Abstract
Topical microbicides may prove to be an important strategy for preventing human immunodeficiency virus type 1 (HIV-1) transmission. We examined the safety and efficacy of sequence-nonspecific phosphorothioate 2′ deoxyribose oligomers as potential novel microbicides. A short, 13-mer poly(T) phosphorothioate oligodeoxynucleotide (OPB-T) significantly inhibited infection of primary peripheral blood mononuclear cells (PBMC) by high-titer HIV-1Ba-L and simian immunodeficiency virus mac251 (SIVmac251). Continuous exposure of human vaginal and foreskin tissue explants to OPB-T showed no toxicity. An abasic 14-mer phosphorothioate 2′ deoxyribose backbone (PDB) demonstrated enhanced anti-HIV-1 activity relative to OPB-T and other homo-oligodeoxynucleotide analogs. When PDB was used to pretreat HIV-1, PDB was effective against R5 and X4 isolates at a half-maximal inhibitory concentration (IC50) of <1 μM in both PBMC and P4-R5 MAGI cell infections. PDB also reduced HIV-1 infectivity following the binding of virus to target cells. This novel topical microbicide candidate exhibited an excellent in vitro safety profile in human PBMC and endocervical epithelial cells. PDB also retained activity in hydroxyethylcellulose gel at pH 4.4 and after transition to a neutral pH and was stable in this formulation for 30 days at room temperature. Furthermore, the compound displayed potent antiviral activity following incubation with a Lactobacillus strain derived from normal vaginal flora. Most importantly, PDB can inhibit HIV-1-induced alpha interferon production. Phosphorothioate 2′ deoxyribose oligomers may therefore be promising microbicide candidates that inhibit HIV-1 infection and also dampen the inflammation which is critical for the initial spread of the virus.
The global spread of human immunodeficiency virus type 1 (HIV-1) is largely driven through sexual contact, particularly in sub-Saharan Africa, where 67% of the 33 million individuals who are currently infected with HIV-1 reside (23). In the absence of a vaccine that elicits sterilizing immunity and protects against HIV-1 infection (13, 21), significant effort is being directed to developing other prophylactic modalities that could potentially slow down or ultimately reverse the rate of propagation of the HIV/AIDS pandemic. One such alternative strategy for reducing the transmission of HIV-1 is the use of protective topical compounds that can be applied directly within the genital tract prior to sexual intercourse (10, 24). Candidate anti-HIV-1 microbicides that are under development for intravaginal application include surfactants that inactivate or disrupt the virus (20), inhibitors of viral binding, fusion, entry, or replication (3, 7, 22, 39, 46, 53), and agents that enhance the normal vaginal microflora (54). Investigations with the rhesus macaque animal model have revealed opportunities at the earliest stages of infection in which a microbicide may be protective by directly inhibiting intravaginal simian immunodeficiency virus (SIV) (50) or modulating the innate immune response to limit viral expansion from the portal of entry (18, 33).
Previous studies have suggested that the local inflammation induced by HIV at the mucosal site of entry is an important amplification step for the spread of infection. The innate immune response to viral infection in the genital mucosa is initiated when Toll-like receptors (TLRs) expressed by epithelial cells, as well as antigen-presenting cells, bind virus-specific molecules (double-stranded RNA and single-stranded RNA) in the cytoplasm and endosomes (49). Plasmacytoid dendritic cells (pDC) produce the majority of alpha interferon (IFN-α) in response to TLR7 and TLR9 activation by HIV and SIV (30). Although the elaboration of IFN-α/β and antiviral chemokines by pDC may contribute to the initial suppression of viral replication, it also fulfills the more immediate requirement of HIV-1 for new target cells to establish local expansion upon which systemic infection depends (29). In support of this, it was reported that augmentation of antiviral innate immunity through the use of TLR7 and TLR9 agonists in a rhesus macaque challenge model enhanced vaginal transmission of SIV (49). The class A CpG oligodeoxynucleotide used in that study as a TLR9 agonist is a strong inducer of IFN-α but also elicits the production of low levels of proinflammatory cytokines in rhesus macaque peripheral blood mononuclear cells (PBMC) (1). When the type I interferon response was induced with class A CpG or the TLR7 agonist imiquimod at mucosal surfaces prior to intravaginal challenge with SIV, a marked increase in the level of plasma viral RNA was observed. Increased virus dissemination was attributed to a pronounced infiltrate of mononuclear cells consisting of dendritic cells (DC), beta-chemokine-producing cells, and activated CD4+ T lymphocytes present in the cervicovaginal mucosa of agonist-treated monkeys (49). Another report demonstrated that TLR9 activation enhances HIV-1 replication (12). Furthermore, Li and colleagues (29) recently established that inhibition of the innate response to SIV in the vaginal environment inhibits the influx of susceptible CD4+ T lymphocytes which is required for the systemic dissemination of the virus from a single small focus of infected cells. These studies strongly suggest that inhibition of TLR7/9-induced immunoinflammatory signaling may be a useful strategy for limiting the initial expansion of HIV/SIV from the mucosa to peripheral sites of infection, and such TLR antagonism may be a desirable property for microbicides to possess.
In the context of modulating cervicovaginal innate immune responses against HIV/SIV infection, inhibition of TLR activation may need to be targeted to pattern recognition receptors that are specifically triggered by these viruses. Some polyanionic microbicides have been shown to inhibit the activation of multiple TLRs (TLR1, TLR2, TLR3, and TLR6) that recognize viral or bacterial components, and this inhibits innate immune responses by epithelial cells derived from the human female genital tract (48). Additionally, vaginal microflora abnormalities have been documented in clinical studies that evaluated the safety of the same class of polyanionic microbicide candidates (6, 43). Therefore, nonselective suppression of various TLRs that recognize different pathogen-associated molecular patterns can compromise host control of the vaginal microflora, as well as defense against pathogens, and thus enhance HIV/SIV transmission. In contrast, selective blocking of TLRs that are activated by HIV/SIV may inhibit the local inflammation that is required for viral expansion without impairing protective responses against other pathogens or the microflora of the cervicovaginal mucosa.
The therapeutic potential of oligodeoxynucleotides containing specific immunosuppressive TTAGGG motifs has been described for different inflammatory diseases and other disorders that are characterized by persistent immune activation (25). Additionally, poly(T) oligodeoxynucleotides were reported to differentially modulate the activation of TLR7 and TLR8 by imidazoquinolines (15), and the phosphorothioate backbone can selectively inhibit TLR7/9 signaling (16). Thus, we investigated small molecules with phosphorothioate 2′ deoxyribose backbones as topical microbicides that may inhibit HIV-1-induced TLR triggering and discovered that they also possess antiviral properties. We report here that a 13-mer poly(T) phosphorothioate oligodeoxynucleotide, OPB-T, is effective at inhibiting HIV-1Ba-L or SIVmac251 infection in human or simian PBMC, respectively, and shows no toxicity against human vaginal and foreskin explants. A baseless 14-mer phosphorothioate 2′ deoxyribose backbone, PDB, possesses a higher level of inhibitory activity against HIV-1 than OPB-T. PDB exhibits efficacy against both CCR5-using (R5) and CXCR4-using (X4) HIV-1 isolates and exhibits no toxicity in a sensitive flow cytometric assay of cell death. Herein, we present data demonstrating that PDB is active when formulated in hydroxyethylcellulose (HEC) gel at pH 4.4, retains antiviral activity following transition to neutral pH, and is stable in gel at room temperature for more than a month. Furthermore, the compound was effective at inhibiting HIV-1 infection despite exposure to Lactobacillus jensenii, a predominant bacterial species of the normal vaginal microflora that produces hydrogen peroxide (H2O2), lactic acid, and other factors (42) that could potentially abrogate the function of microbicides. Notably, PDB is a TLR7/9 antagonist, and we show that it potently suppresses HIV-1-induced IFN-α production. Phosphorothioate oligomers may therefore be useful microbicides against HIV-1, as they not only directly inhibit viral infection but also block HIV-1-induced TLR7/9 activation. Such TLR activation drives the cytokine production required for the recruitment and establishment of HIV-1-infected founder cell populations at mucosal sites and promotes a self-propagating infection in secondary lymphoid organs.
MATERIALS AND METHODS
Cells, viruses, and reagents.
