Abstract
While newer antibiotics play a key role in treating methicillin-resistant Staphylococcus aureus (MRSA) infections, knowledge of their real-world clinical impact is limited. We sought to quantify the effectiveness of linezolid compared to that of vancomycin among MRSA-infected patients. This national retrospective cohort study included adult patients admitted to all Veterans Affairs hospitals between January 2002 and June 2008, infected with MRSA, and treated with either linezolid (oral or intravenous [i.v.]) or vancomycin (i.v.). Patients were followed from their treatment initiation date until the event of interest, discharge, death, or December 2008. Utilizing propensity score methods, we estimated the treatment effects of linezolid primarily on time to discharge and secondarily on time to all-cause in-hospital mortality, therapy discontinuation, and all-cause 90-day readmission with Cox proportional-hazard models. We identified 20,107 patients treated with linezolid (3.2%) or vancomycin (96.8%). Baseline covariates were well balanced by treatment group within propensity score quintiles and between propensity score matched patients (626 pairs). The discharge rate was significantly higher among patients treated with linezolid, representing a decreased length of stay, in both the propensity score adjusted (hazard ratio [HR], 1.38; 95% confidence interval [95% CI], 1.27 to 1.50) and matched (HR, 1.70; 95% CI, 1.44 to 2.00) analyses. A significantly decreased rate of therapy discontinuation, indicating longer therapy duration, was observed in the linezolid group (adjusted HR, 0.64; 95% CI, 0.54 to 0.75; matched HR, 0.49; 95% CI, 0.36 to 0.65). In this clinical population of MRSA-infected patients, linezolid therapy was as effective as vancomycin therapy with respect to in-hospital survival and readmission.
Limited treatment options exist for patients infected with methicillin-resistant Staphylococcus aureus (MRSA). Vancomycin has served as the gold standard of care for many years (16, 28). However, the emergence of bacteria with decreased vancomycin susceptibility has prompted the need for novel antibiotics (16, 32). Linezolid, an oxazolidinone antibiotic, was approved by the Food and Drug Administration in April 2000. While a limited number of clinical trials have reported linezolid superiority, many have found efficacy equivalent to that of vancomycin for the treatment of MRSA infections (10, 14, 30, 31, 33, 35-37). The few studies demonstrating significantly higher clinical cure and survival rates with linezolid therapy have been criticized for their limitations and conclusions, particularly the claim of linezolid superiority based on MRSA subgroup analyses (3, 10, 11, 21, 22). Additionally, there are conflicting data regarding length of stay decreases and length of therapy when comparing linezolid and vancomycin therapies (7, 15, 18-20, 30, 36).
Though randomized clinical trials provide key efficacy data on newly approved agents, insight regarding their effectiveness in clinical practice, particularly among diverse patient populations, and their effectiveness compared to that of standard therapies is often lacking. Due to the increasing complexity of treating MRSA infections, knowledge of the real-world clinical impact of newer agents is needed for informed decision making. We therefore sought to quantify the effectiveness of linezolid compared to that of vancomycin on clinical outcomes among a national cohort of MRSA-infected patients admitted to Veterans Affairs (VA) facilities.
(This work was presented in part at the 25th International Conference on Pharmacoepidemiology and Therapeutic Risk Management in Providence, RI, on 19 August 2009.)
MATERIALS AND METHODS
Data sources.
We utilized standardized Veterans Health Administration national inpatient datasets, which contain International Classification of Diseases, 9th ed. (ICD-9), discharge diagnosis (up to 13 entries per admission) and procedure (up to 5 entries per day) codes (17). National extracts of inpatient and outpatient records for prescriptions, laboratory tests, and select laboratory results were also included. This study was reviewed and approved by the Providence Veterans Affairs Medical Center and University of Rhode Island institutional review boards.
Study design and population identification.
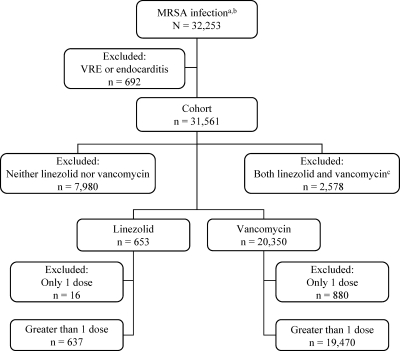
We conducted a retrospective cohort study among adult (≥18 years of age) patients admitted to VA hospitals between 1 January 2002 and 30 June 2008 with a MRSA infection diagnosis code (ICD-9 V09.0). If patients had more than one admission with a MRSA diagnosis code, the first admission occurring during the study period was selected for inclusion. We excluded patients with a concomitant diagnosis code for vancomycin-resistant enterococcus (ICD-9 V09.8) or endocarditis (ICD-9 421.0, 421.1, 421.9, or 996.61), due to vancomycin nonutilization with the former and reported linezolid treatment failure with the latter (26). From this eligible population, we identified two groups of patients initiating therapy during the admission: those receiving oral or intravenous (i.v.) linezolid (exposed group), as the oral formulation is 100% bioavailable, and those treated with i.v. vancomycin (comparison group). Patients receiving greater than one dose of linezolid or vancomycin therapy, but not both, during the admission were selected for inclusion. Figure 1 illustrates the inclusion and exclusion criteria applied for the identification of the final study population.
