Abstract
Quinine resistance (QNR) in Plasmodium falciparum has been detected in many regions of the world where malaria is endemic. Genetic polymorphisms in at least four genes are implicated in QN susceptibility, and their significance often depends on the genetic background of the parasites. In this study, we have culture-adapted 60 P. falciparum clinical isolates from the China-Myanmar border and assessed their in vitro responses to QN. Our results showed that >50% of the parasite isolates displayed reduced sensitivity to QN, with a half-maximal inhibitory concentration (IC50) above 500 nM. Genotyping of pfcrt found that an overwhelming proportion of the parasite population had the chloroquine-resistant genotype, whereas pfmdr1 mutation genotypes and gene amplification were rare. Genotyping of the P. falciparum Na+/H+ exchanger gene (pfnhe1) at the minisatellite ms4760 locus identified 10 haplotypes. Haplotype 7, which harbors three copies of the DNNND repeat, was the most predominant, accounting for nearly half of the parasite isolates. Correlation studies did not reveal significant associations of the polymorphisms in pfcrt and pfmdr1 genes with QN response. However, the ms4760 haplotypes were highly associated with in vitro QN responses. In particular, parasite isolates with an increased DNNND copy number tended to have significantly reduced QN susceptibility, whereas parasite isolates with a higher NHNDNHNNDDD copy number had increased QN susceptibility. This study provided further support for the importance of pfnhe1 polymorphisms in influencing QNR in P. falciparum.
According to the World Malaria Report 2009, malaria caused an estimated 243 million clinical cases, resulting in nearly 0.9 million deaths, in 2008 (43). While most of the malaria burden is in Africa, it has been estimated that Southeast Asia accounts for 30 and 8% of the global malaria morbidity and mortality, respectively. In the Greater Mekong subregion, malaria epidemiology is characterized by immense geographical heterogeneity in disease distribution with many areas of high endemicity (38). Effective chemotherapy is essential for malaria control, but the emergence and spread of drug resistance in malaria parasites have led to a sharp rise in malaria-related morbidity and mortality (20, 41). This situation is particularly grave in Southeast Asia, where multidrug-resistant (MDR) Plasmodium falciparum poses a major challenge to the control of malaria (39). Therefore, for effective and sustainable malaria management, resistance monitoring and mechanism studies are of high priority, particularly in the era of artemisinin-based combination therapy (42).
Quinine (QN) has been a critical antimalarial drug because of its efficacy against chloroquine (CQ)-resistant parasites. In many regions where malaria is endemic, QN is still a primary drug of choice for the treatment of complicated malaria (45). Through its long history in malaria treatment, QN has remained largely effective, and the evolution of QN resistance (QNR) in P. falciparum appears to be slow. However, the observation of reduced sensitivity of P. falciparum to QN in Southeast Asia, South America, and Africa has raised considerable concern (14, 21, 26, 33, 51). In vitro drug assays have found complex patterns of cross-resistance with other quinoline drugs, such as CQ and mefloquine (MQ), suggesting shared resistance mechanisms. Recent genetic and molecular studies indicate that QNR is multifactorial and involves at least four genes: P. falciparum multidrug resistance 1 (pfmdr1), P. falciparum CQ resistance transporter (pfcrt), P. falciparum multidrug resistance-associated protein (pfmrp), and P. falciparum Na+/H+ exchanger 1 gene (pfnhe1). As its name implies, pfmdr1 is involved in resistance to a number of antimalarials. Global isolates of the parasite show that PfMDR1 harbors a large number of point mutations (11). Genetic studies have found that some PfMDR1 mutations, particularly those that are highly prevalent in South America (S1034C/N1042D/D1246Y), where QN has the longest history of use, are associated with increased QNR (30, 35). In addition, increased copy numbers of pfmdr1 increase resistance not only to MQ (8, 22, 24, 25, 44) but also to other arylamino alcohol drugs, such as QN, halofantrine, and lumefantrine (34). Some mutations in PfCRT, the major CQ resistance (CQR) determinant, are found to be associated with stereo-specific changes in responses to QN and quinidine (6, 10). Recently, a genetic study identified PfMRP as playing a role in the efflux of glutathione, CQ, and QN and contributing to parasite responses to multiple antimalarial drugs (27). In addition to these genes, quantitative trait loci analysis of the Dd2 × HB3 cross further mapped QNR to the pfnhe1 gene, which harbors the minisatellite ms4760 (9). Sequence analysis of laboratory-adapted parasite isolates found that increased copy numbers of the minisatellite repeat (DNNND) are associated with reduced susceptibility to QN (9, 13). Consistent with the role of pfnhe1 in QN response, the Dd2 × HB3 progeny clones with higher levels of QNR also exhibited significantly elevated PfNHE activity (4). Direct evidence of the pfnhe1 involvement in QNR came from transfection studies, where reduced pfnhe1 expression was associated with a significant decrease in QN sensitivity (19). It is noteworthy that the effect of pfnhe1 knockdown is strain specific, providing further support for the complex, multifactorial nature of QNR in P. falciparum. Yet, none of these genes studied so far has accounted for high-level QNR.
Southeast Asia has been an epicenter for MDR P. falciparum. Parasites in this region are notorious for their propensity to develop resistance to multiple antimalarial drugs (29). To counter the rapid emergence and spread of drug resistance, malaria drug policies of the countries in Southeast Asia where malaria is endemic have undergone constant changes. CQ and antifolate drugs were abandoned a long time ago (46); resistance to other antimalarial drugs such as MQ has emerged soon after deployment, and QN can no longer be used for malaria monotherapy in this region (17, 26). Although four genes are implicated in reduced QN sensitivity in parasite field isolates, their validity as molecular markers for predicting QNR has been evaluated in only a small number of parasite isolates from diverse regions of the world (13). Therefore, in this study we further investigated the potential association between in vitro QN susceptibility of P. falciparum isolates collected from the China-Myanmar border with genetic polymorphisms in the pfnhe1, pfcrt, and pfmdr1 genes.
MATERIALS AND METHODS
Collection of parasite clinical samples.
A total of 260 P. falciparum field isolates were collected in 2007 to 2009 from symptomatic patients presenting with uncomplicated P. falciparum infections at a malaria clinic in Laiza Township near the China-Myanmar border. The human subject protocol for this study was approved by the Institutional Review Board of Kunming Medical University. Malaria infections were diagnosed by microscopic examination of Giemsa-stained thick and thin blood films. If P. falciparum infection was confirmed, 0.2 ml of blood was spotted on a piece of Whatman 3MM filter paper and used for molecular studies. An additional 0.5 ml of whole blood was collected in heparinized tubes, stored in liquid nitrogen, and transported to the laboratory for culturing.
Establishment of parasite cultures.