P4-R5 MAGI cells were obtained from the NIH AIDS Research and Reference Reagent Program. HEC-1-A human uterine/endocervical cells were purchased from the American Type Culture Collection (ATCC; Manassas, VA). Human blood samples were collected from healthy individuals following Drexel University College of Medicine Institutional Review Board (IRB) approval and acquisition of informed consent. PBMC were isolated from heparinized venous blood by Ficoll-Hypaque (Amersham Pharmacia Biotech, Uppsala, Sweden) density centrifugation. Blood was processed within 1 h of venipuncture. Indian rhesus macaques (Macaca mulatta) were housed at the Bioqual Animal Facility (Rockville, MD) according to the standards and guidelines set forth in the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals and in conjunction with the animal care standards deemed acceptable by the Association for the Assessment and Accreditation of Laboratory Animal Care—International. Blood collection procedures were performed in accordance with institutional animal care and use committee approval. PBMC were freshly isolated from healthy animals by Percoll (Amersham Pharmacia Biotech) density gradient centrifugation of heparinized venous blood and stored at −80°C. Sucrose gradient-purified high-titer stocks of HIV-1Ba-L and HIV-1IIIB were obtained from Advanced Biotechnologies, Inc. (Columbia, MD). The SIVmac251 stock used for in vitro viral infection assays has been described elsewhere (28) and was a kind gift from Ronald Desrosiers (New England Primate Research Center, Harvard Medical School, Southborough, MA). Phosphorothioate homo-oligodeoxynucleotides (OPB-A [5′-AAAAAAAAAAAAA-3′], OPB-G [5′-GGGGGGGGGGGGG-3′], and OPB-T [5′-TTTTTTTTTTTTT-3′]) were synthesized by Invitrogen Corporation (Carlsbad, CA). The abasic phosphorothioate 2′ deoxyribose backbone (PDB) was prepared by TIB Molbiol (Adelphia, NJ). All phosphorothioate compounds were reconstituted in phosphate-buffered saline (PBS) as 9 mM stocks and diluted appropriately in cell culture medium.
Assessment of the anti-HIV/SIV activity of homo-oligodeoxynucleotides in primary PBMC.
Freshly isolated human PBMC (1 × 106 cells/ml) were cultured in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum (FBS), 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin sulfate (Cellgro, Manassas, VA). Cells were activated for 48 h prior to HIV-1Ba-L infection by adding 10 μg/ml phytohemagglutinin (PHA-P; Sigma, St. Louis, MO) and 20 U/ml interleukin-2 (IL-2; Roche Applied Science, Indianapolis, IN) to cultures and incubating them at 37°C in 5% CO2. HIV-1Ba-L at a final titer of 105 50% tissue culture infectious doses (TCID50)/ml was preincubated at 37°C for 30 min with medium alone or phosphorothioates in medium at 1 μM, 5 μM, or 25 μM. One million PBMC were spun down and resuspended in 200 μl of virus-plus-inhibitor solutions for 1 h, washed three times, and cultured for a further 48 h. Supernatants were assayed for HIV-1 p24 content by an enzyme-linked immunosorbent assay (ELISA; ZeptoMetrix Corp., Buffalo, NY). Rhesus macaque PBMC were activated as described above for 96 h prior to SIVmac251 infection. SIVmac251 at a final titer of 3.7 × 106 TCID50/ml was combined with medium or 25 μM OPB-T in medium for 1 h at 37°C in 5% CO2. One million activated simian PBMC were pelleted by centrifugation and resuspended in 200 μl of OPB-T-plus-virus solution. The infections proceeded for 1 h at 37°C in 5% CO2, after which cells were washed, transferred into 24-well plates, and cultured for 48 h in the presence of 20 U/ml IL-2. At 48 h postinfection, culture supernatants were collected and assayed for SIV p27 antigen by ELISA (ZeptoMetrix Corp.).
Determination of phosphorothioate oligomer toxicity in human genital tissue explants.
All experiments were conducted in accordance with Drexel University IRB rules and regulations. Genital tissues were obtained from patients undergoing surgical procedures as part of their care. Tissue samples were deidentified and thus exempt from IRB review. Human neonatal foreskin and adult vaginal tissues were used in an organ tissue culture model system (9). Briefly, vaginal tissues were obtained from women undergoing reconstructive surgeries for noncancerous conditions, and neonatal foreskins were procured during routine circumcision procedures. All tissues were collected immediately prior to the experiments. Only grossly normal samples were released and used for assays. Tissue from each donor was divided into 6 contiguous 6.0-mm circular pieces by use of an Acu-punch biopsy scalpel (Acuderm, Inc., Fort Lauderdale, FL). The cut tissue sections were soaked in an antibiotic wash containing amphotericin B (Fungizone) (250 μg/ml; Life Technologies, Grand Island, NY), nystatin (120 U/ml; Sigma), and 20,000 U/ml penicillin and streptomycin (Cellgro) for 5 to 8 min and washed three times in serum-free and antibiotic-free Dulbecco's modified Eagle's medium (DMEM; Mediatech, Herndon, VA). The circular pieces of tissue with the epithelial layer oriented on top were placed into the upper chamber of a 12-well Transwell dish. A sterile 5% solution of agarose in Hanks' medium was added to the area surrounding the tissue and allowed to solidify, creating a tight seal. To supply nutrients to the tissues, 1 ml of DMEM supplemented with 10% FBS was added to the bottom chamber. Tissues were then treated in triplicate with 300 μl of either 25 μM OPB-T in 10% FBS-DMEM or 10% FBS-DMEM alone and incubated at 37°C with 5% CO2 for 24 h. Following the incubation period, the tissue was harvested, immediately fixed in 10% neutral-buffered formalin, and processed using standard histological techniques for staining with hematoxylin and eosin (H&E). The tissue sections were examined using a Nikon Eclipse 80i microscope (Nikon Instruments, Inc., Melville, NY) at a magnification of ×20.
Inhibition of viral infection/replication with X4- and R5-tropic HIV-1 strains.
At 24 h prior to infection, 8 × 104 P4-R5 MAGI cells/2 ml/well were seeded into 12-well plates and incubated overnight at 37°C in 5% CO2. The cells used in this assay, P4-R5 cells, are HeLa cells that have been engineered to express HIV-1 receptors (CD4, CCR5, and CXCR4) and have an integrated HIV-1 long terminal repeat (LTR) fused to a β-galactosidase (β-Gal) reporter gene (8). This allows for β-Gal expression to be measured when the HIV-1 promoter has been activated by the virus-encoded transactivator of transcription (Tat) protein. On the day of the assay, HIV-1Ba-L at a final titer of 5 × 105 TCID50/ml or HIV-1IIIB at final titers of 104, 105, and 5 × 105 TCID50/ml was either untreated or treated with OPB-T or PDB at different concentrations and incubated for 30 min at 37°C. Volume was subsequently brought up to 1 ml with 10% FBS-RPMI medium, and 300 μl of this solution was applied to the P4-R5 MAGI cells in duplicate. Following a 2-h incubation at 37°C in 5% CO2, 2 ml of fresh DMEM (supplemented with 10% FBS and 125 μg/ml puromycin) was added to each well. After incubation for 48 h at 37°C in 5% CO2, cells were washed twice with PBS, lysed, and incubated for 1 h at room temperature with the reaction buffer supplied in the Galacto-Star β-galactosidase reporter gene assay system (Applied Biosystems, Foster City, CA). Following the incubation, β-Gal expression was quantified utilizing a Fluoroskan Ascent FL luminometer (Thermo Scientific, Waltham, MA).
HIV-1 postbinding inhibition assay.
P4-R5 MAGI cells were infected with 104 TCID50/ml of cell-free HIV-1IIIB for 30 min at 37°C in 5% CO2 and then washed twice with PBS to remove unbound virus. PDB was subsequently added to cultures at a final concentration of 5 μM. At 48 h postinfection, viral infectivity was determined by measuring the total enzymatic activity of β-Gal in cells, as described above.
Cell-free HIV-1IIIB inhibition assay with PDB-HEC gel.
HEC gel was made by adding 2.7% Natrosol 250 Pharm HEC base (Hercules, Inc., Wilmington, DE) to 96.35% H2O, 0.85% NaCl, and 0.1% sorbic acid. The pH of the formulation was adjusted to pH 4.4 with sodium hydroxide, as previously described (47). PDB was added to the HEC gel during gel preparation. The microbicide gel formulations were stored at room temperature for at least 24 h prior to use. Some of the PDB gel samples were assayed after a 30-day storage period at room temperature. The activity of 25 μM PDB gel against HIV-1IIIB at titers of 104, 105, and 5 × 105 TCID50/ml was assessed by using the P4-R5 MAGI assay.
Assessment of PDB-mediated antiviral activity following incubation with a commensal Lactobacillus species.