FIG. 1.
Study inclusion and exclusion criteria applied for sample identification. a, Patients aged 18 years and older, admitted to medical units in Veterans Affairs hospitals between 1 January 2002 and 30 June 2008, with a MRSA diagnosis code. b, If a patient had multiple admissions with a MRSA diagnosis during the study period, only the first admission was included. c, Patients who received both vancomycin (i.v.) and linezolid (i.v. or oral) during the admission. VRE, vancomycin-resistant enterococcus.
Outcome definitions.
The primary outcome of interest was time to discharge, and the secondary endpoints evaluated were time to all-cause in-hospital mortality, therapy discontinuation, and all-cause 90-day readmission. Therapy initiation was used to define the index date of treatment. Time calculations were made from the index date to the event date for each endpoint. Patients who died during the admission were censored on their date of death, and those alive were censored on their date of discharge. If the end of inpatient therapy occurred on the date of discharge or death, patients were censored at these time points. Patients who died during the index admission (n = 1,537) were not included in the follow-up for all-cause 90-day readmission to a medical unit in a VA hospital. Patients without readmission records were censored on their date of death, 90 days from their discharge date, or on 31 December 2008.
Propensity score development.
The Charlson comorbidity index and chronic comorbidities were captured from ICD-9 codes in the year prior to admission and during the index admission (23). Infection type was defined by the following ICD-9 codes: bacteremia, 038.10, 038.11, 038.19, 038.8, 038.9, and 790.7; pneumonia, 482.40, 482.41, 482.49, 482.89, 482.9, 484.8, 485, 486, 510.0, 510.9, 513.0, and 513.1; and skin and soft tissue, 680.0 to 680.9, 681.00 to 681.02, 681.10, 681.11, 681.9, 682.0 to 682.9, 684, 686.9, 704.8, 707.0 to 707.9, 998.31, 998.32, 998.51, 998.59, and 998.83 (8). To assess baseline differences between the two study groups, we utilized Fisher's exact or the χ2 test for categorical data. For continuous variables of interest, we used a t test for normally distributed data, and the nonparametric Wilcoxon rank-sum test was used otherwise.
We employed propensity score methods, where the predicted probability of treatment with linezolid was derived from unconditional logistic regression utilizing a manual backward-elimination approach (1, 5, 25). Propensity scores provide a means of balancing baseline covariates predictive of treatment, mitigating the unequal chance of receiving linezolid versus vancomycin, and are an efficient method to control for confounding in pharmacoepidemiologic analyses (1, 25). Our final model demonstrated fit (Hosmer and Lemeshow, P = 0.477), discrimination (C statistic, 0.784), and an absence of multicollinearity (5). Propensity score stratification into quintiles and 1:1 matching within a 0.01 propensity score caliper range were implemented, related assumptions were assessed, and subsequent covariate balance was reviewed.
Time-to-event analyses.
We developed separate Cox proportional-hazard regression models to quantify the effect of linezolid therapy compared to that of vancomycin therapy for each of the aforementioned outcomes. In propensity score adjustment, indicators of propensity score quintile (reference lowest quintile) were included in the Cox models, while propensity score matching accounted for matched linezolid- and vancomycin-treated pairs. We evaluated Cox proportional-hazard model assumptions, including that of proportionality, with formal tests and graphical displays (6). A hazard ratio (HR) greater than 1 indicated an increased probability of the event occurring sooner in the linezolid group than in the reference vancomycin group. In terms of the study outcomes, an HR greater than 1 would represent higher rates of discharge, mortality, therapy discontinuation, and readmission among patients treated with linezolid. In subgroup analyses, we assessed variations by infection type (24). We evaluated various follow-up periods for all-cause readmission (30, 60, 180, and 365 days) in sensitivity analyses. All analyses were performed using SAS software (version 9.1; SAS Institute Inc., Cary, NC).
