Abstract
We report the discovery of novel boron-containing molecules, exemplified by N-(1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)-2-trifluoromethylbenzamide (AN3520) and 4-fluoro-N-(1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)-2-trifluoromethylbenzamide (SCYX-6759), as potent compounds against Trypanosoma brucei in vitro, including the two subspecies responsible for human disease T. b. rhodesiense and T. b. gambiense. These oxaborole carboxamides cured stage 1 (hemolymphatic) trypanosomiasis infection in mice when administered orally at 2.5 to 10 mg/kg of body weight for 4 consecutive days. In stage 2 disease (central nervous system [CNS] involvement), mice infected with T. b. brucei were cured when AN3520 or SCYX-6759 were administered intraperitoneally or orally (50 mg/kg) twice daily for 7 days. Oxaborole-treated animals did not exhibit gross signs of compound-related acute or subchronic toxicity. Metabolism and pharmacokinetic studies in several species, including nonhuman primates, demonstrate that both SCYX-6759 and AN3520 are low-clearance compounds. Both compounds were well absorbed following oral dosing in multiple species and also demonstrated the ability to cross the blood-brain barrier with no evidence of interaction with the P-glycoprotein transporter. Overall, SCYX-6759 demonstrated superior pharmacokinetics, and this was reflected in better efficacy against stage 2 disease in the mouse model. On the whole, oxaboroles demonstrate potent activity against all T. brucei subspecies, excellent physicochemical profiles, in vitro metabolic stability, a low potential for CYP450 inhibition, a lack of active efflux by the P-glycoprotein transporter, and high permeability. These properties strongly suggest that these novel chemical entities are suitable leads for the development of new and effective orally administered treatments for human African trypanosomiasis.
Human African trypanosomiasis (HAT), perhaps better known as sleeping sickness, is a major threat to the health of over 60 million people in 36 countries in sub-Saharan Africa (11). The estimated number of cases is 50,000 to 70,000 (38, 44). Sleeping sickness is caused by the trypanosomatid protozoan parasite Trypanosoma brucei and is spread by the bite of a tsetse fly. If left untreated, HAT is inevitably fatal (22). There are two main subspecies, T. b. rhodesiense (East Africa) and T. b. gambiense (West Africa), which result in human disease. Currently, treatment is limited to four drugs which are highly toxic and complicated to administer and require lengthy treatment regimens (23, 44). Treatment for stage 1, when the parasites are limited to the hemolymphatic system, is administration of pentamidine (T. b. gambiense) or suramin (T. b. rhodesiense). In stage 2, or central nervous system (CNS) disease, either melarsoprol (T. b. gambiense and T. b. rhodesiense) or eflornithine (T. b. gambiense) is used (14). Melarsoprol kills 5 to 10% of patients (32), and treatment failures of up to 25% have been reported (12, 13). Difluoromethylornithine (DFMO) (Ornidyl) requires a 2-week therapy regimen which is difficult to administer in rural clinics (34). A new combination therapy with DFMO and oral nifurtimox (NECT) reduces the dose time to 1 week but still requires intravenous (i.v.) dosing (35).
Antigenic variation is frequent, rendering prospects for vaccine development impracticable (22, 27). What is urgently needed is a safe, orally (p.o.) administered drug, effective against both stages 1 and 2 of HAT, which then eliminates the need for staging and raises the potential for the eradication of sleeping sickness. Toward this aspiration, we have identified a class of boron-containing compounds, oxaborole carboxamides, as novel leads that show potent and selective trypanocidal activity in vitro. Two examples chosen from this lead series are AN3520 and SCYX-6759. These compounds exhibit rapid, time-dependent cidal activity against T. brucei in vitro and in vivo and are curative in mice with acute or neurological forms of the disease. These oxaborole carboxamides are highly permeable and metabolically stable. Pharmacokinetic analysis demonstrates that AN3520 and SCYX-6759 are orally bioavailable and are able to cross the blood-brain barrier (BBB) at sufficient levels to cure mice in stage 2 of disease. Development of oxaborole carboxamides represents the first time within the past 30 years that a novel lead compound class with activity against the CNS disease model has been identified.
MATERIALS AND METHODS
Test compounds.
A library of proprietary small molecule therapeutics derived from a boron chemistry platform provides the basis for this drug discovery effort. This library of oxaborole compounds was jointly screened by Anacor Pharmaceuticals, Inc. (Palo Alto, CA) and Scynexis, Inc. (Research Triangle Park, NC). Additional analogues were synthesized in-house (Scynexis, Inc., Research Triangle Park, NC) during lead optimization. AN3520 [N-(1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)-2-trifluoromethylbenzamide] and SCYX-6759 [4-fluoro-N-(1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)-2-trifluoro-methylbenzamide] are representative oxaborole 6-carboxamides that demonstrate potent activity against Trypanosoma brucei, including the clinically relevant subspecies T. b. rhodesiense and T. b. gambiense. AN3520 and SCYX-6759 were prepared as 10-mg/ml stocks in dimethyl sulfoxide (DMSO) for in vitro biological and absorption, distribution, metabolism, and excretion (ADME) assays. All compounds were synthesized to a purity of more than 95%, and their identities were confirmed by proton nuclear magnetic resonance (NMR) and high-pressure liquid chromatography (HPLC) with mass spectrometry.
Trypanosome and cell culture.
The bloodstream form T. brucei brucei 427 strain was obtained from Ken Stuart (Seattle Biomedical Research Institute, Seattle, WA) and was used for routine assessment of compound sensitivity in vitro. Additional strains used in vitro include T. b. rhodesiense STIB900 and T. b. gambiense STIB930, a derivative of strain THI/78E (031). T. b. brucei EATRO 110 was a generous gift from the late William Trager of Rockefeller University. The T. b. brucei TREU 667 strain was kindly provided by F. W. Jennings at the University of Glasgow. Both T. b. brucei EATRO 110 and TREU 667 were cultured according to previously described conditions (19, 42) and used for in vivo studies.
T. b. brucei was cultured in complete HMI-9 medium (17) with 10% fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA), 10% Serum Plus medium (SAFC Biosciences, Lenexa, KS), and 100 units/ml penicillin and 0.1 mg/ml streptomycin. To ensure log growth phase, trypanosomes were subcultured at the appropriate dilutions (typically 1:100) every 2 to 3 days in fresh HMI-9 medium. T. b. rhodesiense parasites were grown in minimum essential medium (MEM) with Earle's salts supplemented with 15% horse serum. For T. b. gambiense, the MEM was supplemented with 5% heat-inactivated fetal calf serum (FCS) and 15% human serum (8). L929 mouse fibroblast cells (ATCC CCL1; American Type Culture Collection, Rockville, MD) were used to determine parasite versus mammalian cell selectivity. MDCKII-hMDR1 cells were generously provided by Pieter Borst at the Netherlands Cancer Institute. Mammalian cells were cultured in Dulbecco's modified Eagle medium (DMEM) containing 10% (vol/vol) FBS, 100 units/ml penicillin, and 0.1 mg/ml streptomycin, and Glutamax was added to MDCKII-hMDR1 cultures.
In vitro compound sensitivity assays.
Compounds to be tested were serially diluted in DMSO and added to 96-well plates to give final concentrations ranging from 0.01 to 5 μg/ml. T. b. brucei parasites in the log phase of growth were diluted in HMI-9 medium and added to each well for a final concentration of 1 × 104 parasites per well. For the sensitivity assays using T. b. rhodesiense and T. b. gambiense, parasites were cultured in MEM supplemented with Baltz components (8), diluted in the aforementioned culture medium, and added to each well at a density of 1 × 103 cells/well. The final concentration of DMSO was 0.5%. After 72 h of incubation, resazurin (Sigma-Aldrich, St. Louis, MO) (36) was added to each well (20 μl of 25-mg/100-ml stock in phosphate buffered saline [PBS]) and incubated for an additional 4 to 6 h. To assess cell viability, fluorescence was quantified using an EnVision multilabel plate reader (Perkin Elmer, Waltham, MA) at an excitation wavelength of 530 nm and an emission of 590 nm. Triplicate data points were averaged to generate sigmoidal dose response curves and determine 50% inhibitory concentration (IC50) or IC90 values using XLfit curve-fitting software from IDBS (Guilford, United Kingdom). The IC50 is defined as the amount of compound required to decrease parasite or cell viability by 50% compared to the number of parasites grown in the absence of the test compound. The MIC, defined as the lowest concentration of a compound that completely inhibits visible parasite growth, was determined by visual inspection of 96-well plates after 48 to 72 h of incubation with the test compounds. To evaluate the effects of serum on trypanocidal activity, assays were performed in the presence of increasing concentrations of FCS (Invitrogen, Carlsbad, CA), from 2.5 to 50%. The results were expressed as fold changes in IC50s relative to standard conditions (10% FCS).
An evaluation of mammalian cell cytotoxicity was carried out in parallel with the trypanosome sensitivity assays. L929 mouse fibroblast cells were seeded at 2 × 103 per well and handled as described above for the trypanosome sensitivity assay.
Time-kill assays.