L. jensenii (ATCC 25258) was grown in MRS broth (Moltox, Inc., Boone, NC) under facultative anaerobic conditions. To assess the effect of L. jensenii on the antiviral activity of PDB, the bacterial concentration was adjusted to an optical density of 0.06 at a wavelength of 670 nm in antibiotic-free RPMI 1640 containing 10% FBS. This bacterial density corresponds to 108 CFU/ml (26). PDB was then added to the L. jensenii suspension or medium alone at a final concentration of 50 μM and incubated overnight at 37°C in 5% CO2. Following the incubation period, PDB that had been unexposed or exposed to bacteria was filtered through a 0.2-μm-nominal-pore-size cellulose acetate membrane (Thermo Fisher Scientific, Rochester, NY) to remove lactobacilli and used at a final concentration of 25 μM to inhibit 105 TCID50/ml of cell-free HIV-1IIIB. The antiviral activity of each filtrate was determined with the P4-R5 MAGI assay.
Human endocervial/uterine epithelial cell toxicity assay.
One day prior to performing the assay, HEC-1-A cells were seeded into 96-well flat-bottom plates at a concentration of 105 cells/200 μl/well and maintained at 37°C in 5% CO2. PDB was added to cells at final concentrations ranging from 25 to 250 μM. Following 24 h of continuous exposure, cells were washed three times with PBS and incubated with 1-(4,5-dimethylthiazol-2-yl)-3,5-diphenylformazan (MTT) (7.5 mg/ml; Sigma) for 2 h at 37°C in 5% CO2. After the removal of MTT, intracellular formazan crystals were solubilized for 15 min in 70% isopropyl alcohol and read at 570 nm using a SpectraMax Plus384 spectrophotometer (Molecular Devices, Sunnyvale, CA), as previously described (38). Readings were also recorded at 690 nm in order to quantify nonspecific absorbance.
Primary human PBMC cytotoxicity assay.
PBMC from healthy donors were freshly isolated by Ficoll-Hypaque centrifugation of heparinized venous blood. In order to activate cells, PBMC were first cultured for 48 h at 37°C in 5% CO2 in the presence of 10 μg/ml PHA-P and 20 U/ml IL-2. PDB was added to resting or activated PBMC at final concentrations ranging from 25 to 250 μM. Following 24 h of continuous exposure, cells were washed and stained for apoptosis using annexin V Cy5.5 (BD Biosciences, San Diego, CA). PBMC were stained with annexin V in Hanks' balanced salt solution (HBSS; Cellgro), 3% heat-inactivated horse serum (Invitrogen Corporation), 0.02% NaN3, and 2.5 mM CaCl2 for 30 min on ice, washed two times, and fixed with 1% paraformaldehyde. Samples were collected on a FACSAria (BD Biosciences) and analyzed using FlowJo software (Tree Star, San Carlos, CA).
IFN-α inhibition assay.
PDB at a final concentration of 5 μM was added to freshly isolated healthy donor PBMC plated at 1 × 106 cells/ml/well in 24-well plates. PDB was added to cultures immediately before the addition of 105 TCID50/ml of HIV-1Ba-L. Following a 24-h incubation at 37°C in 5% CO2, IFN-α levels in cell-free supernatants were measured by ELISA (PBL, Piscataway, NJ).
Statistical analysis.
The Student t test and the nonparametric Wilcoxon signed-rank test for paired samples were used for statistical analysis with the JMP data analysis program (SAS, Cary, NC). P values of <0.05 were considered significant.
RESULTS
Phosphorothioate 2′ deoxyribose oligomers inhibit HIV-1 and SIV infection/replication.
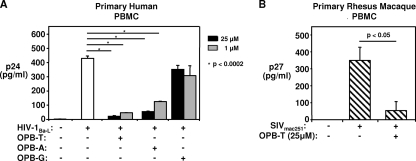
Phosphorothioate oligodeoxynucleotide homopolymers were tested by preincubating HIV-1Ba-L with poly(A) (OPB-A), poly(G) (OPB-G), or poly(T) (OPB-T) 13-mers prior to infection of activated PBMC. We found that phosphorothioate oligodeoxynucleotide homopolymers consisting of different nucleotide bases exhibited anti-HIV activity, but at various degrees. OPB-T exhibited the highest level of anti-HIV-1 activity. Human PBMC infected with 105 TCID50/ml HIV-1Ba-L that was preincubated with 25 μM OPB-T had a 91% ± 2% reduction in HIV p24 levels at 48 h postinfection (Fig. 1A). The same concentration of OPB-A was less effective than OPB-T and inhibited cell-free infection of human PBMC by 105 TCID50/ml of HIV-1Ba-L by 79% ± 5%, while OPB-G was ineffective at reducing viral infectivity.
FIG. 1.
Phosphorothioate 2′ deoxyribose homo-oligodeoxynucleotides inhibit HIV-1 and SIV infection/replication. (A) OPB-T and OPB-A (13-mer) at 1 μM and 25 μM inhibit 48-h p24 production by PBMC infected with 105 TCID50/ml of HIV-1Ba-L. PBMC were activated for 48 h. A total of 105 TCID50/ml of HIV-1Ba-L were exposed for 30 min to 1 μM and 25 μM OPB-T, OPB-A, or OPB-G. Cells were then infected with an HIV/OPB mixture for 1 h, washed twice, and cultured in fresh medium. Supernatants were collected at 2 days postinfection and tested for p24 by ELISA. The bar graph depicts means ± standard errors (SE) of results from triplicate cultures. Representative data from 1 out of 3 donors tested are shown. (B) OPB-T at 25 μM inhibits >85% of 48-h SIV p27 production by PBMC infected with a high dose of 3.7 × 106 TCID50/ml of SIVmac251. PBMC were activated for 96 h before infection. SIV was exposed for 30 min to 25 μM OPB-T before addition to PBMC, and supernatants were collected after 48 h. Means ± SE of results from triplicates are shown. Representative data from 1 out of 3 animals tested are depicted.
We next tested whether OPB-T could inhibit SIVmac251 infection. As shown in Fig. 1B, OPB-T also demonstrated efficacy against cell-free SIVmac251 infection of rhesus macaque PBMC at a high viral titer of 3.7 × 106 TCID50/ml. In rhesus macaque PBMC infected with SIVmac251, 25 μM OPB-T reduced SIV p27 levels by 85% (Fig. 1B).
OPB-T does not cause physical disruption of intact human vaginal and foreskin tissue at the histological level.
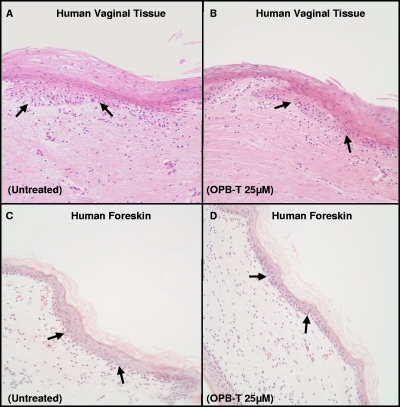
To exclude any toxicity associated with phosphorothioate 2′ deoxyribose-based microbicide compounds on genital epithelium, intact human vaginal and newborn foreskin tissues were incubated ex vivo with either medium alone or OPB-T at a final concentration of 25 μM for 24 h. There was no visible disruption of the squamous epithelium observed following treatment with OPB-T (Fig. 2B and D). The tissue architecture appeared normal subsequent to OPB-T exposure, and no evidence of denuded epithelium or focal necrosis was present.
FIG. 2.
A 13-mer phosphorothioate 2′ deoxyribose oligomer does not cause physical disruption of intact human vaginal or foreskin tissue at the histological level. Histological H&E staining shows that treatment with 25 μM OPB-T did not disrupt squamous epithelium in human vaginal (A, B) or foreskin (C, D) tissue explants compared to the level for the untreated controls. The untreated controls (A, C) show tissue integrity, and the 25 μM OPB-T-treated tissues (B, D) do not exhibit epithelial disruption from a histological perspective. Tissues were treated or untreated with OPB-T for 24 h. Arrows indicate the basal layer. Above the basal layer is the squamous epithelium, and below is the stroma, the connective tissue. All magnifications shown are ×20. Representative data from 2 vaginal and 2 foreskin tissues tested are shown.
PDB demonstrates enhanced anti-HIV-1 activity over phosphorothioate oligodeoxynucleotides.