RESULTS
We identified 20,107 patients treated with linezolid (3.2%) or vancomycin (96.8%). The majority of MRSA infections occurred in Southern facilities. Several significant variations in demographics and comorbidities, including gender, race, amputation, and para- or quadriplegia, were observed by treatment group (Table 1). Patients treated with linezolid were exposed to a greater number of unique antibiotics in the 90 days before admission than those treated with vancomycin. Linezolid use was more common in recent years, with concurrent decreases in vancomycin utilization over time. Previous antibiotic exposure, facility region, surgery during the hospitalization, presence of a catheter while hospitalized, infection type, time to treatment initiation, and treating specialty also varied significantly between the linezolid and vancomycin treatment groups (Table 2). No differences in baseline white blood cell count, blood urea nitrogen, serum creatinine, or creatinine clearance were observed by treatment group.
TABLE 1.
Demographics and comorbid conditions by treatment group
| Covariate | Resulta for patients treated with: |
P valueb | |
|---|---|---|---|
| Linezolid (n = 637) | Vancomycin (n = 19,470) | ||
| Age in yr, mean (SDc) | 65.1 (14.0) | 64.2 (13.6) | 0.089 |
| Race | 0.032 | ||
| White | 371 (58.2) | 11,250 (57.8) | |
| African-American | 72 (11.3) | 2,955 (15.2) | |
| Other | 7 (1.1) | 186 (0.9) | |
| Unknown/missing | 187 (29.4) | 5,079 (26.1) | |
| Gender | 0.010 | ||
| Male | 611 (95.9) | 18,992 (97.5) | |
| Female | 26 (4.1) | 478 (2.5) | |
| Charlson comorbidity index, median (IQRd) | 2 (1-4) | 2 (1-4) | 0.135 |
| Comorbid condition | |||
| Diabetes | 251 (39.4) | 7,966 (40.9) | 0.445 |
| Renal disease | 191 (30.0) | 5,214 (26.8) | 0.073 |
| Chronic respiratory disease | 186 (29.2) | 5,255 (27.0) | 0.217 |
| Coronary heart disease | 185 (29.0) | 5,464 (28.1) | 0.589 |
| Heart failure | 127 (19.9) | 3,774 (19.4) | 0.728 |
| Cancer | 93 (14.6) | 2,405 (12.4) | 0.091 |
| Amputation | 48 (7.5) | 1,013 (5.2) | 0.010 |
| Para- or quadriplegia | 43 (6.8) | 914 (4.7) | 0.017 |
| Hepatic disease | 36 (5.7) | 961 (4.9) | 0.413 |
| Cerebrovascular disease | 32 (5.0) | 1,322 (6.8) | 0.080 |
| HIV/AIDS | 12 (1.9) | 438 (2.3) | 0.539 |
| Transplant | 9 (1.4) | 193 (1.0) | 0.294 |
| Burns | 4 (0.6) | 83 (0.4) | 0.358 |
Data represent numbers of subjects, with percentages in parentheses, unless otherwise indicated.
Determined by t test, χ2 test, Fisher's exact test, or Wilcoxon rank-sum test as appropriate.
SD, standard deviation.
IQR, interquartile range.
TABLE 2.
Health care and antibiotic exposures and hospitalization-related characteristics by treatment group
| Covariate | Resulta for patients treated with: |
P valueb | |
|---|---|---|---|
| Linezolid (n = 637) | Vancomycin (n = 19,470) | ||
| Previous hospitalizationc | 380 (59.7) | 10,935 (56.2) | 0.081 |
| Previous surgeryd | 164 (25.8) | 4,481 (23.0) | 0.108 |
| Previous antibioticse, mean no. (SD) | 1.9 (2.2) | 1.4 (1.7) | <0.001 |
| Previous linezolid or vancomycin | <0.001 | ||
| Linezolid | 22 (3.5) | 58 (0.3) | |
| Vancomycin | 163 (25.6) | 6,039 (31.0) | |
| Linezolid and vancomycin | 80 (12.6) | 225 (1.2) | |
| Origin of admission | 0.155 | ||
| Home | 518 (81.3) | 16,149 (82.9) | |
| Hospital | 60 (9.4) | 1,438 (7.4) | |
| Nursing home | 59 (9.3) | 1,883 (9.7) | |
| Region of facilityf | <0.001 | ||
| North | 78 (12.2) | 1,948 (10.0) | |
| South | 307 (48.2) | 7,995 (41.1) | |
| Midwest | 105 (16.5) | 4,351 (22.3) | |
| West | 147 (23.1) | 5,176 (26.6) | |
| Procedure during hospitalization | |||
| Surgery | 146 (22.9) | 5,705 (29.3) | 0.001 |
| Catheterization | 93 (14.6) | 6,062 (31.1) | <0.001 |
| Mechanical ventilation | 47 (7.4) | 1,593 (8.2) | 0.467 |
| Dialysis | 27 (4.2) | 1,010 (5.2) | 0.287 |
| MRSA infection type | <0.001 | ||
| Bacteremia | 82 (12.9) | 4,498 (23.1) | |
| Pneumonia | 126 (19.8) | 2,718 (14.0) | |
| Skin and soft tissue | 232 (36.4) | 6,965 (35.8) | |
| Other/not specified | 197 (30.9) | 5,289 (27.1) | |
| Treating specialty | 0.002 | ||
| Intensive care | 82 (12.9) | 2,985 (15.3) | |
| Surgery | 96 (15.1) | 2,032 (10.4) | |
| General medicine | 362 (56.8) | 11,384 (58.5) | |
| Other | 97 (15.2) | 3,069 (15.8) | |
| Treatment initiation ≤3 daysg | 390 (61.2) | 14,802 (76.0) | <0.001 |
| Yr of treatment | <0.001 | ||
| 2002-2004 | 185 (29.0) | 7,665 (39.4) | |
| 2005-2006 | 233 (36.6) | 6,352 (32.6) | |
| 2007-2008 | 219 (34.4) | 5,453 (28.0) | |
Data represent numbers of subjects, with percentages in parentheses, unless otherwise indicated.