Since our earlier work found that up to 30% of patient samples from this region contained mixed strain infections (50), we first wanted to identify samples with single P. falciparum infections. For genotyping, parasite genomic DNA was extracted from the filter papers by using a QIAamp DNA microkit (Qiagen, Germany) following the manufacturer's instructions. DNA was eluted in 80 μl of elution buffer. Parasite samples were then genotyped at three polymorphic genes, merozoite surface protein 1 (msp1), msp2, and glutamate-rich protein (glurp), by previously described methods (15, 31, 37). Parasite cultures were initiated only from samples containing monoclonal infections. To adapt parasite samples to continuous cultures, frozen parasite stocks were thawed, washed twice with RPMI 1640 medium at 37°C, and mixed with fresh type O+ human erythrocytes suspended at a 5% hematocrit in complete medium containing HEPES (5.94 g/liter), hypoxanthine (50 mg/liter), Albumax II (5 g/liter), RPMI 1640 (10.4 g/liter), gentamicin (5 mg/liter), NaHCO3 (2.1 g/liter), and 6% AB+ human serum. Parasite cultures were maintained routinely at 37°C in 75-cm2 flasks (Costar) under a gas environment of 92% N2, 5% CO2, and 3% O2.
In vitro QN sensitivity assay.
Parasite sensitivity to QN was determined using a SYBR green I-based fluorescence assay (36). The QN stock solution (25.6 μM) was prepared in ethanol and diluted in complete medium to the desired final concentrations, ranging from 10 to 2,560 nM. Each synchronized parasite culture at the late ring or early trophozoite stage was diluted with complete medium and fresh human erythrocytes to a starting 4% hematocrit and 0.3% parasitemia. For the drug assays, 90 μl of the parasite suspension was dispensed into the test wells of a 96-well microtiter plate, to which 10 μl of QN solution was added to each well to obtain a final concentration of 0, 10, 40, 160, 320, 640, 1,280, or 2,560 nM. The microtiter plates were incubated at 37°C for 72 h as described previously (2). Afterwards, the plates were frozen and thawed, and 100 μl of lysis buffer was added (36). The contents were mixed thoroughly until erythrocyte sediment was not visible. After incubation in the dark at room temperature for 1 h, fluorescence was measured using the Fluoroskan Ascent FL microplate fluorometer (Thermo Scientific, Waltham, MA) with excitation and emission wavelengths centered at 485 and 538 nm, respectively. For each QN concentration, three replicates were performed. To ensure consistency and reproducibility of the results, two biological replicates of the drug assay were performed on separate days. To further enhance comparability of our study results, six laboratory strains that display a wide range of QN sensitivity (3D7, K1, HB3, Dd2, W2, and 7G8) obtained from the Malaria Research and Reference Reagent Resource Center (MR4) and two Chinese laboratory isolates (SM and FCC1/HN) were similarly cultured and assayed.
Analysis of genetic polymorphisms at pfnhe1, pfmdr1, and pfcrt.
Two pfmdr1 fragments (bp 19 to 606 and bp 2902 to 3867 of the open reading frame) were amplified by PCR with two primer pairs as described previously to include the codons 86, 184, 1034, 1042, and 1246 (3). To determine the polymorphisms in codons 72 to 76 and codon 220 of the pfcrt gene, a nested PCR was performed with primers described previously (16). A fragment containing the ms4760 minisatellite in the pfnhe1 gene was amplified using primers pfnhe-3802F and pfnhe-4322R (9). All PCR products were verified on 1% agarose gels. Amplified DNA was purified using the High Pure PCR cleanup microkit (Roche) and sequenced using BigDye Terminator v3.1. Sequences were aligned with the Clustal X 2.0.12 with manual editing.
Statistical analysis.
All statistical analyses were performed using SPSS for Windows, version 8. The in vitro drug response data were entered into the SPSS data editor, and the geometric mean of the half-maximal inhibitory concentration (IC50) and standard deviation (SD) were calculated for all isolates from a regression-probit analysis. The associations between IC50s and polymorphisms in pfmdr1, pfcrt, and pfnhe1 were assessed using a nonparametric Kruskal-Wallis test with Bonferroni correction, and the significance cutoff level was set at P = 0.0045. The differences between IC50s of parasite isolates with different pfnhe1 ms4760 haplotypes were compared using a t test.
RESULTS
In vitro QN responses.
We have collected a total of 260 clinical P. falciparum isolates at the China-Myanmar border. Genotyping the parasites at three highly polymorphic loci (msp1, msp2, and glurp) identified 118 samples with monoclonal infections, of which 60 were successfully culture adapted and assayed for in vitro sensitivity to QN. In addition, QN sensitivity of eight laboratory strains was also measured in order to define the cutoff values of QN responses. The IC50s of QN-sensitive (QNS) strains 3D7 and HB3 were 108 and 319 nM, respectively, whereas the laboratory QNR strains 7G8, W2, and Dd2 had much higher QN IC50s, 619, 720, and 602 nM, respectively. This result was consistent with the earlier separation of these strains into the QNS and QNR categories. The K1 strain that originated from Thailand showed an intermediate level of QN sensitivity (IC50, 407 nM). The two laboratory parasite strains (FCC1/HN and SM) collected in China in 1983 and 1998, respectively, had in vitro QN IC50s among the lowest in this study, below 200 nM. In comparison, a substantial proportion of the isolates (>50%) from the China-Myanmar border showed reduced QN sensitivity, with IC50s above 500 nM. Remarkably, one China-Myanmar border isolate exhibited significantly elevated in vitro resistance to QN, with an IC50 above 2,000 nM (Table 1).
TABLE 1.
