc. Surg. 42:152-157. [DOI] [PubMed] [Google Scholar]
- 6.Cox, K. L., J. N. Isenberg, and M. E. Ament. 1982. Gastric acid hypersecretion in cystic fibrosis. J. Pediatr. Gastroenterol. Nutr. 1:559-565. [DOI] [PubMed] [Google Scholar]
- 7.Denning, D. W., P. Ribaud, N. Milpied, D. Caillot, R. Herbrecht, E. Thiel, A. Haas, M. Ruhnke, and H. Lode. 2002. Efficacy and safety of voriconazole in the treatment of acute invasive aspergillosis. Clin. Infect. Dis. 34:563-571. [DOI] [PubMed] [Google Scholar]
- 8.Eggermont, E., and K. De Boeck. 1991. Small-intestinal abnormalities in cystic fibrosis patients. Eur. J. Pediatr. 150:824-828. [DOI] [PubMed] [Google Scholar]
- 9.Ferguson, J. H., and A. B. Chang. 2009. Vitamin D supplementation for cystic fibrosis. Cochrane Database Syst. Rev. 2009(4):CD007298. [DOI] [PubMed]
- 10.Fondacaro, J. D., J. E. Heubi, and F. W. Kellogg. 1982. Intestinal bile acid malabsorption in cystic fibrosis: a primary mucosal cell defect. Pediatr. Res. 16:494-498. [DOI] [PubMed] [Google Scholar]
- 11.Freifeld, A., S. Arnold, W. Ooi, F. Chen, T. Meyer, L. J. Wheat, M. Smedema, A. Lemonte, and P. Connolly. 2007. Relationship of blood level and susceptibility in voriconazole treatment of histoplasmosis. Antimicrob. Agents Chemother. 51:2656-2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han, E. E., P. M. Beringer, S. G. Louie, M. A. Gill, and B. J. Shapiro. 2004. Pharmacokinetics of Ibuprofen in children with cystic fibrosis. Clin. Pharmacokinet. 43:145-156. [DOI] [PubMed] [Google Scholar]
- 13.Han, K., R. Bies, H. Johnson, B. Capitano, D. Blisard, S. Husain, P. K. Linden, A. Marcos, E. J. Kwak, B. Potoski, D. L. Paterson, and R. Venkataramanan. 2009. Population pharmacokinetic analysis of voriconazole in liver transplant patients. Abstracts of the ASCPT Annual Meeting. ASCPT, Washington, DC.
- 14.Herbrecht, R., D. W. Denning, T. F. Patterson, J. E. Bennett, R. E. Greene, J. W. Oestmann, W. V. Kern, K. A. Marr, P. Ribaud, O. Lortholary, R. Sylvester, R. H. Rubin, J. R. Wingard, P. Stark, C. Durand, D. Caillot, E. Thiel, P. H. Chandrasekar, M. R. Hodges, H. T. Schlamm, P. F. Troke, and B. de Pauw. 2002. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N. Engl. J. Med. 347:408-415. [DOI] [PubMed] [Google Scholar]
- 15.Johnson, H. J., K. Han, B. Capitano, D. Blisard, S. Husain, P. K. Linden, A. Marcos, E. J. Kwak, B. Potoski, D. L. Paterson, M. Romkes, and R. Venkataramanan. 2010. Voriconazole pharmacokinetics in liver transplant recipients. Antimicrob. Agents Chemother. 54:852-859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalivianakis, M., and H. J. Verkade. 1999. The mechanisms of fat malabsorption in cystic fibrosis patients. Nutrition 15:167-169. [DOI] [PubMed] [Google Scholar]
- 17.Karlsson, M. O., I. Lutsar, and P. A. Milligan. 2009. Population pharmacokinetic analysis of voriconazole plasma concentration data from pediatric studies. Antimicrob. Agents Chemother. 53:935-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kennedy, J. M., and A. M. Riji. 1998. Effects of surgery on the pharmacokinetic parameters of drugs. Clin. Pharmacokinet. 35:293-312. [DOI] [PubMed] [Google Scholar]
- 19.Lin, S. J., J. Schranz, and S. M. Teutsch. 2001. Aspergillosis case-fatality rate: systematic review of the literature. Clin. Infect. Dis. 32:358-366. [DOI] [PubMed] [Google Scholar]