Determined by χ2 test or Wilcoxon rank-sum test as appropriate.
All-cause hospitalization to a Veterans Affairs medical unit in the previous year.
Any surgical procedure in the previous year.
Previous exposures to unique antibiotics, at least one dose, in the 90 days before admission.
U.S. Census Bureau-defined regions.
Treatment initiated within 3 days of the admission date.
The propensity score was derived from an unconditional logistic regression model controlling for age, gender, race, region of facility, renal disease, amputation, para- or quadriplegia, cerebrovascular disease, previous linezolid and/or vancomycin exposure, catheterization, surgery, infection type, treating specialty, treatment year, time to treatment initiation, age by renal disease, age by previous linezolid and/or vancomycin exposure, age by infection type, age by treatment year, age by time to treatment initiation, race by region, race by treating specialty, race by time to treatment initiation, region by amputation, region by previous linezolid and/or vancomycin exposure, region by treating specialty, amputation by time to treatment initiation, catheter by infection type, catheter by treating specialty, infection type by treatment year, infection type by time to treatment initiation, and treatment year by time to treatment initiation. Propensity score overlap between the linezolid and vancomycin treatment groups was observed within quintiles. Propensity score matching yielded 626 matched pairs, identifying a vancomycin-treated match for 98.3% of linezolid-treated patients. Baseline covariates were well balanced by treatment group within propensity score quintiles and between the matched linezolid- and vancomycin-treated pairs.
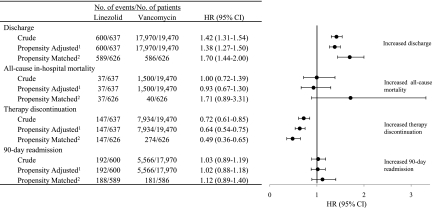
The results of propensity score adjusted and propensity score matched analyses were comparable for each study outcome (Fig. 2). Based on unadjusted Kaplan-Meier estimates of event-free distribution functions, the median time to discharge from treatment initiation was 6 days in the linezolid group and 9 days in the vancomycin group (likelihood ratio test, P < 0.001). The discharge rate was significantly higher among patients treated with linezolid in both the propensity score adjusted (HR, 1.38; 95% confidence interval [95% CI], 1.27 to 1.50) and matched (HR, 1.70; 95% CI, 1.44 to 2.00) analyses. The median time to therapy discontinuation was 16 days in the linezolid group and 13 days in the vancomycin group (P < 0.001). A significantly decreased rate of therapy discontinuation was observed in the linezolid group (adjusted HR, 0.64; 95% CI, 0.54 to 0.75; matched HR, 0.49; 95% CI, 0.36 to 0.65).
FIG. 2.
Hazard ratios of study outcomes for linezolid compared to vancomycin therapy. 1, Adjusted by propensity score quintiles (reference quintile I). 2, Propensity score matched within a 0.01 caliper range.
In the overall population, 7.6% of patients died during the hospitalization. The median survival times (P = 0.381) and 90-day readmission event-free days (P = 0.677) did not differ significantly by treatment group. No associations between treatment group and time to death or time to 90-day readmission were observed. These findings were consistent in sensitivity analyses of all-cause readmission, including 30-, 60-, 180-, and 365-day follow-up periods. Of the total patients followed after discharge, 9.2% had a MRSA infection diagnosis code listed in a readmission occurring within the year after discharge (9.2% for the linezolid group and 9.2% for the vancomycin group).