QN responses of P. falciparum strains and polymorphisms in the pfnhe1, pfmdr1, and pfcrt genes
| Parasite isolate | IC50 (nM) (mean ± SD) |
pfnhe1 ms4760a |
pfmdr1 codonb |
pfcrt codon(s)b |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Haplotype | Rep1 | Rep2 | 86 | 184 | 1034 | 1042 | 1246 | 72-76 | 220 | ||
| F07-7 | 456 ± 33 | 1 | 2 | 2 | N | Y | S | N | D | CVIET | S |
| F07-10 | 306 ± 16 | 1 | 2 | 2 | N | Y | S | N | D | CVIET | S |
| F07-23 | 447 ± 71 | 1 | 2 | 2 | N | Y | S | N | D | CVIET | S |
| F07-50 | 204 ± 13 | 1 | 2 | 2 | N | F | S | N | D | CVIET | S |
| F07-59 | 197 ± 15 | 1 | 2 | 2 | N | F | S | N | D | CVIET | S |
| F08B53 | 468 ± 68 | 1 | 2 | 2 | N | Y | S | N | D | CVIET | S |
| F09N1 | 191 ± 46 | 1 | 2 | 2 | N | Y | S | D | D | CVIET | S |
| F09N18 | 284 ± 45 | 1 | 2 | 2 | N | Y | S | N | D | CVIET | S |
| F07-13 | 365 ± 61 | 3 | 1 | 2 | N | Y | S | N | D | CVIET | S |
| F09A54 | 243 ± 32 | 3 | 1 | 2 | N | F | S | N | D | CVIET | S |
| F09A55 | 149 ± 26 | 3 | 1 | 2 | N | F | S | N | D | CVIET | S |
| F09A9 | 562 ± 128 | 5 | 4 | 1 | N | Y | S | N | D | CVIET | S |
| F09N72 | 500 ± 47 | 5 | 4 | 1 | N | Y | S | N | D | CVIET | S |
| F07-3 | 875 ± 44 | 6 | 2 | 1 | N | Y | S | N | D | CVIET | S |
| F07-9 | 321 ± 101 | 6 | 2 | 1 | N | F | S | N | D | CVIET | S |
| F07-11 | 629 ± 81 | 6 | 2 | 1 | N | Y | S | N | D | CVIET | S |
| F07-46 | 894 ± 56 | 6 | 2 | 1 | N | Y | S | N | D | CVIET | S |
| F07-56 | 427 ± 52 | 6 | 2 | 1 | N | Y | S | N | D | CVIET | S |
| F08B32 | 302 ± 45 | 6 | 2 | 1 | N | F | S | N | D | CVIET | S |
| F09N35 | 905 ± 28 | 6 | 2 | 1 | N | F | S | N | D | CVIET | S |
| F09N58 | 448 ± 109 | 6 | 2 | 1 | N | F | S | N | D | CVIET | S |
| F09N66 | 711 ± 78 | 6 | 2 | 1 | N | F | S | N | D | CVIET | S |
| F09N68 | 288 ± 82 | 6 | 2 | 1 | N | F | S | N | D | CVIET | S |
| F07-6 | 543 ± 39 | 7 | 3 | 1 | N | Y | S | N | D | CVIET | S |
| F07-8 | 874 ± 12 | 7 | 3 | 1 | N | F | S | D | D | CVIET | S |
| F07-25 | 502 ± 45 | 7 | 3 | 1 | N | F | S | N | D | CVIET | S |
| F07-27 | 650 ± 44 | 7 | 3 | 1 | N | Y | S | N | D | CVIET | S |
| F07-28 | 473 ± 25 | 7 | 3 | 1 | N | Y | S | N | D | CVIET | S |
| F07-29 | 282 ± 5 | 7 | 3 | 1 | N | Y | S | N | D | CVIET | S |
| F07-31 | 433 ± 32 | 7 | 3 | 1 | N | F | S | N | D | CVIET | S |
| F07-35 | 821 ± 224 | 7 | 3 | 1 | N | Y | S | N | D | CVIET | S |
| F07-40 | 298 ± 53 | 7 | 3 | 1 | Y | Y | S | N | D | CVIET | A |
| F07-42 | 388 ± 95 | 7 | 3 | 1 | N | Y | S | N | D | CVIET | S |
| F07-58 | 680 ± 91 | 7 | 3 | 1 | N | Y | S | N | D | CVIET | S |
| F08B2 | 1,016 ± 196 | 7 | 3 | 1 | N | F | S | N | D | CVIET | S |
| F08B9 | 218 ± 31 | 7 | 3 | 1 | N | F | S | N | D | CVIET | S |
| F08B27 | 870 ± 111 | 7 | 3 | 1 | N | Y | S | N | D | CVIET | S |
| F08B41 | 886 ± 61 | 7 | 3 | 1 | N | Y | S | N | D | CVIET | S |
| F08B61 | 1,128 ± 106 | 7 | 3 | 1 | N | Y | S | N | D | CVIET | S |
| F08B63 | 579 ± 20 | 7 | 3 | 1 | N | Y | S | N | D | CVIET | S |
| F08B72 | 583 ± 78 | 7 | 3 | 1 | N | Y | S | N | D | CVIET | S |
| F09A10 | 716 ± 8 | 7 | 3 | 1 | N | Y | S | N | D | CVIET | S |
| F09A41 | 1,295 ± 99 | 7 | 3 | 1 | N | Y | S | N | D | CVIET | S |
| F09A61 | 373 ± 27 | 7 | 3 | 1 | N | Y | S | N | D | CVIET | S |
| F09N6 | 710 ± 45 | 7 | 3 | 1 | N | Y | S | D | D | CVIET | S |
| F09N22 | 535 ± 37 | 7 | 3 | 1 | N | F | S | N | D | CVIET | S |
| F09N29 | 853 ± 45 | 7 | 3 | 1 | N | Y | S | D | D | CVIET | S |
| F09N33 | 490 ± 28 | 7 | 3 | 1 | N | Y | S | N | D | CVIET | S |
| F09N40 | 2,123 ± 93 | 7 | 3 | 1 | N | Y | S | N | D | CVIET | S |
| F09N44 | 514 ± 26 | 7 | 3 | 1 | N | Y | S | N | D | CVIET | S |
| F09N64 | 475 ± 66 | 7 | 3 | 1 | N | Y | S | N | D | CVIET | S |
| F09N78 | 513 ± 18 | 7 | 3 | 1 | N | Y | S | N | D | CVIET | S |
| Feng | 371 ± 44 | 7 | 3 | 1 | N | Y | S | N | D | CVIET | |
| PF18 | 300 ± 48 | 7 | 3 | 1 | N | Y | S | N | D | SVMNT | S |
| F07-34 | 574 ± 33 | 9 | 3 | 2 | N | Y | S | N | D | CVIET | S |
| F08B7 | 1,099 ± 283 | 9 | 3 | 2 | N | Y | S | N | D | CVIET | S |
| F08B60 | 767 ± 32 | 14 | 3 | 1 | N | Y | S | N | D | CVIET | S |
| F09A21 | 258 ± 26 | 18 | 2 | 2 | N | Y | S | N | D | CVIET | S |
| F07-47 | 324 ± 21 | 20 | 4 | 1 | N | F | S | N | D | CVIET | S |
| F08B40 | 260 | 21 | 1 | 1 | N | F | S | N | D | CVIET | S |
| SM | 188 ± 33 | 6 | 2 | 1 | Y | Y | S | N | D | CVIET | S |
| FCC1/HN | 179 ± 68 | 6 | 2 | 1 | N | Y | S | N | D | CVIET | S |
| W2 | 720 ± 221 | 1 | 2 | 2 | Y | Y | S | N | D | CVIET | S |
| 3D7 | 108 ± 48 | 2 | 1 | 2 | N | Y | S | N | D | CVMNK | A |
| 7G8 | 619 ± 73 | 1 | 2 | 2 | N | F | C | D | Y | SVMNT | S |
| Dd2 | 602 ± 87 | 1 | 2 | 2 | Y | Y | S | N | D | CVIET | S |
| K1 | 407 ± 75 | 5 | 4 | 1 | Y | Y | S | N | D | CVIET | S |
| HB3 | 319 ± 55 | 5 | 4 | 1 | N | F | S | D | D | CVMNK | A |
The Rep 1 column indicates the number of DNNND repeats; the Rep 2 column shows the number of NHNDNHNNDDD repeats.
Letters in bold indicate point mutations.
Molecular polymorphisms at pfcrt, pfmdr1, and pfnhe1.
To study the genetic polymorphisms in the pfcrt, pfmdr1, and pfnhe1 genes in the parasite population at the China-Myanmar border, we genotyped these three genes in 60 fresh parasite isolates and eight reference strains. For pfcrt, genotypes around codons 76 and 220 were determined. The major CQR determinant PfCRT 76T was present in all of these field samples. The majority (98%) of the parasite isolates around codon 76 had a mutant genotype of CVIET, which is most often associated with CQR in Southeast Asia. All but 1 parasite isolate from the 59 isolates successfully genotyped at codon 220 had the A220S mutation.