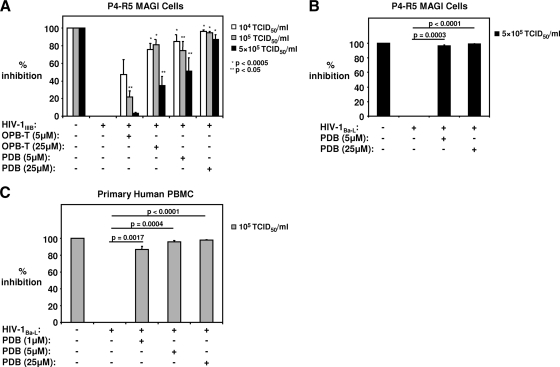
Since phosphorothioate oligodeoxynucleotides showed various degrees of HIV-1 inhibition, we sought to determine whether the abasic phosphorothioate 2′ deoxyribose backbone had antiviral activity. At a concentration of 25 μM, PDB inhibited 104 and 105 TCID50/ml HIV-1IIIB infection by 97% ± 1% and 95% ± 2%, respectively (Fig. 3 A). The magnitudes of viral inhibition produced by 25 μM OPB-T were 76% ± 7% for 104 TCID50/ml HIV-1IIIB and 81% ± 6% for 105 TCID50/ml HIV-1IIIB (Fig. 3A). When both compounds were tested against HIV-1IIIB at a high titer of 5 × 105 TCID50/ml, 25 μM PDB inhibited infection/replication by 87% ± 6%, while 25 μM OPB-T reduced infectivity by only 35% ± 10% (Fig. 3A). At titers of 104 and 105 TCID50/ml, 5 μM PDB was more effective than 5 μM OPB-T at reducing viral infection/replication, with reductions of approximately 2-fold and 3-fold, respectively (Fig. 3A). PDB at concentrations of 5 μM and 25 μM also significantly inhibited 5 × 105 TCID50/ml HIV-1Ba-L infections of P4-R5 MAGI cells by 96% ± 2% and 99% ± 1%, respectively (Fig. 3B). When tested for activity against infection of primary cells, PDB, even at a low concentration of 1 μM, inhibited 105 TCID50/ml HIV-1Ba-L infection of human PBMC by 87% ± 4% (Fig. 3C), while at 5 μM and 25 μM, PDB completely inhibited 105 TCID50/ml HIV-1Ba-L infections (Fig. 3C). These data suggest that the 50% inhibitory concentration (IC50) of PDB for the inhibition of 105 TCID50/ml of HIV-1Ba-L is well below 1 μM and that PDB is more effective than phosphorothioate oligodeoxynucleotides.
FIG. 3.
PDB more effectively inhibits infection/replication of cell-free HIV-1IIIB infection than OPB-T and is also a potent inhibitor of HIV-1Ba-L. (A) HIV-1IIIB inhibition by PDB and OPB-T is shown. (B) Inhibition of 5 × 105 TCID50/ml HIV-1Ba-L by PDB is shown. (A, B) HIV was exposed for 30 min to 5 μM and 25 μM PDB or OPB-T before the HIV/compound mixture was added to P4-R5 MAGI cells. β-Gal expression was measured 48 h later. (C) PDB inhibits infection of PBMC by 105 TCID50/ml HIV-1Ba-L. Following exposure of HIV to 1 μM, 5 μM, or 25 μM PDB, the HIV-compound mixture was added to activated PBMC for 1 h. Viral antigen levels in cell supernatants were measured at 48 h postinfection by ELISA. Means ± SE of results from 3 independent experiments are shown.
PDB exhibits postbinding inhibitory effects on HIV-1 infectivity.
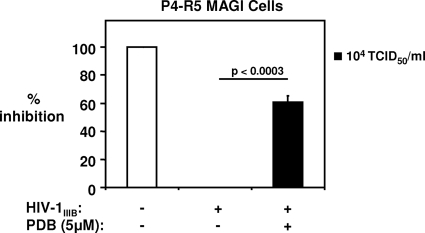
To explore the concept that an abasic phosphorothioate oligomer may reduce viral infection/replication following the initial attachment of HIV-1 to target cells, we performed a modified P4-R5 MAGI assay in which the virus inoculum was washed out after a 30-min infection, followed by treatment of cells with 5 μM PDB for 48 h. As shown in Fig. 4, PDB decreased the postbinding infectivity of HIV-1IIIB at a titer of 104 TCID50/ml by 61% ± 5%. When the same experiment was performed with a 1-h virus adsorption period, PDB reduced β-Gal production in P4-R5 MAGI cells by 33% ± 4% and did not exert a postbinding inhibitory effect on HIV-1 infectivity following a 2-h inoculation with HIV-1IIIB (data not shown).
FIG. 4.
PDB exhibits anti-HIV-1 activity even after exposure of target cells to virus. P4-R5 MAGI cells were infected with HIV-1IIIB at a titer of 104 TCID50/ml for 30 min, followed by washout of the virus inoculum, and then PDB (5 μM) was added for 48 h. Cells were harvested, and the total enzymatic activity of β-Gal in lysates was measured. Means ± SE of results from 3 independent experiments are shown.
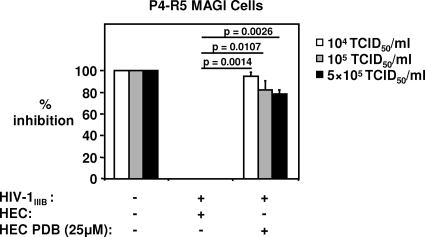
A PDB-HEC gel formulation inhibits cell-free HIV-1 infection of P4-R5 MAGI cells.
Formulation of a microbicide into a gel is required for topical application, as this allows for a more uniform intravaginal dispersion and increases retention at the application site. For these experiments, HEC gel was selected as a vehicle. This gel has been studied extensively and does not induce toxicity or inflammation in the vaginal mucosa (47). In order to determine if PDB microbicide gel is likely to be active in vivo, the pH of the gel formulation was initially adjusted to pH 4.4, the pH of the vaginal environment. Furthermore, the addition of medium to the virus-gel mixture following the preincubation period results in a pH change from pH 4.4 to pH 6.8 to 7.0, similar to the transition that occurs when semen is introduced into the vaginal canal. Despite this pH transition and inclusion in HEC gel, PDB at a concentration of 25 μM reduced the infectivity of cell-free HIV-1IIIB at titers of 104 TCID50/ml, 105 TCID50/ml, and 5 × 105 TCID50/ml by 95% ± 4%, 82% ± 9%, and 78% ± 4%, respectively (Fig. 5).
FIG. 5.
PDB-HEC gel formulation can inhibit cell-free HIV-1IIIB infection of P4-R5 MAGI cells. PDB-25 μM HEC gel formulation (pH 4.4) inhibits β-Gal expression by P4-R5 MAGI cells infected with 104, 105, and 5 × 105 TCID50/ml of HIV-1IIIB. HIV-1 was exposed for 30 min to 25 μM gel-formulated PDB, and the HIV-PDB mixture was added to P4-R5 MAGI cells. β-Gal expression was measured 48 h later. Pooled data from 3 experiments are shown. Bars depict mean percents inhibition of β-Gal expression ± SE.
An important aspect of an effective microbicide is shelf-life/stability over prolonged periods of time. In order to assess stability for up to 30 days, a 25 μM PDB-loaded HEC gel (pH 4.4) was stored at room temperature, and at different time intervals (day 0, day 15, and day 30), anti-HIV efficacy was assessed using the P4-R5 MAGI assay. Storage of 25 μM PDB topical gel at room temperature for up to 1 month does not appear to influence its activity against HIV-1IIIB. At day 30, 25 μM PDB gel inhibited HIV-1IIIB at a titer of 104 TCID50/ml by 88% ± 1% and HIV-1IIIB at a titer of 105 TCID50/ml by 91% ± 2%. Physical changes that may occur during long-term storage of topical microbicide gels, including discoloration, settling, and/or phase separation, were not observed in 25 μM PDB-HEC gel (data not shown).
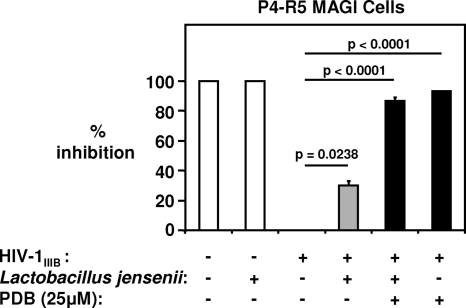
PDB retains activity against HIV-1 following exposure to a Lactobacillus strain derived from normal vaginal flora.
