Abstract
Pentraxin 3 (PTX3) is an acute-phase glycoprotein with a nonredundant function in the host resistance to Aspergillus fumigatus. PTX3 activity was evaluated against pulmonary aspergillosis in rats immunosuppressed with cortisone acetate. PTX3 enhanced the survival rate and reduced the lung fungal burden of infected rats in both therapeutic and prophylactic modalities. Thus, we extended the protective activity of PTX3 in pulmonary aspergillosis to corticosteroid-induced immunodeficiency, which is a relevant clinical condition in graft-versus-host disease and in solid organ transplant.
Pentraxin 3 (PTX3) is a multimeric protein composed of eight subunits covalently associated by disulfide bonds and N-glycosylated with complex-type oligosaccharides (7, 8). PTX3 was identified as a nonredundant factor in the host resistance to the opportunistic fungus Aspergillus fumigatus (5). The effective pharmacological role of PTX3 against A. fumigatus infection was initially evaluated in mice with bone marrow transplants (5, 6). The combination of PTX3 with first-line antifungal treatments (amphotericin B [Ambisome] and voriconazole) indicated that PTX3 is able to enhance the therapeutic index of these drugs in mice (3, 6). PTX3 was also effective by itself against pulmonary aspergillosis in p47phox−/− mice, a model of chronic granulomatous disease (3).
Corticosteroid treatment is one of the most relevant pharmacological conditions that predispose humans to a high risk of infection with Aspergillus (9, 10, 12). To further characterize PTX3, we evaluated the protective activity of this protein against A. fumigatus in corticosteroid-induced immunodeficiency.
A rat model of invasive pulmonary aspergillosis (IPA) was set up. Immunodeficiency was induced in 8- to 10-week-old Sprague-Dawley rats (Harlan), fed with a low-protein diet (8% protein), by subcutaneous injections of cortisone acetate (CA) (Sigma Chemical Co., St. Louis, MO). CA was administered at a dose of 150 mg/kg of body weight 6 days before and then every other day up to the day of infection and was maintained after infection by administering 80 mg/kg every other day up to the end of the experiment (2). Conidia were obtained from 4- and 5-day cultures in Sabouraud agar medium at 28°C and scraped in a Sabouraud broth medium (0.05% Tween 80). Rats were intratracheally inoculated with a single administration of 5 × 107 conidia of A. fumigatus in 0.2 ml of sterile saline (6). This inoculum led to mortality within 12 to 13 days and provided an extensive lung infection (Fig. 1). In order to establish whether this model of pulmonary infection was reversible by a clinically approved antifungal, voriconazole was administered orally (30 mg/kg) the same day of infection and then daily for 8 days. In all the experiments, voriconazole-treated rats showed a mean survival time (MST) of >28 days and a survival rate in the range of 50 to 90%.
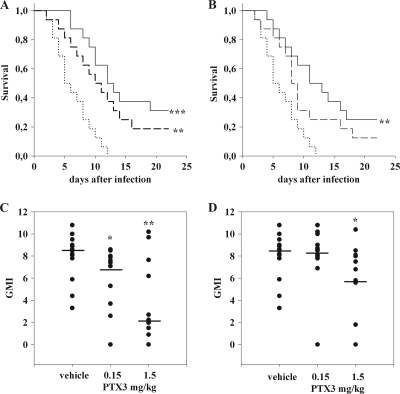
FIG. 1.
Survival rates and lung fungal burdens of rats infected with A. fumigatus and treated with PTX3 (n = 16). (A) Survival curves of rats treated daily with PTX3 3 days before the day of infection and then for an additional 3 days (schedule ii). (B) Survival curves of rats treated with PTX3 the same day of infection and then daily for an additional 3 days (schedule iii). Unbroken line, 1.5 mg/kg PTX3; dashed line, 0.15 mg/kg PTX3; dotted line, saline. (C) Fungal burden of rats treated with PTX3 according to schedule ii. (D) Fungal burden of rats treated with PTX3 according to schedule iii. The fungal burden was measured by evaluation of galactomannan content in the lung and was expressed as a positivity index (GMI). Dots represent the GMI from a single animal. Means are represented by bars. *, P < 0.05; **, P < 0.005; and ***, P < 0.001 (compared to the vehicle). The experiments were repeated at least twice.