We genotyped pfmdr1 at codons 86, 184, 1034, 1042, and 1246. For the 60 isolates collected from the China-Myanmar border area, most pfmdr1 point mutations except that at codon 184 were rare (Table 1). Only one isolate harbored two mutations, at codons 184 and 1042, whereas the rest of the isolates from this area had no more than one mutation at these five codons. The frequencies of the 86Y, 184F, and 1042D mutations were 1.7, 30, and 6.7%, respectively. All these field isolates were wild type at codons 1034 and 1246 (Table 1).
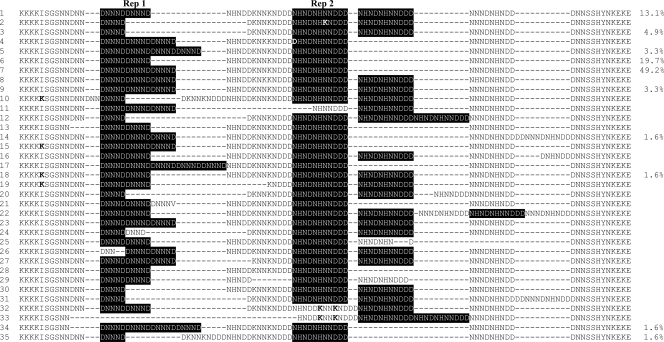
We sequenced the ms4760 fragment of the pfnhe1 gene and found 10 ms4760 haplotypes (Fig. 1). These included two new haplotypes (ms4760-34 and -35) and eight haplotypes (ms4760-1, -3, -5, -6, -7, -9, -14, and -18) that were described previously in P. falciparum samples collected from different regions of the world (1, 9, 40). The most prevalent ms4760 haplotype was ms4760-7, found in nearly half of the isolates (Fig. 1). The ms4760 haplotypes 6 and 1 were relatively abundant, accounting for 16.7 and 13.3% of the isolates, respectively. In comparison, the two new haplotypes, ms4760-34 and -35, were found in only one isolate each (Fig. 1). In the 60 field samples, the copy number of the DNNND sequence varied from one to four repeats, with two and three copies being more common and accounting for 32 and 57% of parasite samples, respectively. In addition, all parasite isolates studied possessed either one copy (76.7%) or two copies (23.3%) of the longer repeat, NHNDNHNNDDD (Fig. 1).
FIG. 1.
Alignment of the 35 pfnhe1 ms4760 haplotypes identified to date. Two types of repeats (Rep 1, DNNND, and Rep 2, NHNDNHNNDDD) are highlighted. Bold letters indicate mutated amino acids.
Associations of genetic polymorphisms with QN response.
Genetic studies have detected associations of polymorphisms in pfcrt, pfmdr1, and pfnhe1 genes with QN susceptibility (9, 13). To determine whether the observed polymorphisms in these three genes are associated with QN responses in parasite isolates examined in this study, we tested for a correlation between each polymorphic allele and the QN IC50, and the results are summarized in Table 2. For the 60 newly established parasite isolates from the China-Myanmar border area, the QN IC50 was not significantly associated with any polymorphism in pfmdr1 (P = 0.022 to 0.501) or pfcrt (P = 0.040 to 0.433). In contrast, when the more common pfnhe1 ms4760 haplotypes (profiles 1, 3, 5, 6, and 7) were used for statistical analysis, a significant association was detected between the QN IC50 and ms4760 haplotype (P = 0.003). These five ms4760 haplotypes accounted for more than 90% of the isolates included in this study. Based on the in vitro QN IC50s, these five haplotypes could be divided into two groups. Isolates with ms4760 haplotype 1 or 3 (group 1) were more susceptible to QN, with IC50s of 319 ± 122 nM (mean ± SD) and 223 ± 106 nM, respectively. In comparison, isolates with ms4760 haplotype 5, 6, or 7 (group 2) had reduced QN sensitivity, with IC50s of 531 ± 44 nM, 580 ± 254 nM, and 661 ± 374 nM, respectively. The IC50s were not significantly different between haplotypes within each group (P > 0.05; t test), whereas they were significantly different between the two groups (P < 0.05; t test).
TABLE 2.
Summary of the association of in vitro QN response (IC50) with polymorphisms in the pfnhe1, pfmdr1, and pfcrt genes from P. falciparum isolates
| Genotype and basis of analysis |
P valuea |
Significance | |
|---|---|---|---|
| All samplesb | 60 field isolates | ||
| pfnhe1 | |||
| ms4760 haplotype | 0.010 | 0.003 | S |
| His/Asp ratio | 0.003 | 0.001 | S |
| No. of DNNND repeats | 0.001 | 0.003 | S |
| No. of DDNHNDNHNND repeats | 0.012 | 0.001 | S |
| pfmdr1 | |||
| Codon 86 | 0.573 | 0.400 | NS |
| Codon 184 | 0.067 | 0.022 | NS |
| Codon 1034 | 0.647 | NS | |
| Codon 1042 | 0.519 | 0.501 | NS |
| Codon 1246 | 0.647 | NS | |
| pfcrt | |||
| Codons 72 to 76 | 0.198 | 0.433 | NS |
| Codon 220 | 0.407 | 0.040 | NS |
P values were determined with the Kruskal-Wallis test. The variables analyzed were the QN IC50s. S, significant; NS, not significant (cutoff of P = 0.0045 [Bonferroni correction]).
All samples included 60 new isolates, 8 laboratory standard strains, and 2 strains established earlier in China.
Two types of repeats in ms4760 have been associated with the in vitro QN response (1, 9, 13). In parasite isolates collected from the China-Myanmar border, we have also observed significant associations between the copy numbers of two repeat types and the QN response (for the DNNND repeat, P = 0.003; for the NHNDNHNNDDD repeat, P = 0.001) (Table 2; Fig. 2). Four isolates had one DNNND repeat, and they tended to be more susceptible to QN (254 ± 89 nM). In comparison, isolates with two or three DNNND repeats tended to have significantly reduced susceptibility to QN (IC50, 453 ± 239 nM for two repeats [n = 19]; IC50, 674 ± 365 nM for three repeats [n = 34]; P < 0.0045, Kruskal-Wallis test). However, the three isolates with four DNNND repeats showed intermediate susceptibility to QN (IC50, 462 ± 123 nM) (Fig. 2). As for the longer repeat, a parasite isolates having one copy of the repeat was associated with reduced QN sensitivity (IC50, 624 ± 337 nM [n = 46 isolates]), whereas an isolate having two copies of this longer pfnhe1 repeat was associated with intermediate QN sensitivity (IC50, 374 ± 244 nM [n = 14 isolates]; P < 0.0045, Kruskal-Wallis test).
FIG. 2.
