Abstract
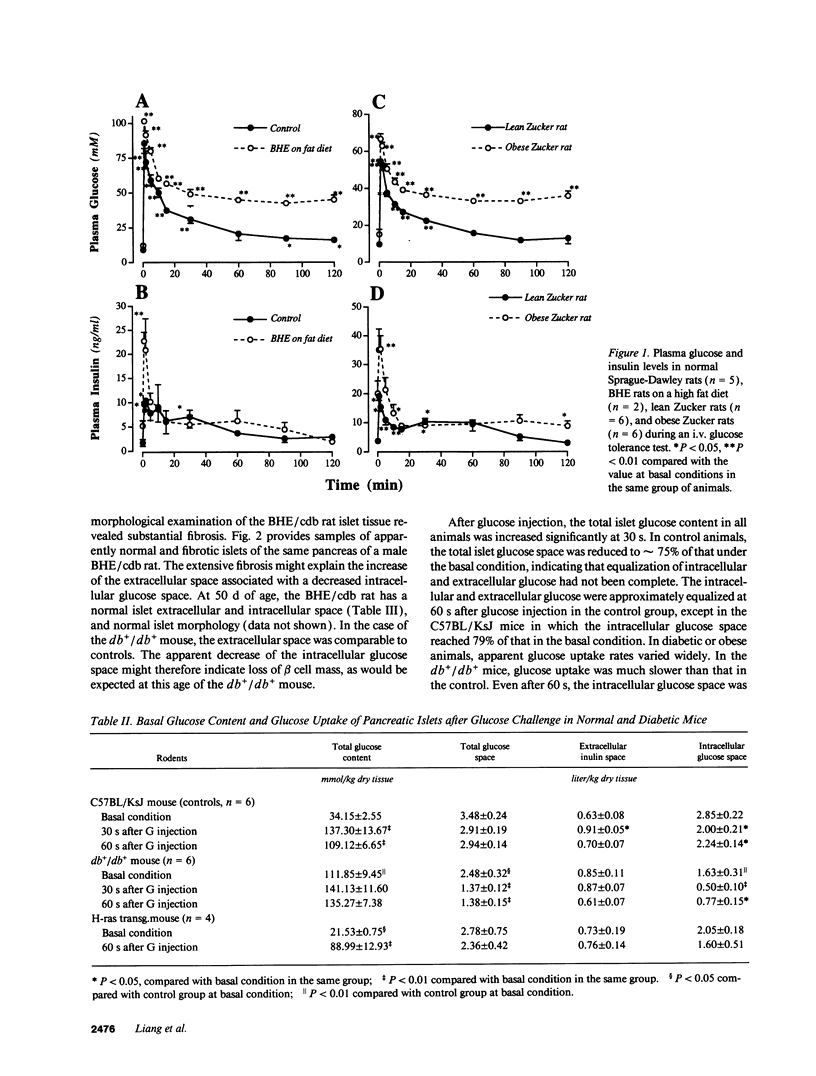
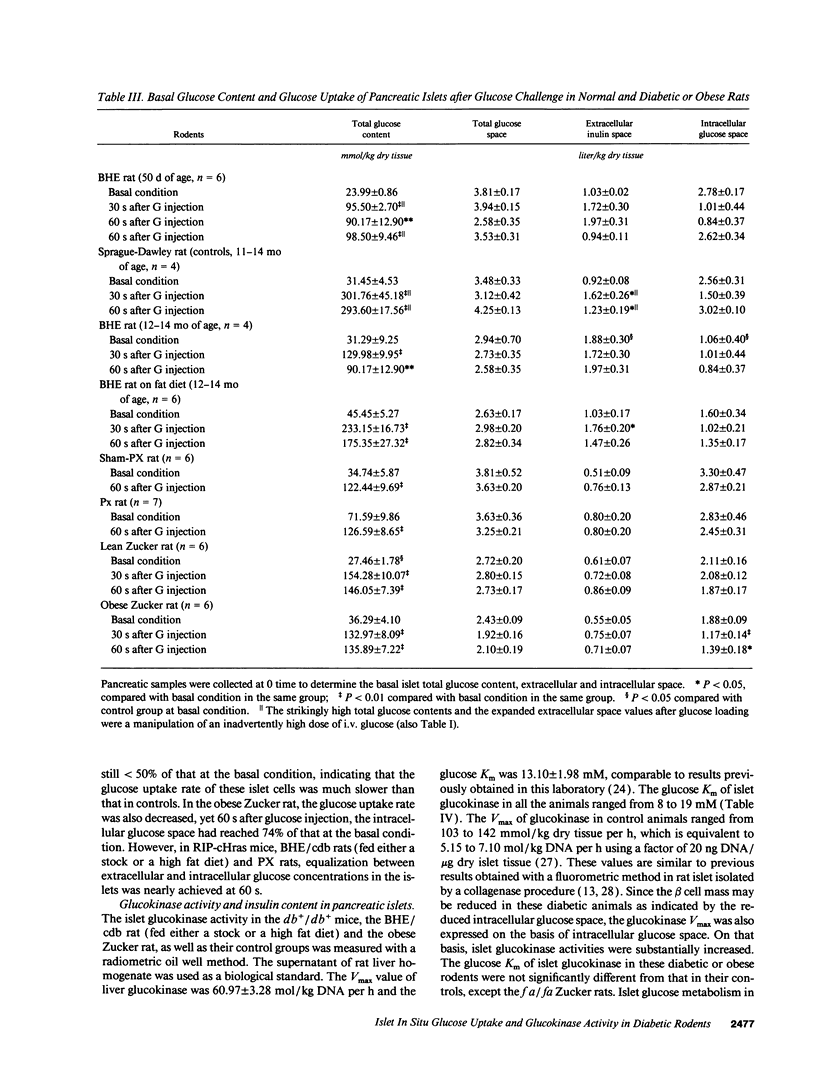
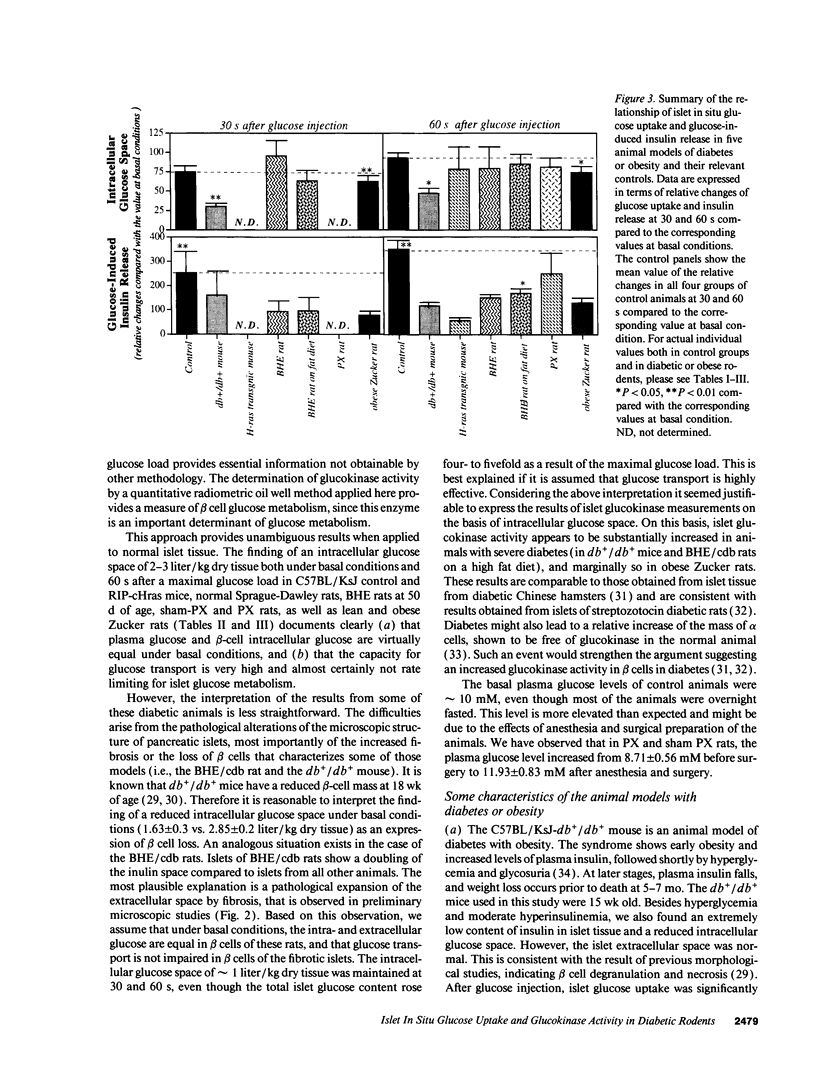
The present study evaluated the involvement of glucose transport and phosphorylation in glucose-stimulated insulin release from pancreatic islets. Using quantitative histochemical techniques, we investigated basal islet glucose content, islet glucose uptake in situ during acute extreme experimental hyperglycemia, and islet glucokinase activity in several animal models of diabetes and obesity. The basal islet glucose content in anaesthetized diabetic or obese rodents was either the same or higher than that in their relevant controls. The rate of glucose uptake of islet tissue in these animals after an i.v. glucose injection was different. The db+/db+ mouse and the obese Zucker rat exhibited significantly reduced islet glucose uptake rates. RIP-cHras transgenic mice, BHE/cdb rats and partially pancreatectomized rats showed normal islet glucose uptake rates. The activity of islet glucokinase was increased to a different degree related to the blood glucose level. All five animal models of diabetes or obesity exhibited either a delay or a reduction of insulin release in response to supra maximal glucose stimulation. Our results indicate that the impairment of glucose-induced insulin release in diabetes is not consistently associated with a reduction of islet glucose uptake nor a change of glucokinase activity.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bedoya F. J., Matschinsky F. M., Shimizu T., O'Neil J. J., Appel M. C. Differential regulation of glucokinase activity in pancreatic islets and liver of the rat. J Biol Chem. 1986 Aug 15;261(23):10760–10764. [PubMed] [Google Scholar]
- Bedoya F. J., Meglasson M. D., Wilson J. M., Matschinsky F. M. Radiometric oil well assay for glucokinase in microscopic structures. Anal Biochem. 1985 Feb 1;144(2):504–513. doi: 10.1016/0003-2697(85)90147-2. [DOI] [PubMed] [Google Scholar]
- Bedoya F. J., Oberholtzer J. C., Matschinsky F. M. Glucokinase in B-cell-depleted islets of Langerhans. J Histochem Cytochem. 1987 Oct;35(10):1089–1093. doi: 10.1177/35.10.3305701. [DOI] [PubMed] [Google Scholar]