Human recombinant PTX3 (UniProtKB accession no. P26022), herein reported as PTX3, was produced in HEK293 cells and purified as described previously (11). Administered doses of PTX3 (0.15 and 1.5 mg/kg) were suggested from previous experiments in murine models of IPA (3, 5, 6). PTX3 was intraperitoneally administered in rats once daily by following three different schedules: (i) 3 days before and the day of infection, (ii) 3 days before, the day of infection, and then for an additional 3 days, and (iii) the day of infection and then daily for an additional 3 days. Control animals were administered a vehicle (saline) by following administration schedule ii. Survival analysis was conducted using a log-rank test followed by Holm-Sidak pairwise tests.
All vehicle-treated rats died within 12 days, showing an MST of 6.4 days (95% confidence interval [CI], 4.9 to 7.9 days) (Fig. 1). A dose- and schedule-related protection was observed in rats treated with PTX3. When PTX3 was administered according to schedule i, no significant effect on mortality rate was observed. MST was 9.3 days (95% CI, 6.9 to 11.7 days) and 10.0 days (95% CI, 6.7 to 13.3 days) for the doses of 0.15 and 1.5 mg/kg, respectively. When PTX3 was administered according to schedule ii, PTX3 significantly reduced mortality, with an MST of 11.4 days (95% CI, 6.7 to 13.3 days; P value of <0.005 compared to the vehicle) and 14.3 days (95% CI, 11.2 to 17.4 days; P value of <0.001 compared to the vehicle) for the doses of 0.15 and 1.5 mg/kg, respectively (Fig. 1A). When PTX3 was administered according to schedule iii, the dose of 1.5 mg/kg was effective in reducing the mortality rate of rats (MST, 13.21 days; 95% CI, 9.8 to 16.3 days; P value of <0.005 compared to the vehicle), while 0.15 mg/kg was ineffective (MST, 10.2 days; 95% CI, 7.2 to 13.2 days; P value of 0.06) (Fig. 1B).
Fungal burden in the lung was evaluated by measuring galactomannan (GM) (1) and expressed as the galactomannan index (GMI). Lungs were collected from rats on the day of death and at the end of the experiment from the rats who survived. GM was detected in lung homogenates by a commercially available kit (Platelia Aspergillus; Bio-Rad Laboratories) (Fig. 1C and D). Statistical comparisons among groups were performed by the Kruskal-Wallis one-way analysis of variance (ANOVA) on ranks followed by Dunn's test.
No differences in lung GMI were observed between saline- and PTX3-treated rats when PTX3 was administered according to schedule i. If PTX3 was administered according to schedule ii, both doses reduced the lung fungal burden, and when PTX3 was administered according to schedule iii, only the dose of 1.5 mg/kg decreased the lung fungal burden.
In order to evaluate the role of PTX3 on neutrophil activity in the early postinfection phase, another experiment was performed. Rats were immunosuppressed and infected as described above and treated daily with saline or PTX3 at a dose of 1.5 mg/kg according to administration schedule iii. On day 4, rats were sacrificed and neutrophil activity in the lungs was evaluated by determining the levels of myeloperoxidase (MPO) by enzyme-linked immunosorbent assay (ELISA) (Hbt catalog no. HK105). Serum and lung keratinocyte chemoattractant (KC) was evaluated by ELISA (R&D Systems catalog no. RCN100), and the serum/lung ratio was calculated in order to evaluate the gradient for neutrophil recruitment. Statistics were calculated by a nonparametric ANOVA Kruskal-Wallis test.
CA-treated rats showed higher levels of MPO in the lung (53.5 ± 4.9 μg/g [mean ± standard deviation] of tissue) than untreated rats (sham) (31.2 ± 10.2 μg/g of tissue; P value of <0.05 compared to CA-treated rats). The infection (the CA plus A. fumigatus group) caused a further increase in lung MPO (67.7 ± 0.7 μg/g of tissue; P value of <0.05 compared to CA-treated rats). Treatment with PTX3 reduced lung MPO compared to that in rats treated with CA plus A. fumigatus (56.6 ± 10.3 μg/g of tissue; P value of <0.05 compared to rats treated with CA plus A. fumigatus) (Table 1). In spite of a certain variability in lung and serum KC levels, we observed, consistent with MPO titer in the lung, a slight increase in serum/lung KC ratio in PTX3-treated rats (Table 1).