The production of H2O2, lactic acid and other factors by the lactobacilli that dominate a healthy vaginal microflora provides broad-spectrum protection against a host of different pathogens (35) but may also significantly diminish the function of topical microbicides designed for preexposure prophylaxis against HIV-1. To investigate whether PDB retained antiviral activity following exposure to L. jensenii, one of the most prevalent microorganisms in the healthy human female genital tract (45), the compound was incubated overnight in a turbid bacterial suspension and subsequently filtered for use in the P4-R5 MAGI assay. The resultant filtrate containing 25 μM PDB inhibited 105 TCID50/ml of cell-free HIV-1IIIB by 87% ± 2% (Fig. 6). Filtered PDB that had not been exposed to L. jensenii and was used to pretreat virus inhibited the same titer by 93% ± 0.5%, while the bacterial filtrate alone reduced infectivity by 30% ± 3% (Fig. 6).
FIG. 6.
PDB retains potent antiviral activity against HIV-1 following exposure to a predominant commensal Lactobacillus species of the normal vaginal microflora. PDB at a final concentration of 50 μM was unexposed or exposed overnight to 108 CFU/ml of L. jensenii and subsequently filtered. The resultant filtrates containing PDB (25 μM) were used to pretreat HIV-1IIIB at a final titer of 105 TCID50/ml before addition to P4-R5 MAGI cells. Viral infectivity was determined after 48 h by measuring β-Gal activity in cell lysates. Pooled results from 3 independent experiments are depicted. Bars represent the average percents inhibition of viral infectivity ± SE.
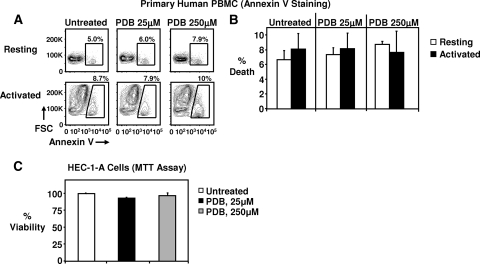
PDB exhibits no toxicity on primary human PBMC or endocervical/uterine epithelial cells.
In order to further exclude any possible toxicity associated with PDB, we incubated resting or 48-h-PHA-P-activated human PBMC with PDB for 24 h (continuous exposure) and subsequently stained them for cell death using annexin V conjugated to a fluorochrome. Cells were then analyzed by flow cytometry. Annexin V binds to phosphatidyl serine that is exposed on the surfaces of early and late apoptotic and necrotic cells. In this very sensitive assay, which can detect even small increases in cell death, PDB at 25 and 250 μM concentrations did not induce apoptosis/necrosis in PBMC (Fig. 7A and B). PDB also exhibited no toxicity against human endocervical/uterine epithelial cells. Continuous exposure of HEC-1-A cells to PDB at concentrations of 25 μM and 250 μM did not affect viability after 24 h (Fig. 7C).
FIG. 7.
PDB induces no death in human PBMC or HEC-1-A cells exposed continuously to the compound for 24 h. (A) A continuous 24-h exposure to PDB had no effect on the viability of resting or activated human PBMC. PBMC from healthy donors were either unstimulated (resting) or stimulated with PHA-P and IL-2 for 48 h (activated). Cells were then harvested, resuspended in fresh medium, and treated with 25 μM and 250 μM PDB for 24 h. Cell death was measured by annexin V staining and flow cytometry. Percentages in plots indicate the percent death. Annexin V and forward scatter (FSC) are shown. (B) Pooled data from 3 donors are shown. (C) A continuous 24-h exposure of HEC-1-A cells to PDB at concentrations of 25 μM and 250 μM had no effect on their viability. Viability was measured by an MTT assay. Pooled data from 3 experiments are shown.
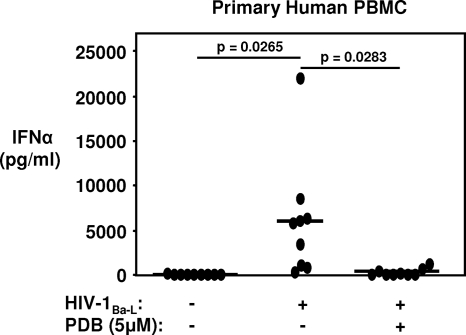
PDB inhibits HIV-1-induced IFN-α production from primary human PBMC.
Abasic phosphorothioate deoxyribose backbones have been reported to be TLR7/9-specific antagonists (16). HIV-1-induced IFN-α production by pDC results from TLR7 and TLR9 signaling triggered by HIV-1 recognition (4, 31). Since the elicitation of a type I interferon response at the portal of entry may drive local expansion and virus dissemination to peripheral sites of infection (29, 49), the ability of PDB to suppress HIV-1Ba-L-induced IFN-α production by PBMC in vitro was assessed. At a concentration of 5 μM, PDB reduced the generation of IFN-α from PBMC exposed to 105 TCID50/ml of HIV-1Ba-L by 96% ± 3% (Fig. 8). PDB alone, tested at a range of 5 to 25 μM, did not induce IFN-α when PBMC from 3 donors were exposed to the oligomer continuously for 24 h (data not shown). Thus, in addition to having potent anti-HIV activity, PDB also possesses strong TLR7/9 antagonistic properties.
FIG. 8.
PDB inhibits HIV-1-induced IFN-α production from primary human PBMC. Following isolation, PBMC were resuspended in fresh medium, treated with 5 μM PDB, and immediately infected with 105 TCID50/ml HIV-1Ba-L. At 24 h postinfection, IFN-α in cell-free supernatants was measured by ELISA. Data depict results from individual donors (n = 9). Horizontal lines indicate means.
DISCUSSION
Traditionally, the development of microbicides to prevent the transmission of HIV-1 has been aimed exclusively at inhibiting viral infection/replication. We have focused on the development of phosphorothioate 2′ deoxyribose compounds as nontoxic topical microbicides that directly inhibit viral infection while simultaneously blocking TLR signaling and the downstream inflammatory responses which are thought to be crucial for the early expansion of infected founder cell populations in the cervicovaginal mucosa. Immune cell populations are known to be present in the epithelium and stroma of the vagina during all stages of reproduction and can include resident macrophages, DC, and T and B lymphocytes (5, 37). These cells may defend against sexually transmitted disease-related pathogens via specific and nonspecific mechanisms and also play a role in tissue inflammation. Additionally, some of these cells can serve as targets for infection by viruses, such as HIV-1. During the earliest stages of HIV-1 infection, the mucosal innate immune response may be deleterious instead of protective to the host by inducing the influx of new target cells for dissemination of the virus (29, 49). The sources of HIV-1 dissemination following the initial stages of infection are likely to be small foci of infected CD4+ T lymphocytes that are present within the mucosa (18). Therefore, inhibition of the virus, as well as suppression of HIV-1-induced innate immune responses at the site of entry, may enhance the efficacy of microbicides and potentially prevent transmission.
Entry of HIV-1 in the vaginal tract has been postulated to occur by several mechanisms, including passage of virus or infected cells into ulcerations or microabrasions, capture and transport of virus by Langerhans cells or circulating DC, transcytosis of virus through the epithelium into the lamina propria, direct infection of the epithelium, and/or trafficking of infected cells (for a review, see reference 27). In addition to cell-free virus present in seminal fluid, immune cells in the vagina can fuse with incoming semen-borne HIV-1-infected leukocytes, which represents viral infection through direct cell-to-cell contact (40). The establishment of a systemic SIV infection following intravaginal challenge with cell-free virus is well documented for nonhuman primates (32, 34, 44). Animal models of HIV transmission have also demonstrated successful vaginal infection with cell-associated virus (14, 22). However, these studies are confounded by the possible presence of cell-free virus in the inocula or virions being released from live or dying infected cells postinoculation into the genital tract. At present, the form of virus that is dominant in human sexual transmission is not known. Vaginal tissues exhibit greater expression of CCR5 than the CXCR4 coreceptor (36), and it is generally considered that monocyte-tropic strains of HIV-1 are more likely to be transmitted during heterosexual intercourse. This assumption is further supported by the isolation of primarily monocyte-tropic strains early in infection (55), the relative abundance of monocytes over lymphocytes in semen and vaginal lavage mononuclear cell populations (2, 52), and the virtual absence of seminal T lymphocytes in HIV-infected men (41). The potential vaginal target cells for the virus include CD4+ T lymphocytes, monocytes/macrophages, Langerhans cells and circulating DC, and possibly epithelial cells. In the experiments presented in this report, low concentrations of PDB completely inhibited cell-free X4 and R5 HIV-1 after brief exposure to the compound at physiologic temperatures. PDB retained its antiviral efficacy following incorporation into HEC, a topical gel that is suitable for intravaginal application (47).