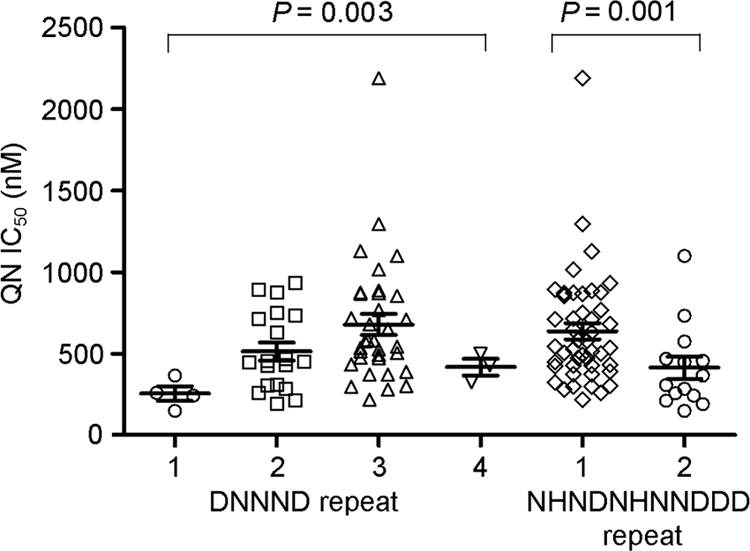
Scatter plot of mean IC50 values (± SD) of the parasite isolates for QN, categorized by the number of copies Rep 1 (1, 2, 3, or 4) or Rep 2 (1 or 2). For Rep 1 and Rep 2, the P values were determined by using a nonparametric Kruskal-Wallis test with Bonferroni correction.
The copy numbers of the two types of repeats influence the ratios of two charged amino acids, histidine and aspartic acid, in the C-terminal domain of pfnhe1. The 10 ms4760 haplotypes found in the parasite population from the China-Myanmar border could be grouped according to eight His/Asp ratios (Table 3). Statistical analysis of the more abundant His/Asp groups found that the His/Asp ratio was significantly associated with QN response (P = 0.001). Increased His/Asp ratios (5:2 or 6:2) were associated with higher QN sensitivity, whereas strains with lower His/Asp ratios (5:4 or lower) had intermediate or reduced QN susceptibility (Table 3).
TABLE 3.
Summary of pfnhe-1 ms4760 haplotypes, His/Asp ratios, DNNND copy numbers, and in vitro QN responses (IC50) of 60 field P. falciparum isolates
| His/Asp ratioa | ms4760 haplotype(s) | No. of isolates | No. of DNNND copies | IC50(nM) (mean ± SD) |
|---|---|---|---|---|
| 5:8 | 5, 34 | 3 | 4 | 462 ± 123 |
| 5:6 | 7 | 31 | 3 | 661 ± 374 |
| 6:6 | 14 | 1 | 3 | 767 |
| 7:6 | 9 | 2 | 3 | 837 ± 372 |
| 5:4 | 6 | 10 | 2 | 580 ± 254 |
| 7:4 | 1, 18 | 9 | 2 | 312 ± 115 |
| 5:2 | 35 | 1 | 1 | 260 |
| 6:2 | 3 | 3 | 1 | 223 ± 106 |
ms4760 haplotypes found among the China-Myanmar border parasite population are grouped here by His/Asp ratios, which are arranged from low to high values.
DISCUSSION
QN has been used to treat malaria for centuries, and development of resistance in P. falciparum is slow. However, a decrease in efficacy and emergence of clinical resistance in P. falciparum in some areas of endemicity have been observed (12, 26). In Thailand, QN was used extensively in the 1980s due to the spread of parasites resistant to CQ and antifolates (32). Although QN remains an effective treatment for severe MDR falciparum malaria in this area, there is evidence of a decline in its therapeutic response (26). In China's Yunnan province and bordering malarious regions, in vitro drug resistance surveys have also detected reduced QN responses in P. falciparum (48, 49). Further, there are significant geographical variations in in vitro QN responses reported: parasites from western Yunnan bordering Myanmar (IC50, 608 nM) exhibited much lower QN susceptibility than parasites from southern Yunnan (480 nM) and southeastern Yunnan (352 nM) (47). This appears to agree with the malaria epidemiology in this region, where Myanmar is the country with the highest malaria endemicity in the Greater Mekong subregion and remains 1 of the 31 high-burden malarious countries in the world (43). In this study, we culture adapted 60 parasite clinical isolates from the China-Myanmar border area and grew parasites in vitro for 10 to 14 days before performing in vitro drug assays. While different in vitro studies have used different cutoff values for QNR without clinical verification (5, 28), the range of the IC50s of the three laboratory QNR strains 7G8, W2, and Dd2 (602 to 720 nM) suggests that 500 nM is an appropriate cutoff IC50 for QNR. Our results showed that 50% of the parasite samples had an in vitro QN IC50 above 500 nM, among which 13 parasites had IC50s of >800 nM. It is noteworthy that an African parasite isolate recently obtained from a malaria patient with QN treatment failure had an in vitro QN IC50 of 829 nM (23). Therefore, our findings suggest that a large proportion of parasite isolates from the China-Myanmar border P. falciparum population may have developed increased resistance to QN.
While many drug resistance phenotypes are complex and involve multiple genes, a small number of genes usually play more important roles in determining the phenotype. In vitro QN responses in cloned P. falciparum parasites showed a smooth, continuous distribution of IC50s, suggesting that QNR is a multigene trait (18). In vitro correlation studies of putative transporter genes in P. falciparum have found evidence of associations of mutations in pfmdr1, pfcrt, and three additional genes with higher QN IC50s (18). One such transporter, pfmrp, was later shown to modulate the responses to multiple antimalarial drugs, including QN (27). Analysis of the Dd2 × HB3 cross further confirmed the contribution of pfcrt and pfmdr1 to QN susceptibility and identified pfnhe1 as another candidate associated with the QN response (9). While these four genes are associated with QN susceptibility, they do not appear to be the major genetic determinants of QNR. Besides, the degrees of their contributions to QNR seem to depend on the genetic background of the parasite (6, 7, 19, 30, 35), which varies greatly among regions of malaria endemicity due to different drug selection pressures. To validate these genes as genetic markers of QNR, Henry et al. performed an evaluation of 23 P. falciparum laboratory clones and found a strong association of pfnhe1 polymorphism with QN response (13). Our study using 60 parasite samples collected from a region of malaria hyperendemicity at the China-Myanmar border further corroborated this finding (9, 13). However, a similar study using 40 samples from Madagascar and 36 samples from Africa did not identify such a correlation but found a significant positive correlation between in vitro QN response and number of the ms4760 long repeat (1). Those authors speculated that this discrepancy might be due to differences in parasite origins, since most of the strains with the highest QN IC50s examined in earlier studies originated from Southeast Asia (9, 13).
The frequency of the ms4760 haplotype displays regional variations, which may be a result of geographically different natural histories of drug selection. In India, haplotype 6 is the predominant one, followed by haplotypes 7 and 3 (40). In Madagascar, hapotypes 1 and 7 are the most predominant (1). The predominant pfnhe1 haplotype at the China-Myanmar border was haplotype 7, accounting for almost 50% of the parasite isolates. Parasites with this most abundant ms4760 haplotype were associated with an increased QN IC50 (>600 nM). The ms4760 polymorphism depends largely on the numbers of two types of repeats, DNNND and NHNDNHNNDDD. Our study showed that an increased number of DNNND repeats was correlated with increased QN IC50, whereas parasites with one copy of the NHNDNHNNDDD repeat tended to have higher QN IC50s than those with two copies of this repeat. Besides, 10 of the 13 parasite isolates for which the IC50 was over 800 nM had three DNNND repeats, and 12 had one NHNDNHNNDDD repeat. With regard to the influence of the ms4760 repeats on QN response, Bennett et al. speculated that the number of DNNND repeats, together with the proximal C-terminal poly-His region, might affect the His/Asp ratio and the pI of PfNHE1 and lead to altered Na+/H+ set point regulation in QNR (4). In support of this hypothesis, we found a significant association of the His/Asp ratio with the QN IC50. More specifically, parasites with higher His/Asp ratios were likely to have increased QN sensitivity.