The assessment of oxaborole-mediated killing of T. b. brucei in vitro was conducted using the CellTiter Glo kit (Promega, Inc., Madison, WI) to measure trypanosome ATP content as a real-time indicator of viability. Test compounds were serially diluted from 5 to 0.01 μg/ml into white-wall/clear-bottom 96-well plates (Corning Inc., Lowell, MA) containing HMI-9 media. Trypanosomes (1 × 104) were added to each well. At specified intervals, the CellTiter Glo reagent was added to lyse the parasites, and the plates were incubated for 10 min in the dark. Luminescence was quantified using an EnVision plate reader. All determinations were done in duplicate. Time-kill parameters were determined from plots of parasite viability versus incubation time for each concentration tested. To study drug-induced morphological changes, treated parasites were observed under the 100× objective on a Nikon TE2000 equipped with a DS-Di1 cooled monochrome camera and NIS-Elements imaging software (Nikon Instruments, Lewisville, TX). Several microscope fields were observed, and drug effects on parasites from each of the test concentrations were documented.
Examination of reversibility of trypanocidal effects.
To establish the time required to cause persistent or irreversible effects by oxaboroles, T. b. brucei parasites were assessed for their ability to recover from transient exposure to test compounds. Trypanosomes were seeded in clear 96-well V-bottom plates at a density of 1 × 105 parasites per well and incubated with the serially diluted test compound (from 0.02 to 10 μg/ml). One plate was prepared for each time point. At the designated time, a plate was removed from the incubator and spun at 4.4 × 103 rpm for 5 min to sediment the parasites. The supernatant was aspirated, and 100 μl of warmed HMI-9 medium was added to each well. The wash was repeated twice more. The parasites were resuspended in 100 μl of warmed medium, and 20 μl of this suspension was added to 80 μl of HMI-9 medium in triplicate plates. Following 72 h of incubation, resazurin was added and trypanocidal activity determined as described for the in vitro sensitivity assay.
Efficacy in the mouse model for acute HAT.
For efficacy studies against acute infections, groups of 3 to 5 female Swiss Webster mice (Ace Animals, Boyertown, PA) were injected intraperitoneally (i.p.) with freshly drawn infected rat blood containing 2.5 × 105 trypanosomes (T. b. brucei EATRO 110 strain). Oxaborole compounds were formulated in 2% ethanol-5% dextrose and were given at a dose volume of 200 μl for a 25-g animal starting 24 h after infection. Animals remaining parasite free for more than 30 days beyond the end of the treatment period were considered cured (3, 4).
Efficacy in a mouse model for chronic (CNS) HAT.
Evaluation of AN3520 and SCYX6759 against stage 2 CNS infections was conducted with a chronic disease-inducing strain (TREU 667) of T. b. brucei (3, 4, 5). Female Swiss Webster mice were infected intraperitoneally with 1 × 104 parasites (200 μl) followed by treatment with a single 10 mg/kg i.p. dose of diminazene aceturate (Berenil) on day 4 postinfection to serve as the positive control. Infection in the remaining groups of animals was allowed to proceed for 21 days prior to treatment with either diminazene aceturate (single i.p. dose at 10 mg/kg) or the test compound given i.p. or p.o. twice daily for 7 or 14 days. Animals were immediately removed from the cages and euthanized upon recrudescence. Animals remaining aparasitemic for at least 180 days after the end of the treatment period were considered cured (3, 4, 5). Brain homogenates or the blood of oxaborole-cured animals failed to generate infection when injected into naive, cyclophosphamide-immunosuppressed mice.
Plasma protein and brain tissue binding.
Binding of AN3520 and SCYX-6759 to human or mouse plasma proteins or mouse brain homogenate was determined by rapid equilibrium dialysis (RED) (Pierce, Rockford, IL) using a 48-well-plate-based format according to the manufacturer's instructions. Briefly, 0.5, 2.0, or 5.0 μM AN3520 and SCYX-6759 was added to fresh human or mouse plasma (Bioreclamation, Liverpool, NY) or mouse brain homogenate. Duplicate aliquots of each plasma or brain sample were transferred into the sample chambers of the RED devices, and dialysis buffer (BupH PBS) was added to the buffer chambers. The plates were sealed and incubated at 37°C for 4 h. After dialysis, samples collected from the buffer and tissue chambers were treated with ice-cold methanol (3 volumes for plasma, 4 volumes for brain) to precipitate proteins. The treated samples were centrifuged for 10 min at 3,000 × g at 15°C. The supernatants were assayed for the test compound by liquid chromatography-tandem mass spectrometry (LC-MS/MS). Calibration standards and quality-control samples were prepared in a matched matrix and assayed with samples. Values for unbound and bound fractions and mass balance were calculated. Concordance of binding for each batch of plasma was confirmed by assay of warfarin, imipramine, and carbamazepine.
In vitro metabolism.
The metabolic stability of AN3520 and SCYX-6759 was evaluated using CD-1 mouse or human liver microsomal fractions (from males and females) (XenoTech, Lenexa, KS). The method was adapted from a previously published procedure (25). Briefly, AN3520 and SCYX-6759 (1 μM) were incubated with microsomes (1.05 mg/ml protein) for 0, 10, 15, and 30 min at 37°C in an oxygen-enriched environment in the presence of an NADPH-regenerating system. Each compound was also incubated, under similar conditions, but with uridine-5′ diphosphoglucoronosyltransferase and liver S9 subcellular fractions from various species (2.5 mg/ml protein) (10) for 0, 15, 30, and 60 min. The reactions were quenched with 3 volumes of ice-cold methanol, and supernatants were analyzed for the parent compound by LC-MS/MS. Metabolic competencies of microsomal and S9 fractions were confirmed using control compounds 7-ethoxycoumarin, propranolol, and verapamil. Intrinsic clearance (CLint) and/or half-life (t1/2) values were determined for each compound.
In vitro prediction of BBB permeability and P-glycoprotein (P-gp)-mediated efflux transport.
The propensity for AN3520 and SCYX-6759 to cross the blood-brain barrier (BBB) was examined using an in vitro MDCKII-hMDR1 Transwell assay (33). MDCKII-hMDR1 cells were seeded at a density of 3 × 105 cells per well onto microporous polycarbonate membranes in 12-well Costar Transwell plates (Corning Inc., Lowell, MA). The cells were used for permeability studies 3 days later. Trans-epithelial resistance (TEER) was measured for each insert to ensure the integrity of the monolayer (acceptable TEER values were >50 Ωcm2).
The permeability and propensity for P-gp-mediated efflux of AN3520 and SCYX-6759 were evaluated by adding each compound at a concentration of 3 μM, in the presence or absence of 2 μM GF120918, to the apical compartment. Competency of the P-gp efflux transporter was confirmed by assay of propranolol (nonsubstrate) and amprenavir (substrate). Cell monolayers were incubated in triplicate with shaking (160 rpm) at 37°C in a 5% CO2-enriched humidified atmosphere for 1 h. Samples were removed from the apical and basolateral compartments after incubation and assayed for test compound concentrations by LC-MS/MS. Values for mass balance, PappA→B (apparent permeability value in nm/s for the apical to the basolateral direction), PappA→B+GF918 (apparent permeability value in nm/s for the apical to the basolateral direction in the presence of an MDR1 [Pgp] inhibitor), and absorption quotient (AQ) (40, 41, 43) were calculated for each compound. Acceptance criterion for mass balance was 70 to 120%.
In vitro stability in mouse brain homogenate.
The stability of each oxaborole in CNS tissue was evaluated by incubation in fresh rodent brain homogenate. AN3520 and SCYX-6759 (0.1 μM or 5 μM) were incubated in freshly prepared homogenates of mouse brain tissue for 5 h at 37°C with gentle shaking. After incubation, the reactions were quenched with 4 volumes of ice-cold methanol. Supernatants from the incubation mixtures were analyzed for the parent compound by LC-MS/MS.
In vitro inhibition of cytochrome P450 enzymes.
The potential for AN3520 and SCYX-6759 to exert drug-drug interactions which are mediated through inhibition of cytochrome P450 activities was assessed using P450-Glo assay kits (Promega Inc., Madison, WI). Assays for human cytochrome P450 isoforms 3A4, 1A2, 2C9, 2C19, and 2D6 were performed in triplicate according to the manufacturer's instructions for the concentration range 1 to 100 μM (6 levels). Briefly, AN3520 and SCYX-6759 were added to membrane preparations containing human recombinant CYP450 enzymes together with luminogenic substrates specific to each isoform. Specific CYP450 inhibitors were included as positive controls (ketoconazole, CYP3A4; alpha-naphthoflavone, 1A2; sulfaphenazole, CYP2C9; troglitazone, 2C19; and quinidine, CYP2D6). Reactions were initiated by the addition of NADPH-regenerating solution and were allowed to incubate an additional 15 to 30 min (isoform dependent). Luciferin detection reagent, containing the luciferin-labeled probe substrate, was then added. Luminescence was recorded on an EnVision multilabel plate reader. Calculation of IC50s for each enzyme was determined using GraphPad Prism (V5.01). The potential for drug-drug interactions was characterized as high (IC50 < 1 μM), moderate (1 μM < IC50 < 10 μM), or low (IC50 > 10 μM) (25).
Pharmacokinetic studies in rodents and nonhuman primates.
