Abstract
High-dose cefepime therapy is recommended for febrile neutropenia. Safety issues have been raised in a recent meta-analysis reporting an increased risk of mortality during cefepime therapy. Cefepime-related neurological toxicity has been associated with overdosing due to severe renal dysfunction. This study aimed to investigate the association between cefepime plasma concentrations and neurological toxicity in febrile neutropenic patients. Cefepime trough concentrations (by high-performance liquid chromatography) were retrospectively analyzed for 30 adult febrile neutropenic patients receiving the recommended high-dose regimen (6 g/day for a glomerular filtration rate [GFR] of >50 ml/min). The dose adjustment to renal function was evaluated by the ratio of the cefepime daily dose per 100 ml/min of glomerular filtration. The association between cefepime plasma concentrations and neurological toxicity was assessed on the basis of consistent neurological symptoms and/or signs (by NCI criteria). The median cefepime concentration was 8.7 mg/liter (range, 2.1 to 38 mg/liter) at a median of 4 days (range, 2 to 15 days) after the start of therapy. Neurological toxicity (altered mental status, hallucinations, or myoclonia) was attributed to cefepime in 6/30 (20%) patients (median GFR, 45 ml/min; range, 41 to 65 ml/min) receiving a median dose of 13.2 g/day per 100 ml/min GFR (range, 9.2 to 14.3 g/day per 100 ml/min GFR). Cefepime discontinuation resulted in complete neurological recovery for five patients and improvement for one patient. A multivariate logistic regression model confirmed high cefepime concentrations as an independent predictor of neurological toxicity, with a 50% probability threshold at ≥22 mg/liter (P = 0.05). High cefepime plasma concentrations are associated with neurological toxicity in febrile neutropenic patients with mild renal dysfunction. Careful adherence to normalized dosing per 100 ml/min GFR is crucial. Monitoring of plasma concentrations may contribute to preventing neurological toxicity of high-dose therapy for this life-threatening condition.
Cefepime, an extended-spectrum cephalosporin, is recommended for the empirical therapy of febrile neutropenia (11). The time during which the antibiotic plasma concentration is higher than the MIC for the causative pathogen is crucial for efficacy under this life-threatening condition (16). High-dose cefepime therapy (6 g/day for a glomerular filtration rate [GFR] of >50 ml/min) is recommended to achieve coverage of the most frequent bacterial pathogens in febrile neutropenia, including Pseudomonas aeruginosa (9, 26). Despite success rates of up to 60 to 80%, similar to those of other broad-spectrum beta-lactams, cefepime therapy has recently been found to be associated with higher mortality rates (29). These findings indirectly suggest that the excess mortality might be due to toxicity. In vitro and in vivo studies have attributed the proconvulsive effect of cephalosporins to the drug-induced suppression of inhibitory neurotransmission via a concentration-dependent modulation of the γ-amino-butyric acid [(GABA)A] receptors (24). Cefepime-related neurological toxicity, including encephalopathy, confusion, myoclonia, seizures, or nonconvulsive status epilepticus, has been reported for patients with severe renal dysfunction (2, 6, 7, 13, 17, 23). Coma leading to death has been observed in some cases (1, 6, 23). However, cefepime plasma concentrations were reported for only a minority of these clinical observations (2, 6, 14).
Pharmacokinetic analyses have demonstrated a linear relationship between cefepime dose and exposure and between the clearance of creatinine and the clearance of cefepime (3, 4, 25). Dose adjustment to renal function is recommended by the manufacturer if the GFR is lower than 50 ml/min (8). However, dose adjustment may be difficult to achieve during the therapy of patients requiring maximal drug doses for life-threatening infections and experiencing acute changes in the volume of distribution (capillary leak syndrome, volume overload, or hypoalbuminemia) and drug clearance (renal dysfunction or renal replacement therapy) (22). The unpredictable variability of cefepime blood concentrations may result in drug overexposure (6, 15, 22).
The objective of the present study was to assess the association between cefepime plasma concentrations and neurological toxicity of high-dose therapy in febrile neutropenic patients.
(Results of this analysis have been presented in an oral session [14a] at the 48th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy [ICAAC]-Infectious Diseases Society of America [IDSA] 46th Annual Meeting, Washington, DC, 27 October 2008.)
MATERIALS AND METHODS
Setting.
A therapeutic drug-monitoring program for anti-infective agents has been developed at the University Hospital of Lausanne (Switzerland) to improve the management of patients with severe infections. This study was conducted with patients hospitalized in the isolation unit of the Infectious Diseases Service for myeloablative chemotherapy of acute leukemia or intensive chemotherapy with autologous hematopoietic stem cell transplantation. All consecutive patients for whom cefepime trough concentrations had been measured during empirical therapy for febrile neutropenia over a 2-year period were retrospectively studied.
Management of febrile neutropenia.
Neutropenia was defined as a neutrophil count of <500/mm3 and fever as a single temperature of ≥38.5°C or two temperatures of ≥38°C. The management of febrile neutropenia, including the diagnosis of infection and prompt empirical broad-spectrum antibacterial therapy, was based on international guidelines (11, 12).
Clinical data.
Clinical and laboratory assessments (including serum creatinine levels) had been performed daily and reported in the medical records by the treating physicians. For the present analysis, patient demographics and characteristics (age, sex, weight, underlying disease, chemotherapy, serum creatinine values at baseline and during follow-up, etiology of fever, and comedications) were retrospectively extracted. The GFR was calculated by the Cockroft-Gault formula (creatinine clearance derived from age, sex, total body weight, and serum creatinine level). In addition, GFR normalized to a standard body weight of 70 kg was assessed in order to account for the potential imbalance due to interindividual weight variations when comparing different clinical characteristics.