We consider our samples as not ideal for vigorously testing the contributions of pfcrt and pfmdr1 polymorphisms to QN responses, since the overwhelming majority of our parasite isolates contained the CQR pfcrt genotype (CVIET at codons 72 to 76 and codon 220S) and wild-type pfmdr1. The CQR genotype was consistent with our earlier study in two neighboring counties in Yunnan Province (50), which all indicated that CQR parasites were still highly prevalent in this large region despite the withdrawal of CQ in treatment for P. falciparum decades ago. Since pfcrt point mutations have strong associations with QNR (18), it has been argued that CQ and QN may both interact with pfcrt. It is thus plausible that the homogeneous CQR pfcrt genotype in our studied parasite population may be partially responsible for the observed prevalence of reduced QN sensitivity. However, with regard to the point mutations at the pfmdr1 C-terminal domain, all parasite isolates from the study area were wild type with 1034F and 1246D, while <7% of the parasite isolates had the 1042D mutation. The triple mutation 1034C/1042D/1246Y, commonly found in South America and which is associated with reduced QN response (35), was not found in our studied parasite isolates. Despite the Y184F mutation being present in ∼30% of the field parasite isolates, no association with QN response was detected. Furthermore, in areas where MQ has been heavily deployed, increased pfmdr1 copy number is associated with decreased susceptibility to MQ and a number of other antimalarials, including QN (24). However, pfmdr1 amplification was found in only 1 of the 60 parasite isolates (data not shown), suggesting neither pfmdr1 polymorphisms nor the copy number is likely to play a major role in the reduced QN response observed in this region.
The association of the two ms4760 haplotypes with decreased QN susceptibility requires further investigation, as the sample size of our study was relatively small. Since our samples were collected from a relatively small geographic region, the influence of population structure should be minimal. Yet, we cannot rule out this possibility, because we did not test the studied parasite population. We also genotyped polymorphic sites in msp1, msp2, and glurp, and none of the sites was associated with the parasite response to QN (data not shown). Similarly, substitutions in pfcrt and pfmdr1 were not associated with the response to QN. These data suggest that the association of the repeats in pfnhe1 and QN response is unlikely to be the result of a structured population.
Molecular techniques are increasingly being used for drug resistance surveillance, because many of the target gene polymorphisms are predictive of drug resistance and clinical drug failures. The combination of in vitro drug assay and molecular work will generate a more detailed picture of the epidemiology of drug resistance. Yet, the molecular targets for a number of drugs are not completely understood, making molecular diagnosis of drug resistance difficult. In this study, we evaluated the validity of three candidate genes for QN susceptibility in the China-Myanmar border area. Our result found further evidence of an association between pfnhe1 polymorphisms and in vitro QN response. However, conflicting results obtained from another study have cast doubts on the validity of pfnhe1 polymorphisms for predicting in vitro QN response (1). Nonetheless, these studies have detected relationships for either the short or long repeat in ms4760 with QN response, suggesting a possibility that the pfnhe1 polymorphisms might be associated with the QN susceptibility phenotype by a hitchhiking effect. Taken together, the degree of contribution of pfcrt, pfmdr1, and pfnhe1 to QN susceptibility seems to vary geographically and should be examined in diverse parasite populations. Given that the China-Myanmar border area has a totally different drug use history from other areas where P. falciparum is endemic and that drug resistance surveillance efforts are very weak in this region, focused research on drug resistance mechanisms and extensive surveillance are highly desired to guide regional malaria control.
Acknowledgments
We thank the staff at the Laiza malaria clinic for help with the collection of the parasite samples, and we thank other laboratory members for technical assistance with the parasite cultures.
This work was supported by 1R01AI075429 from NIAID, NIH, by the National Natural Science Foundation of China (no. 30960050).
Footnotes
Published ahead of print on 19 July 2010.
REFERENCES
- 1.Andriantsoanirina, V., D. Menard, S. Rabearimanana, V. Hubert, C. Bouchier, M. Tichit, J. L. Bras, and R. Durand. 2010. Association of microsatellite variations of Plasmodium falciparum Na+/H+ exchanger (Pfnhe-1) gene with reduced in vitro susceptibility to quinine: lack of confirmation in clinical isolates from Africa. Am. J. Trop. Med. Hyg. 82:782-787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baniecki, M. L., D. F. Wirth, and J. Clardy. 2007. High-throughput Plasmodium falciparum growth assay for malaria drug discovery. Antimicrob. Agents Chemother. 51:716-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basco, L. K., and P. Ringwald. 2002. Molecular epidemiology of malaria in Cameroon. X. Evaluation of PFMDR1 mutations as genetic markers for resistance to amino alcohols and artemisinin derivatives. Am. J. Trop. Med. Hyg. 66:667-671. [DOI] [PubMed] [Google Scholar]
- 4.Bennett, T. N., J. Patel, M. T. Ferdig, and P. D. Roepe. 2007. Plasmodium falciparum Na+/H+ exchanger activity and quinine resistance. Mol. Biochem. Parasitol. 153:48-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brasseur, P., J. Kouamouo, R. Moyou-Somo, and P. Druilhe. 1992. Multi-drug resistant falciparum malaria in Cameroon in 1987-1988. I. Stable figures of prevalence of chloroquine- and quinine-resistant isolates in the original foci. Am. J. Trop. Med. Hyg. 46:1-7. [DOI] [PubMed] [Google Scholar]
- 6.Cooper, R. A., M. T. Ferdig, X. Z. Su, L. M. Ursos, J. Mu, T. Nomura, H. Fujioka, D. A. Fidock, P. D. Roepe, and T. E. Wellems. 2002. Alternative mutations at position 76 of the vacuolar transmembrane protein PfCRT are associated with chloroquine resistance and unique stereospecific quinine and quinidine responses in Plasmodium falciparum. Mol. Pharmacol. 61:35-42. [DOI] [PubMed] [Google Scholar]