- Bedoya F. J., Wilson J. M., Ghosh A. K., Finegold D., Matschinsky F. M. The glucokinase glucose sensor in human pancreatic islet tissue. Diabetes. 1986 Jan;35(1):61–67. doi: 10.2337/diab.35.1.61. [DOI] [PubMed] [Google Scholar]
- Berdanier C. D. The BHE rat: an animal model for the study of non-insulin-dependent diabetes mellitus. FASEB J. 1991 May;5(8):2139–2144. doi: 10.1096/fasebj.5.8.2022312. [DOI] [PubMed] [Google Scholar]
- Bonner-Weir S., Trent D. F., Weir G. C. Partial pancreatectomy in the rat and subsequent defect in glucose-induced insulin release. J Clin Invest. 1983 Jun;71(6):1544–1553. doi: 10.1172/JCI110910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bue J. M., Hausman D. B., Berdanier C. D. Gestational diabetes in the BHE rat: influence of dietary fat. Am J Obstet Gynecol. 1989 Jul;161(1):234–240. doi: 10.1016/0002-9378(89)90272-x. [DOI] [PubMed] [Google Scholar]
- Burant C. F., Bell G. I. Mammalian facilitative glucose transporters: evidence for similar substrate recognition sites in functionally monomeric proteins. Biochemistry. 1992 Oct 27;31(42):10414–10420. doi: 10.1021/bi00157a032. [DOI] [PubMed] [Google Scholar]
- Burch P. T., Berner D. K., Leontire A., Vogin A., Matschinsky B. M., Matschinsky F. M. Metabolic adaption of pancreatic islet tissue in aging rats. J Gerontol. 1984 Jan;39(1):2–6. doi: 10.1093/geronj/39.1.2. [DOI] [PubMed] [Google Scholar]
- Coleman D. L., Hummel K. P. Studies with the mutation, diabetes, in the mouse. Diabetologia. 1967 Apr;3(2):238–248. doi: 10.1007/BF01222201. [DOI] [PubMed] [Google Scholar]
- Efrat S., Fleischer N., Hanahan D. Diabetes induced in male transgenic mice by expression of human H-ras oncoprotein in pancreatic beta cells. Mol Cell Biol. 1990 Apr;10(4):1779–1783. doi: 10.1128/mcb.10.4.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froguel P., Zouali H., Vionnet N., Velho G., Vaxillaire M., Sun F., Lesage S., Stoffel M., Takeda J., Passa P. Familial hyperglycemia due to mutations in glucokinase. Definition of a subtype of diabetes mellitus. N Engl J Med. 1993 Mar 11;328(10):697–702. doi: 10.1056/NEJM199303113281005. [DOI] [PubMed] [Google Scholar]