The blood distribution, CNS disposition, and pharmacokinetics of AN3520 and SCYX-6759 were evaluated in infected or noninfected rodents following intravenous or oral administration of compounds. Pharmacokinetics and bioavailability of SCYX-6759 were also evaluated in noninfected cynomolgus monkeys. All rodent procedures were performed following Institutional Animal Care and Use Committee (IACUC) review and in Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC)-approved facilities. In vivo phases of rodent studies were performed at ViviSource (Waltham, MA); studies in nonnaïve cynomolgus monkeys were performed by BioDuro (Beijing, China).
Male CD-1 mice (approximately 25 g), male Sprague-Dawley rats (approximately 225 g), or male cynomolgus monkeys (approximately 3 to 5 kg) were administered the test article by either bolus intravenous injection or oral gavage. Animals in the i.v. groups received a single 2-mg/kg i.v. dose. Animals received oral doses of the test articles either once or twice daily, with doses ranging from 8 mg/kg to 50 mg/kg. All doses were administered as clear colorless solutions in either 50% (vol/vol) PEG 400-20% (vol/vol) ethanol-30% (vol/vol) carboxymethylcellulose (0.5% wt/vol in sterile water for injection) or 2% (vol/vol) ethanol-5% (wt/vol) dextrose in sterile water for injection. Doses were administered in volumes of 4 ml/kg, 2 ml/kg, and 1 mg/kg for mice, rats, and cynomolgus monkeys, respectively. For pharmacokinetic analysis, blood samples were collected from mice via cardiocentesis. Serial blood samples were collected from rats via a vascular access port located in the lateral saphenous vein. Mice and rats were euthanized in a CO2 chamber before collection of terminal blood samples or brain tissue. Serial blood samples from monkeys were collected without anesthesia via a peripheral vein. Blood samples were collected into polypropylene tubes containing K2EDTA anticoagulant and stored on ice until centrifuged for the preparation of plasma. Plasma was stored at −70°C. Whole brains were collected following decapitation, blotted dry, placed into polypropylene containers, and then immediately frozen at approximately −70°C. Cerebrospinal fluid was collected from rats via cisterna magna puncture, placed in sterile polypropylene tubes, and stored at approximately −70°C.
Brain tissue homogenization.
Whole-tissue homogenization of brain tissue was performed on the day of analysis with either a Precellys 24 bead mill homogenizer (Bertin Technologies, France) for mouse tissues or an Ultra-Turrax T25 tissue grinder (IKA-Werke, Wilmington, NC) for rat tissues. Brains were blotted dry, weighed, and homogenized in 1 volume of PBS. After homogenization, an additional single volume of PBS was added to reduce viscosity. Homogenization of mouse tissues was performed in 1.5-ml Precellys tubes containing ceramic CK14 beads (1.4-mm diameter) for 1 cycle, 6.5 × 103 rpm, for 23 s. Rat tissues were processed in 15-ml Costar tubes (Corning Inc., Lowell, MA) at speed setting 4 for 30 s. The density of brain tissue used for the determination of volume was 1.043 g/ml. All tissue homogenates were treated in a bath sonicator for 5 min at approximately 22°C to remove air bubbles.
Analysis of test compounds in biological samples.
Samples of plasma (25 μl) and cerebrospinal fluid (CSF) (10 μl) were treated with 3 volumes of ice-cold methanol, containing 25 ng/ml of 2-chloro-4-fluoro-N-(1-hydroxy-1,3-dihyrobenzo[c][1,2]oxaborol-6-yl)benzamide as an internal standard, to precipitate proteins. Homogenates of brain tissue were treated similarly, except that 4 volumes of ice-cold methanol and internal standard solutions were added. Treated samples were gently mixed at ambient temperature for 10 min and then centrifuged at approximately 3,000 × g for 15 min at 15°C. The supernatants were transferred to 96-well plates or HPLC vials for analysis by LC-MS/MS, employing a qualified, two-dimensional chromatographic separation to overcome ion suppression caused by coelution of endogenous components in brain tissue with the oxaboroles. In summary, treated samples were loaded onto a Phenomenex (Torrance, CA) Synergi Polar RP 50- by 2-mm (3-μm) extraction column and then back eluted onto a Phenomenex Luna C8(2) 50- by 2-mm (3-μm) analytical HPLC column. Mobile phases for extraction and analytical columns comprised 5 mM ammonium formate and 0.1% (vol/vol) formic acid in deionized water (A) and 5 mM ammonium formate and 0.1% (vol/vol) formic acid in methanol (B). The samples were loaded onto the extraction column in 40% B and back eluted onto the analytical column using a step gradient to 50% B between 0.55 to 1.5 min after loading the sample. A linear gradient from 5% B to 95% B over 4.5 min was employed for analytical chromatography. Test articles eluted at approximately 3 min. The total run time was 6 min. The LC-MS/MS instrumentation comprised either a Sciex (Applied Biosystems, Foster City, CA) API-3000 or API-4000 triple quadrupole mass spectrometer interfaced to an Agilent HPLC system via a TurboIonSpray electrospray source operated in the positive ionization mode. Instrumental conditions and precursors to product ion transitions for tandem mass spectrometry were optimized for sensitivity. The MS/MS transitions for AN3520 and SCYX-6759 were 322.1/302.1 and 340.1/191.1 atomic mass units, respectively.
Pharmacokinetic parameters were calculated from composite mean plasma or tissue data using noncompartmental (oral and intravenous routes) and biexponential (intravenous route) analyses in Microsoft Excel.
RESULTS
Structures of study compounds.
Oxaborole carboxamides represent a novel class of lead compounds which exhibit potent efficacy against T. brucei both in vitro and in vivo. Figure 1 shows the structural formulas of AN3520, SCYX-6759, and SCYX-9296. Boron, which has thus far been an unexploited atom in medicinal chemistry, was placed in a benzoxaborole scaffold containing a fused aromatic ring. SCYX-6759 contains fluorine meta to the trifluoromethyl group in the pendant phenyl ring. SCYX-9296 is a direct analogue of AN3520, differing only in replacement of the −B-OH moiety with a carbonyl (C = O) group.
FIG. 1.
Structures of AN3520, SCYX-6759, and SCYX-9296.
Inhibition of T. brucei in vitro.
Oxaborole carboxamides AN3520 and SCYX-6759 exhibited potent activity against T. b. brucei, T. b. rhodesiense, and T. b. gambiense in vitro. Parasite-mediated reduction of resazurin was used as an indicator of trypanosome viability. IC50s of approximately 0.04 μg/ml and 0.07 μg/ml for AN3520 and SCYX-6759, respectively, were achieved against T. b. brucei (Table 1). Similar IC50s were attained using T. b. rhodesiense and T. b. gambiense. The MIC values, defined as the concentration at which no visible growth of trypanosomes occurred 48 h postexposure to the drug, were 0.08 μg/ml and 0.16 μg/ml for AN3520 and SCYX-6759, respectively, against T. b. brucei. Additionally, the oxaboroles exhibited a >90-fold selective inhibition of parasite versus mammalian cell (L929 mouse cell line) proliferation in vitro (Table 1).
TABLE 1.
Activity of oxaborole compounds against T. brucei subspecies in vitro
| Compound | IC50 (μg/ml) |
|||
|---|---|---|---|---|
| T. b. brucei 427 | T. b. rhodesiense STIB900 | T. b. gambiense STIB930 | L929 | |
| AN3520 | 0.04 | 0.038 | 0.012 | 3.77 |
| SCYX-6759 | 0.07 | 0.038 | 0.030 | 9.51 |
| SCYX-9296 | >50 | NDa | ND | >50 |
ND, not determined.
A parallel series of experiments was done to explore the possible role of boron in trypanocidal activity. SCYX-9296, a derivative of AN3520 in which the boron was replaced with a carbon (Fig. 1), was incubated with T. b. brucei. No inhibition of trypanosomes was observed when SCYX-9296 was tested in vitro at concentrations up to 50 μg/ml, suggesting that the cyclic boronic acid is essential for trypanocidal activity.
Time-kill assays and examination of reversibility of effects of oxaboroles.
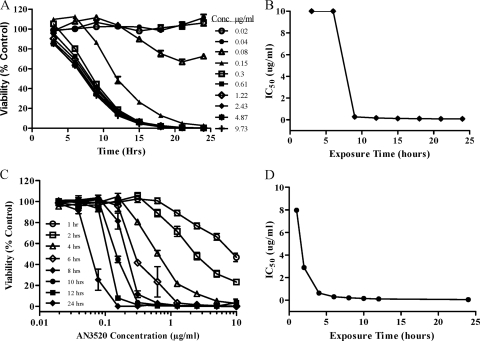
In order to ascertain a further understanding of the pharmacokinetic/pharmacodynamic parameters of the in vitro trypanocidal activity of oxaboroles, time-kill assays (29, 30) were performed using AN3520. The results are presented in Fig. 2. Trypanosome survival was measured at various times with continuous drug pressure. The onset of trypanocidal activity was very rapid, with IC50s decreasing more than 100-fold within the first 18 h (Fig. 2B). When measured 24 h after exposure, the IC50 for AN3520 killing of T. brucei is very close to that measured in the standard 72-h assay, 0.09 μg/ml versus 0.04 μg/ml. In summary, the viability of T. brucei exposed to AN3520 decreased with time and was dependent on a compound concentration up to 0.3 μg/ml, or approximately 4 times the MIC. Overall, there was a >3-log reduction in T. brucei viability within 24 h following exposure to oxaboroles.