- 7.Cooper, R. A., K. D. Lane, B. Deng, J. Mu, J. J. Patel, T. E. Wellems, X. Su, and M. T. Ferdig. 2007. Mutations in transmembrane domains 1, 4 and 9 of the Plasmodium falciparum chloroquine resistance transporter alter susceptibility to chloroquine, quinine and quinidine. Mol. Microbiol. 63:270-282. [DOI] [PubMed] [Google Scholar]
- 8.Cowman, A. F., D. Galatis, and J. K. Thompson. 1994. Selection for mefloquine resistance in Plasmodium falciparum is linked to amplification of the pfmdr1 gene and cross-resistance to halofantrine and quinine. Proc. Natl. Acad. Sci. U. S. A. 91:1143-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferdig, M. T., R. A. Cooper, J. Mu, B. Deng, D. A. Joy, X. Z. Su, and T. E. Wellems. 2004. Dissecting the loci of low-level quinine resistance in malaria parasites. Mol. Microbiol. 52:985-997. [DOI] [PubMed] [Google Scholar]
- 10.Fidock, D. A., T. Nomura, A. K. Talley, R. A. Cooper, S. M. Dzekunov, M. T. Ferdig, L. M. Ursos, A. B. Sidhu, B. Naude, K. W. Deitsch, X. Z. Su, J. C. Wootton, P. D. Roepe, and T. E. Wellems. 2000. Mutations in the P. falciparum digestive vacuole transmembrane protein PfCRT and evidence for their role in chloroquine resistance. Mol. Cell 6:861-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foote, S. J., D. E. Kyle, R. K. Martin, A. M. Oduola, K. Forsyth, D. J. Kemp, and A. F. Cowman. 1990. Several alleles of the multidrug-resistance gene are closely linked to chloroquine resistance in Plasmodium falciparum. Nature 345:255-258. [DOI] [PubMed] [Google Scholar]
- 12.Giboda, M., and M. B. Denis. 1988. Response of Kampuchean strains of Plasmodium falciparum to antimalarials: in-vivo assessment of quinine and quinine plus tetracycline; multiple drug resistance in vitro. J. Trop. Med. Hyg. 91:205-211. [PubMed] [Google Scholar]
- 13.Henry, M., S. Briolant, A. Zettor, S. Pelleau, M. Baragatti, E. Baret, J. Mosnier, R. Amalvict, T. Fusai, C. Rogier, and B. Pradines. 2009. Plasmodium falciparum Na+/H+ exchanger 1 transporter is involved in reduced susceptibility to quinine. Antimicrob. Agents Chemother. 53:1926-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jelinek, T., P. Schelbert, T. Loscher, and D. Eichenlaub. 1995. Quinine resistant falciparum malaria acquired in east Africa. Trop. Med. Parasitol. 46:38-40. [PubMed] [Google Scholar]
- 15.Kaneko, O., M. Kimura, F. Kawamoto, M. U. Ferreira, and K. Tanabe. 1997. Plasmodium falciparum: allelic variation in the merozoite surface protein 1 gene in wild isolates from southern Vietnam. Exp. Parasitol. 86:45-57. [DOI] [PubMed] [Google Scholar]
- 16.Mittra, P., S. Vinayak, H. Chandawat, M. K. Das, N. Singh, S. Biswas, V. Dev, A. Kumar, M. A. Ansari, and Y. D. Sharma. 2006. Progressive increase in point mutations associated with chloroquine resistance in Plasmodium falciparum isolates from India. J. Infect. Dis. 193:1304-1312. [DOI] [PubMed] [Google Scholar]
- 17.Mockenhaupt, F. P. 1995. Mefloquine resistance in Plasmodium falciparum. Parasitol. Today 11:248-253. [DOI] [PubMed] [Google Scholar]
- 18.Mu, J., M. T. Ferdig, X. Feng, D. A. Joy, J. Duan, T. Furuya, G. Subramanian, L. Aravind, R. A. Cooper, J. C. Wootton, M. Xiong, and X. Z. Su. 2003. Multiple transporters associated with malaria parasite responses to chloroquine and quinine. Mol. Microbiol. 49:977-989. [DOI] [PubMed] [Google Scholar]
- 19.Nkrumah, L. J., P. M. Riegelhaupt, P. Moura, D. J. Johnson, J. Patel, K. Hayton, M. T. Ferdig, T. E. Wellems, M. H. Akabas, and D. A. Fidock. 2009. Probing the multifactorial basis of Plasmodium falciparum quinine resistance: evidence for a strain-specific contribution of the sodium-proton exchanger PfNHE. Mol. Biochem. Parasitol. 165:122-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olliaro, P. 2005. Drug resistance hampers our capacity to roll back malaria. Clin. Infect. Dis. 41(Suppl. 4):S247-257. [DOI] [PubMed] [Google Scholar]
- 21.Pettinelli, F., M. E. Pettinelli, P. Eldin de Pecoulas, J. Millet, D. Michel, P. Brasseur, and P. Druilhe. 2004. Short report. High prevalence of multidrug-resistant Plasmodium falciparum malaria in the French territory of Mayotte. Am. J. Trop. Med. Hyg. 70:635-637. [PubMed] [Google Scholar]
- 22.Pickard, A. L., C. Wongsrichanalai, A. Purfield, D. Kamwendo, K. Emery, C. Zalewski, F. Kawamoto, R. S. Miller, and S. R. Meshnick. 2003. Resistance to antimalarials in Southeast Asia and genetic polymorphisms in pfmdr1. Antimicrob. Agents Chemother. 47:2418-2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pradines, B., T. Pistone, K. Ezzedine, S. Briolant, L. Bertaux, M. C. Receveur, D. Parzy, P. Millet, C. Rogier, and D. Malvy. 2010. Quinine-resistant malaria in traveler returning from Senegal, 2007. Emerg. Infect. Dis. 16:546-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Price, R. N., C. Cassar, A. Brockman, M. Duraisingh, M. van Vugt, N. J. White, F. Nosten, and S. Krishna. 1999. The pfmdr1 gene is associated with a multidrug-resistant phenotype in Plasmodium falciparum from the western border of Thailand. Antimicrob. Agents Chemother. 43:2943-2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Price, R. N., A. C. Uhlemann, A. Brockman, R. McGready, E. Ashley, L. Phaipun, R. Patel, K. Laing, S. Looareesuwan, N. J. White, F. Nosten, and S. Krishna. 2004. Mefloquine resistance in Plasmodium falciparum and increased pfmdr1 gene copy number. Lancet 364:438-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pukrittayakamee, S., W. Supanaranond, S. Looareesuwan, S. Vanijanonta, and N. J. White. 1994. Quinine in severe falciparum malaria: evidence of declining efficacy in Thailand. Trans. R. Soc. Trop. Med. Hyg. 88:324-327. [DOI] [PubMed] [Google Scholar]
- 27.Raj, D. K., J. Mu, H. Jiang, J. Kabat, S. Singh, M. Sullivan, M. P. Fay, T. F. McCutchan, and X. Z. Su. 2009. Disruption of a Plasmodium falciparum multidrug resistance-associated protein (PfMRP) alters its fitness and transport of antimalarial drugs and glutathione. J. Biol. Chem. 284:7687-7796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ralaimazava, P., R. Durand, N. Godineau, A. Keundjian, Z. Jezic, B. Pradines, O. Bouchaud, and J. Le Bras. 2002. Profile and evolution of the chemosusceptibility of falciparum malaria imported into France in 2000. Euro Surveill. 7:113-118. [DOI] [PubMed] [Google Scholar]
