Abstract
Respiratory disease is the main cause of morbidity and mortality in patients with cystic fibrosis (CF). In particular, patients suffer from chronic infection due to biofilm formation by opportunistic Pseudomonas aeruginosa (32). Therefore, there is an urgent need to develop alternative ways to treat biofilm-associated clinical infections. The aim of this study was to compare the antimicrobial effects in vitro of the combinations tobramycin-clarithromycin and tobramycin-azithromycin against five P. aeruginosa biofilms and to establish the most effective combination. We performed a kinetic study over a period of 28 days of a twice-daily coadministration of the combinations tobramycin-clarithromycin and tobramycin-azithromycin on 12-day-old, mature P. aeruginosa biofilms formed on microplate pegs for 4 clinical isolates and one laboratory strain (PAO1) to simulate the treatment of CF patients with tobramycin inhalation solution (TOBI) through aerosolization. A synergy between tobramycin and clarithromycin was recorded for 3/5 biofilms, with a bacterial decrease of more than 5 log. Conversely, we found an antagonistic activity when 4 μg/ml tobramycin was administered with azithromycin at 2 μg/ml for P. aeruginosa PAO1 and with azithromycin at 2, 20, 50, 100, and 200 μg/ml for P. aeruginosa PYO1. Treatment with tobramycin at 4 μg/ml combined with clarithromycin at 200 μg/ml eradicated all five biofilms, while tobramycin-azithromycin at the same concentrations eradicated only three biofilms. Results of this study suggest that local administration of tobramycin and clarithromycin into the respiratory tract represents a better strategy than the combination tobramycin-azithromycin for the treatment of P. aeruginosa-associated pulmonary infections.
Cystic fibrosis (CF) is a major exocrinopathy caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene (26). The dysfunction of this protein provokes abnormal electrolyte transport, which often affects the respiratory tract. The airways of patients afflicted with CF are colonized by many pathogens, among which the Gram-negative bacterium Pseudomonas aeruginosa is predominant. After repeated episodes of mainly P. aeruginosa-associated respiratory tract infections, patients start to develop pulmonary insufficiency that gradually worsens and finally leads to end-stage respiratory failure and death (3, 29).
Chronic P. aeruginosa infection affects 80 to 90% of patients with CF (6) and is difficult to treat with conventional antibiotic therapies. Indeed, tobramycin is one of the most effective antibiotics against P. aeruginosa infections in patients with CF, but persistent usage enables the pathogen to activate defense mechanisms (18), making eradication rarely successful. Biofilms are one of these defense mechanisms. They are complex and organized in bacterial communities, able to grow in association with different biological or abiotic surfaces. Biofilms play a relevant role in persistent infections, significantly increasing antibiotic resistance (29). They are now thought to be involved in 65 to 80% of all microbial infections (8). In the thick CF airway mucus, P. aeruginosa can form biofilms, which might greatly impede its eradication during treatment and could promote recurrent infections in these patients. Conventional antibiotic susceptibility testing examines the efficacy of antibiotics against planktonically grown cultures of bacteria under aerobic conditions. This may not be the most appropriate approach, considering that bacteria grow in CF airways as a biofilm. This might explain the difficulty in predicting the outcome of a treatment by using in vitro testing. Anwar et al. have therefore proposed that in these clinical infections microbiological laboratories should use the bacterial strain to establish biofilms and use these biofilms to test susceptibility of the strain to antibacterial agents (1). This conclusion prompted us to develop an in vitro model of mature biofilms formed on microplate pegs in order to find antibiotic associations able to fully eradicate P. aeruginosa under these growth conditions. This model simulates the in vivo growth conditions of P. aeruginosa in the upper respiratory airways of patients with CF better than planktonic and immature biofilm models. Using this model, we have previously demonstrated that the combination tobramycin-clarithromycin might be a good antibiotic association to treat P. aeruginosa in patients with CF (35).
In a previous work (36), we observed synergistic activity of a tobramycin and clarithromycin combination on 9/23 biofilms of P. aeruginosa, but no correlation between drug susceptibility profiles and the phenotype or genotype of the isolates was observed. We suggested that macrolides should offer considerable potential for the treatment of biofilm-forming P. aeruginosa strains in CF airways. Considering that azithromycin is widely used to treat lung inflammation of patients with CF (31), the aim of this paper was to compare the antimicrobial in vitro effect on mature biofilms of the combination tobramycin-clarithromycin versus that of tobramycin-azithromycin and to establish and confirm the most effective combination.
Clarithromycin belongs to the class of macrolides which are grouped according to the number of atoms comprising the lactone ring, e.g., 12-, 14-, 15-, and 16-membered rings. The 14-membered-ring group includes clarithromycin. Only azithromycin has a 15-membered ring, and it is characterized by a stronger antibacterial activity than that of the other macrolides on Gram-negative organisms. We used our own model of mature biofilm (36), and the antibiotics were tested on four clinical isolates and one laboratory strain of P. aeruginosa. Three mucoid phenotypes were chosen, because mucoid strains have increased resistance to various antibiotics (14).