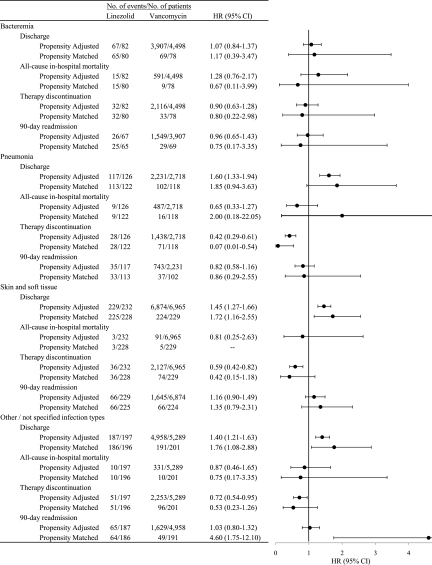
In subgroup analyses by infection type (Fig. 3), no associations between treatment group and any of the study outcomes were observed among patients with bacteremia. In the pneumonia subgroup, the discharge rate was significantly higher for linezolid-treated patients in propensity adjusted (HR, 1.60; 95% CI, 1.33 to 1.94) but not matched (HR, 1.85; 95% CI, 0.94 to 3.63) analyses. The therapy discontinuation rate among those with skin and soft tissue infections was significantly higher for linezolid-treated patients in propensity adjusted (HR, 0.59; 95% CI, 0.42 to 0.82) but not matched (HR, 0.42; 95% CI, 0.15 to 1.18) analyses. In propensity score matched analyses among patients with “other/not specified” infection types, the 90-day readmission rate was significantly higher in the linezolid group (HR, 4.60; 95% CI, 1.75 to 12.10).
FIG. 3.
Hazard ratios of study outcomes by infection type for linezolid compared to vancomycin therapy.
DISCUSSION
We assessed the real-world effectiveness of linezolid compared to that of vancomycin for the treatment of MRSA infections among a large cohort of patients admitted to VA hospitals. To our knowledge, this is the first national observational cohort study evaluating the impact of linezolid therapy on time to discharge, in-hospital mortality, therapy discontinuation, and readmission. Time-to-event analyses revealed significant differences between the linezolid and vancomycin treatment groups in two of the outcomes evaluated.
Compared to vancomycin therapy, we found linezolid therapy to be associated with a decreased length of stay after treatment initiation, as indicated by the significantly higher discharge rate. While length of stay was generally lower (1.0 to 3.5 days) for patients treated with linezolid compared to vancomycin in randomized controlled trials, it was uncertain whether these decreases would be experienced in clinical practice, particularly due to the small efficacy trial sample sizes and MRSA subgroup analyses (7, 15, 18, 19, 30). In our pharmacoepidemiologic effectiveness study of patients hospitalized with MRSA infections in VA facilities throughout the country, the median length of stay was 3 days shorter among those treated with linezolid compared to those treated with vancomycin. We observed a decreased rate of therapy discontinuation in the linezolid group, representing an increased length of therapy. The reasons behind longer therapy duration in the linezolid group compared to the vancomycin group are not clear but may be related to prescribing practices as patients transition out of the hospital.
Interpreting survival with linezolid treatment compared to vancomycin in clinical trials has been complicated by conflicting results, subgroup analyses, and small study populations (14, 31, 33, 35, 37). Two trials reported similar death rates by treatment group in the overall study population but did not describe the mortality rates in the MRSA subset (33, 35). A MRSA subgroup analysis of two nosocomial pneumonia clinical trials reported a higher survival rate among patients treated with linezolid compared to those treated with vancomycin (60/75 versus 54/85, P = 0.030), which was not observed in the overall study population (37). Alternatively, MRSA bacteremia survival did not vary by treatment group in a pooled analysis of five randomized S. aureus bacteremia trials (odds ratio [OR], 1.08; 95% CI, 0.41 to 2.85) (31). In our retrospective cohort study, treatment with linezolid therapy did not significantly reduce the risk of death. Readmission rates were similar by treatment group in clinical trials, although few studies reported such rates with very short follow-up times (≤35 days) (7, 19). In the overall MRSA cohort and subgroups of pneumonia, skin and soft tissue infections, and bacteremia, we did not find readmission rates to vary by treatment group.
This study has several limitations. There is always the potential for residual confounding by unobserved covariates. While the propensity score methodology successfully balanced the baseline covariates assessed, it does not ensure subsequent balance of unobserved covariates. In sensitivity analyses of residual confounding, strong confounders, with a significant confounder-outcome association and unequal distribution by treatment group, could change the lower 95% confidence limit of the hazard ratio to include one for the primary outcome. The therapeutic impact of vancomycin could not be determined in this study, as peak and trough results were not available for evaluation. The mean baseline creatinine clearance in the vancomycin group was 59 ml/min, suggesting that a typical dosing regimen of 1 g every 12 h would be sufficient to achieve a therapeutic trough (12, 27). Additionally, we expect the average MIC of our national VA cohort to correspond with the national average MIC of 1 mg/liter (9, 27, 34). Patients receiving both linezolid and vancomycin during the admission were excluded, as patients failing treatment with vancomycin may have been switched to linezolid.