FIG. 2.
Time-kill plots demonstrating concentration-dependent effects and irreversibility of AN3520 in T. b. brucei in vitro. Parasite survival was measured at various times in the presence of continuous drug pressure (A) or after compound washout at specified times and viability assessment at 72 h (C). Experiments were conducted in triplicate, and plots represent the mean survival of treated parasites expressed as percentages of the control, untreated parasites. (B and D) IC50s calculated for time-kill and compound washout procedures, respectively.
Pulse incubation of T. brucei with AN3520 shows that only a short period of exposure to the compound is required to produce irreversible effects on trypanosome survival (Fig. 2C). Persistency or irreversibility of the oxaborole effect is time and, to a lesser degree, concentration dependent. As observed for time-kill experiments, increasing the oxaborole concentration above 0.3 μg/ml did not lead to more robust or faster killing after washout and incubation in compound-free medium. IC50s determined following compound washout at various time points after exposure are displayed in Fig. 2D. SCYX-6759 displayed time-kill and reversibility kinetics that were similar to those observed with AN3520-treated T. brucei parasites (data not shown).
Microscopic observations of treated parasites revealed a clear time-dependent alteration in trypanosome morphology (see Data S1 in the supplemental material). Within the first 6 h after exposure, T. brucei parasites exhibited reduced motility. AN3520-induced changes included gradual loss of shape, resulting in parasites appearing round and reduced in size. This was accompanied by the appearance of multiple, often elongated or detached flagella. Parasites were completely lysed within 24 h. Control parasites incubated with the nonboron-containing analogue SCYX-9296, or medium alone, maintained a normal cellular shape and viability throughout the observation period.
Activity of oxaboroles in murine models of HAT.
The potential in vivo activity of oxaboroles was initially conducted with the mouse model of acute trypanosomiasis. Female Swiss Webster mice were inoculated intraperitoneally with 2.5 × 105 parasites of the T. b. brucei EATRO 110 strain. Beginning at 24 h postinfection, the mice were given a twice-daily dose of either AN3520 or SCYX-6729, at 5 to 10 mg/kg (intraperitoneal or oral dose) for 4 days. Mice showed 67% or 100% cure rates, respectively, with no evidence of compound-related toxicity (Table 2). Treated animals were monitored weekly for parasitemia through day 30 postinfection. Animals remaining parasite free for more than 30 days beyond the end of the treatment period were considered cured. Untreated animals succumbed to the disease within 4 to 5 days following infection.
TABLE 2.
Treatment of T. b. brucei EATRO110 acute mouse infections with oxaborolesa
| Compound | Dose (mg/kg) | Route | No. of cured mice/total no. of mice | Avg. survival (no. of days) | % cured |
|---|---|---|---|---|---|
| AN3520 | 5 | p.o. | 3/3 | >30 | 100 |
| 10 | p.o. | 2/3 | 11b | 67 | |
| SCYX-6759 | 1.25 | p.o. | 3/5 | 10.5b | 60 |
| 2.5 | p.o. | 5/5 | >30 | 100 | |
| 5 | p.o. | 3/3 | >30 | 100 | |
| 10 | p.o. | 3/3 | >30 | 100 | |
| Diminazene aceturate | 2 | i.p. | 3/3 | >30 | 100 |
| Untreated | 0/5 | 4 | 0 |
Mice were infected with 250,000 T. b. brucei EATRO 110 parasites, and the infection was allowed to progress for 24 h prior to initiation of treatment. Parasitemia was examined weekly, and cures were determined as described in Materials and Methods. Infected animals were dosed twice daily for 4 consecutive days. Diminazene aceturate was administered as a single i.p. dose to serve as a positive control. Cured animals are defined as those that remained aparasitemic for >30 days after the start of the dosing period.
Average survival of mice showing relapse.
The results of the time-kill assay had demonstrated complete parasite clearance in vitro. To determine whether a single high dose would produce a similar effect in vivo, T. b. brucei-infected mice were given a single intraperitoneal dose of AN3520 at 50 mg/kg at 24, 48, or 72 h postinfection. The single dose given at 24 or 48 h after inoculation with trypanosomes resulted in complete cures with mice with acute T. b. brucei infection. When dosing was delayed until 72 h postinfection, the cure rate dropped to 30%; however, the animals that succumbed to the disease showed significantly increased survival times compared to the untreated controls (data not shown).
Successful treatment of stage 2 HAT requires compounds to cross the BBB and kill T. brucei parasites located within the CNS. To determine if oxaborole compounds could clear a CNS infection in mice, animals infected with chronic disease-inducing T. b. brucei TREU 667 strain parasites were dosed intraperitoneally or orally with AN3520 or SCYX-6759 (Table 3). When given at 50 mg/kg twice daily for 7 or 14 days, AN3520 was curative to 71% or 78% of mice, respectively. Cure rates dropped to 20% or less at doses of 25 mg/kg or less. Cure rates for SCYX-6759 were 83% and 100%, respectively, for 7 and 14 days of treatment. Dosing SCYX-6759 at 25 mg/kg or lower did not provide substantial protection or cure rates in the CNS model. Diminazene aceturate was also included in the study. When given early in the infection, prior to CNS involvement, diminazene aceturate cures the hemolymphatic infection and prevents the occurrence of a CNS form of the disease in mice (18, 19). When given at day 21 postinfection, diminazene aceturate clears only parasites from peripheral blood, leading to a CNS disease and reemergence of parasitemia due to the migration of parasites from the nervous system back into the vascular and lymphatic system (3, 4, 18, 19). In this study, diminazene aceturate prevented a CNS infection when given as a single dose of 10 mg/kg at day 4. In contrast, diminazene aceturate was unable to resolve the infection when given 21 days after infection (Table 3). Brain homogenates or blood obtained from AN3520- and SCYX-6759-treated animals that had survived for more than 180 days after dosing did not produce an infection when injected into uninfected mice.
TABLE 3.
Treatment of T. b. brucei TREU667 CNS infections with oxaborolesa
| Compound tested | Dose (mg/kg) | Dosing route | Duration (no. of days) | Avg. no. of days until relapse | No. of cured mice/total no. of mice | % cured |
|---|---|---|---|---|---|---|
| AN3520 | 50 | i.p. | 14 | 52.5 | 7/9 | 78 |
| 50 | i.p. | 7 | 57 | 12/17 | 71 | |
| 25 | i.p. | 14 | 51.6 | 2/10 | 20 | |
| 25 | i.p. | 7 | 39.1 | 1/17 | 6 | |
| 12.5 | i.p. | 7 | 35 | 0/8 | 0 | |
| SCYX-6759 | 50 | i.p. | 14 | 10/10 | 100 | |
| 50 | p.o. | 7 | 56 | 5/6 | 83 | |
| 25 | p.o. | 7 | 38.8 | 2/10 | 20 | |
| 12.5 | p.o. | 7 | 35.7 | 1/10 | 10 | |
| 6 | p.o. | 7 | 36.6 | 1/10 | 10 | |
| Diminazene aceturate | 10 | i.p. | 1 (D4) | 10/10 | 100 | |
| 10 | i.p. | 1 (D21) | 48.3 | 0/10 | 0 |
Mice were infected with 10,000 T. b. brucei TREU667 parasites, and the infection was allowed to progress for 21 days prior to initiation of treatment. Parasitemia was examined weekly, and cures were determined as described in Materials and Methods. Compounds were administered twice daily for 7 or 14 consecutive days. Diminazene aceturate was administered as a single i.p. dose on day 4 (D4) or day 21 (D21) to serve as positive and negative controls, respectively. Cured animals are defined as those that remained aparasitemic for >180 days after the start of the dosing period.
Binding to human and mouse plasma proteins and brain tissue.
Binding of AN3520 and SCYX-6759 to human and mouse plasma proteins was determined by rapid equilibrium dialysis (RED; Pierce). AN3520 and SCYX-6759 bound extensively to mouse plasma proteins (99.5% and 99.8%, respectively) and more modestly to human proteins (95.3% and 97.7%, respectively). Acceptable mass balances were demonstrated (97 to 110%).
To assess the possible impact of protein binding on in vitro potency demonstrated in the assay described above, mouse or bovine serum was added to the in vitro T. b. brucei inhibition assay. The resulting in vitro IC50 was attenuated by less than 3- to 4-fold in the presence of 25% mouse serum or 50% bovine serum. These results suggest a low affinity for protein binding (data not shown). In contrast, the in vitro potency of suramin, the current standard of care for stage 1 HAT (hemolymphatic stage), when tested under the same conditions, was attenuated by more than 25-fold.
The binding of AN3520 and SCYX-6759 to mouse brain tissue was examined over the therapeutically relevant concentration range 0.5 μM to 5.0 μM. Binding of both compounds was less extensive to mouse brain tissue than to mouse plasma proteins and, in contrast to plasma, was generally independent of concentration (87.6% to 85.3% and 89.6% to 88.6% for 0.5 μM to 5.0 μM AN3520 and SCYX-6759, respectively). Consequently, it is unlikely that binding to brain tissue would result in attenuated potency.
AN3520 and SCYX-6759 are low-clearance compounds when incubated with liver subcellular fractions.