MATERIALS AND METHODS
Antimicrobial agents.
Micronized tobramycin, clarithromycin, and azithromycin were purchased from Teva, Petach Tikva, Israel. Stock solutions (10 mg/ml) of tobramycin were prepared in sterile water. Clarithromycin and azithromycin were first dissolved in methanol, and stock solutions (2 mg/ml) were prepared with pH 6.5 phosphate buffer (23). Stock solutions were stored at −20°C until use. We previously confirmed that methanol at the concentration used had no bactericidal activity. The purpose of our work was to test for a potential effect of macrolides on the response to tobramycin. To this end, tobramycin was used at its MIC, i.e., 4 μg/ml (37). Clarithromycin and azithromycin were tested at 2, 20, 50, 100, and 200 μg/ml. This range of concentrations was chosen because the macrolides seem to accumulate in bronchial secretions. According to Conte et al. (7) and Rodvold (27), the concentration of clarithromycin can reach 40 μg/ml 6 h after oral intake of 500 mg of the drug. No data are yet available, but it is very likely that much higher concentrations of macrolide in the upper airways should be reached after delivery of the drugs in a nebulized form (16).
Testing of susceptibility to each drug was performed on planktonic cultures using the 2-fold dilution method according to the CLSI (6a). Determination of MICs was performed in 96-well microplates (650-185; Greiner), and results were recorded after incubation at 35°C for 18 h (Table 1) .
TABLE 1.
MICs of antimicrobial agents on planktonic cultures of P. aeruginosa PAO1 and clinical isolates
| Strain | MIC (μg/ml) of antimicrobial agenta: |
||
|---|---|---|---|
| CLR | AZM | TOB | |
| PAO1 | 250-500 | 128 | 2-4 |
| PYO1 | >1,000 | >500 | 8 |
| PYO2 | >1,000 | >500 | 2 |
| MC99 | >1,000 | 64 | ≤1 |
| MC75 | 1,000 | 64 | ≤1 |
CLR, clarithromycin; AZM, azithromycin; TOB, tobramycin. All MIC values were measured in triplicate and are presented as suggested by the CLSI (6a).
Bacterial strains.
The P. aeruginosa reference strain (PAO1) and four clinical isolates from sputum of CF patients from the University Hospital Erasme in Brussels, Belgium (PYO1 and PYO2), and from the University Hospital of Ghent, Belgium (MC75 and MC99), were tested. The strains included three mucoid (PYO2, MC99, and MC75) and two nonmucoid (PAO1 and PYO1) phenotypes. Identification of the strains was confirmed by an API 20 NE system (bioMérieux, Marcy-l'Etoile, France).
Bacterial suspensions were prepared from fresh subcultures, and aliquots were stored at −20°C in glycerol until use. Before use, bacterial suspensions were spread onto Mueller-Hinton solid medium and incubated at 35°C for 24 h.
Inoculum preparation for biofilm study.
The bacterial suspension was prepared as previously described (36) in Mueller-Hinton broth adjusted with the cations Ca2+ and Mg2+ (CAMHB) at a concentration of ∼108 CFU/ml.
Kinetic study of the coadministration of antimicrobial agents to 12-day-old biofilms.
Biofilm was formed in 96-well microplates (Nunc Micro Wells), according to a previously described protocol (36). The medium was changed daily from day 1 to day 12 in order to promote biofilm formation and maturation. We have shown previously that such a mature biofilm (12 days old) is a more realistic in vitro model than 24-hour-old biofilm (35).
After formation of mature biofilms, the pegs were removed, rinsed, and put in contact continuously for 28 days with the antibiotic solutions, freshly replenished twice daily. This in vitro kinetic model of mature biofilm mimics the treatment of patients with tobramycin inhalation solution (TOBI) (25), during which TOBI is inhaled twice a day for 28 days at intervals as close as possible to 12 h and no less than 6 h apart. Prior to enumeration of the bacteria, some biofilms (clinical isolate MC75) formed on the pegs were photographed. Enumeration of the bacteria was carried out after starting antibiotic administration at 0, 2, 5, 7, 10, 14, and 28 days. For enumeration of bacteria, the pegs were rinsed in a new microplate containing isotonic 0.01 M potassium phosphate buffer, pH 7.5, to remove unbound cells. Thereafter, the pegs were put in fresh CAMHB in a new microplate. The pegs and microplate were then sealed and sonicated at 35°C for 5 min to resuspend the biofilm from the pegs into the CAMHB. Aliquots of these suspensions of biofilm were then plated on petri dishes containing a solid medium and incubated overnight at 35°C. Then, CFUs from antibiotic-treated and antibiotic-untreated biofilms were counted and compared to each other. The logarithmic decrease in CFU from antibiotic-treated biofilms was calculated using the following formula: logarithmic decrease = [log(CFUpositive control) − log(CFUx)], where x corresponds to the tested antimicrobial agent used alone or in combination. A bacterial reduction to a level below the detection limit was defined as a decrease in the number of CFU/ml of at least 5 log.