Cefepime therapy.
Cefepime had been prescribed by the treating physicians at the dosing schedule for febrile neutropenia, i.e., 6 g/day (2 g every 8 h), with dosing adjustment for patients with a GFR of <50 ml/min according to the manufacturer's recommendations (8). Due to the retrospective design of the study, the investigators had no influence on drug dosing. Data on cefepime therapy were collected: unitary dose administered in a 30-min infusion, dosing interval, time of trough plasma concentration measurement after the start of therapy, and any dosing modifications. The dose adjustment to renal function was retrospectively evaluated by the ratio cefepime daily dose in g/day per 100 ml/min GFR and per 100 ml/min GFR normalized to a standard 70-kg body weight.
Measurement of cefepime plasma concentrations.
Trough plasma concentrations had been measured upon the request of the treating physicians for a suspicion of toxicity or impairment of renal function. Five-milliliter blood samples were collected into tubes containing sodium citrate 5 min before the administration of the subsequent dose. Tubes were centrifuged at 4°C within 1 h and stored at −80°C until analysis. Cefepime plasma concentrations were measured by a UV high-performance liquid chromatography method validated according to international guidelines (5). The analytical range was 0.25 to 200 mg/liter, and the intrarun/interrun accuracy and coefficient of variation were >95% and <10%, respectively. Cefepime trough plasma concentrations had been interpreted in real time by infectious disease specialists, and cefepime discontinuation or dosing adjustment had been proposed according to the clinical context (e.g., in the presence of neurological signs consistent with cefepime-associated toxicity).
Cefepime-associated neurological toxicity.
Medical records of patients with a measurement of cefepime plasma concentrations were retrospectively analyzed by the investigators. Cefepime-associated neurological toxicity was defined according to the following criteria: (i) consistent neurological symptoms and/or signs according to the National Cancer Institute (NCI) classification (confusion, depressed level of consciousness, cognitive disturbances, myoclonia, seizures, or hallucinations) occurring after ≥2 days of cefepime therapy, (ii) the absence of an alternative cause for neurological symptoms and/or signs, and (iii) improvement (complete or partial resolution) of neurological symptoms and/or signs within 2 days after cefepime discontinuation or dose reduction in the absence of a concomitant intervention (18). Severity and the probability of an association with cefepime therapy were graded according to the recommended NCI scale (18). Data on the following chemotherapeutic agents and drugs were collected due to their potential association with neurological toxicity (i.e., reported at an incidence of >0.1%): cytarabine (high dose, i.e., a cumulative dose of >3,000 mg/m2), methotrexate (parenteral or intrathecal), opioids (morphine, tramadol, and hydromorphone), amphotericin B, and voriconazole (8).
Statistical analysis.
Patients were divided in two distinct groups: (i) those with the presence of neurological signs and/or symptoms consistent with cefepime-associated neurological toxicity according to the above-described definition and (ii) those with an absence of neurological signs and/or symptoms. Fisher's exact test and nonparametric tests (Mann-Whitney) were used for the univariate comparison of proportions and continuous variables in these two groups, respectively. Two-sided P values of <0.05 were considered statistically significant. The relationship between cefepime dose adjusted to the creatinine clearance rate and drug plasma concentration was analyzed by Spearman's correlation. A logistic regression analysis (Stata software, version 10; Stata Corp., College Station, TX) assessed the probability of cefepime-associated neurological toxicity (defined according to the above-described criteria) as a function of the cefepime trough concentration. The role of the following covariates was assessed by a multiple logistic regression model forced to include cefepime plasma concentration, age, sex, weight, GFR, cefepime dose, duration of therapy, and opioid comedication; both stepwise forward selection and stepwise backward elimination of covariates were applied.
RESULTS
Cefepime plasma concentrations in 30 febrile neutropenic patients were measured at a median of 4 days after the start of therapy (range, 2 to 15 days). Demographics, clinical characteristics, and etiologies of febrile episodes are summarized in Table 1. No relevant comorbidity other than malignancy, no uremia, and no underlying central nervous system (CNS) disorder were present. All patients received the recommended cefepime daily dose of 6 g (2 g every 8 h) for a GFR of >50 ml/min, with the exception of two patients with a GFR of <50 ml/min at baseline, for whom the initial dose was adjusted appropriately according to the manufacturer's recommendations. At the time of measurement, a large variability of the ratio of the cefepime daily dose per GFR was observed: with a median of 7.5 g/day per 100 ml/min GFR and a range of 2.7 to 14.3 g/day per 100 ml/min GFR (median of 8.0 g/day per 100 ml/min GFR and range of 3.2 to 16.7 g/day per 100 ml/min GFR normalized to a 70-kg body weight). The ratio of the daily dose per GFR (and GFR normalized to a 70-kg body weight) was significantly higher for patients with mild renal impairment (i.e., GFR of 40 to 70 ml/min) than for those with normal renal function (GFR of >70 ml/min) or moderate or severe renal dysfunction (GFR of <40 ml/min) (P < 0.001) (Table 2).
TABLE 1.