TABLE 1.
Levels of lung MPO and ratios of serum/lung KC in rats with different conditions of CA immunosuppression, A. fumigatus infection, and PTX3 treatmenta
| Group (n = 4) | MPO (μg/g lung) | KC serum/lung ratio |
|---|---|---|
| Sham | 31.2 ± 10.2 | 0.201 ± 0.103 |
| CA | 53.5 ± 4.9b | 0.027 ± 0.004 |
| CA plus A. fumigatus | 67.7 ± 0.7c | 0.010 ± 0.001 |
| CA plus A. fumigatus plus PTX3 | 56.6 ± 10.3d | 0.024 ± 0.019 |
Lung MPO reported as micrograms per gram of lung, and KC level in serum and lung reported as a ratio. In the Sham group, rats remained untreated. In the CA group, rats were immunosuppressed with CA. In the group CA plus A. fumigatus, rats were immunosuppressed and infected with A. fumigatus conidia and treated with saline. In the group CA plus A. fumigatus plus PTX3, rats were immunosuppressed, infected, and treated with PTX3 at 1.5 mg/kg of body weight. Saline and PTX3 were administered according to schedule iii. Means ± standard deviations are reported for each group. The analysis was performed the 4th day after infection.
P < 0.05 compared to Sham.
P < 0.05 compared to CA.
P < 0.05 compared to CA plus A. fumigatus.
The activity of PTX3 on the fungal burden was also investigated at early time points postinfection. CFU were evaluated in 8 rats immunosuppressed and infected as described above. Four rats were treated with saline and the other four with PTX3 at a dose of 1.5 mg/kg by following administration schedule iii. Four days after infection, rats were sacrificed and lungs were aseptically removed and disrupted in 5 ml of sterile saline and treated with a tissue homogenizer (Stomacher; Seward, Ltd., London, United Kingdom). Serial dilutions of the homogenates were spread on Sabouraud dextrose agar plates and incubated at 37°C. CFU were counted after 24, 48, and 72 h of incubation. The number of CFU from the lungs of rats that received PTX3 was 1.7 ± 0.5 log10, while the number of CFU from rats treated with saline was 3.6 ± 0.5 log10 (Student's t test, P value of <0.05).
Here, we showed that PTX3 is effective against IPA in rats immunosuppressed with CA. These data confirm the ability of PTX3 to enhance conidial phagocytosis by innate immunity cells (5). In fact, a significant reduction of the lung fungal burden of PTX3-treated rats was observed, evaluated either as the amount of galactomannan or the number of CFU. The recently described ability of PTX3 to mitigate the primary inflammatory response (4) might explain why we found that PTX3 treatment reduced MPO and KC concentrations in the lungs of immunosuppressed or infected rats, restoring them to levels of uninfected immunosuppressed rats.
PTX3 may provide a new therapeutic option in clinical cases of antifungal drug resistance or in those cases where the severity of side effects on particularly compromised patients might discourage the use of traditional antifungal treatments.
Acknowledgments
Giovanni Salvatori is the author, as the inventor, of two patent applications on PTX3: WO200603774 and WO03072603.
Footnotes
Published ahead of print on 12 July 2010.