Synergistic activity between antibiotics was defined as a decrease in the number of CFU/ml of at least 2 logarithmic scales with regard to the activity of each antibiotic alone.
Antagonism between antibiotics was defined as an increase in the number of CFU/ml of at least 2 logarithmic scales with regard to the activity of each antibiotic alone.
Indifference was defined as an increase in the number of CFU/ml of less than 2 logarithmic scales with regard to the activity of each antibiotic alone.
Statistical analysis.
All tests were done in triplicate. Results were analyzed with the Mann-Whitney nonparametric test. All graphical representations were made using GraphPad Prism 4.03 (GraphPad Software, Inc., San Diego, CA).
RESULTS
Kinetic study of the coadministration of antimicrobial agents to 12-day-old biofilms during 28 days of treatment.
The activity of each antibiotic used alone during 28 days of treatment was assessed initially. Three different strains, one laboratory strain (PAO1) and one mucoid (PYO2) and one nonmucoid (PYO1) clinical strain, were used to establish the most effective concentrations of the two tested macrolides, clarithromycin and azithromycin. Tested concentrations ranged from 2 to 200 μg/ml. By comparing the antimicrobial activities of clarithromycin and azithromycin after 28 days of treatment, we observed that no bacteria could be cultured from the pegs when PAO1 biofilm was exposed to azithromycin at 200 μg/ml (Table 2). This macrolide had no effect on the other two strains. Clarithromycin alone had no effect on any strain. In a further experiment, the highest concentration (200 μg/ml) of each of the two macrolides was tested on the biofilms of MC99 and MC75. As shown in Fig. 1, the biofilm formed by strain MC75 completely disappeared after treatment with 200 μg/ml azithromycin. At this concentration, the bactericidal activity of the macrolide was also confirmed. Clarithromycin had an antimicrobial activity on MC99 biofilm, with a bacterial decrease of more than 2 log (Table 2). The PAO1, PYO2, and MC75 biofilms were resistant to 4 μg/ml tobramycin (Table 2; Fig. 1A and 2B). At this concentration, tobramycin had a bactericidal activity on PYO1 and MC99 biofilms (Table 2). The number of bacteria in the biofilm was 500 CFU/ml (the limit of detection) at the end of the 28-day treatment.
TABLE 2.
Bacterial logarithmic decrease after 28 days of a twice-daily treatment of clarithromycin, azithromycin, or tobramycin alone
| Strain | Decrease in bacteria (log CFU/ml) after treatment with antibiotic at indicated concn (μg/ml)a |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CLR |
AZM |
TOB |
|||||||||
| 2 | 20 | 50 | 100 | 200 | 2 | 20 | 50 | 100 | 200 | 4 | |
| PAO1 | ≤1 | ≤1 | ≤1 | ≤1 | ≤1 | ≤1 | ≤1 | ≤1 | ≤1 | >5 | ≤1 |
| PYO1 | ≤1 | ≤1 | ≤1 | ≤1 | ≤1 | ≤1 | 1.5 | 1.7 | 1.7 | ≤1 | >5 |
| PYO2 | ≤1 | ≤1 | ≤1 | ≤1 | ≤1 | 1.1 | 1.3 | 1.2 | ≤1 | ≤1 | ≤1 |
| MC99 | NDb | ND | ND | ND | >2 | ND | ND | ND | ND | >5 | >5 |
| MC75 | ND | ND | ND | ND | 1.4 | ND | ND | ND | ND | >5 | 1.3 |
CLR, clarithromycin; AZM, azithromycin; TOB, tobramycin. Boldface type indicates a bacterial reduction to a level below the detection limit.
ND, not determined.
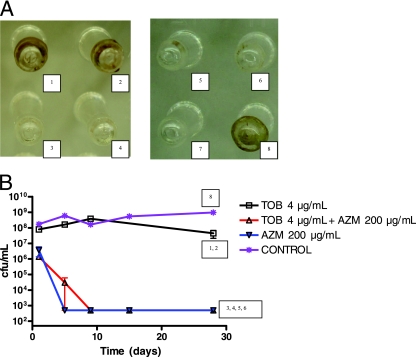
FIG. 1.
(A) Observation of biofilms on pegs after 28 days of a twice-daily repeated treatment with tobramycin and azithromycin, alone or combined, on clinical isolate MC75. Panels 1 and 2 represent duplicates, as do panels 3 and 4 and panels 5 and 6. Panels 1 and 2, persistent biofilm after 28 days of treatment with tobramycin at 4 μg/ml (1.3 log bacterial decrease). Panels 3 and 4, biofilm destruction after 28 days of treatment with azithromycin at 200 μg/ml (>5 log bacterial decrease). Panels 5 and 6, biofilm destruction after 28 days of treatment with the antibiotic combination of tobramycin at 4 μg/ml plus azithromycin at 200 μg/ml (>5 log bacterial decrease). Panel 7, positive control. Panel 8, negative control (no antibiotic). (B) Kinetic study of the effect of administration of the antimicrobial agents tobramycin (TOB) and azithromycin (AZM), alone or in combination, on 12-day-old biofilms of strain MC75. The numbering corresponds to the panels described above. Short vertical bars represent standard errors.