Patient demographics, clinical characteristics, and episodes of febrile neutropenia
| Parameter for cefepime therapy courses (n = 30) | Value |
|---|---|
| No. (%) of male patients/no. (%) of female | |
| patients | 16 (53)/14 (47) |
| Median age (yr) (range) | 58 (32-78) |
| No. (%) of patients with type of malignancy | |
| Acute leukemiaa | 17 (57) |
| Non-Hodgkin lymphoma | 6 (20) |
| Multiple myeloma | 4 (13) |
| Solid tumors | 3 (10) |
| No. (%) of patients with type of chemotherapy | |
| High-dose cytarabine regimen (acute leukemia)b | 2 (7) |
| Low-dose cytarabine regimen (acute leukemia)c | 12 (40) |
| Autologous stem cell transplantationd | 11 (37) |
| Other | 5 (17) |
| Median duration of neutropenia (days) (range) | 14 (2-58) |
| No. of patients (%) with episodes of febrile neutropeniaf | |
| MDI | 13 (43) |
| Bacteremic | 12 (40) |
| Nonbacteremic | 1 (3) |
| CDI | 9 (30) |
| FUO | 8 (27) |
| No. (%) of patients who survived at discharge | 29 (97)e |
Fifteen patients with acute myeloid leukemia and two patients with acute lymphoblastic leukemia.
High-dose cytarabine (1,000 to 2,000 mg/m2/day for 3 to 6 days with mitoxantrone, aracytin, or amsacrin).
Low-dose cytarabine (200 mg/m2/day for 7 days with daunorubicin, idarubicin, or amsacrin).
Conditioning regimens for autologous stem cell transplantation include (i) cytarabine (400 mg/m2/day for 4 days) with carmustine, etoposide phosphate, and melphalan; (ii) melphalan (200 mg/m2, single dose); and (iii) cyclophosphamide and etoposide-phosphate.
One patient died due to a toxic encephalopathy probably associated with intrathecal methotrexate therapy.
No severe sepsis or septic shock was observed. MDI, microbiologically documented infections; CDI, clinically documented infections; FUO, fever of undetermined origin.
TABLE 2.
Cefepime doses and trough concentrations according to renal functiona
| Parameter | Value for group |
P value | ||
|---|---|---|---|---|
| GFR >70 ml/min (n = 15) | GFR 40-70 ml/min (n = 13) | GFR <40 ml/min (n = 2) | ||
| Median cefepime dose (g/day) (range) | 6 (all) | 6 (4-6) | 2 (all) | 0.0004 |
| Median cefepime dose (g/day) per 100 ml/min GFR (range) | 5.6 (2.7-8.1) | 10.7 (7.4-14.3) | 7.2 (7.1-7.4) | <0.0001 |
| Normalized for 70 kg body wtb | 5.4 (3.2-9.8) | 12.8 (6.0-16.7) | 8.2 (7.7-8.7) | 0.0003 |
| Median cefepime trough concn (mg/liter) (range) | 7 (2.1-21) | 20 (2.4-38) | 11.4 (6.8-16) | 0.02 |
Renal function is defined as the GFR at the time of measurement of cefepime plasma concentrations. Normal renal function, GFR of >70 ml/min; mild renal dysfunction, GFR of 40 to 70 ml/min; moderate to severe renal dysfunction, GFR of <40 ml/min.
The GFR has been normalized for a body weight of 70 kg in order to account for the potential imbalance due to body weight variations.
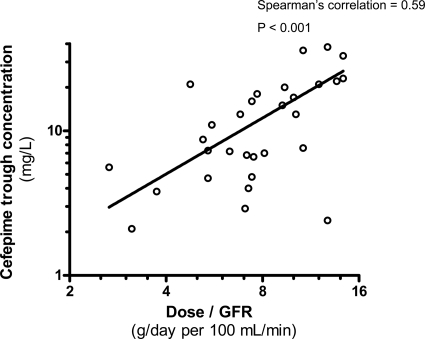
The median cefepime trough concentration was 8.7 mg/liter (range, 2.1 to 38 mg/liter). Figure 1 shows the log-log correlation between the cefepime daily dose adjusted to the GFR and the cefepime trough concentrations (Spearman's correlation coefficient of 0.59; P < 0.001).
FIG. 1.
Correlation between the ratio of the cefepime daily dose to the GFR (calculated creatinine clearance) and cefepime trough plasma concentrations. The continuous line represents a log-log correlation model.
The following neurological symptoms and signs were observed for 8 of 30 (27%) patients: altered mental status (1 patient), decreased level of consciousness (1 patient), hallucinations and cognitive disturbances (3 patients), and myoclonia (3 patients). These symptoms were attributed to cefepime for 6/30 (20%) patients fulfilling the NCI criteria of neurological toxicity described in Materials and Methods; for this subgroup of patients, the median cefepime trough concentration was 28 mg/liter (range, 15 to 38 mg/liter). For the two remaining patients with neurological symptoms and/or signs, hallucinations and cognitive disturbances were not attributed to cefepime therapy, as they did not fulfill the above-described NCI criteria. Opioid medication (tramadol and hydromorphone, respectively) was identified as the causal agent in these cases, as neurological signs resolved within 1 day after the discontinuation of opioids in the absence of any concomitant intervention. Cefepime therapy had been previously interrupted for one patient without significant improvement and was continued at an unchanged dose for the second patient, for whom a complete resolution of symptoms was observed after the discontinuation of the opioid. Cefepime trough concentrations were 2.4 and 8.7 mg/liter, respectively. These two patients were excluded from further analyses.