Intrinsic clearance (CLint) and half-life (t1/2) were determined for AN3520 and SCYX-6759 with human and CD-1 mouse liver microsomes. The intrinsic clearance value for both compounds was <5 μl/min/mg, and half-life was >350 min. AN3520 and SCYX-6759 were also incubated with mouse, rat, dog, cynomolgus monkey, and human liver S9 fractions (from males and females) to evaluate stability against a broader range of phase I and II cytosolic-metabolizing enzymes. The half-life for both compounds in liver S9 from all species was >350 min. The results in liver microsomes and S9 fractions indicate that both compounds are low-clearance compounds with the potential for good in vivo pharmacokinetic properties.
Both AN3520 and SCYX-6759 were incubated in freshly homogenized CD-1 mouse brain tissue. The results suggest that SCYX-6759 is more stable than AN3520. Following an incubation of 0.1 μM and 5 μM of either compound at 37°C for 5 h, 91% and 99%, respectively, of SCYX-6759 remained; comparatively, only 49% and 68%, respectively, of AN3520 remained at the end of the incubation period.
AN3520 and SCYX-6759 are highly permeable across MDCKII-hMDR1 monolayers and are not substrates for P-gp.
MDCKII-hMDR1 cell monolayers were used to assess the in vitro intrinsic permeability of AN3520 and SCYX-6759 and whether either could serve as a substrate for P-gp. Cells were incubated with 3 μM of either compound. Mean PappA→B values for AN3520 and SCYX-6759 of 424 nm/s and 379 nm/s, respectively, were achieved (Table 4). In an additional experiment, the P-gp modulator GF120918 was included in the incubations (16). AN3520 and SCYX-6759 achieved mean PappA→B+GF918 values of 434 nm/s and 386 nm/s, respectively. The corresponding absorption quotient (AQ) was 0.02 for both AN3520 and SCYX-6759, indicating that neither compound was a substrate for P-gp (an AQ of ≥0.3 is predictive for P-gp substrates [41]). The potential for a compound to be readily permeable across the intestine or BBB is classified as high when either the PappA→B+GF918 is >50 nm/s for the gut (40) or is >150 nm/s and is a non-P-gp substrate for the BBB (26). Consequently, the MDCKII-hMDR1 data, complemented with modest plasma protein binding, predicts that both compounds should cross the BBB. Furthermore, in light of low metabolic clearance, both compounds should achieve good systemic exposure following oral administration.
TABLE 4.
MDCKII-hMDR1 permeability
| Compound | Papp A→B (nm/s) | Papp A→B+GF918 (nm/s) | Mass balance (%) |
Absorption quotient | P-gp classification | |
|---|---|---|---|---|---|---|
| A→B | A→B+GF918 | |||||
| AN3520 | 424 | 434 | 92 | 90 | 0.02 | Nonsubstrate |
| SCYX-6759 | 379 | 386 | 89 | 95 | 0.02 | Nonsubstrate |
AN3520 and SCYX-6759 present a low risk for CYP450-based drug-drug interactions.
The potential for AN3520 and SCYX-6759 to exert drug-drug interactions mediated through inhibition of cytochrome P450 activities was assessed using the P450-Glo assay (Promega) for recombinant human CYP450 isoforms 3A4, 1A2, 2C19, 2C9, and 2D6 (Table 5). The potential for drug-drug interactions can be quantified as high (IC50 < 1 μM), moderate (1 μM < IC50 < 10 μM), or low (IC50 > 10 μM) according to the observed level of enzyme inhibition (24). Both AN3520 and SCYX-6759 exhibited IC50s that were >10 μM for all CYP isoforms studied, indicating a low potential for CYP450-based adverse drug-drug interactions.
TABLE 5.
Potential for AN3520 and SCYX-6759 to inhibit cytochrome P450 enzyme activities
| CYP isoform | IC50 (μM)a |
|
|---|---|---|
| AN3520 | SCYX-6759 | |
| 3A4 | >100 | >100 |
| 2D6 | 38.7 | 30.3 |
| 2C9 | 29.6 | 30.6 |
| 2C19 | 69.7 | 47.4 |
| 1A2 | >100 | >100 |
The potential of drug-drug interactions was classified as high (IC50 < 1 μM), moderate (1 μM < IC50 < 10 μM), or low (IC50 > 10 μM), according to the observed level of enzyme inhibition. The values are expressed as the means from triplicate determinations.
Pharmacokinetics following single intravenous and oral administration to rodents and primates.
Tables 6 and 7 present summaries of the values of the pharmacokinetic parameters determined following the administration of single doses of SCYX-6759 and AN3520 by either an intravenous or oral route. Plasma, rather than whole blood, was selected for the studies because of the limited distribution of AN3520 in erythrocytes (data not shown). Analysis of plasma concentration was considered to be more pharmacologically relevant for the systemic clearance of parasites.
TABLE 6.
Pharmacokinetic parameters for AN3520 and SCYX-6759 in male CD-1 mice, male Sprague-Dawley rats, and male nonnaïve cynomolgus monkeys in plasma, following intravenous administration
| Animal | Compound | Dose (mg/kg) | AUC0→last (μg·h/ml) | tlast (h) | AUC0→∞ (μg·h/ml) | CL (liters/h/kg) | V (liters/kg) | t1/2 (h) |
|---|---|---|---|---|---|---|---|---|
| Mouse | AN3520 | 2 | 17.95 | 16 | 18.1 | 0.1115 | 0.4745 | 2.875 |
| SCYX-6759 | 3.3 | 44.7 | 24 | 56.4 | 0.059 | 0.97 | 11.4 | |
| Rat | SCYX-6759 | 13.4 | 26.4 | 18 | 26.4 | 0.371 | 4.61 | 13.8 |
| Monkey | SCYX-6759 | 2 | 20.1 | 48 | 20.3 | 0.104 | 0.741 | 8.8 |
TABLE 7.
Pharmacokinetic parameters for AN3520 and SCYX-6759 in male CD-1 mice, male Sprague-Dawley rats, and male nonnaïve cynomolgus monkeys in plasma following oral administrationa
| Species | Compound | Dose (mg/kg) | AUC0→last (μg·h/ml) | tlast (h) | AUC0→∞ (μg·hrml) | CL/F (liters/h/kg) | t1/2 (h) | Tmax (h) | Cmax (μg/ml) | F (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Mouse | AN3520 | 8.2 | 28.42 | 24 | 28.7 | 0.289 | 3.67 | 0.17 | 4.35 | 39 |
| SCYX-6759 | 9 | 56.9 | 24 | 96.3 | 0.093 | 20.2 | 0.5 | 5.3 | 69 | |
| 20.8 | 58.7 | 24 | 90.4 | 0.23 | 17.1 | 0.5 | 8.3 | 54 | ||
| 29.2 | 86.6 | 24 | 133 | 0.219 | 18.2 | 0.5 | 19.2 | 54 | ||
| Rat | SCYX-6759 | 13.4 | 49.6 | 18 | ND | ND | ND | 2 | 6.28 | ND |
| 23.5 | 92.4 | 12 | 95.5 | 0.261 | 8.1 | 2 | 11.6 | 123 | ||
| 50.2 | 132 | 12 | 150 | 0.334 | 5.4 | 1 | 21.1 | 90 | ||
| Monkey | SCYX-6759 | 10 | 84.4 | 48 | 85.6 | 0.125 | 9 | 1.3 | 12.3 | 85.6 |
Tmax, time to maximum concentration of drug in serum; Cmax, maximum concentration of drug in serum; ND, not determined (the elimination phase could not be determined; consequently, there are no values for t1/2 or F).
Following intravenous administration of 2 mg/kg to mice, AN3520 demonstrated an apparent elimination half-life of 2.9 h, a systemic clearance value (CL) of 0.12 liters/h/kg, and a volume of distribution (V) of 0.47 liters/kg (Table 6). Comparatively, SCYX-6759 achieved a superior pharmacokinetic profile, most notably an extended t1/2 of 11.4 h, a lower systemic clearance of 0.06 liters/h/kg, and a V of 0.97. In light of these results, the decision was made to advance SCYX-6759 for evaluation in Sprague-Dawley rats and cynomolgus monkeys. In rats, following intravenous administration of 13.4 mg/kg of SCYX-6759, an apparent elimination t1/2 value of 13.8 h was achieved, along with a systemic clearance value of 0.37 liters/h/kg and a V value of 4.61. Intravenous administration of SCYX-6759 of 2 mg/kg in cynomolgus monkeys resulted in an apparent elimination t1/2 value of 8.8 h, a systemic clearance value of 0.104 liters/h/kg, and a V value of 0.741 liters/kg. Systemic clearance was low in both species, approaching 5% of hepatic blood flow (15). Values for the volume of distribution were low in mice and monkeys, suggesting that the compounds remained in the plasma compartment rather than concentrating in tissues; surprisingly, V appeared markedly higher in rats.