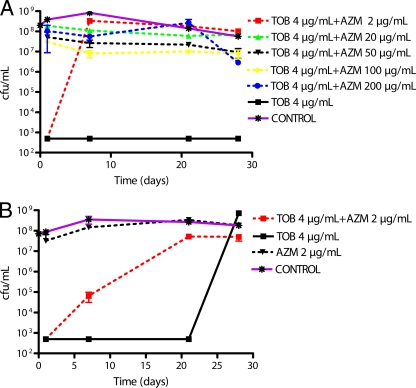
FIG. 2.
Kinetic study of the antagonistic effect of administration of the antimicrobial agents tobramycin (TOB) and azithromycin (AZM) on 12-day-old biofilms of (A) P. aeruginosa PYO1 and (B) P. aeruginosa PAO1.
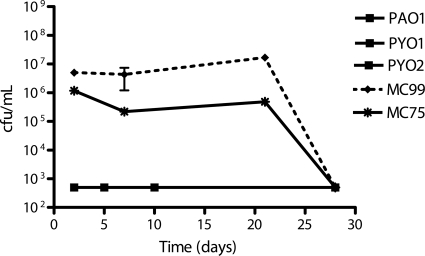
The biofilms were next exposed to 4 μg/ml tobramycin in combination with different concentrations of either clarithromycin or azithromycin. The results of these drug combinations were classified as synergistic, antagonistic, or without influence on antimicrobial activity if the effect of the combined drugs was better than, worse than, or the same as that of the individual drug activities, respectively. Only the 28-day treatment with 4 μg/ml tobramycin combined with 200 μg/ml clarithromycin eradicated all 5 biofilms (Table 3). This combination had a synergistic activity on PAO1, PYO2, and MC75 biofilms, with an important decrease of more than 5 log. It had no obvious influence on the biofilms of PYO1 and MC99 strains, which were completely eradicated by tobramycin alone.
TABLE 3.
Comparison of the antimicrobial activities of the combinations of tobramycin-clarithromycin and tobramycin-azithromycin after 28 days of a twice-daily treatmenta
| Strain | TOB (4 μg/ml) plus CLR (200 μg/ml) |
TOB (4 μg/ml) plus AZM (200 μg/ml) |
||
|---|---|---|---|---|
| Effectb | Decrease in bacteria (log CFU/ml) | Effectb | Decrease in bacteria (log CFU/ml) | |
| PAO1 | S | >5 | N | >5 |
| PYO1 | N | >5 | A | >1 |
| PYO2 | S | >5 | N | >1 |
| MC99 | N | >5 | N | >5 |
| MC75 | S | >5 | N | >5 |
TOB, tobramycin; CLR, clarithromycin; AZM, azithromycin.
S, synergy; N, neutral, or no synergy (no influence on antimicrobial activity); A, antagonism.
The combination of 4 μg/ml tobramycin and 200 μg/ml azithromycin showed an important logarithmic decrease (more than 5 log) in the number of CFU/ml in 3 biofilms (PAO1, MC99, and MC75). This treatment had no effect on PYO1 and PYO2 biofilms, which was surprising, considering that by itself 4 μg/ml tobramycin eradicated the PYO1 biofilm, and this result suggested an antagonism between tobramycin and azithromycin. This was further explored by testing the combination of tobramycin and various concentrations of azithromycin. As shown in Fig. 2 A, 200 μg/ml azithromycin fully inhibited the bactericidal activity of tobramycin within 2 days. Similar results were obtained with concentrations of azithromycin as low as 2 μg/ml. At a concentration of 2 μg/ml, azithromycin also reversed the effect of tobramycin but with a 1-week delay. Considering that by itself 200 μg/ml azithromycin had a strong bactericidal activity on the PAO1 strain, the interaction between tobramycin and azithromycin on this biofilm was reexamined with 2 μg/ml azithromycin. As shown in Fig. 2B, this concentration of the macrolide had no effect on bacterial viability. Tobramycin, at least at the beginning of the experiment (from day 2 until day 21), was bactericidal. Bacteria slowly became resistant to the combination of tobramycin and azithromycin. The resistance was already significant after 7 days, with a difference of 2 log (P < 0.05 compared to tobramycin alone), and only 105 CFU/ml were counted. The antimicrobial effect of tobramycin disappeared completely after 21 days (107 CFU/ml) (P > 0.1 compared to control).