The maintenance of a healthy vaginal environment depends upon low pH resulting from the production of lactic acid via glycogen metabolism by lactobacilli and the presence of H2O2-producing lactobacilli that prevent the colonization of bacterial vaginosis flora (11). Since HIV-1 is readily inactivated at pHs of <4.5 (27), metabolic products of Lactobacillus spp. that decrease vaginal pH to lie within the acidic range may confer some degree of virucidal activity. This is supported by the results presented in this study, which demonstrate that an L. jensenii culture supernatant filtrate reduced HIV-1 infectivity. The efficacy of polymeric HIV-1 inhibitors can also be affected by acidic pH, as this may alter the secondary or tertiary structures of these compounds and neutralize their negative charge, thereby drastically reducing antiviral activity (26). Notably, the PDB gel formulation maintained its activity at pH 4.4, which is comparable to the pH of the vaginal canal. Stability testing was expanded through the use of an antiviral assay designed to include a main constituent of the indigenous microflora that normally colonizes vaginal mucous membranes. The fact that PDB potently suppressed HIV-1 infectivity, despite incubation with 108 CFU/ml of L. jensenii, indicates that PDB may potentially prevent HIV-1 acquisition in the presence of the bioactive commensal microflora that normally line the female urogenital tract. Furthermore, when the reaction between PDB gel formulation and virus was blocked by the addition of medium in a standard P4-R5 MAGI assay, a transition from pH 4.4 to pH 6.8 to 7.0 was observed. The PDB gel therefore retains activity at a low pH and following changes that mimic the pH transition which occurs when the human vaginal environment is exposed to seminal plasma. Finally, the topical gel formulation at pH 4.4 was stable and retained anti-HIV activity after storage for 30 days at room temperature, which further underscores its outstanding potential for development as a topical microbicide.
Members of the current class of polysaccharide microbicides, including polystyrene sulfonate, dextran sulfate, cellulose sulfate, and naphthalene sulfonated polymer (PRO 2000), possess similar chemical features (sulfation or sulfonation) as well as anionic charge (19). A recent report suggests that certain polyanionic compounds belonging to this group can interfere with TLR ligand-triggered innate immune responses by epithelial cells derived from the human female genital tract (48). Polyanionic microbicide-mediated suppression of the activities of multiple TLRs that recognize viral or bacterial components was observed (48). Nonselective suppression of various TLRs that recognize different pathogen-associated molecular patterns may compromise host immune mechanisms that regulate the vaginal microflora and defense against pathogens in the genital mucosa. Short, base-free phosphorothioate 2′ deoxyribose oligomers function as TLR7/9 antagonists (16, 17) and do not cross-react with other TLR family members (16). In this regard, PDB may hold an advantage over polyanion-based microbicides since it not only directly inhibits HIV-1 but also selectively suppresses TLR7/9 signaling and can potently block production of HIV-1-induced IFN-α by PBMC. TLR7 and TLR9 are involved in the induction of cytokine production from pDC exposed to HIV and SIV (30). Furthermore, TLR7/9 stimulation can enhance the vaginal transmission of SIV in vivo, and such TLR triggering by HIV-1 in the cervicovaginal tract most likely facilitates infection (49). TLR7/9 triggering by HIV-1 may induce inflammation and an influx of target cells at the site of infection, something previously seen in human skin, where TLR7/9 activation resulted in the generation of local inflammatory responses and subsequent recruitment of immune cells to the affected area (51). Therefore, inhibition of HIV-1-mediated TLR7/9 stimulation and IFN-α production by PDB may impede the initial spread of infection by impairing the recruitment of target cells to the vaginal mucosa.
PDB also exhibits an excellent safety profile in vitro. In our studies, PDB at concentrations that were much higher than the effective antiviral and TLR7/9-specific immunomodulatory doses was nontoxic to primary human PBMC as well as human endocervical epithelial cells. Furthermore, optimal antiviral concentrations of phosphorothioate 2′ deoxyribose oligonucleotides did not cause physical disruption of human neonatal foreskin or vaginal tissue. This is an important predictor of microbicide safety, as mucosal barrier disruption may augment the risk for vaginal transmission of HIV-1 and other pathogens. Further investigations are required to determine whether phosphorothioate 2′ deoxyribose-based compounds affect the natural microflora present in the genital tract and the viability or function of human sperm. Although the above-mentioned in vitro toxicity studies are important, in vivo safety remains to be established. Experiments are required to investigate whether repeated local TLR7/9 inhibition induces physical or immunological perturbations in the mucosal epithelial barrier of the genital tract, as this may impair mechanisms that prevent pathogen entry. The safety endpoints presented in this report possess obvious limitations, as they also do not exclude the possibility of systemic toxicity in vivo. Pharmacokinetic absorption and safety testing will be necessary to determine if PDB or similar compounds are viable microbicide candidates that merit further study.
The mechanism of HIV-1 inhibition by PDB remains to be elucidated. Phosphorothioates may function through their polyanionic properties and inhibit the virus prior to cell association or block virus-cell interaction at the cell surface. Previous studies have suggested that phosphorothioate oligodeoxynucleotides may interact directly with a TLR agonist to inhibit TLR activation (15). Our findings indicate that PDB exerts an inhibitory effect on HIV-1 infectivity, even following binding of the virus to target cells. It is therefore possible that the baseless phosphorothioate oligomer may interact with HIV-1 RNA, interfering with TLR activation and inhibiting the virus. This antiviral activity may not be restricted to HIV-1, as preliminary studies suggest that phosphorothioate 2′ deoxyribose oligomers can prevent infection of mice with influenza type A virus in vivo (data not shown). Therefore, phosphorothioate 2′ deoxyribose-based compounds that suppress TLR7 activity (15) may also be active against herpes simplex virus type 2 and other sexually transmitted viruses. Finally, our data indicate that the anti-HIV activity of oligodeoxynucleotides is primarily mediated by the phosphorothioate 2′ deoxyribose backbone. Indeed, PDB possessed more-potent antiviral activity than poly(T), poly(A), or poly(G) oligodeoxynucleotide analogs of similar length.
On the basis of our findings, PDB possesses effective inhibitory activity against HIV-1 at an IC50 of <1 μM, which is readily attainable in topical formulation. PDB is chemically compatible with a universal gel-based delivery vehicle, retains activity at a low vaginal pH as well as after a pH transition, and exhibits no toxicity in tissue cultures of primary human PBMC or endocervical/uterine epithelial cells. Unlike other microbicides that failed clinical trails and were subsequently shown to induce either local toxicity or global dampening of mucosal TLR signaling, PDB is a specific inhibitor of the TLR7/9-triggered innate immune response which may be critical for the local expansion of HIV-1 from the genital tract to systemic sites of infection. The potent antiviral activity and excellent toxicity profile of PDB, coupled with its immunomodulatory characteristics for activity against HIV-1, make it a promising candidate microbicide for preclinical development.
Acknowledgments
This work was supported by grants NIH R21 AI082680, NIH R01 AI062437, NIH R01 AI046719, and R01 AI066215 to P.D.K. from the National Institutes of Health.
P4-R5 MAGI cells were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, from Nathaniel Landau.
Footnotes
Published ahead of print on 12 July 2010.