REFERENCES
- 1.Becker, M. J., S. de Marie, D. Willemse, H. A. Verbrugh, and I. A. Bakker-Woudenberg. 2000. Quantitative galactomannan detection is superior to PCR in diagnosing and monitoring invasive pulmonary aspergillosis in an experimental rat model. J. Clin. Microbiol. 38:1434-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cicogna, C. E., M. H. White, E. M. Bernard, T. Ishimura, M. Sun, W. P. Tong, and D. Armstrong. 1997. Efficacy of prophylactic aerosol amphotericin B lipid complex in a rat model of pulmonary aspergillosis. Antimicrob. Agents Chemother. 41:259-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D'Angelo, C., A. De Luca, T. Zelante, P. Bonifazi, S. Moretti, G. Giovannini, R. G. Iannitti, S. Zagarella, S. Bozza, S. Campo, G. Salvatori, and L. Romani. 2009. Exogenous pentraxin 3 restores antifungal resistance and restrains inflammation in murine chronic granulomatous disease. J. Immunol. 183:4609-4618. [DOI] [PubMed] [Google Scholar]
- 4.Deban, L., R. C. Russo, M. Sironi, F. Moalli, M. Scanziani, V. Zambelli, I. Cuccovillo, A. Bastone, M. Gobbi, S. Valentino, A. Doni, C. Garlanda, S. Danese, G. Salvatori, M. Sassano, V. Evangelista, B. Rossi, E. Zenaro, G. Constantin, C. Laudanna, B. Bottazzi, A. Mantovani. 2010. Regulation of leukocyte recruitment by the long pentraxin PTX3. Nat. Immunol. 11:328-334. [DOI] [PubMed] [Google Scholar]
- 5.Garlanda, C., E. Hirsch, S. Bozza, A. Salustri, M. De Acetis, R. Nota, A. Maccagno, F. Riva, B. Bottazzi, G. Peri, A. Doni, L. Vago, M. Botto, R. De Santis, P. Carminati, G. Siracusa, F. Altruda, A. Vecchi, L. Romani, A. Mantovani. 2002. Nonredundant role of the long pentraxin PTX3 in anti-fungal innate immune response. Nature 420:182-186. [DOI] [PubMed] [Google Scholar]
- 6.Gaziano, R., S. Bozza, S. Bellocchio, K. Perruccio, C. Montagnoli, L. Pitzurra, G. Salvatori, R. De Santis, P. Carminati, A. Mantovani, and L. Romani. 2004. Anti-Aspergillus fumigatus efficacy of pentraxin 3 alone and in combination with antifungals. Antimicrob. Agents Chemother. 48:4414-4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inforzato, A., V. Rivieccio, A. P. Morreale, A. Bastone, A. Salustri, L. Scarchilli, A. Verdoliva, S. Vincenti, G. Gallo, C. Chiapparino, L. Pacello, E. Nucera, O. Serlupi-Crescenzi, A. J. Day, B. Bottazzi, A. Mantovani, R. De Santis, and G. Salvatori. 2008. Structural characterization of PTX3 disulfide bond network and its multimeric status in cumulus matrix organization. J. Biol. Chem. 283:10147-10161. [DOI] [PubMed] [Google Scholar]
- 8.Inforzato, A., G. Peri, A. Doni, C. Garlanda, A. Mantovani, A. Bastone, A. Carpentieri, A. Amoresano, P. Pucci, A. Roos, M. R. Daha, S. Vincenti, G. Gallo, P. Carminati, R. De Santis, G. Salvatori. 2006. Structure and function of the long pentraxin PTX3 glycosidic moiety: fine-tuning of the interaction with C1q and complement activation. Biochemistry 45:11540-11551. [DOI] [PubMed] [Google Scholar]
- 9.Jantunen, E., A. Nihtinen, and V. J. Anttila. 2008. Changing landscape of invasive aspergillosis in allogeneic stem cell transplant recipients. Transpl. Infect. Dis. 10:156-161. [DOI] [PubMed] [Google Scholar]
- 10.Paczesny, S., D. Hanauer, Y. Sun, and P. Reddy. 2010. New perspectives on the biology of acute GVHD. Bone Marrow Transplant. 45:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rivieccio, V., A. Esposito, P. Bellofiore, P. Palladino, M. Sassano, M. Colombo, and A. Verdoliva. 2007. High-level expression and efficient purification of recombinant human long pentraxin PTX3 in Chinese hamster ovary cells. Protein Expr. Purif. 51:49-58. [DOI] [PubMed] [Google Scholar]
- 12.Salmasian, H., M. Rohanizadegan, S. Banihosseini, R. Rahimi Darabad, M. Rabbani-Anari, A. Shakiba, and J. L. Ferrara. 2010. Corticosteroid regimens for treatment of acute and chronic graft versus host disease (GvHD) after allogenic stem cell transplantation. Cochrane Database Syst. Rev. 20:CD005565. [DOI] [PMC free article] [PubMed] [Google Scholar]