When considering the global response to the treatments, the combination of tobramycin and clarithromycin showed better antimicrobial activity than did tobramycin alone, with synergy on 3 out of 5 strains and equivalence on the other 2 strains (PYO1 and MC99) (Fig. 3) . Treatment with tobramycin at 4 μg/ml combined with clarithromycin at 200 μg/ml over 28 days eradicated all biofilms (5/5), while the combination tobramycin-azithromycin at the same concentrations eradicated only 3 biofilms (Table 3).
FIG. 3.
Study of the synergistic effect of administration of the antimicrobial agents tobramycin at 4 μg/ml and clarithromycin at 200 μg/ml on 12-day-old biofilms of P. aeruginosa PAO1, PYO1, PYO2, MC99, and MC75. The graphics for PAO1, PYO1, and PYO2 are combined. Results for the 3 strains PAO1, PYO1, and PYO2 were the same.
DISCUSSION
Once established, P. aeruginosa chronic infection in cystic fibrosis patients is very difficult to cure, and it is considered the major cause of morbidity and mortality (21). Therefore, a treatment able to eradicate chronic P. aeruginosa infections would significantly increase the survival of CF patients (12, 13). However, this goal is still beyond reach because drug therapy for CF patients is compromised by a number of bacterial factors that render infectious agents resistant to antibiotics. These factors include, among others, the increased expression of efflux pumps that remove antibiotics and the lack of penetration of antibiotics into bacterial biofilms.
Aggressive antibiotic therapy, based on nebulized antibiotics such as tobramycin or colistin used alone or in combination with oral ciprofloxacin for the treatment of patients, has been developed. This therapeutic approach has proven successful for the eradication of new colonizations with P. aeruginosa and currently enables clinicians to postpone chronic infection in many patients. In addition, antibiotic therapy during acute exacerbations in chronically infected patients helps to reduce clinical symptoms, and long-term treatment slows down the loss of lung function. Thus, antibiotic treatment is useful in different aspects in CF therapy, but eradication of chronically infecting P. aeruginosa is still beyond reach because the exact optimal protocol is yet to be determined. Recent clinical trials have demonstrated the clinical efficacy of long-term macrolide therapy (33). In the present study, we observed that the effect of the combination of tobramycin and a macrolide was affected by the structure of the macrolide. The interaction between clarithromycin and tobramycin was often synergistic, while azithromycin had sometimes antagonistic activities with respect to tobramycin.
Tobramycin is one of the most effective antibiotics against P. aeruginosa infections in CF patients. The development of efficient nebulizing devices (20) promoted the use of aerosolized tobramycin, which allowed much higher concentrations to reach the upper respiratory tract and proved more efficient than other drug delivery forms. However, in spite of this major improvement, eradication of the infection remains barely achievable because P. aeruginosa forms biofilms and becomes resistant to tobramycin. Slow penetration due to electrostatic interactions with the mucus and extracellular polysaccharide matrix and lack of activity against cells with a slow growth rate and a low metabolic activity are particularly important (5). Some authors have reported that subinhibitory levels of aminoglycosides help to induce biofilm formation (9, 18). It has also been reported that P. aeruginosa responds to anaerobic conditions by increased production of alginate, which poses a physical barrier to antibiotics (38). Treerat et al. suggested that the chemotherapy for CF patients might be compromised not only by antibiotic-resistant pathogens but also by a lack of penetration of antibiotics caused either by bacterial biofilms or by the high sodium flux in the CF lung (34). They also found that Na+ and K+, but not Cl−, are the chief antagonists of tobramycin efficacy. Recently, Mah et al. discovered that periplasmic glucans interact with tobramycin in P. aeruginosa biofilms. This observation led them to suggest that glucans delay, by sequestration, the effect of the antibiotic (22). Another mechanism involved in biofilm resistance to tobramycin involves the overexpression of a cluster of 4 genes, PA1874 to PA1877. This sequence encodes membrane proteins acting like a multidrug efflux pump for tobramycin, gentamicin, and rifampin (39). All of these results might explain the resistance to tobramycin observed for PAO1, PYO2, and MC75 biofilms. In order to prevent or at least delay tobramycin resistance and to impede the formation of a biofilm, drug association with tobramycin rather than monotherapy is now advocated extensively. Tobramycin has been associated with various antibiotics or even nonantibiotic agents, such as amino acids (2) or amiloride (34). The association of tobramycin with macrolides is rather unexpected considering that P. aeruginosa is insensitive to therapeutic concentrations of macrolides. The positive interaction is probably secondary to the anti-inflammatory properties of the macrolides and due to their ability to prevent both the formation of the biofilm (19) and the expression of quorum sensing (4). This probably explains why macrolides improve lung function in CF patients (and prevent resistance to other antibiotics active on P. aeruginosa) in spite of their lack of bactericidal activity.