Following oral administration, both AN3520 and SCYX-6759 were absorbed rapidly into the systemic circulation (Table 7). Maximum blood concentrations (Cmax) were observed at approximately 10 min (AN3520) and 30 min (SCYX-6759) in rodents. Maximum blood concentrations were observed at 1.3 h in monkeys (SCYX-6759 only). After a single 8.2-mg/kg dose in mice, AN3520 demonstrated an apparent elimination t1/2 value of 3.7 h and an oral clearance (CL/F) value of 0.29 liters/h/kg. SCYX-6759 was administered to mice at dose levels of 9, 20.8, or 29.2 mg/kg. The values for the apparent elimination t1/2 were 20.2, 17.1, and 18.2 h, respective of dose level; oral clearance (CL/F) values of 0.093, 0.230, and 0.219 liters/h/kg were achieved. Exposure generally increased with dose. Notably, a comparison of SCYX-6759 and AN3520 reveals that following a 9-mg/kg dose of SCYX-6759, the oral clearance of SCYX-6759 (0.093 liters/h/kg) was approximately a 3-fold improvement over the value achieved for AN3520 (0.289 liters/h/kg).
In rats, following administration of a single oral dose of SCYX-6759 at levels of 13.4, 23.5, or 50.2 mg/kg, the values of maximum blood concentrations (Cmax) increased in direct proportion to dose. The area under the concentration-time curve to infinity (AUC0-∞) increased in a less-than-proportional manner. The median oral clearance rates in rats for dose levels 13.4, 23.4, and 50.2 mg/kg were 0.117, 0.216, and 0.334 liters/h/kg, respectively. The values attained for the apparent elimination t1/2 were 8.1 and 5.4 h. Absolute bioavailability for SCYX-6759 was excellent, with values of 90% and 123%.
Oral administration of a single dose of SCYX-6759 in nonnaïve cynomolgus monkeys at a dose level of 10 mg/kg attained 84% bioavailability, as well as an apparent elimination half-life of 9 h, similar to that observed in rats. Oral clearance (CL/F) of 0.125 liters/h/kg was obtained, a modest 2-fold improvement over that obtained in rats. A maximum blood concentration (Cmax) of 12.3 μg/ml was achieved at 1.3 h. The AUC0-∞ was calculated as 85.6 μg·h/ml, a 2- to 3-fold increase in the AUC0-∞ observed in rats (based on normalizing doses to 10 mg/kg).
Pharmacokinetic parameters in brain tissue and CSF were evaluated in male Sprague-Dawley rats after oral administration of 13.4, 23.5, and 50.2 mg/kg (Table 8). Values for the brain/plasma ratio based on the area under the concentration-time curve from 0 minutes postdose to the concentration of the last quantifiable time point (AUC0-last) were between 8% and 20% for the low (13.4 mg/kg)- and high (50.2 mg/kg)-dose groups, respectively. The corresponding brain/plasma ratios, based on Cmax, were between 16% and 26%. SCYX-6759 also partitioned into CSF, achieving CSF/brain ratios between 24% and 49% based on AUC0-last and between 22% and 49% based on Cmax.
TABLE 8.
Pharmacokinetic parameters for SCYX-6759 in male Sprague-Dawley rats in brain and CSF following oral administration
| Matrix | Dose (mg/kg) | AUC0→last (μg·h/ml) | tlast (h) | AUC0→∞ (μg·h/ml) | t1/2 (h) | Tmax (h) | Cmax (μg/m) |
|---|---|---|---|---|---|---|---|
| Brain | 13.4 | 4.1 | 12 | 4.4 | 6 | 1 | 0.99 |
| 23.5 | 11.9 | 18 | 11.9 | 2.6 | 2 | 2.32 | |
| 50.2 | 26.8 | 12 | 27.2 | 2.9 | 2 | 5.5 | |
| CSF | 13.4 | 2.02 | 12 | 2.02 | 4.6 | 1 | 0.364 |
| 23.5 | 3.61 | 12 | 3.65 | 3.1 | 4 | 1.13 | |
| 50.2 | 6.33 | 12 | 6.33 | 2.5 | 6 | 1.22 |
Pharmacokinetics following repeat oral administration to infected mice.
The impact of exposure in plasma and brain compartments on efficacy in a murine model of stage 2 (CNS) HAT was determined by dosing an additional group of infected mice with the same regimen as that of infected animals undergoing treatment with SCYX-6759. Infected female Swiss Webster mice received SCYX-6759 as a twice-daily (q12h) oral dose for 7 days. Dose levels were 6, 12.5, 25, and 50 mg/kg. Blood samples and complete brain tissues were harvested from the animals after the last dose on study day 7. Remaining infected animals were monitored for recurrence of parasitemia, as described in Materials and Methods.
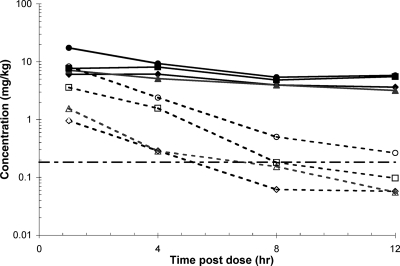
Concentration versus time profiles and pharmacokinetic parameters are presented in Fig. 3 and Table 9. SCYX-6759 demonstrated increases in Cmax and AUC0-last in plasma and whole-brain tissue in a generally dose-dependent manner. The average plasma concentrations at steady state were determined from the AUC0-last. The results show that even for the animals in the low-dose group (6 mg/kg), plasma concentrations of SCYX-6759 were approximately 25-fold more than the in vitro MIC. This concentration of compound is considered to be at a level sufficient to clear blood-borne parasites. Values for the brain/plasma ratio based on AUC0-last were between approximately 6% and 27% for the low (6 mg/kg)- and high (50 mg/kg)-dose groups, respectively. The corresponding brain/plasma ratios based on Cmax were between 16% and 47% for the low (6 mg/kg)- and high (50 mg/kg)-dose groups, respectively. Exposure in brain exceeded the in vitro MIC only in animals receiving 50 mg/kg SCYX-6759.
FIG. 3.
Concentration-time profiles for SCYX-6759 in plasma (solid lines) or whole brain tissue (broken lines) from infected female Swiss Webster mice receiving either 6 mg/kg (open diamond), 12.5 mg/kg (vertical open rectangle), 25 mg/kg (horizontal open rectangle), or 50 mg/kg (open circle) oral doses of SCYX-6759, twice daily (q12h) for 7 days. The horizontal broken line represents the in vitro MIC. Plasma and brain data are presented as either micrograms per milliliter or micrograms per gram of wet tissue, respectively.
TABLE 9.
Pharmacokinetic parameters for SCYX-6759 in plasma and brain tissue of infected female Swiss Webster micea
| SCYX-6759 in: | Dose (mg/kg) | Cmax (μg/ml) | Cmin (μg/ml) | AUC0→12 h (μg·h/ml) | t1/2 |
|---|---|---|---|---|---|
| Plasma | 6 | 6.1 | 3.65 | 57 | 10.8 |
| 12.5 | 7.1 | 3.20 | 55 | 11.6 | |
| 25 | 8.1 | 5.50 | 74 | 14.7 | |
| 50 | 17.5 | 5.80 | 101 | 11.6 | |
| Complete brain tissue | 6 | 1.0 | 0.06 | 3.3 | 3.4 |
| 12.5 | 1.6 | 0.05 | 4.8 | 3.4 | |
| 25 | 3.6 | 0.10 | 13.6 | 2.0 | |
| 50 | 8.3 | 0.26 | 27.5 | 2.5 |
Infected female Swiss Webster mice received 6, 12.5, 25, or 50 mg/kg oral doses of SCYX-6759, twice daily (q12h) over 7 days.
DISCUSSION
Current treatment regimens for HAT are comprised of 4 drugs: pentamidine, suramin, melarsoprol, or eflornithine, and the combination nifurtimox-eflornithine. These drugs are toxic and cumbersome in their administration (44). The need for a safe, effective, easily administered treatment is critical, with over 60 million people at risk for HAT in sub-Saharan Africa. SCYX-6759 and AN3520 belong to a novel class of boron-containing compounds which are potent inhibitors of T. brucei. Boron is an unexploited atom in medicinal chemistry. The utility of boron has been recently highlighted with the development of new antifungal, antibacterial, and anti-inflammatory compounds (2, 6, 7, 9), some of which are currently undergoing clinical trials (1, 37). Replacement of the boron atom with carbon (SCYX-9296) results in the loss of trypanocidal activity. Recent studies show that boron and the oxaborole ring are required for the inhibition of yeast cytoplasmic leucyl-tRNA synthetase by 5-fluoro-1,3-dihydro-1-hydroxy-2,1-benzoxaborole (AN2690) (37). Conversely, replacement of the boron atom of the peptide boronate Z-Leu-Leu-Leu-B(OH)2 proteosome inhibitor (or other related boronic acids, such as Velcade) shows a modest impact on trypanocidal activity in vitro (31). This suggests that the two classes of boron-containing compounds have different mechanisms of action and potentially different clinical profiles.
The antitrypanosomal activities of AN3520 and SCYX-6759 were examined via time-kill studies. Both compounds yielded a concentration- and time-dependent decrease in ATP content. These data suggest that oxaboroles are primarily time-dependent trypanocidal agents whose efficacy is only partly dependent on concentration. Limited studies have been conducted in the past to investigate the time-kill kinetics of trypanocidal agents in vitro to generate models for the evaluation of the activity of compounds for the treatment of HAT. One such example demonstrated T. brucei killing in vitro by pentamidine to be both time and concentration dependent, but several days were required to see this trend, even at concentrations far in excess of the MIC (28). In contrast, time-kill kinetics of antibacterial agents have been explored extensively against a variety of bacterial pathogens. Based on such studies, the killing action of antibacterial agents has been classified as being dependent either on exposure (maximum concentration/MIC), the area under the concentration-time curve/MIC, or simply the time that the antibiotic concentration is kept above the MIC (29, 30). Antibacterial agents are generally classified as cidal when they produce a 3-log reduction in bacterial viability after exposure to the test agent, a result observed with both oxaborole compounds in this study.