We identified patients with MRSA infections from the V09.0 ICD-9 code, as microbiological culture results could not be obtained. Little information regarding the accuracy of this diagnosis code exists. Despite this limitation, the code is used in research to identify MRSA infections with a reportedly high positive predictive value (92%) (2, 4, 29). Further, increased sensitivity is expected when antibiotic treatment is taken into account and when greater numbers of diagnosis entries are available (4, 13, 29). While the possibility of MRSA infection underascertainment exists, differential variation by treatment group is doubtful. MRSA infections requiring inpatient treatment may be captured with more consistency, which would indicate better ascertainment among those treated with anti-MRSA therapies.
In our study population, linezolid was utilized much less frequently than vancomycin (3.2% versus 96.8%). In the linezolid group, few patients died during the hospitalization (37/637) and subgroup analyses by infection type resulted in small numbers, which affected our ability to discern differences by treatment group. As more patients are treated with linezolid in the future, a clearer picture of its effectiveness by infection type and its impact on mortality will emerge. The generalizability of this study is limited to VA patients.
In summary, linezolid was associated with a significantly shorter length of stay and significantly longer duration of therapy compared to vancomycin for the treatment of MRSA infections. Among our national cohort of MRSA-infected patients, linezolid was as effective as vancomycin, with similar in-hospital survival and readmission rates by treatment group. Future research should include comprehensive pharmacoeconomic analyses assessing costs related to length of stay and duration of therapy comparing linezolid and vancomycin.
Acknowledgments
We gratefully acknowledge the Center on Systems, Outcomes and Quality in Chronic Disease and Rehabilitation, a REAP of the Health Services Research and Development Service, Providence Veterans Affairs Medical Center Research Service, for data storage and software assistance.
The views expressed are those of the authors and do not necessarily reflect the position or policy of the United States Department of Veterans Affairs.
No financial support for this study was received.
After the completion of this study and manuscript, A.R.C., K.L.L., and B.J.Q. received collaborative research funding from Pfizer Inc., makers of linezolid, for separate research. K.L.L. has received unrestricted investigator initiated research grants and/or served on a drug advisory board for Astellas, Cubist, Forrest, Merck, and Pfizer. B.J.Q. has received research funding from Takeda Pharmaceuticals North America.
Footnotes
Published ahead of print on 26 July 2010.
REFERENCES
- 1.D'Agostino, R. B., Jr. 1998. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat. Med. 17:2265-2281. [DOI] [PubMed] [Google Scholar]
- 2.Elixhauser, A., and C. Steiner. 2007. Infections with methicillin-resistant Staphylococcus aureus (MRSA) in U.S. hospitals, 1993-2005. Healthcare Cost and Utilization Project (HCUP) statistical brief no. 35. Agency for Healthcare Research and Quality, Rockville, MD. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb35.pdf. [PubMed]
- 3.Estes, L., and R. Orenstein. 2007. Cost-effectiveness analysis of linezolid compared with vancomycin for the treatment of nosocomial pneumonia caused by methicillin-resistant Staphylococcus aureus. Clin. Ther. 29:381-384. [DOI] [PubMed] [Google Scholar]
- 4.Gerber, J. S., S. E. Coffin, S. A. Smathers, and T. E. Zaoutis. 2009. Trends in the incidence of methicillin-resistant Staphylococcus aureus infection in children's hospitals in the United States. Clin. Infect. Dis. 49:65-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hosmer, D. W., and S. Lemeshow. 2000. Applied logistic regression, 2nd ed. John Wiley & Sons, Inc., New York, NY.
- 6.Hosmer, D. W., and S. Lemeshow. 1999. Applied survival analysis: regression modeling of time to event data. John Wiley & Sons, Inc., New York, NY.
- 7.Itani, K. M., J. Weigelt, J. Z. Li, and S. Duttagupta. 2005. Linezolid reduces length of stay and duration of intravenous treatment compared with vancomycin for complicated skin and soft tissue infections due to suspected or proven methicillin-resistant Staphylococcus aureus (MRSA). Int. J. Antimicrob. Agents 26:442-448. [DOI] [PubMed] [Google Scholar]
- 8.Jhung, M. A., S. N. Banerjee, S. Fridkin, F. C. Tenover, and L. C. McDonald. 2008. Enhanced detection of Staphylococcus aureus-related hospitalizations using administrative databases, United States—1999-2005. 18th Annu. Sci. Meet. Soc. Healthcare Epidemiol. Am., Orlando, FL. http://www.cdc.gov/ncidod/dhqp/SHEA_EnhanDetecS_aureus_textonly.html.