In our hands, the combination of tobramycin and clarithromycin also proved beneficial. Clarithromycin alone had no effect when tested on either the biofilm or the planktonic form of the five strains. However, biofilms formed by three of the strains (PAO1, PYO2, and MC75), which were initially found to be resistant to tobramycin alone, were subsequently completely eradicated after a 28-day exposure to the combination of tobramycin plus clarithromycin. Azithromycin is a macrolide with a structure slightly different from that of clarithromycin and with distinct pharmacokinetic properties. It can easily penetrate tissues and is found at high concentrations in airway secretions (31). These properties probably account for the beneficial effects of azithromycin in treating chronic infections with P. aeruginosa (10). Using the in vitro mature biofilm model, we also observed that azithromycin alone has a slightly greater effect than clarithromycin alone on biofilms and the embedded bacteria. The responses to azithromycin varied among the strains and required azithromycin concentrations never reached in the upper airways after oral or systemic delivery of the drug. In contrast to clarithromycin, azithromycin never synergized with the aminoglycoside tobramycin but rather antagonized its bactericidal effect. Indeed, two of the strains tested (PAO1 and PYO1), which at the beginning of the assay were sensitive to tobramycin, became resistant to the aminoglycoside after treatment of the biofilm with azithromycin and tobramycin. This antagonism was already observed at a very low concentration (2 μg/ml) of azithromycin, i.e., a concentration which has been reported in bronchial secretions after oral intake of the drug. The antagonism between tobramycin and azithromycin seemed to be time and dose dependent. These results led us to suspect that subinhibitory concentrations of azithromycin accelerated the occurrence of resistance to tobramycin. A similar result has recently been reported by Hill et al. These authors showed that azithromycin (0.4 μg/ml) was antagonistic in up to 19% of combinations with 200 μg/ml tobramycin among anaerobically grown or biofilm-grown isolates. Antagonism occurred with the combination tobramycin (200 μg/ml)-azithromycin (0.4 μg/ml)-ciprofloxacin (2 μg/ml) (17). The mechanism of this antagonism has not yet been explained. Skindersoe et al. observed that at low, subinhibitory concentrations, azithromycin modified the expression of more than 300 genes (30). The transcription of a vast majority of these genes (more than 270 genes) was decreased by 2 μg/ml azithromycin. A comparison between the genes regulated by azithromycin and the 135 genes of the tobramycin resistome of P. aeruginosa (28) revealed that several genes belong to both groups. This is consistent with the current view that antibiotic resistance is multifactorial (11). A recent study suggested that MexAB-OprM and MexCD-OprJ are involved in biofilm resistance to azithromycin (15). Mulet et al. showed that mutations in nfxB induce hyperexpression of the multidrug efflux pump MexCD-OprJ, which is also responsible for cross-resistance to other, unrelated antipseudomonal agents, such as ciprofloxacin or cefepime (24). Chronic exposure to azithromycin could thus increase the expression of an efflux pump able to export tobramycin.
In conclusion, our results reveal an important difference in the effects of combinations of tobramycin and various macrolides. At first consideration, azithromycin alone may be seen as a better choice than clarithromycin in clinical practice, as it more readily has access to the sites of infection. However, the genomic effects of azithromycin, which are beneficial with regard to affecting biofilm formation and quorum sensing, may in fact turn out to be deleterious with regard to its antagonistic influence on other antibiotics, such as tobramycin. The combination of tobramycin and clarithromycin did not seem to have this drawback; on the contrary, clarithromycin prevented or even reversed the resistance of biofilms to tobramycin. Therefore, the tobramycin-clarithromycin combination appears as a potential association to be assessed clinically for the treatment of chronic pulmonary infections caused by P. aeruginosa.
Acknowledgments
Clinical isolates were kindly provided by Olivier Denis, Erasme Hospital, Brussels, Belgium. We thank Séverine Tandel for technical assistance.
This work was supported by Laboratoires SMB SA, Belgium.
We declare no conflict of interest for this study.
The clinical isolates tested were part of a routine sampling taken at Hospital Gent, Belgium, for the characterization of CF-associated P. aeruginosa strains by different studies, including this one, and did not require ethical clearance.
Footnotes
Published ahead of print on 9 August 2010.
REFERENCES
- 1.Anwar, H., M. K. Dasgupta, and J. W. Costerton. 1990. Testing the susceptibility of bacteria in biofilms to antibacterial agents. Antimicrob. Agents Chemother. 34:2043-2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borriello, G., L. Richards, G. D. Ehrlich, and P. S. Stewart. 2006. Arginine or nitrate enhances antibiotic susceptibility of Pseudomonas aeruginosa in biofilms. Antimicrob. Agents Chemother. 50:382-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boucher, R. C. 2004. New concepts of the pathogenesis of cystic fibrosis lung disease. Eur. Respir. J. 23:146-158. [DOI] [PubMed] [Google Scholar]
- 4.Bush, A., and B. K. Rubin. 2003. Macrolides as biological response modifiers in cystic fibrosis and bronchiectasis. Semin. Respir. Crit. Care Med. 24:737-748. [DOI] [PubMed] [Google Scholar]
- 5.Chambless, J. D., S. M. Hunt, and P. S. Stewart. 2006. A three-dimensional computer model of four hypothetical mechanisms protecting biofilms from antimicrobials. Appl. Environ. Microbiol. 72:2005-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, X., S. Schauder, N. Potier, A. Van Dorsselaer, I. Pelczer, B. L. Bassler, and F. M. Hughson. 2002. Structural identification of a bacterial quorum-sensing signal containing boron. Nature 415:545-549. [DOI] [PubMed] [Google Scholar]
- 6a.Clinical and Laboratory Standards Institute. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 6th ed. Approved standard M7-A6. CLSI, Wayne, PA.