The potential for the reversibility of trypanocidal effects revealed that exposure of T. brucei to oxaborole compounds at concentrations 5- to 10-fold above MICs for 8 to 12 h results in elimination of parasites from culture, even after the compounds have been washed away. The inability of T. brucei parasites to recover from transient exposure to compounds in vitro suggests that oxaboroles are retained within the parasites or that they exert irreversible effects on the potential target(s) within the parasite during this time frame. This early “commitment to death” phenomenon has implications for in vivo efficacy, as it suggests that serum concentrations above the MIC do not need to be maintained for extended periods of time to achieve cure or parasite elimination. The “commitment to death” phenomenon following a short exposure has been reported for trypanosome killing by diminazene aceturate, trybizine hydrochloride, or S-adenosylmethionine decarboxylase inhibitors, but much higher IC50/MIC ratios were used and extended periods were required to observe full suppression of parasite growth (3, 20, 21). We have classified oxaboroles as cidal agents against T. brucei because they demonstrated a greater-than-3-log reduction in parasite viability over a 24-h period with no evidence of visible growth of organisms after this period. Animals infected with T. b. brucei and treated twice daily, either intraperitoneally or orally for 4 days, were cured at rates of 67% to 100%. These preliminary results indicated that the use of oxaboroles for the treatment of HAT was tenable and merited further study.
Metabolism and pharmacokinetic studies revealed that SCYX-6759 and AN3520 are low-clearance compounds (<5 μl/min/mg) with a long half-life in vitro (>350 min), as demonstrated during incubations with liver microsomal or S9 subcellular fractions from multiple animal species. Concordant with the in vitro metabolic stability data, both compounds achieved low clearance, approaching only 5% of liver blood flow when administered to mice. SCYX-6759 was advanced to further pharmacokinetic studies in rats and nonhuman primates, where it maintained intravenous clearance values below 5% of liver blood flow. Inhibition studies with recombinant human CYP450 enzymes afforded IC50s greater than 10 μM for all isoforms studied, indicative of a low risk for metabolism-based drug-drug interactions.
SCYX-6759 and AN3520 were well absorbed following oral administration in multiple animal species. SCYX-6759 appeared to achieve marginally higher bioavailability. Both oxaborole compounds demonstrate propensity to cross the blood-brain barrier, as revealed in results from a transport assay predicative for non-P-gp substrates (absorption quotient less than 0.3) (26). Each compound exceeded the predicative value by 2.5-fold. The capacity of SCYX-6759 and AN3520 for intestinal permeability was shown to be 7-fold greater than the threshold value of 50 nm/s (40).
While the in vitro potency against T. b. brucei was similar for both compounds (Table 1) and both achieved complete cures in the murine hemolymphatic model of HAT (Table 2), our studies revealed that SCYX-6759 possessed superior pharmacokinetics. These were beneficial in the stage 2 (CNS) murine HAT model where, in contrast to AN3520, SCYX-6759 was able to achieve complete cures following a twice-daily intraperitoneal treatment with 50-mg/kg doses (a 100% cure for SCYX-6759 versus a 78% cure for AN3520). Cure rates were also modestly superior for SCYX-6759 following oral delivery (Table 3). For SCYX-6759, this was associated with maintenance of a whole-brain concentration (Fig. 3) above the in vitro MIC (0.156 μg/ml). The importance of sustained exposure to oxaborole concentrations above the MIC for efficacy is consistent with time-kill data.
Exposure in rodent brain tissue increased with dose. This is consistent with saturation of an efflux transporter at the blood-brain barrier or saturation of plasma protein binding. Binding to proteins, despite being approximately 99% in mice, appeared to be low affinity, because the addition of 50% serum only attenuated potency in the in vitro T. b. brucei assay by 2- to 3-fold. In contrast, the in vitro potency of suramin, the current standard-of-care medication for hemolymphatic HAT, was attenuated by more than 25-fold under similar conditions. Interestingly, binding to brain tissue was less extensive than to plasma proteins, suggesting brain levels of these highly permeable compounds may be greatly influenced by clearance of free drug from plasma. In vitro biodistribution and in vitro transporter studies are under way to provide further insight (39).
In summary, we have identified a class of boron-containing compounds, oxaborole carboxamides, as novel leads with potent and selective activity against T. brucei. Oxaboroles display rapid time-dependent killing of parasites and demonstrate efficacy against T. brucei in murine models of acute and chronic infections. Pharmacokinetic analysis in rodent and primate models shows that oxaboroles are highly bioavailable and readily cross the blood-brain barrier to account for their efficacy against late-stage HAT. The anti-parasitic and pharmacological profiles of oxaborole carboxamides provide a unique opportunity for the development of safe and effective treatments that could serve as key tools for the eradication of sleeping sickness as a major public health issue in sub-Saharan Africa.
Supplementary Material
Acknowledgments
We thank Annmarie Kowalczyk for her invaluable assistance with assembling the data contained in this report.
Footnotes
Published ahead of print on 26 July 2010.
Supplemental material for this article may be found at http://aac.asm.org/.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1.Akama, T., S. J. Baker, Y. K. Zhang, V. Hernandez, H. Zhou, V. Sanders, Y. Freund, R. Kimura, K. R. Maples, and J. J. Plattner. 2009. Discovery and structure-activity study of a novel benzoxaborole anti-inflammatory agent (AN2728) for the potential topical treatment of psoriasis and atopic dermatitis. Bioorg. Med. Chem. Lett. 19:2129-2132. [DOI] [PubMed] [Google Scholar]
- 2.Alley, M. R., S. J. Baker, K. R. Beutner, and J. Plattner. 2007. Recent progress on the topical therapy of onychomycosis. Expert Opin. Invest. Drugs 16:157-167. [DOI] [PubMed] [Google Scholar]
- 3.Bacchi, C. J., R. Brun, S. L. Croft, K. Alicea, and Y. Bühler. 1996. In vivo trypanocidal activities of new S-adenosylmethionine decarboxylase inhibitors. Antimicrob. Agents Chemother. 40:144-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bacchi, C. J., H. C. Nathan, A. B. Clarkson, Jr., E. J. Bienen, A. J. Bitonti, P. P. McCann, and A. Sjoerdsma. 1987. Effects of the ornithine decarboxylase inhibitors DL-alpha-difluoromethylornithine and alpha-monofluoromethyldehydroornithine methyl ester alone and in combination with suramin against Trypanosoma brucei brucei central nervous system models. Am. J. Trop. Med. Hyg. 36:46-52. [DOI] [PubMed] [Google Scholar]
- 5.Bacchi, C. J., H. C. Nathan, N. Yarlett, B. Goldberg, P. P. McCann, A. Sjoerdsma, M. Saric, and A. B. Clarkson, Jr. 1994. Combination chemotherapy of drug-resistant Trypanosoma brucei rhodesiense infections in mice using DL-alpha-difluoromethylornithine and standard trypanocides. Antimicrob. Agents Chemother. 38:563-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker, S. J., T. Akama, Y. K. Zhang, V. Sauro, C. Pandit, R. Singh, M. Kully, J. Khan, J. J. Plattner, S. J. Benkovic, V. Lee, and K. R. Maples. 2006. Identification of a novel boron-containing antibacterial agent (AN0128) with anti-inflammatory activity, for the potential treatment of cutaneous diseases. Bioorg. Med. Chem. Lett. 16:5963-5967. [DOI] [PubMed] [Google Scholar]
- 7.Baker, S. J., Y. K. Zhang, T. Akama, A. Lau, H. Zhou, V. Hernandez, W. Mao, M. R. Alley, V. Sanders, and J. J. Plattner. 2006. Discovery of a new boron-containing antifungal agent, 5-fluoro-1,3-dihydro-1-hydroxy-2,1-benzoxaborole (AN2690), for the potential treatment of onychomycosis. J. Med. Chem. 49:4447-4450. [DOI] [PubMed] [Google Scholar]
- 8.Baltz, T., D. Baltz, C. Giroud, and J. Crockett. 1985. Cultivation in a semi-defined medium of animal infective forms of Trypanosoma brucei, T. equiperdum, T. evansi, T. rhodiense and T. gambiense. EMBO J. 4:1273-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barak, O., and D. S. Loo. 2007. AN-2690, a novel antifungal for the topical treatment of onychomycosis. Curr. Opin. Invest. Drugs 8:662-668. [PubMed] [Google Scholar]