Among the remaining 22 patients, no other neurological symptoms and/or signs potentially related to drug adverse events (such as seizures, dizziness, ototoxicity, or neuropathy) were reported: the median cefepime trough concentration was 7.2 mg/liter, with a range of 2.1 to 21 mg/liter (P < 0.0001 versus patients with cefepime-associated neurological toxicity).
Patients with cefepime-associated neurological toxicity (n = 6) are compared with those without any neurological signs (n = 22) (control group) in Table 3.
TABLE 3.
Comparison of patients with cefepime-associated neurological toxicity and those without any neurological signs and/or symptoms
| Parameter | Value for group |
P value | |
|---|---|---|---|
| Cefepime-associated neurological toxicity (n = 6) | No neurological signs/symptoms (n = 22) | ||
| No. (%) of male patients | 6 (100) | 9 (41) | 0.02 |
| Median age (yr) (range) | 63 (56-74) | 56 (32-78) | 0.08 |
| Median wt (kg) (range) | 80 (66-85) | 72 (40-111) | 0.21 |
| No. (%) of patients with malignancy | |||
| Hematological | 6 (100) | 19 (86) | 1.00 |
| Solid tumor | 0 (0) | 3 (14) | 1.00 |
| No. (%) of patients with infection | |||
| Bacteremia | 1 (17) | 10 (45) | 0.36 |
| Other than bacteremia | 5 (83) | 12 (55) | 0.36 |
| No. (%) of patients taking comedications with potential neurological toxicity | |||
| High-dose cytarabinea | 0 (0) | 2 (9) | 1.00 |
| Intrathecal chemotherapyb | 2 (33) | 5 (23) | 0.62 |
| Opioid comedications | 2 (33) | 6 (27) | 1.00 |
| Amphotericin B-voriconazole | 1 (17)-0 (0) | 0 (0)-1 (5) | 0.39 |
| No. (%) of patients taking medications with potential nephrotoxicity | |||
| Vancomycin | 3 (50)c,d | 4 (18)c,d | 0.14 |
| Aminoglycosides | 0 (0) | 1 (5)d | 1.00 |
| Amphotericin B | 1 (17) | 0 (0) | 0.21 |
| GFR (ml/min) (range) | |||
| At start of cefepime therapy | 57 (52-70) | 72 (23-196) | 0.06 |
| Normalized for 70-kg body wte | 54 (43-67) | 72 (19-184) | 0.04 |
| At time of cefepime trough measurement | 45 (41-65) | 81 (27-226) | 0.02 |
| Normalized for 70-kg body wte | 43 (36-58) | 89 (23-189) | 0.006 |
| Median cefepime dose (g/day) per 100 ml/min GFR (range) | |||
| At start of cefepime therapy | 10.5 (8.6-11.5) | 8.2 (3.1-15.4) | 0.02 |
| Normalized for 70-kg body wte | 11.3 (9.0-14.0) | 7.9 (3.3-16.7) | 0.006 |
| At time of cefepime trough measurement | 13.2 (9.2-14.3) | 7.1 (2.7-12.0) | 0.002 |
| Normalized for 70-kg body wte | 14.2 (10.3-16.7) | 6.8 (3.2-12.8) | 0.0003 |
| Median cefepime trough (mg/liter) (range) | 28 (15-38) | 7.2 (2.1-21.0) | <0.0001 |
Cytarabine at 1,000 to 2,000 mg/m2/day for 3 to 6 days (within 14 days before the start of cefepime therapy).
Methotrexate at 15 mg, cytarabine at 30 mg, and hydrocortisone at 30 mg (within 14 days before the start of cefepime therapy).
Vancomycin trough blood concentrations were in the recommended range, 5 to 10 mg/liter.
No ototoxicity was detected clinically in patients receiving short-course therapies (i.e., <7 days) with aminoglycosides or glycopeptides.
The GFR has been normalized for a body weight of 70 kg in order to account for the potential imbalance due to body weight variations.
The six patients with cefepime-associated neurological toxicity were all males and had received the recommended cefepime daily dose of 6 g/day for a GFR of >50 ml/min at baseline (median, 57 ml/min; range, 52 to 70 ml/min). The median GFR at the time of measurement had slightly declined (45 ml/min; range, 41 to 65 ml/min) and was below 50 ml/min in 4/6 (67%) cases, whereas cefepime dosing had not been adjusted accordingly by the treating physicians (median dose, 13.2 g/day per 100 ml/min GFR; range, 9.2 to 14.3 g/day per 100 ml/min GFR; P < 0.002 versus patients without any neurological manifestations).
The clinical characteristics of these patients are presented in Table 4. Neurological toxicity had appeared at a median of 3 days (range, 2 to 14 days) after the start of cefepime therapy. In 5/6 (83%) cases, dose adjustment or therapy discontinuation resulted in complete neurological recovery within 1 day. One patient presenting with myoclonia and confusion experienced a transient partial improvement (resolution of myoclonia) 24 h after the discontinuation of cefepime. However, neurological signs (confusion and depressed level of consciousness) recurred 2 days later and progressed to coma, resulting in death after 20 days. Cerebral magnetic resonance imaging (MRI) showed a multifocal leukoencephalopathy, and the electroencephalogram showed a diffuse slow-wave activity consistent with a toxic etiology. The result of the analysis of the cerebrospinal fluid (including negative bacterial cultures and PCR assays for bacteria, herpes simplex virus, and JC virus) was normal with the exception of a slightly increased protein level. Among comedications, methotrexate (intrathecal injection 2 days before the onset of neurological symptoms) was considered a potential cause of this recurrent toxic encephalopathy. Two additional patients received concomitant opioid comedications. However, neurological signs persisted unchanged despite their interruption and resolved only after cefepime discontinuation.