- 20.Neely, M., T. Rushing, A. Kovacs, R. Jelliffe, and J. Hoffman. 2010. Voriconazole pharmacokinetics and pharmacodynamics in children. Clin. Infect. Dis. 50:27-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nomura, K., Y. Fujimoto, Y. Kanbayashi, K. Ikawa, and M. Taniwaki. 2008. Pharmacokinetic-pharmacodynamic analysis of voriconazole in Japanese patients with hematological malignancies. Eur. J. Clin. Microbiol. Infect. Dis. 27:1141-1143. [DOI] [PubMed] [Google Scholar]
- 22.Panackal, A. A., A. Dahlman, K. T. Keil, C. L. Peterson, L. Mascola, S. Mirza, M. Phelan, B. A. Lasker, M. E. Brandt, J. Carpenter, M. Bell, D. W. Warnock, R. A. Hajjeh, and J. Morgan. 2003. Outbreak of invasive aspergillosis among renal transplant recipients. Transplantation 75:1050-1053. [DOI] [PubMed] [Google Scholar]
- 23.Pascual, A., T. Calandra, S. Bolay, T. Buclin, J. Bille, and O. Marchetti. 2008. Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin. Infect. Dis. 46:201-211. [DOI] [PubMed] [Google Scholar]
- 24.Pfizer. VFEND® IV (voriconazole) for injection, VFEND® tablets, VFEND® for oral suspension.
- 25.Potoski, B. A., and J. Brown. 2002. The safety of voriconazole. Clin. Infect. Dis. 35:1273-1275. [DOI] [PubMed] [Google Scholar]
- 26.Scott, L. J., and D. Simpson. 2007. Voriconazole: a review of its use in the management of invasive fungal infections. Drugs 67:269-298. [DOI] [PubMed] [Google Scholar]
- 27.Smith, J., N. Safdar, V. Knasinski, W. Simmons, S. M. Bhavnani, P. G. Ambrose, and D. Andes. 2006. Voriconazole therapeutic drug monitoring. Antimicrob. Agents Chemother. 50:1570-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan, K., N. Brayshaw, K. Tomaszewski, P. Troke, and N. Wood. 2006. Investigation of the potential relationships between plasma voriconazole concentrations and visual adverse events or liver function test abnormalities. J. Clin. Pharmacol. 46:235-243. [DOI] [PubMed] [Google Scholar]
- 29.Theuretzbacher, U., F. Ihle, and H. Derendorf. 2006. Pharmacokinetic/pharmacodynamic profile of voriconazole. Clin. Pharmacokinet. 45:649-663. [DOI] [PubMed] [Google Scholar]
- 30.Tsang, V. T., A. Johnston, F. Heritier, N. Leaver, M. E. Hodson, and M. Yacoub. 1994. Cyclosporin pharmacokinetics in heart-lung transplant recipients with cystic fibrosis. Effects of pancreatic enzymes and ranitidine. Eur. J. Clin. Pharmacol. 46:261-265. [DOI] [PubMed] [Google Scholar]
- 31.Walsh, T. J., E. J. Anaissie, D. W. Denning, R. Herbrecht, D. P. Kontoyiannis, K. A. Marr, V. A. Morrison, B. H. Segal, W. J. Steinbach, D. A. Stevens, J. A. van Burik, J. R. Wingard, and T. F. Patterson. 2008. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin. Infect. Dis. 46:327-360. [DOI] [PubMed] [Google Scholar]
- 32.Walsh, T. J., M. O. Karlsson, T. Driscoll, A. G. Arguedas, P. Adamson, X. Saez-Llorens, A. J. Vora, A. C. Arrieta, J. Blumer, I. Lutsar, P. Milligan, and N. Wood. 2004. Pharmacokinetics and safety of intravenous voriconazole in children after single- or multiple-dose administration. Antimicrob. Agents Chemother. 48:2166-2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheyu, C., and Y. Lunan. 2006. Early changes of small intestine function in rats after liver transplantation. Transplant Proc. 38:1564-1568. [DOI] [PubMed] [Google Scholar]