- 7.Conte, J. E., Jr., J. A. Golden, S. Duncan, E. McKenna, and E. Zurlinden. 1995. Intrapulmonary pharmacokinetics of clarithromycin and of erythromycin. Antimicrob. Agents Chemother. 39:334-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 9.Drenkard, E., and F. M. Ausubel. 2002. Pseudomonas biofilm formation and antibiotic resistance are linked to phenotypic variation. Nature 416:740-743. [DOI] [PubMed] [Google Scholar]
- 10.Equi, A., I. M. Balfour-Lynn, A. Bush, and M. Rosenthal. 2002. Long term azithromycin in children with cystic fibrosis: a randomised, placebo-controlled crossover trial. Lancet 360:978-984. [DOI] [PubMed] [Google Scholar]
- 11.Fajardo, A., N. Martinez-Martin, M. Mercadillo, J. C. Galan, B. Ghysels, S. Matthijs, P. Cornelis, L. Wiehlmann, B. Tummler, F. Baquero, and J. L. Martinez. 2008. The neglected intrinsic resistome of bacterial pathogens. PLoS One 3:e1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frederiksen, B., C. Koch, and N. Hoiby. 1997. Antibiotic treatment of initial colonization with Pseudomonas aeruginosa postpones chronic infection and prevents deterioration of pulmonary function in cystic fibrosis. Pediatr. Pulmonol. 23:330-335. [DOI] [PubMed] [Google Scholar]
- 13.Frederiksen, B., S. Lanng, C. Koch, and N. Hoiby. 1996. Improved survival in the Danish center-treated cystic fibrosis patients: results of aggressive treatment. Pediatr. Pulmonol. 21:153-158. [DOI] [PubMed] [Google Scholar]
- 14.George, A. M., P. M. Jones, and P. G. Middleton. 2009. Cystic fibrosis infections: treatment strategies and prospects. FEMS Microbiol. Lett. 300:153-164. [DOI] [PubMed] [Google Scholar]
- 15.Gillis, R. J., K. G. White, K. H. Choi, V. E. Wagner, H. P. Schweizer, and B. H. Iglewski. 2005. Molecular basis of azithromycin-resistant Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 49:3858-3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hickey, A. J., D. Lu, E. D. Ashley, and J. Stout. 2006. Inhaled azithromycin therapy. J. Aerosol Med. 19:54-60. [DOI] [PubMed] [Google Scholar]
- 17.Hill, D., B. Rose, A. Pajkos, M. Robinson, P. Bye, S. Bell, M. Elkins, B. Thompson, C. Macleod, S. D. Aaron, and C. Harbour. 2005. Antibiotic susceptibilities of Pseudomonas aeruginosa isolates derived from patients with cystic fibrosis under aerobic, anaerobic, and biofilm conditions. J. Clin. Microbiol. 43:5085-5090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffman, L. R., D. A. D'Argenio, M. J. MacCoss, Z. Zhang, R. A. Jones, and S. I. Miller. 2005. Aminoglycoside antibiotics induce bacterial biofilm formation. Nature 436:1171-1175. [DOI] [PubMed] [Google Scholar]
- 19.Ichimiya, T., K. Takeoka, K. Hiramatsu, K. Hirai, T. Yamasaki, and M. Nasu. 1996. The influence of azithromycin on the biofilm formation of Pseudomonas aeruginosa in vitro. Chemotherapy 42:186-191. [DOI] [PubMed] [Google Scholar]
- 20.Kesser, K. C., and D. E. Geller. 2009. New aerosol delivery devices for cystic fibrosis. Respir. Care 54:754-767; discussion, 767-768. [DOI] [PubMed] [Google Scholar]
- 21.Lyczak, J. B., C. L. Cannon, and G. B. Pier. 2002. Lung infections associated with cystic fibrosis. Clin. Microbiol. Rev. 15:194-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mah, T. F., B. Pitts, B. Pellock, G. C. Walker, P. S. Stewart, and G. A. O'Toole. 2003. A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature 426:306-310. [DOI] [PubMed] [Google Scholar]
- 23.Mor, N., and L. Heifets. 1993. MICs and MBCs of clarithromycin against Mycobacterium avium within human macrophages. Antimicrob. Agents Chemother. 37:111-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mulet, X., M. D. Macia, A. Mena, C. Juan, J. L. Perez, and A. Oliver. 2009. Azithromycin in Pseudomonas aeruginosa biofilms: bactericidal activity and selection of nfxB mutants. Antimicrob. Agents Chemother. 53:1552-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramsey, B. W., M. S. Pepe, J. M. Quan, K. L. Otto, A. B. Montgomery, J. Williams-Warren, K. M. Vasiljev, D. Borowitz, C. M. Bowman, B. C. Marshall, S. Marshall, and A. L. Smith. 1999. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. Cystic Fibrosis Inhaled Tobramycin Study Group. N. Engl. J. Med. 340:23-30. [DOI] [PubMed] [Google Scholar]