TABLE 4.
Clinical characteristics of patients with cefepime-associated neurological toxicityd
| Patient characteristics (sex, age, wt, GFR at baseline)a | Malignancy, chemotherapy, IT (days from start of CFP) | CFP daily dosing schedule (dose [g/day per 100 ml/min GFR at start of therapy]) | Other comedication with potential neurological toxicity (days from start of CFP) | Time of AE (days from start of CFP) (GFR [ml/min]) | CFP trough concn (mg/liter) | Assessment of AE (NCI grading); causality of CFP overdosing | Modification of CFP dosing schedule and CFP trough concn in follow-up (days from intervention) | Final assessment (days after dose adjustment) |
|---|---|---|---|---|---|---|---|---|
| M, 63 yr, 84 kg, 52 ml/min | Acute myeloid leukemia, low-dose cytarabineb (−7) | Cefepime, 2 g every 8 h (11.5) | 3 (56) | 36 | Myoclonia (III); probable | Discontinuation | Complete resolution (+1) | |
| M, 64 yr, 78 kg, 57 ml/min | Acute myeloid leukemia, low-dose cytarabineb (−5), ITc (−11) | Cefepime, 2 g every 8 h (10.5) | 2 (65) | 15 | Myoclonia (II); probable | Discontinuation | Complete resolution (+1) | |
| M, 56 yr, 82 kg, 57 ml/min | Lymphoma, etoposide-phosphate, cyclophosphamide (−4) | Cefepime, 2 g every 8 h (10.5) | Tramadol (−5) | 3 (47) | 38 | Confusion (II); probable | 16-h interruption and then 33% decrease of daily dose; 13 mg/liter (+5) | Complete resolution (+1) |
| M, 73 yr, 85 kg, 69 ml/min | Acute myeloid leukemia, low-dose cytarabineb (ongoing) | Cefepime, 2 g every 8 h (8.7) | 3 (44) | 22 | Depressed level of consciousness (III); probable | 16-h interruption and then 33% decrease of daily dose | Complete resolution (+1) | |
| M, 61 yr, 73 kg, 70 ml/min | Multiple myeloma, melphalan (−9) | Cefepime, 2 g every 8 h (8.6) | Morphine (−1) | 6 (42) | 33 | Hallucinations, cognitive disturbances (III); probable | 16-h interruption and then 33% decrease of daily dose | Complete resolution (+1) |
| M, 74 yr, 66 kg, 53 ml/min | Acute myeloid leukemia, low-dose cytarabineb(−8), ITc (−2) | Cefepime, 2 g every 8 h (11.3) | Amphotericin B (+4) | 14 (41) | 23 | Myoclonia, confusion (III); probable | 16-h interruption and then 33% decrease of daily dose; 16 mg/liter (+1) | Partial improvement (+1) and then recurrent encephalopathy resulting in death (+20) |
The GFR was calculated by the Cockroft-Gault formula. M, male.
Low-dose cytarabine of 200 mg/m2/day for 7 days.
IT, intrathecal chemotherapy of methotrexate (15 mg), cytarabine (30 mg), and hydrocortisone (30 mg).
AE, adverse event; CFP, cefepime.
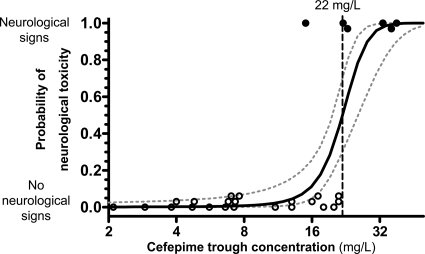
The logistic regression analysis showed an independent association of high cefepime trough concentrations with neurological adverse events (odds ratio [OR], 1.42 per mg/liter; 95% confidence interval [CI], 1.00 to 2.03; P = 0.05). Although male sex, GFR, and cefepime doses at the start of therapy and at the time of measurement of the cefepime trough concentration were significantly different between patients with and those without neurological toxicity (Table 3), these and other covariates (listed in “Statistical analysis” in Materials and Methods) tested in the multiple-regression model did not significantly improve the prediction of neurological toxicity by the cefepime trough concentration. The logistic regression model predicted a 50% probability of cefepime-associated neurological toxicity at ≥22 mg/liter (8% probability at ≥15 mg/liter, 34% at ≥20 mg/liter, 75% at ≥25 mg/liter, and 95% at ≥30 mg/liter) (Fig. 2). McFadden's pseudo-R2 value was 0.59.
FIG. 2.
Probability of cefepime-associated neurological toxicity as a function of the cefepime trough plasma concentration (logistic regression model). The symbols represent cefepime trough plasma concentrations in each individual patient with neurological toxicity (altered mental status, confusion, or myoclonia) attributed to cefepime according to NCI criteria (black symbols) (top) or without any neurological symptoms and/or signs (white symbols) (bottom). The continuous line represents the logistic regression model predicting the probability of neurological toxicity as a function of the cefepime trough plasma concentration; 1-standard-error confidence bands are reported within dashed lines. The vertical dotted line indicates the trough concentration of cefepime with a 50% increase of the risk of neurological toxicity.