- 29.Rathod, P. K., T. McErlean, and P. C. Lee. 1997. Variations in frequencies of drug resistance in Plasmodium falciparum. Proc. Natl. Acad. Sci. U. S. A. 94:9389-9393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reed, M. B., K. J. Saliba, S. R. Caruana, K. Kirk, and A. F. Cowman. 2000. Pgh1 modulates sensitivity and resistance to multiple antimalarials in Plasmodium falciparum. Nature 403:906-909. [DOI] [PubMed] [Google Scholar]
- 31.Roper, C., W. Richardson, I. M. Elhassan, H. Giha, L. Hviid, G. M. Satti, T. G. Theander, and D. E. Arnot. 1998. Seasonal changes in the Plasmodium falciparum population in individuals and their relationship to clinical malaria: a longitudinal study in a Sudanese village. Parasitology 116:501-510. [DOI] [PubMed] [Google Scholar]
- 32.Sattabongkot, J., T. Tsuboi, G. E. Zollner, J. Sirichaisinthop, and L. Cui. 2004. Plasmodium vivax transmission: chances for control? Trends Parasitol. 20:192-198. [DOI] [PubMed] [Google Scholar]
- 33.Segurado, A. A., S. M. di Santi, and M. Shiroma. 1997. In vivo and in vitro Plasmodium falciparum resistance to chloroquine, amodiaquine and quinine in the Brazilian Amazon. Rev. Inst. Med. Trop. Sao Paulo 39:85-90. [DOI] [PubMed] [Google Scholar]
- 34.Sidhu, A. B., A. C. Uhlemann, S. G. Valderramos, J. C. Valderramos, S. Krishna, and D. A. Fidock. 2006. Decreasing pfmdr1 copy number in Plasmodium falciparum malaria heightens susceptibility to mefloquine, lumefantrine, halofantrine, quinine, and artemisinin. J. Infect. Dis. 194:528-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sidhu, A. B., S. G. Valderramos, and D. A. Fidock. 2005. pfmdr1 mutations contribute to quinine resistance and enhance mefloquine and artemisinin sensitivity in Plasmodium falciparum. Mol. Microbiol. 57:913-926. [DOI] [PubMed] [Google Scholar]
- 36.Smilkstein, M., N. Sriwilaijaroen, J. X. Kelly, P. Wilairat, and M. Riscoe. 2004. Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob. Agents Chemother. 48:1803-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Snounou, G., X. Zhu, N. Siripoon, W. Jarra, S. Thaithong, K. N. Brown, and S. Viriyakosol. 1999. Biased distribution of msp1 and msp2 allelic variants in Plasmodium falciparum populations in Thailand. Trans. R. Soc. Trop. Med. Hyg. 93:369-374. [DOI] [PubMed] [Google Scholar]
- 38.Socheat, D., M. B. Denis, T. Fandeur, Z. Zhang, H. Yang, J. Xu, X. Zhou, S. Phompida, R. Phetsouvanh, S. Lwin, K. Lin, T. Win, S. W. Than, Y. Htut, S. Prajakwong, C. Rojanawatsirivet, R. Tipmontree, S. Vijaykadga, S. Konchom, D. Cong le, N. T. Thien, K. Thuan le, P. Ringwald, A. Schapira, E. Christophel, K. Palmer, P. R. Arbani, C. Prasittisuk, R. Rastogi, F. Monti, C. Urbani, R. Tsuyuoka, S. Hoyer, L. Otega, K. Thimasarn, S. Songcharoen, J. P. Meert, F. Gay, L. Crissman, N. Cho Min, W. Chansuda, D. Darasri, K. Indaratna, P. Singhasivanon, S. Chuprapawan, S. Looareesuwan, S. Supavej, C. Kidson, V. Baimai, S. Yimsamran, and K. Buchachart. 2003. Mekong malaria. II. Update of malaria, multi-drug resistance and economic development in the Mekong region of Southeast Asia. Southeast Asian J. Trop. Med. Public Health 34(Suppl. 4):1-102. [PubMed] [Google Scholar]
- 39.Uhlemann, A. C., and S. Krishna. 2005. Antimalarial multi-drug resistance in Asia: mechanisms and assessment. Curr. Top. Microbiol. Immunol. 295:39-53. [DOI] [PubMed] [Google Scholar]
- 40.Vinayak, S., M. T. Alam, M. Upadhyay, M. K. Das, V. Dev, N. Singh, A. P. Dash, and Y. D. Sharma. 2007. Extensive genetic diversity in the Plasmodium falciparum Na+/H+ exchanger 1 transporter protein implicated in quinine resistance. Antimicrob. Agents Chemother. 51:4508-4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.White, N. 1999. Antimalarial drug resistance and combination chemotherapy. Philos. Trans. R. Soc. Lond. B Biol. Sci. 354:739-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.WHO. 2009. Development of a strategy towards elimination of Plasmodium falciparum parasites with altered response to artemisinins. World Health Organization, Geneva, Switzerland.
- 43.WHO. 2009. World malaria report 2009. World Health Organization, Geneva, Switzerland.
- 44.Wilson, C. M., S. K. Volkman, S. Thaithong, R. K. Martin, D. E. Kyle, W. K. Milhous, and D. F. Wirth. 1993. Amplification of pfmdr 1 associated with mefloquine and halofantrine resistance in Plasmodium falciparum from Thailand. Mol. Biochem. Parasitol. 57:151-160. [DOI] [PubMed] [Google Scholar]
- 45.Winstanley, P. 2001. Modern chemotherapeutic options for malaria. Lancet Infect. Dis. 1:242-250. [DOI] [PubMed] [Google Scholar]
- 46.Wongsrichanalai, C., A. L. Pickard, W. H. Wernsdorfer, and S. R. Meshnick. 2002. Epidemiology of drug-resistant malaria. Lancet Infect. Dis. 2:209-218. [DOI] [PubMed] [Google Scholar]
- 47.Yang, H. 1997. Plasmodium falciparum malaria in Yunnan: surveillance and control. Chin. J. Parasit. Dis. Control 10:63-64. [Google Scholar]
- 48.Yang, H., P. Yang, and Y. Yang. 1994. In vitro sensitivity of Plasmodium falciparum to mefloquine, quinine, amodiaquine, chloroquine, pyronaridine and sulfadoxine/pyrimethamine in south Yunnan. Chin. J. Parasitol. Parasit. Dis. 12:140-142. [PubMed] [Google Scholar]
- 49.Yang, H. L., D. Q. Liu, Y. M. Yang, K. G. Huang, Y. Dong, P. F. Yang, M. Z. Liao, and C. Y. Zhang. 1997. In vitro sensitivity of Plasmodium falciparum to eight antimalarials in China-Myanmar and China-Lao PDR border areas. Southeast Asian J. Trop. Med. Public Health 28:460-464. [PubMed] [Google Scholar]
- 50.Yang, Z., Z. Zhang, X. Sun, W. Wan, L. Cui, X. Zhang, D. Zhong, G. Yan, and L. Cui. 2007. Molecular analysis of chloroquine resistance in Plasmodium falciparum in Yunnan Province, China. Trop. Med. Int. Health 12:1051-1060. [DOI] [PubMed] [Google Scholar]
- 51.Zalis, M. G., L. Pang, M. S. Silveira, W. K. Milhous, and D. F. Wirth. 1998. Characterization of Plasmodium falciparum isolated from the Amazon region of Brazil: evidence for quinine resistance. Am. J. Trop. Med. Hyg. 58:630-637. [DOI] [PubMed] [Google Scholar]