- 9.Jones, R. N. 2006. Microbiological features of vancomycin in the 21st century: minimum inhibitory concentration creep, bactericidal/static activity, and applied breakpoints to predict clinical outcomes or detect resistant strains. Clin. Infect. Dis. 42(Suppl. 1):S13-S24. [DOI] [PubMed] [Google Scholar]
- 10.Kalil, A. C., S. Puumala, and J. Stoner. 2006. Is linezolid superior to vancomycin for complicated skin and soft tissue infections due to methicillin-resistant Staphylococcus aureus? Antimicrob. Agents Chemother. 50:1910-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalil, A. C., S. E. Puumala, and J. Stoner. 2004. Unresolved questions with the use of linezolid vs vancomycin for nosocomial pneumonia. Chest 125:2370-2371. [DOI] [PubMed] [Google Scholar]
- 12.Karam, C. M., P. S. McKinnon, M. M. Neuhauser, and M. J. Rybak. 1999. Outcome assessment of minimizing vancomycin monitoring and dosing adjustments. Pharmacotherapy 19:257-266. [DOI] [PubMed] [Google Scholar]
- 13.Klompas, M., and D. S. Yokoe. 2009. Automated surveillance of health care-associated infections. Clin. Infect. Dis. 48:1268-1275. [DOI] [PubMed] [Google Scholar]
- 14.Kollef, M. H., J. Rello, S. K. Cammarata, R. V. Croos-Dabrera, and R. G. Wunderink. 2004. Clinical cure and survival in Gram-positive ventilator-associated pneumonia: retrospective analysis of two double-blind studies comparing linezolid with vancomycin. Intensive Care Med. 30:388-394. [DOI] [PubMed] [Google Scholar]
- 15.Li, Z., R. J. Willke, L. A. Pinto, B. E. Rittenhouse, M. J. Rybak, A. M. Pleil, C. W. Crouch, B. Hafkin, and H. A. Glick. 2001. Comparison of length of hospital stay for patients with known or suspected methicillin-resistant Staphylococcus species infections treated with linezolid or vancomycin: a randomized, multicenter trial. Pharmacotherapy 21:263-274. [DOI] [PubMed] [Google Scholar]
- 16.Lodise, T. P., Jr., and P. S. McKinnon. 2007. Burden of methicillin-resistant Staphylococcus aureus: focus on clinical and economic outcomes. Pharmacotherapy 27:1001-1012. [DOI] [PubMed] [Google Scholar]
- 17.Maynard, C., and M. K. Chapko. 2004. Data resources in the Department of Veterans Affairs. Diabetes Care 27(Suppl. 2):B22-B26. [DOI] [PubMed] [Google Scholar]
- 18.McCollum, M., S. V. Sorensen, and L. Z. Liu. 2007. A comparison of costs and hospital length of stay associated with intravenous/oral linezolid or intravenous vancomycin treatment of complicated skin and soft-tissue infections caused by suspected or confirmed methicillin-resistant Staphylococcus aureus in elderly US patients. Clin. Ther. 29:469-477. [DOI] [PubMed] [Google Scholar]
- 19.McKinnon, P. S., S. V. Sorensen, L. Z. Liu, and K. M. Itani. 2006. Impact of linezolid on economic outcomes and determinants of cost in a clinical trial evaluating patients with MRSA complicated skin and soft-tissue infections. Ann. Pharmacother. 40:1017-1023. [DOI] [PubMed] [Google Scholar]
- 20.Mullins, C. D., A. Kuznik, F. T. Shaya, N. A. Obeidat, A. R. Levine, L. Z. Liu, and W. Wong. 2006. Cost-effectiveness analysis of linezolid compared with vancomycin for the treatment of nosocomial pneumonia caused by methicillin-resistant Staphylococcus aureus. Clin. Ther. 28:1184-1198. [DOI] [PubMed] [Google Scholar]
- 21.Powers, J. H., D. Lin, and D. Ross. 2005. FDA evaluation of antimicrobials: subgroup analysis. Chest 127:2298-2301. [DOI] [PubMed] [Google Scholar]
- 22.Powers, J. H., D. B. Ross, D. Lin, and J. Soreth. 2004. Linezolid and vancomycin for methicillin-resistant Staphylococcus aureus nosocomial pneumonia: the subtleties of subgroup analyses. Chest 126:314-316. [DOI] [PubMed] [Google Scholar]