DISCUSSION
Neurological toxicity attributed to documented cefepime overdosing in the context of a mild impairment of renal function was observed for febrile neutropenic cancer patients. Despite initially appropriate cefepime doses according to the manufacturer's recommendations, patients with a subsequent borderline impairment of renal function (i.e., GFR between 40 and 70 ml/min) showed a cefepime accumulation that resulted in neurological toxicity, mainly myoclonia and/or confusion. In most cases, a reduction in the dose of cefepime or discontinuation resulted in complete neurological recovery. However, one patient died due to a severe encephalopathy with tetraspasticity and coma following a transient improvement after cefepime discontinuation. An independent association between high cefepime plasma concentrations and neurological toxicity was found, with a 50% probability of neurological toxicity at cefepime plasma concentrations of ≥22 mg/liter. Due to the small sample size of this retrospective observation, this threshold and the frequency of these potentially severe adverse events remain to be confirmed with larger prospective data sets.
The monitoring of cefepime plasma concentrations contributed to the identification of the cause of neurological toxicity. In contrast, despite an important variability, cefepime trough concentrations observed in this analysis (range, 2.1 to 38 mg/liter) were all exceeding the MIC90 for the most frequent pathogens of febrile neutropenia (i.e., 2 mg/liter), which confirms the appropriateness of high-dose cefepime therapy for efficacy for this life-threatening condition (9).
Safety data from North American and European clinical trials of cefepime have suggested a low incidence of neurological toxicity (0.1% for seizures in a probable or possible relationship with cefepime therapy) comparable with those of other cephalosporins (19). More recent observations have reported a higher incidence of cefepime-associated encephalopathy (1%) and have suggested that the frequency of insidious neurological toxicity other than seizures, such as confusion, depressed level of consciousness, altered mental status, or myoclonia, is underestimated during therapy with cefepime or other cephalosporins (7, 10, 13, 17). This neurological toxicity was described mainly for patients with severe renal dysfunction, but data on cefepime plasma concentrations are lacking in the majority of these cases. Signs of encephalopathy have been reported for small case series, with cefepime trough concentrations of >30 mg/liter (2, 6, 14). The present study suggests that the neurotoxic threshold may be lower (e.g., 15 to 20 mg/liter). Whether concomitant medications such as opioids or previous chemotherapy may have contributed to lowering this threshold is unknown. Neurological toxicity, in particular myelopathy or cerebellar dysfunction, has been described for high-dose cytarabine regimens (cumulative doses of at least 3,000 mg/m2) (28). However, the six patients with cefepime-attributed neurological toxicity in the present study received lower cumulative cytarabine doses, i.e., <3,000 mg/m2. Similarly, the intrathecal administration of methotrexate has been associated with aseptic meningitis or subacute neurological signs (ataxia or seizures) (28). Delayed irreversible leukoencephalopathy has also been reported and was the most probable etiology of the fatal coma in one case in the present analysis. However, the transient neurological improvement after cefepime discontinuation suggests that this drug contributed to the initial signs of neurological toxicity in this patient. Some cases of coma and death associated with cefepime therapy were also reported for patients with severe renal dysfunction (1, 6, 23). However, few data on cefepime plasma concentrations were reported, and the causal role of cefepime therapy in irreversible encephalopathy leading to death remained unclear. A recent meta-analysis of febrile neutropenic patients reported a significant increase in the overall mortality rate for those receiving cefepime therapy compared with the mortality rate for those receiving other beta-lactam antibiotics (relative risk [RR], 1.42; 95% CI, 1.09 to 1.84) (29). The absence of differences in the success of antibiotic therapy (RR, 1.00; 95% CI, 0.96 to 1.05) indirectly suggests that this higher risk of mortality might be due to toxicity. However, source data on the incidence of neurological toxic events and causes of death were not available for this meta-analysis. Postmarketing investigations for the reassessment of the safety profile of cefepime requested by the Food and Drug Administration (FDA) failed to demonstrate an increased mortality rate for cefepime-treated patients (27). Nevertheless, experts have emphasized the need for an awareness of the signs and symptoms of neurological toxicity occurring during cefepime therapy and the importance of cefepime dose adjustment to renal function (20). The original data of the present study provide a rationale for substantiating these recent recommendations.
Dose adjustment is recommended for patients with impaired renal function when the GFR is lower than 50 ml/min (8, 26). The majority of cases of cefepime-related neurological toxicity reported in the literature occurred in the context of a severe impairment of renal function. Interestingly, in the present experience, this complication occurred only for patients with borderline renal dysfunction. Despite an appropriate initial cefepime dose according to the manufacturer's recommendations (i.e., 6 g/day for a GFR of >50 ml/min), a slight decrease of the GFR was observed in some cases but was not detected by the treating physicians, which possibly contributed to cefepime accumulation in the absence of a subsequent dose adjustment. However, cefepime-related neurological toxicity has also been observed for two patients with a stable GFR of >50 ml/min (at baseline and at the time of measurement), suggesting that the GFR threshold for the adjustment of high-dose cefepime therapy may be higher (e.g., 60 to 70 ml/min). Normalized dosing according to the GFR (i.e., 6 g/day per 100 ml/min GFR) might be suggested for the individualized optimization of the therapy regimen. These results were confirmed by normalizing the GFR for a standard 70-kg body weight in order to control the potential bias due to important interindividual variations of weight. Our observation reflects the difficulty in applying the dosing recommendations during therapy of febrile neutropenia because of frequent variations of the pharmacokinetic parameters (volume of distribution and renal clearance) that may influence drug exposure (21, 22). Moreover, renal function may be overestimated by the Cockroft-Gault formula under these debilitating conditions that are often associated with denutrition and amyotrophy, resulting in low serum creatinine concentrations. In this small subset of patients, we were unable to identify the role of potentially nephrotoxic comedications in the mild decline of renal function during cefepime therapy. In addition to a systematic check of the GFR, monitoring of cefepime concentrations may thus be useful during high-dose therapy for this life-threatening condition.