- Gidh-Jain M., Takeda J., Xu L. Z., Lange A. J., Vionnet N., Stoffel M., Froguel P., Velho G., Sun F., Cohen D. Glucokinase mutations associated with non-insulin-dependent (type 2) diabetes mellitus have decreased enzymatic activity: implications for structure/function relationships. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1932–1936. doi: 10.1073/pnas.90.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- Inman L. R., McAllister C. T., Chen L., Hughes S., Newgard C. B., Kettman J. R., Unger R. H., Johnson J. H. Autoantibodies to the GLUT-2 glucose transporter of beta cells in insulin-dependent diabetes mellitus of recent onset. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1281–1284. doi: 10.1073/pnas.90.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jetton T. L., Magnuson M. A. Heterogeneous expression of glucokinase among pancreatic beta cells. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2619–2623. doi: 10.1073/pnas.89.7.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. H., Crider B. P., McCorkle K., Alford M., Unger R. H. Inhibition of glucose transport into rat islet cells by immunoglobulins from patients with new-onset insulin-dependent diabetes mellitus. N Engl J Med. 1990 Mar 8;322(10):653–659. doi: 10.1056/NEJM199003083221003. [DOI] [PubMed] [Google Scholar]
- Johnson J. H., Newgard C. B., Milburn J. L., Lodish H. F., Thorens B. The high Km glucose transporter of islets of Langerhans is functionally similar to the low affinity transporter of liver and has an identical primary sequence. J Biol Chem. 1990 Apr 25;265(12):6548–6551. [PubMed] [Google Scholar]
- Johnson J. H., Ogawa A., Chen L., Orci L., Newgard C. B., Alam T., Unger R. H. Underexpression of beta cell high Km glucose transporters in noninsulin-dependent diabetes. Science. 1990 Oct 26;250(4980):546–549. doi: 10.1126/science.2237405. [DOI] [PubMed] [Google Scholar]