- 26.Riordan, J. R., J. M. Rommens, B. Kerem, N. Alon, R. Rozmahel, Z. Grzelczak, J. Zielenski, S. Lok, N. Plavsic, J. L. Chou, et al. 1989. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 245:1066-1073. [DOI] [PubMed] [Google Scholar]
- 27.Rodvold, K. A. 1999. Clinical pharmacokinetics of clarithromycin. Clin. Pharmacokinet. 37:385-398. [DOI] [PubMed] [Google Scholar]
- 28.Schurek, K. N., A. K. Marr, P. K. Taylor, I. Wiegand, L. Semenec, B. K. Khaira, and R. E. Hancock. 2008. Novel genetic determinants of low-level aminoglycoside resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 52:4213-4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh, P. K., A. L. Schaefer, M. R. Parsek, T. O. Moninger, M. J. Welsh, and E. P. Greenberg. 2000. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature 407:762-764. [DOI] [PubMed] [Google Scholar]
- 30.Skindersoe, M. E., M. Alhede, R. Phipps, L. Yang, P. O. Jensen, T. B. Rasmussen, T. Bjarnsholt, T. Tolker-Nielsen, N. Hoiby, and M. Givskov. 2008. Effects of antibiotics on quorum sensing in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 52:3648-3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Southern, K. W., and P. M. Barker. 2004. Azithromycin for cystic fibrosis. Eur. Respir. J. 24:834-838. [DOI] [PubMed] [Google Scholar]
- 32.Staab, D. 2004. Cystic fibrosis—therapeutic challenge in cystic fibrosis children. Eur. J. Endocrinol. 151(Suppl. 1):S77-S80. [DOI] [PubMed] [Google Scholar]
- 33.Tateda, K., Y. Ishii, S. Kimura, M. Horikawa, S. Miyairi, and K. Yamaguchi. 2007. Suppression of Pseudomonas aeruginosa quorum-sensing systems by macrolides: a promising strategy or an oriental mystery? J. Infect. Chemother. 13:357-367. [DOI] [PubMed] [Google Scholar]
- 34.Treerat, P., F. Widmer, P. G. Middleton, J. Iredell, and A. M. George. 2008. In vitro interactions of tobramycin with various nonantibiotics against Pseudomonas aeruginosa and Burkholderia cenocepacia. FEMS Microbiol. Lett. 285:40-50. [DOI] [PubMed] [Google Scholar]
- 35.Tre-Hardy, M., C. Mace, N. El Manssouri, F. Vanderbist, H. Traore, and M. J. Devleeschouwer. 2009. Effect of antibiotic co-administration on young and mature biofilms of cystic fibrosis clinical isolates: the importance of the biofilm model. Int. J. Antimicrob. Agents 33:40-45. [DOI] [PubMed] [Google Scholar]
- 36.Tre-Hardy, M., H. Traore, N. El Manssouri, F. Vanderbist, M. Vaneechoutte, and M. J. Devleeschouwer. 2009. Evaluation of long-term co-administration of tobramycin and clarithromycin in a mature biofilm model of cystic fibrosis clinical isolates of Pseudomonas aeruginosa. Int. J. Antimicrob. Agents 34:370-374. [DOI] [PubMed] [Google Scholar]
- 37.Tre-Hardy, M., F. Vanderbist, H. Traore, and M. J. Devleeschouwer. 2008. In vitro activity of antibiotic combinations against Pseudomonas aeruginosa biofilm and planktonic cultures. Int. J. Antimicrob. Agents 31:329-336. [DOI] [PubMed] [Google Scholar]
- 38.Worlitzsch, D., R. Tarran, M. Ulrich, U. Schwab, A. Cekici, K. C. Meyer, P. Birrer, G. Bellon, J. Berger, T. Weiss, K. Botzenhart, J. R. Yankaskas, S. Randell, R. C. Boucher, and G. Doring. 2002. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J. Clin. Invest. 109:317-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang, L., and T. F. Mah. 2008. Involvement of a novel efflux system in biofilm-specific resistance to antibiotics. J. Bacteriol. 190:4447-4452. [DOI] [PMC free article] [PubMed] [Google Scholar]