The limitations of this study are the retrospective assessment, the small sample size, and the lack of routine monitoring of cefepime plasma concentrations resulting in a possible selection bias, as cefepime measurements were triggered by specific clinical conditions.
In conclusion, the present study supports the utility of pharmacodynamic analyses of the relationship between antibiotic plasma concentrations and toxicity. These observations provide relevant information for improving the safety of high-dose cefepime therapy, which is standard for the treatment of febrile neutropenia. Cefepime accumulation may be associated with neurological toxicity in patients with a borderline impairment of renal function. Upfront drug dosing adjustment (e.g., if the GFR is below 60 to 70 ml/min) at the start of therapy and careful monitoring for a decline of renal function during therapy are required. Therapeutic drug monitoring for patients with suspected neurological toxicity or at risk of drug accumulation due to an impaired renal elimination may identify patients with a drug exposure exceeding the neurotoxic threshold (e.g., 15 to 20 mg/liter) and facilitate the diagnosis or prevention of this serious adverse event by individualized dose adjustment.
Acknowledgments
We thank Bristol-Myers Squibb Switzerland and Astra Zeneca Switzerland for unrestricted grant support.
We have no conflict of interest.
Footnotes
Published ahead of print on 12 July 2010.
REFERENCES
- 1.Abanades, S., J. Nolla, A. Rodriguez-Campello, C. Pedro, A. Valls, and M. Farre. 2004. Reversible coma secondary to cefepime neurotoxicity. Ann. Pharmacother. 38:606-608. doi: 10.1345/aph.1D322. [DOI] [PubMed] [Google Scholar]
- 2.Barbey, F., D. Bugnon, and J. P. Wauters. 2001. Severe neurotoxicity of cefepime in uremic patients. Ann. Intern. Med. 135:1011. [DOI] [PubMed] [Google Scholar]
- 3.Barbhaiya, R. H., S. T. Forgue, C. R. Gleason, C. A. Knupp, K. A. Pittman, D. J. Weidler, H. Movahhed, J. Tenney, and R. R. Martin. 1992. Pharmacokinetics of cefepime after single and multiple intravenous administrations in healthy subjects. Antimicrob. Agents Chemother. 36:552-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbhaiya, R. H., C. A. Knupp, S. T. Forgue, G. R. Matzke, D. R. Guay, and K. A. Pittman. 1990. Pharmacokinetics of cefepime in subjects with renal insufficiency. Clin. Pharmacol. Ther. 48:268-276. [DOI] [PubMed] [Google Scholar]
- 5.Bugnon, D., E. Giannoni, P. Majcherczyk, M. P. Glauser, and P. Moreillon. 2002. Pitfalls in cefepime titration from human plasma: plasma- and temperature-related drug degradation in vitro. Antimicrob. Agents Chemother. 46:3654-3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chatellier, D., M. Jourdain, J. Mangalaboyi, F. Ader, C. Chopin, P. Derambure, and F. Fourrier. 2002. Cefepime-induced neurotoxicity: an underestimated complication of antibiotherapy in patients with acute renal failure. Intensive Care Med. 28:214-217. doi: 10.1007/s00134-001-1170-9. [DOI] [PubMed] [Google Scholar]
- 7.Chow, K. M., C. C. Szeto, A. C. Hui, T. Y. Wong, and P. K. Li. 2003. Retrospective review of neurotoxicity induced by cefepime and ceftazidime. Pharmacotherapy 23:369-373. [DOI] [PubMed] [Google Scholar]
- 8.Compendium Suisse des Médicaments. 2009. Arzneimittel-Kompendium der Schweiz. Documed SA, Basel, Switzerland. http://www.kompendium.ch.
- 9.European Committee on Antimicrobial Susceptibility Testing. 30 June 2008, accession date. Antimicrobial wild type distributions of microorganisms. European Committee on Antimicrobial Susceptibility Testing. http://www.eucast.org.