- Kurtz T. W., Morris R. C., Pershadsingh H. A. The Zucker fatty rat as a genetic model of obesity and hypertension. Hypertension. 1989 Jun;13(6 Pt 2):896–901. doi: 10.1161/01.hyp.13.6.896. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H. The quantitative histochemistry of the brain; histological sampling. J Histochem Cytochem. 1953 Nov;1(6):420–428. doi: 10.1177/1.6.420. [DOI] [PubMed] [Google Scholar]
- Liang Y., Najafi H., Smith R. M., Zimmerman E. C., Magnuson M. A., Tal M., Matschinsky F. M. Concordant glucose induction of glucokinase, glucose usage, and glucose-stimulated insulin release in pancreatic islets maintained in organ culture. Diabetes. 1992 Jul;41(7):792–806. doi: 10.2337/diab.41.7.792. [DOI] [PubMed] [Google Scholar]
- Like A. A., Chick W. L. Studies in the diabetic mutant mouse. I. Light microscopy and radioautography of pancreatic islets. Diabetologia. 1970 Jun;6(3):207–215. doi: 10.1007/BF01212231. [DOI] [PubMed] [Google Scholar]
- Magnuson M. A. Tissue-specific regulation of glucokinase gene expression. J Cell Biochem. 1992 Feb;48(2):115–121. doi: 10.1002/jcb.240480202. [DOI] [PubMed] [Google Scholar]
- Matschinsky F. M., Ellerman J. E. Metabolism of glucose in the islets of Langerhans. J Biol Chem. 1968 May 25;243(10):2730–2736. [PubMed] [Google Scholar]
- Matschinsky F. M. Glucokinase as glucose sensor and metabolic signal generator in pancreatic beta-cells and hepatocytes. Diabetes. 1990 Jun;39(6):647–652. doi: 10.2337/diab.39.6.647. [DOI] [PubMed] [Google Scholar]
- Meglasson M. D., Burch P. T., Berner D. K., Najafi H., Vogin A. P., Matschinsky F. M. Chromatographic resolution and kinetic characterization of glucokinase from islets of Langerhans. Proc Natl Acad Sci U S A. 1983 Jan;80(1):85–89. doi: 10.1073/pnas.80.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meglasson M. D., Matschinsky F. M. Pancreatic islet glucose metabolism and regulation of insulin secretion. Diabetes Metab Rev. 1986;2(3-4):163–214. doi: 10.1002/dmr.5610020301. [DOI] [PubMed] [Google Scholar]