REFERENCES
- 1.Abel, K., Y. Wang, L. Fritts, E. Sanchez, E. Chung, P. Fitzgerald-Bocarsly, A. M. Krieg, and C. J. Miller. 2005. Deoxycytidyl-deoxyguanosine oligonucleotide classes A, B, and C induce distinct cytokine gene expression patterns in rhesus monkey peripheral blood mononuclear cells and distinct alpha interferon responses in TLR9-expressing rhesus monkey plasmacytoid dendritic cells. Clin. Diagn. Lab. Immunol. 12:606-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, D. J., J. A. Politch, L. D. Tucker, R. Fichorova, F. Haimovici, R. E. Tuomala, and K. H. Mayer. 1998. Quantitation of mediators of inflammation and immunity in genital tract secretions and their relevance to HIV type 1 transmission. AIDS Res. Hum. Retroviruses 14(Suppl. 1):S43-S49. [PubMed] [Google Scholar]
- 3.Balzarini, J., H. Pelemans, S. Aquaro, C. F. Perno, M. Witvrouw, D. Schols, E. De Clercq, and A. Karlsson. 1996. Highly favorable antiviral activity and resistance profile of the novel thiocarboxanilide pentenyloxy ether derivatives UC-781 and UC-82 as inhibitors of human immunodeficiency virus type 1 replication. Mol. Pharmacol. 50:394-401. [PubMed] [Google Scholar]
- 4.Beignon, A. S., K. McKenna, M. Skoberne, O. Manches, I. DaSilva, D. G. Kavanagh, M. Larsson, R. J. Gorelick, J. D. Lifson, and N. Bhardwaj. 2005. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. J. Clin. Invest. 115:3265-3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bjercke, S., H. Scott, L. R. Braathen, and E. Thorsby. 1983. HLA-DR-expressing Langerhans'-like cells in vaginal and cervical epithelium. Acta Obstet. Gynecol. Scand. 62:585-589. [DOI] [PubMed] [Google Scholar]
- 6.Bollen, L. J., K. Blanchard, P. H. Kilmarx, S. Chaikummao, C. Connolly, P. Wasinrapee, N. Srivirojana, J. Achalapong, J. W. Tappero, and J. M. McNicholl. 2008. No increase in cervicovaginal proinflammatory cytokines after Carraguard use in a placebo-controlled randomized clinical trial. J. Acquir. Immune Defic. Syndr. 47:253-257. [DOI] [PubMed] [Google Scholar]
- 7.Boyd, M. R., K. R. Gustafson, J. B. McMahon, R. H. Shoemaker, B. R. O'Keefe, T. Mori, R. J. Gulakowski, L. Wu, M. I. Rivera, C. M. Laurencot, M. J. Currens, J. H. Cardellina II, R. W. Buckheit, Jr., P. L. Nara, L. K. Pannell, R. C. Sowder II, and L. E. Henderson. 1997. Discovery of cyanovirin-N, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: potential applications to microbicide development. Antimicrob. Agents Chemother. 41:1521-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charneau, P., G. Mirambeau, P. Roux, S. Paulous, H. Buc, and F. Clavel. 1994. HIV-1 reverse transcription. A termination step at the center of the genome. J. Mol. Biol. 241:651-662. [DOI] [PubMed] [Google Scholar]
- 9.Collins, K. B., B. K. Patterson, G. J. Naus, D. V. Landers, and P. Gupta. 2000. Development of an in vitro organ culture model to study transmission of HIV-1 in the female genital tract. Nat. Med. 6:475-479. [DOI] [PubMed] [Google Scholar]
- 10.Dhawan, D., and K. H. Mayer. 2006. Microbicides to prevent HIV transmission: overcoming obstacles to chemical barrier protection. J. Infect. Dis. 193:36-44. [DOI] [PubMed] [Google Scholar]
- 11.Dimitonova, S. P., S. T. Danova, J. P. Serkedjieva, and B. V. Bakalov. 2007. Antimicrobial activity and protective properties of vaginal lactobacilli from healthy Bulgarian women. Anaerobe 13:178-184. [DOI] [PubMed] [Google Scholar]
- 12.Equils, O., M. L. Schito, H. Karahashi, Z. Madak, A. Yarali, K. S. Michelsen, A. Sher, and M. Arditi. 2003. Toll-like receptor 2 (TLR2) and TLR9 signaling results in HIV-long terminal repeat trans-activation and HIV replication in HIV-1 transgenic mouse spleen cells: implications of simultaneous activation of TLRs on HIV replication. J. Immunol. 170:5159-5164. [DOI] [PubMed] [Google Scholar]
- 13.Fauci, A. S. 2008. 25 years of HIV. Nature 453:289-290. [DOI] [PubMed] [Google Scholar]
- 14.Girard, M., J. Mahoney, Q. Wei, E. van der Ryst, E. Muchmore, F. Barre-Sinoussi, and P. N. Fultz. 1998. Genital infection of female chimpanzees with human immunodeficiency virus type 1. AIDS Res. Hum. Retroviruses 14:1357-1367. [DOI] [PubMed] [Google Scholar]
- 15.Gorden, K. K., X. Qiu, J. J. Battiste, P. P. Wightman, J. P. Vasilakos, and S. S. Alkan. 2006. Oligodeoxynucleotides differentially modulate activation of TLR7 and TLR8 by imidazoquinolines. J. Immunol. 177:8164-8170. [DOI] [PubMed] [Google Scholar]
- 16.Haas, T., J. Metzger, F. Schmitz, A. Heit, T. Muller, E. Latz, and H. Wagner. 2008. The DNA sugar backbone 2′ deoxyribose determines toll-like receptor 9 activation. Immunity 28:315-323. [DOI] [PubMed] [Google Scholar]
- 17.Haas, T., F. Schmitz, A. Heit, and H. Wagner. 2009. Sequence independent interferon-alpha induction by multimerized phosphodiester DNA depends on spatial regulation of Toll-like receptor-9 activation in plasmacytoid dendritic cells. Immunology 126:290-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haase, A. T. 2005. Perils at mucosal front lines for HIV and SIV and their hosts. Nat. Rev. Immunol. 5:783-792. [DOI] [PubMed] [Google Scholar]
- 19.Herold, B. C., I. Scordi-Bello, N. Cheshenko, D. Marcellino, M. Dzuzelewski, F. Francois, R. Morin, V. M. Casullo, R. A. Anderson, C. Chany II, D. P. Waller, L. J. Zaneveld, and M. E. Klotman. 2002. Mandelic acid condensation polymer: novel candidate microbicide for prevention of human immunodeficiency virus and herpes simplex virus entry. J. Virol. 76:11236-11244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howett, M. K., E. B. Neely, N. D. Christensen, B. Wigdahl, F. C. Krebs, D. Malamud, S. D. Patrick, M. D. Pickel, P. A. Welsh, C. A. Reed, M. G. Ward, L. R. Budgeon, and J. W. Kreider. 1999. A broad-spectrum microbicide with virucidal activity against sexually transmitted viruses. Antimicrob. Agents Chemother. 43:314-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iaccino, E., M. Schiavone, G. Fiume, I. Quinto, and G. Scala. 2008. The aftermath of the Merck's HIV vaccine trial. Retrovirology 5:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khanna, K. V., K. J. Whaley, L. Zeitlin, T. R. Moench, K. Mehrazar, R. A. Cone, Z. Liao, J. E. Hildreth, T. E. Hoen, L. Shultz, and R. B. Markham. 2002. Vaginal transmission of cell-associated HIV-1 in the mouse is blocked by a topical, membrane-modifying agent. J. Clin. Invest. 109:205-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kilmarx, P. H. 2009. Global epidemiology of HIV. Curr. Opin. HIV AIDS 4:240-246. [DOI] [PubMed] [Google Scholar]
- 24.Klasse, P. J., R. J. Shattock, and J. P. Moore. 2006. Which topical microbicides for blocking HIV-1 transmission will work in the real world? PLoS Med. 3:e351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klinman, D. M., D. Tross, S. Klaschik, H. Shirota, and T. Sato. 2009. Therapeutic applications and mechanisms underlying the activity of immunosuppressive oligonucleotides. Ann. N. Y. Acad. Sci. 1175:80-88. [DOI] [PubMed] [Google Scholar]
- 26.Lackman-Smith, C., C. Osterling, K. Luckenbaugh, M. Mankowski, B. Snyder, G. Lewis, J. Paull, A. Profy, R. G. Ptak, R. W. Buckheit, Jr., K. M. Watson, J. E. Cummins, Jr., and B. E. Sanders-Beer. 2008. Development of a comprehensive human immunodeficiency virus type 1 screening algorithm for discovery and preclinical testing of topical microbicides. Antimicrob. Agents Chemother. 52:1768-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lederman, M. M., R. E. Offord, and O. Hartley. 2006. Microbicides and other topical strategies to prevent vaginal transmission of HIV. Nat. Rev. Immunol. 6:371-382. [DOI] [PubMed] [Google Scholar]
- 28.Lewis, M. G., S. Bellah, K. McKinnon, J. Yalley-Ogunro, P. M. Zack, W. R. Elkins, R. C. Desrosiers, and G. A. Eddy. 1994. Titration and characterization of two rhesus-derived SIVmac challenge stocks. AIDS Res. Hum. Retroviruses 10:213-220. [DOI] [PubMed] [Google Scholar]