- 23.Quan, H., V. Sundararajan, P. Halfon, A. Fong, B. Burnand, J. C. Luthi, L. D. Saunders, C. A. Beck, T. E. Feasby, and W. A. Ghali. 2005. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care 43:1130-1139. [DOI] [PubMed] [Google Scholar]
- 24.Rassen, J. A., M. A. Brookhart, and S. Schneeweiss. 2009. Applying propensity scores estimated in a full cohort to achieve balance in subgroup analyses. Pharmacoepidemiol. Drug Saf. 18(Suppl. 1):S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubin, D. B. 1997. Estimating causal effects from large data sets using propensity scores. Ann. Intern. Med. 127:757-763. [DOI] [PubMed] [Google Scholar]
- 26.Ruiz, M. E., I. C. Guerrero, and C. U. Tuazon. 2002. Endocarditis caused by methicillin-resistant Staphylococcus aureus: treatment failure with linezolid. Clin. Infect. Dis. 35:1018-1020. [DOI] [PubMed] [Google Scholar]
- 27.Rybak, M., B. Lomaestro, J. C. Rotschafer, R. Moellering, Jr., W. Craig, M. Billeter, J. R. Dalovisio, and D. P. Levine. 2009. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am. J. Health Syst. Pharm. 66:82-98. [DOI] [PubMed] [Google Scholar]
- 28.Sakoulas, G., and R. C. Moellering, Jr. 2008. Increasing antibiotic resistance among methicillin-resistant Staphylococcus aureus strains. Clin. Infect. Dis. 46(Suppl. 5):S360-S367. [DOI] [PubMed] [Google Scholar]
- 29.Schaefer, M., K. Ellingson, C. Conover, A. Genisca, S. Fridkin, D. Currie, T. Esposito, L. Pantilla, P. Ruesto, K. Martin, D. Cronin, M. Costello, S. Sokalski, and A. Srinivasan. 2008. Evaluation of ICD-9 codes as a mechanism for surveillance of methicillin-resistant Staphylococcus aureus infections in Illinois—United States, 2007, abstr. 383. Abstr. 18th Annu. Sci. Meet. Soc. Healthcare Epidemiol. Am., Orlando, FL.
- 30.Sharpe, J. N., E. H. Shively, and H. C. Polk, Jr. 2005. Clinical and economic outcomes of oral linezolid versus intravenous vancomycin in the treatment of MRSA-complicated, lower-extremity skin and soft-tissue infections caused by methicillin-resistant Staphylococcus aureus. Am. J. Surg. 189:425-428. [DOI] [PubMed] [Google Scholar]
- 31.Shorr, A. F., M. J. Kunkel, and M. Kollef. 2005. Linezolid versus vancomycin for Staphylococcus aureus bacteraemia: pooled analysis of randomized studies. J. Antimicrob. Chemother. 56:923-929. [DOI] [PubMed] [Google Scholar]
- 32.Sievert, D. M., J. T. Rudrik, J. B. Patel, L. C. McDonald, M. J. Wilkins, and J. C. Hageman. 2008. Vancomycin-resistant Staphylococcus aureus in the United States, 2002-2006. Clin. Infect. Dis. 46:668-674. [DOI] [PubMed] [Google Scholar]
- 33.Stevens, D. L., D. Herr, H. Lampiris, J. L. Hunt, D. H. Batts, and B. Hafkin. 2002. Linezolid versus vancomycin for the treatment of methicillin-resistant Staphylococcus aureus infections. Clin. Infect. Dis. 34:1481-1490. [DOI] [PubMed] [Google Scholar]
- 34.Tenover, F. C., and R. C. Moellering, Jr. 2007. The rationale for revising the Clinical and Laboratory Standards Institute vancomycin minimal inhibitory concentration interpretive criteria for Staphylococcus aureus. Clin. Infect. Dis. 44:1208-1215. [DOI] [PubMed] [Google Scholar]
- 35.Weigelt, J., K. Itani, D. Stevens, W. Lau, M. Dryden, and C. Knirsch. 2005. Linezolid versus vancomycin in treatment of complicated skin and soft tissue infections. Antimicrob. Agents Chemother. 49:2260-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weigelt, J., H. M. Kaafarani, K. M. Itani, and R. N. Swanson. 2004. Linezolid eradicates MRSA better than vancomycin from surgical-site infections. Am. J. Surg. 188:760-766. [DOI] [PubMed] [Google Scholar]
- 37.Wunderink, R. G., J. Rello, S. K. Cammarata, R. V. Croos-Dabrera, and M. H. Kollef. 2003. Linezolid vs vancomycin: analysis of two double-blind studies of patients with methicillin-resistant Staphylococcus aureus nosocomial pneumonia. Chest 124:1789-1797. [PubMed] [Google Scholar]