- Orci L., Thorens B., Ravazzola M., Lodish H. F. Localization of the pancreatic beta cell glucose transporter to specific plasma membrane domains. Science. 1989 Jul 21;245(4915):295–297. doi: 10.1126/science.2665080. [DOI] [PubMed] [Google Scholar]
- Robertson R. P., Seaquist E. R., Walseth T. F. G proteins and modulation of insulin secretion. Diabetes. 1991 Jan;40(1):1–6. doi: 10.2337/diab.40.1.1. [DOI] [PubMed] [Google Scholar]
- SALAS J., SALAS M., VINUELA E., SOLS A. GLUCOKINASE OF RABBIT LIVER. J Biol Chem. 1965 Mar;240:1014–1018. [PubMed] [Google Scholar]
- Seaquist E. R., Neal A. R., Shoger K. D., Walseth T. F., Robertson R. P. G-proteins and hormonal inhibition of insulin secretion from HIT-T15 cells and isolated rat islets. Diabetes. 1992 Nov;41(11):1390–1399. doi: 10.2337/diab.41.11.1390. [DOI] [PubMed] [Google Scholar]
- Tal M., Wu Y. J., Leiser M., Surana M., Lodish H., Fleischer N., Weir G., Efrat S. [Val12] HRAS downregulates GLUT2 in beta cells of transgenic mice without affecting glucose homeostasis. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5744–5748. doi: 10.1073/pnas.89.13.5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorens B., Sarkar H. K., Kaback H. R., Lodish H. F. Cloning and functional expression in bacteria of a novel glucose transporter present in liver, intestine, kidney, and beta-pancreatic islet cells. Cell. 1988 Oct 21;55(2):281–290. doi: 10.1016/0092-8674(88)90051-7. [DOI] [PubMed] [Google Scholar]
- Trus M. D., Zawalich W. S., Burch P. T., Berner D. K., Weill V. A., Matschinsky F. M. Regulation of glucose metabolism in pancreatic islets. Diabetes. 1981 Nov;30(11):911–922. doi: 10.2337/diab.30.11.911. [DOI] [PubMed] [Google Scholar]
- Unger R. H. Diabetic hyperglycemia: link to impaired glucose transport in pancreatic beta cells. Science. 1991 Mar 8;251(4998):1200–1205. doi: 10.1126/science.2006409. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Pastan I., Shih T. Y., Scolnick E. M. Localization of the src gene product of the Harvey strain of MSV to plasma membrane of transformed cells by electron microscopic immunocytochemistry. Cell. 1980 Apr;19(4):1005–1014. doi: 10.1016/0092-8674(80)90091-4. [DOI] [PubMed] [Google Scholar]