- 29.Li, Q., J. D. Estes, P. M. Schlievert, L. Duan, A. J. Brosnahan, P. J. Southern, C. S. Reilly, M. L. Peterson, N. Schultz-Darken, K. G. Brunner, K. R. Nephew, S. Pambuccian, J. D. Lifson, J. V. Carlis, and A. T. Haase. 2009. Glycerol monolaurate prevents mucosal SIV transmission. Nature 458:1034-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mandl, J. N., A. P. Barry, T. H. Vanderford, N. Kozyr, R. Chavan, S. Klucking, F. J. Barrat, R. L. Coffman, S. I. Staprans, and M. B. Feinberg. 2008. Divergent TLR7 and TLR9 signaling and type I interferon production distinguish pathogenic and nonpathogenic AIDS virus infections. Nat. Med. 14:1077-1087. [DOI] [PubMed] [Google Scholar]
- 31.Meier, A., G. Alter, N. Frahm, H. Sidhu, B. Li, A. Bagchi, N. Teigen, H. Streeck, H. J. Stellbrink, J. Hellman, J. van Lunzen, and M. Altfeld. 2007. MyD88-dependent immune activation mediated by human immunodeficiency virus type 1-encoded Toll-like receptor ligands. J. Virol. 81:8180-8191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller, C. J., N. J. Alexander, S. Sutjipto, A. A. Lackner, A. Gettie, A. G. Hendrickx, L. J. Lowenstine, M. Jennings, and P. A. Marx. 1989. Genital mucosal transmission of simian immunodeficiency virus: animal model for heterosexual transmission of human immunodeficiency virus. J. Virol. 63:4277-4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller, C. J., Q. Li, K. Abel, E. Y. Kim, Z. M. Ma, S. Wietgrefe, L. La Franco-Scheuch, L. Compton, L. Duan, M. D. Shore, M. Zupancic, M. Busch, J. Carlis, S. Wolinsky, and A. T. Haase. 2005. Propagation and dissemination of infection after vaginal transmission of simian immunodeficiency virus. J. Virol. 79:9217-9227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller, C. J., M. Marthas, J. Torten, N. J. Alexander, J. P. Moore, G. F. Doncel, and A. G. Hendrickx. 1994. Intravaginal inoculation of rhesus macaques with cell-free simian immunodeficiency virus results in persistent or transient viremia. J. Virol. 68:6391-6400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Hanlon, D. E., B. R. Lanier, T. R. Moench, and R. A. Cone. 2010. Cervicovaginal fluid and semen block the microbicidal activity of hydrogen peroxide produced by vaginal lactobacilli. BMC Infect. Dis. 10:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patterson, B. K., A. Landay, J. Andersson, C. Brown, H. Behbahani, D. Jiyamapa, Z. Burki, D. Stanislawski, M. A. Czerniewski, and P. Garcia. 1998. Repertoire of chemokine receptor expression in the female genital tract: implications for human immunodeficiency virus transmission. Am. J. Pathol. 153:481-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patton, D. L., S. S. Thwin, A. Meier, T. M. Hooton, A. E. Stapleton, and D. A. Eschenbach. 2000. Epithelial cell layer thickness and immune cell populations in the normal human vagina at different stages of the menstrual cycle. Am. J. Obstet. Gynecol. 183:967-973. [DOI] [PubMed] [Google Scholar]
- 38.Pauwels, R., J. Balzarini, M. Baba, R. Snoeck, D. Schols, P. Herdewijn, J. Desmyter, and E. De Clercq. 1988. Rapid and automated tetrazolium-based colorimetric assay for the detection of anti-HIV compounds. J. Virol. Methods 20:309-321. [DOI] [PubMed] [Google Scholar]
- 39.Pearce-Pratt, R., and D. M. Phillips. 1996. Sulfated polysaccharides inhibit lymphocyte-to-epithelial transmission of human immunodeficiency virus-1. Biol. Reprod. 54:173-182. [DOI] [PubMed] [Google Scholar]
- 40.Piguet, V., and Q. Sattentau. 2004. Dangerous liaisons at the virological synapse. J. Clin. Invest. 114:605-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Politch, J. A., K. H. Mayer, and D. J. Anderson. 2009. Depletion of CD4+ T cells in semen during HIV infection and their restoration following antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 50:283-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rabe, L. K., and S. L. Hillier. 2003. Optimization of media for detection of hydrogen peroxide production by Lactobacillus species. J. Clin. Microbiol. 41:3260-3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwartz, J. L., C. Mauck, J. J. Lai, M. D. Creinin, V. Brache, S. A. Ballagh, D. H. Weiner, S. L. Hillier, R. N. Fichorova, and M. Callahan. 2006. Fourteen-day safety and acceptability study of 6% cellulose sulfate gel: a randomized double-blind phase I safety study. Contraception 74:133-140. [DOI] [PubMed] [Google Scholar]
- 44.Sodora, D. L., A. Gettie, C. J. Miller, and P. A. Marx. 1998. Vaginal transmission of SIV: assessing infectivity and hormonal influences in macaques inoculated with cell-free and cell-associated viral stocks. AIDS Res. Hum. Retroviruses 14(Suppl. 1):S119-S123. [PubMed] [Google Scholar]
- 45.Spurbeck, R. R., and C. G. Arvidson. 2010. Lactobacillus jensenii surface-associated proteins inhibit Neisseria gonorrhoeae adherence to epithelial cells. Infect. Immun. 78:3103-3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tan, G. T., A. Wickramasinghe, S. Verma, S. H. Hughes, J. M. Pezzuto, M. Baba, and P. Mohan. 1993. Sulfonic acid polymers are potent inhibitors of HIV-1 induced cytopathogenicity and the reverse transcriptases of both HIV-1 and HIV-2. Biochim. Biophys. Acta 1181:183-188. [DOI] [PubMed] [Google Scholar]
- 47.Tien, D., R. L. Schnaare, F. Kang, G. Cohl, T. J. McCormick, T. R. Moench, G. Doncel, K. Watson, R. W. Buckheit, M. G. Lewis, J. Schwartz, K. Douville, and J. W. Romano. 2005. In vitro and in vivo characterization of a potential universal placebo designed for use in vaginal microbicide clinical trials. AIDS Res. Hum. Retroviruses 21:845-853. [DOI] [PubMed] [Google Scholar]
- 48.Trifonova, R. T., G. F. Doncel, and R. N. Fichorova. 2009. Polyanionic microbicides modify Toll-like receptor-mediated cervicovaginal immune responses. Antimicrob. Agents Chemother. 53:1490-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang, Y., K. Abel, K. Lantz, A. M. Krieg, M. B. McChesney, and C. J. Miller. 2005. The Toll-like receptor 7 (TLR7) agonist, imiquimod, and the TLR9 agonist, CpG ODN, induce antiviral cytokines and chemokines but do not prevent vaginal transmission of simian immunodeficiency virus when applied intravaginally to rhesus macaques. J. Virol. 79:14355-14370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weber, J., A. Nunn, T. O'Connor, D. Jeffries, V. Kitchen, S. McCormack, J. Stott, N. Almond, A. Stone, and J. Darbyshire. 2001. ‘Chemical condoms’ for the prevention of HIV infection: evaluation of novel agents against SHIV(89.6PD) in vitro and in vivo. AIDS 15:1563-1568. [DOI] [PubMed] [Google Scholar]
- 51.Wenzel, J., M. Uerlich, O. Haller, T. Bieber, and T. Tueting. 2005. Enhanced type I interferon signaling and recruitment of chemokine receptor CXCR3-expressing lymphocytes into the skin following treatment with the TLR7-agonist imiquimod. J. Cutan. Pathol. 32:257-262. [DOI] [PubMed] [Google Scholar]
- 52.Wolff, H., and D. J. Anderson. 1988. Immunohistologic characterization and quantitation of leukocyte subpopulations in human semen. Fertil. Steril. 49:497-504. [PubMed] [Google Scholar]
- 53.Zaneveld, L. J., D. P. Waller, R. A. Anderson, C. Chany II, W. F. Rencher, K. Feathergill, X. H. Diao, G. F. Doncel, B. Herold, and M. Cooper. 2002. Efficacy and safety of a new vaginal contraceptive antimicrobial formulation containing high molecular weight poly(sodium 4-styrenesulfonate). Biol. Reprod. 66:886-894. [DOI] [PubMed] [Google Scholar]
- 54.Zeitlin, L., T. E. Hoen, S. L. Achilles, T. A. Hegarty, A. E. Jerse, J. W. Kreider, S. S. Olmsted, K. J. Whaley, R. A. Cone, and T. R. Moench. 2001. Tests of Buffergel for contraception and prevention of sexually transmitted diseases in animal models. Sex. Transm. Dis. 28:417-423. [DOI] [PubMed] [Google Scholar]
- 55.Zhu, T., H. Mo, N. Wang, D. S. Nam, Y. Cao, R. A. Koup, and D. D. Ho. 1993. Genotypic and phenotypic characterization of HIV-1 patients with primary infection. Science 261:1179-1181. [DOI] [PubMed] [Google Scholar]