- 10.Garces, E. O., M. F. Andrade de Anzambuja, D. da Silva, J. A. Bragatti, T. Jacoby, and T. F. Saldanha. 2008. Renal failure is a risk factor for cefepime-induced encephalopathy. J. Nephrol. 21:526-534. [PubMed] [Google Scholar]
- 11.Hughes, W. T., D. Armstrong, G. P. Bodey, E. J. Bow, A. E. Brown, T. Calandra, R. Feld, P. A. Pizzo, K. V. Rolston, J. L. Shenep, and L. S. Young. 2002. 2002 guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clin. Infect. Dis. 34:730-751. [DOI] [PubMed] [Google Scholar]
- 12.Immunocompromised Host Society. 1990. The design, analysis, and reporting of clinical trials on the empirical antibiotic management of the neutropenic patient. Report of a consensus panel. J. Infect. Dis. 161:397-401. [DOI] [PubMed] [Google Scholar]
- 13.Jallon, P., L. Fankhauser, R. Du Pasquier, A. Coeytaux, F. Picard, S. Hefft, and F. Assal. 2000. Severe but reversible encephalopathy associated with cefepime. Neurophysiol. Clin. 30:383-386. [DOI] [PubMed] [Google Scholar]
- 14.Lam, S., and I. H. Gomolin. 2006. Cefepime neurotoxicity: case report, pharmacokinetic considerations, and literature review. Pharmacotherapy 26:1169-1174. doi: 10.1592/phco.26.8.1169. [DOI] [PubMed] [Google Scholar]
- 14a.Lamoth, F., T. Buclin, A. Pascual, L. A. Decosterd, T. Calandra, and O. Marchetti. 2008. Association between high cefepime plasmid concentrations and neurotoxicity in febrile neutropenic patients with mild renal dysfunction, abstr. A-3558. Abstr. 48th Intersci. Conf. Antimicrob. Agents Chemother, and 46th Ann. Meet. Infect. Dis. Soc. Am. American Society for Microbiology, Washington, DC.
- 15.Lipman, J., S. C. Wallis, and C. Rickard. 1999. Low plasma cefepime levels in critically ill septic patients: pharmacokinetic modeling indicates improved troughs with revised dosing. Antimicrob. Agents Chemother. 43:2559-2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lortholary, O., A. Lefort, M. Tod, A. M. Chomat, C. Barras-Joly, and C. Cordonnier. 2008. Pharmacodynamics and pharmacokinetics of antibacterial drugs in the management of febrile neutropenia. Lancet Infect. Dis. 8:612-620. doi: 10.1016/S1473-3099(08)70228-7. [DOI] [PubMed] [Google Scholar]
- 17.Martinez-Rodriguez, J. E., F. J. Barriga, J. Santamaria, A. Iranzo, J. A. Pareja, M. Revilla, and C. R. la Rosa. 2001. Nonconvulsive status epilepticus associated with cephalosporins in patients with renal failure. Am. J. Med. 111:115-119. [DOI] [PubMed] [Google Scholar]
- 18.National Cancer Institute. 30 June 2008, accession date. Common terminology criteria for adverse events. National Cancer Institute online guidelines. National Cancer Institute, National Institutes of Health, Bethesda, MD. http://ctep.cancer.gov/reporting/ctc.html.
- 19.Neu, H. C. 1996. Safety of cefepime: a new extended-spectrum parenteral cephalosporin. Am. J. Med. 100:68S-75S. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen, T. D., B. Williams, and E. Trang. 19 February 2009, posting date. Cefepime therapy and all-cause mortality. Clin. Infect. Dis. [Epub ahead of print.] doi: 10.1086/597264. [DOI] [PubMed]
- 21.Pea, F., P. Viale, D. Damiani, F. Pavan, F. Cristini, R. Fanin, and M. Furlanut. 2005. Ceftazidime in acute myeloid leukemia patients with febrile neutropenia: helpfulness of continuous intravenous infusion in maximizing pharmacodynamic exposure. Antimicrob. Agents Chemother. 49:3550-3553. doi: 10.1128/AAC.49.8.3550-3553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pea, F., P. Viale, and M. Furlanut. 2005. Antimicrobial therapy in critically ill patients: a review of pathophysiological conditions responsible for altered disposition and pharmacokinetic variability. Clin. Pharmacokinet. 44:1009-1034. [DOI] [PubMed] [Google Scholar]
- 23.Sonck, J., G. Laureys, and D. Verbeelen. 2008. The neurotoxicity and safety of treatment with cefepime in patients with renal failure. Nephrol. Dial. Transplant. 23:966-970. doi: 10.1093/ndt/gfm713. [DOI] [PubMed] [Google Scholar]
- 24.Sugimoto, M., I. Uchida, T. Mashimo, S. Yamazaki, K. Hatano, F. Ikeda, Y. Mochizuki, T. Terai, and N. Matsuoka. 2003. Evidence for the involvement of GABA(A) receptor blockade in convulsions induced by cephalosporins. Neuropharmacology 45:304-314. [DOI] [PubMed] [Google Scholar]
- 25.Tam, V. H., P. S. McKinnon, R. L. Akins, G. L. Drusano, and M. J. Rybak. 2003. Pharmacokinetics and pharmacodynamics of cefepime in patients with various degrees of renal function. Antimicrob. Agents Chemother. 47:1853-1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.U.S. Food and Drug Administration. 30 June 2008, accession date. Maxipime. U.S. Food and Drug Administration, Washington, DC. http://www.fda.gov/medwatch/SAFETY/2003/03MAR_PI/Maxipime_PI.pdf.
- 27.U.S. Food and Drug Administration. 2009. Cefepime (marketed as Maxipime) update of ongoing safety review. U.S. Food and Drug Administration, Washington, DC. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm167427.htm.
- 28.Verstappen, C. C., J. J. Heimans, K. Hoekman, and T. J. Postma. 2003. Neurotoxic complications of chemotherapy in patients with cancer: clinical signs and optimal management. Drugs 63:1549-1563. [DOI] [PubMed] [Google Scholar]
- 29.Yahav, D., M. Paul, A. Fraser, N. Sarid, and L. Leibovici. 2007. Efficacy and safety of cefepime: a systematic review and meta-analysis. Lancet Infect. Dis. 7:338-348. doi: 10.1016/S1473-3099(07)70109-3. [DOI] [PubMed] [Google Scholar]