- 10.Beaune, P. M., and F. P. Guengerich. 1988. Human drug metabolism in vitro. Pharmacol. Ther. 37:193-211. [DOI] [PubMed] [Google Scholar]
- 11.Brun, R., J. Blum, F. Chappuis, and C. Burri. 2010. Human African trypanosomiasis. Lancet 375:148-159. [DOI] [PubMed] [Google Scholar]
- 12.Brun, R., R. Schumacher, C. Schmid, C. Kunz, and C. Burri. 2001. The phenomenon of treatment failures in human African trypanosomosis. Trop. Med. Int. Health 6:906-914. [DOI] [PubMed] [Google Scholar]
- 13.Burri, C., and J. Keiser. 2001. Pharmacokinetic investigations in patients from northern Angola refractory to melasoprol treatment. Trop. Med. Int. Health 6:412-420. [DOI] [PubMed] [Google Scholar]
- 14.Croft, S. 1999. Pharmacological approaches to antitrypanosomal chemotherapy. Mem. Inst. Oswaldo Cruz 94:215-220. [DOI] [PubMed] [Google Scholar]
- 15.Davies, B., and T. Morris. 1993. Physiological parameters in laboratory animals and humans. Pharm. Res. 10:1093-1095. [DOI] [PubMed] [Google Scholar]
- 16.Edwards, J. E., K. R. Brouwer, and P. J. McNamara. 2002. GF120918, a P-glycoprotein modulator, increases the concentration of unbound amprenavir in the central nervous system in rats. Antimicrob. Agents Chemother. 46:2284-2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirumi, H., and K. Hirumi. 1989. Continuous cultivation of Trypanosoma brucei blood stream forms in a medium containing a low concentration of serum protein without feeder cell layers. J. Parasitol. 75:985-989. [PubMed] [Google Scholar]
- 18.Jennings, F. W., J. Rodgers, B. Bradley, G. Gettinby, P. Kennedy, and M. Murray. 2002. HAT: potential therapeutic benefits of an alternative suramin and melarsoprol regimen. Parasitol. Int. 51:381-388. [DOI] [PubMed] [Google Scholar]
- 19.Jennings, F. W., and A. R. Gray. 1983. Relapsed parasitaemia following chemotherapy of chronic Trypanosoma brucei infections in mice and its relation to cerebral trypanosomes. Contrib. Microbiol. Immunol. 7:147-154. [PubMed] [Google Scholar]
- 20.Kaminsky, R., and R. Brun. 1998. In vitro and in vivo activities of trybizine hydrochloride against various pathogenic trypanosome species. Antimicrob. Agents Chemother. 42:2858-2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaminsky, R., M. Mamman, F. Chuma, and E. Zweygarth. 1993. Time-dose-response of Trypanosoma brucei brucei to diminazene aceturate (Berenil) and in vitro simulation of drug-concentration-time profiles in cattle plasma. Acta Trop. 54:19-30. [DOI] [PubMed] [Google Scholar]
- 22.Kennedy, P. G. E. 2008. The continuing problem of human African trypanosomiasis (sleeping sickness). Ann. Neurol. 64:116-126. [DOI] [PubMed] [Google Scholar]
- 23.Kennedy, P. G. E. 2004. Human African trypanosomiasis of the CNS: current issues and challenges. J. Clin. Invest. 113:496-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krippendorff, B. F., P. Lienau, A. Reichel, and W. Huisinga. 2007. Optimizing classification of drug-drug interaction potential for CYP450 isoenzyme inhibition assays in early drug discovery. J. Biomol. Screen. 12:92-99. [DOI] [PubMed] [Google Scholar]
- 25.Li, X., A. Bjorkman, T. B. Anderson, L. L. Gustafsson, and C. M. Masimirembwa. 2003. Identification of human cytochrome P450s that metabolize anti-parasitic drugs and predictions of in vivo drug hepatic clearance from in vitro data. Eur. J. Clin. Pharmacol. 59:429-442. [DOI] [PubMed] [Google Scholar]
- 26.Mahar Doan, K. M., S. A. Wring, L. J. Shampine, K. H. Jordan, J. P. Bishop, J. Kratz, E. Yang, C. J. Serabjit-Singh, K. K. Adkison, and J. W. Polli. 2004. Steady-state brain concentrations of antihistamines in rats: interplay of membrane permeability, P-glycoprotein efflux and plasma protein binding. Pharmacology 72:92-98. [DOI] [PubMed] [Google Scholar]
- 27.McCulloch, R. 2004. Antigenic variation in African trypanosomes: monitoring progress. Trends Parasitol. 20:117-121. [DOI] [PubMed] [Google Scholar]
- 28.Miézanm, T. W., U. Bronner, F. Doua, P. Cattand, and L. Rombo. 1994. Long term exposure of Trypanosoma brucei gambiense to pentamidine in vitro. Trans. R. Soc. Trop. Med. Hyg. 88:332-333. [DOI] [PubMed] [Google Scholar]
- 29.Mueller, M., A. de la Peña, and H. Derendorf. 2004. Issues in pharmacokinetics and pharmacodynamics of anti-infective agents: kill curves versus MIC. Antimicrob. Agents Chemother. 48:369-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nielsen, E. I., A. Viberg, E. Löwdin, O. Cars, M. O. Karlsson, and M. Sandström. 2007. Semimechanistic pharmacokinetic/pharmacodynamic model for assessment of activity of antibacterial agents from time-kill curve experiments. Antimicrob. Agents Chemother. 15:128-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nkemngu, N. J., V. Rosenkranz, M. Wink, and D. Steverding. 2002. Antitrypanosomal activities of proteasome inhibitors. Antimicrob. Agents Chemother. 46:2038-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pépin, J., and F. Milford. 1994. The treatment of human African trypanosomiasis. Adv. Parasitol. 33:1-47. [DOI] [PubMed] [Google Scholar]
- 33.Polli, J. W., S. A. Wring, J. E. Humphreys, L. Huang, J. B. Morgan, L. O. Webster, and C. S. Serabjit-Singh. 2001. Rational use of in vitro P-glycoprotein assays in drug discovery. J. Pharmacol. Exp. Ther. 299:620-628. [PubMed] [Google Scholar]
- 34.Priotto, G., S. Kaspanan, D. Ngouama, S. Ghorashian, U. Arnold, S. Ghabri, and U. Karunakara. 2007. Nifurtimox-eflornithine combination therapy for second stage Trypanosoma brucei gambiense sleeping sickness: a randomized clinical trial in Congo. Clin. Infect. Dis. 45:1435-1442. [DOI] [PubMed] [Google Scholar]
- 35.Priotto, G., and A.-M. Sevscik. 2008. An improved treatment for sleeping sickness. DNDi Newsl. 17:1-2. [Google Scholar]
- 36.Räz, B., M. Iten, Y. Grether-Bühler, R. Kaminsky, and R. Brun. 1997. The Alamar Blue assay to determine drug sensitivity of African trypanosomes (T. b. rhodesiense and T. b. gambiense) in vitro. Acta Trop. 68:139-147. [DOI] [PubMed] [Google Scholar]
- 37.Rock, F., W. Mao, A. Yaremchuk, M. Tukalo, T. Crépin, H. Zhou, Y. K. Zhang, V. Hernandez, T. Akama, S. J. Baker, J. J. Plattner, L. Shapiro, S. A. Martinis, S. J. Benkovic, S. Cusack, and M. R. Alley. 2007. An antifungal agent inhibits an aminoacyl-tRNA synthetase by trapping tRNA in the editing site. Science 316:1759-1761. [DOI] [PubMed] [Google Scholar]
- 38.Simarro, P. P., J. Jannin, and P. Cattand. 2008. Eliminating human African trypanosomiasis: where do we stand and what comes next? PLoS Med. 5:e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Summerfield, S. G., A. J. Stevens, L. Cutler, M. del Carmen Osuna, B. Hammond, S.-P. Tang, A. Hersey, D. J. Spalding, and P. Jeffrey. 2006. Improving the in vitro prediction of in vivo central nervous system penetration: integrating permeability, P-glycoprotein efflux, and free fractions in blood and brain. J. Pharmacol. Exp. Ther. 316:1282-1290. [DOI] [PubMed] [Google Scholar]
- 40.Thiel-Demby, V. E., J. E. Humphreys, L. A. St John Williams, H. M. Ellens, N. Shah, A. D. Ayrton, and J. W. Polli. 2009. Biopharmaceutics classification system: validation and learnings of an in vitro permeability assay. Mol. Pharmacol. 6:11-18. [DOI] [PubMed] [Google Scholar]
- 41.Thiel-Demby, V. E., T. K. Tippin, J. E. Humphreys, C. J. Serabjit-Singh, and J. W. Polli. 2004. In vitro absorption and secretory quotients: practical criteria derived from a study of 331 compounds to assess for the impact of P-glycoprotein-mediated efflux on drug candidates. J. Pharm. Sci. 93:2567-2572. [DOI] [PubMed] [Google Scholar]
- 42.Trager, W. 1978. Cultivation of parasites in vitro. Am. J. Trop. Med. Hyg. 27:216-222. [DOI] [PubMed] [Google Scholar]
- 43.Troutman, T. D. 2003. Novel experimental parameters to quantify the modulation of absorptive and secretory transport of compounds by P-glycoprotein in cell culture models of intestinal epithelium. Pharm. Res. 20:1210-1224. [DOI] [PubMed] [Google Scholar]
- 44.World Health Organization. 2006. African trypanosomiasis (sleeping sickness), fact sheet N259. WHO, Geneva, Switzerland. http://www.who.int/mediacentre/factsheets/fs259/en/. Accessed 24 March 2010.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.