Abstract
The trypanocidal agents nifurtimox and benznidazole both function as prodrugs and must undergo enzyme-mediated activation, a reaction catalyzed by type I nitroreductase (NTR). In the search for new parasitic therapies, we have utilized this finding to investigate whether aziridinyl nitrobenzamide derivatives have activity against bloodstream-form Trypanosoma brucei and Trypanosoma cruzi amastigotes, parasite stages that replicate in the mammalian host. For T. cruzi drug screening, we generated trypanosomes that expressed the luciferase reporter gene and optimized a mammalian infection model in a 96-well plate format. A subset of compounds having a 5-(aziridin-1-yl)-2,4-dinitrobenzyl structure was shown to be metabolized by purified T. brucei NTR and when screened against both parasite life cycle stages displayed significant growth-inhibitory properties: the most potent compounds generated 50% inhibitory concentrations of <1 μM. The trypanocidal activity was shown to be NTR specific, since parasites overexpressing this enzyme were hypersensitive to the aziridinyl dinitrobenzyl agents. We conclude that members of the aziridinyl nitrobenzamide class of nitroheterocycles provide new lead structures that have the potential to treat trypanosomal infections.
The protozoan parasites Trypanosoma brucei and Trypanosoma cruzi are the causative agents of African and American trypanosomiases, respectively. Both diseases predominately afflict people living in the developing world, infecting an estimated 10 million individuals. Over the past 15 years, coordinated surveillance, treatment, and vector control programs against both trypanosomiases have led to a dramatic reduction in the number of new cases in regions of endemicity (3, 49). However, for African trypanosomiasis, localized epidemics can rapidly arise following political and socioeconomic disruption, killing tens of thousands of people (3, 5). In the case of Chagas' disease, while infection rates are falling in areas of endemicity, migration, tourism, illicit drug usage, and modern medical practices have all contributed to it becoming a problem elsewhere, with an estimated 300,000 patients now living in the United States (4, 18, 46). The only available treatments for Chagas' disease are the nitroheterocyclic compounds nifurtimox and benznidazole, which have been in use for nearly 40 years. Their use is controversial, since both are toxic, may be carcinogenic, and have poor efficacy against the chronic stage (16, 34, 46). Additionally, some T. cruzi strains are reported to be refractory to treatment (17, 37). Despite these problems, nifurtimox, in cotherapy with eflornithine (NECT), has recently been added to the WHO's Essential Medicines List for treatment of West African trypanosomiasis (8, 42-44), and it is currently being subjected to clinical trials as a treatment for pediatric neuroblastoma (47, 48).
In common with other nitroheterocyclic compounds, nifurtimox and benznidazole are characterized by a nitro group linked to an aromatic ring (19). Both function as prodrugs and must undergo activation prior to mediating their cytotoxic effects, a process catalyzed by nitroreductases (NTRs). Based on oxygen sensitivity and flavin cofactors, NTRs can be broadly divided into two groups (41). The activity of type I NTRs is oxygen insensitive, with the enzyme containing flavin mononucleotide (FMN) as a cofactor. They utilize NAD(P)H as an electron donor, transferring reducing equivalents to the substrate in a series of sequential two-electron reduction events. This class of NTR is associated mainly with bacteria and is absent from most eukaryotes, with a subset of protozoan parasites, including trypanosomes, being major exceptions (36, 38, 39, 54). In contrast, the activity of the ubiquitous type II NTRs is oxygen sensitive, with the enzyme containing flavin adenine dinucleotide (FAD) or FMN as a cofactor. These function by catalyzing a one-electron reduction of the nitro group to generate a nitro radical. In a futile cycle, this radical reacts with oxygen to produce superoxide anions, with the subsequent regeneration of the original nitro compound (14, 35). Although some mammalian enzymes, such as NAD(P)H quinone oxidoreductase 1 and nitric oxide synthase, can mediate a two-electron reduction reaction under aerobic conditions, type II NTR activities predominate in most cell types (7, 9).
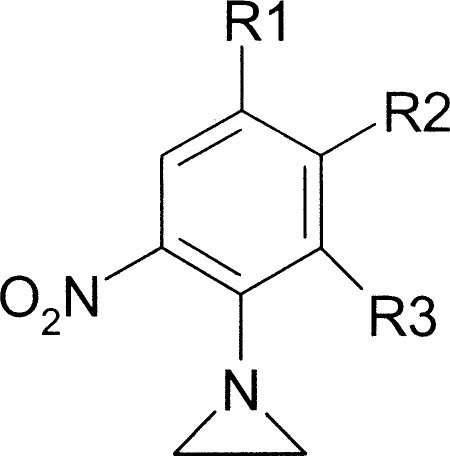
Recently there has been a renaissance in the use of nitroaromatic compounds, with several undergoing evaluation in the treatment of infectious diseases. This includes use of the nitric oxide-generating prodrug PA-824 against Mycobacterium tuberculosis and nitazoxanide against Giardia, Cryptosporidium, and viral hepatitis (1, 32, 51). Others, such as nitrobenzamides, nitrobenzylcarbamates, and nitroindolines, have shown promise as anticancer therapies (10, 13). The best characterized of these is the aziridinyl nitrobenzamide (ANB) compound CB1954 [5-(aziridin-1-yl)-2,4-dinitrobenzamide] (see Table 1). Two distinct systems have been developed to promote preferential activation of this nitroheterocycle in tumors. In one system, CB1954 is coadministered with the synthetic enhancer substrate nicotinamide riboside (NRH), a therapy evaluated in clinical trials under the trade name Prolarix (11, 40). The reducing equivalents supplied by NRH augment the ability of NAD(P)H quinone oxidoreductase 2 to convert the prodrug to its toxic forms (31, 55). The alternative activation system is a two-step strategy termed gene-directed enzyme prodrug therapy (10, 13). In the first phase, a gene encoding a type I NTR, usually Escherichia coli nfsB, is introduced and expressed in the tumor using a selective vector. This is then followed by administration of CB1954, which subsequently undergoes nitro reduction in NTR-expressing cells, leading to the formation of hydroxylamine and amine derivatives (22, 23, 28-30, 52). For both activation pathways, the NTR-generated derivatives mediate their cytotoxic effects through alkylation of target substrates or by formation of DNA adducts (22, 23, 52).
TABLE 1.
Structures of aziridinyl nitrobenzamide compounds
CB1954 has recently been reported to have activity against bloodstream-form (BSF) T. brucei (50). However, its mechanism of action has not been established, and its efficacy against T. cruzi has not been addressed. Here we report the activities of nine ANB prodrugs against BSF T. brucei and amastigote T. cruzi, the parasite stages that replicate in the mammalian host. To facilitate screening of the compounds against T. cruzi, a transgenic cell line constitutively expressing the luciferase reporter was generated. This work identifies three structurally related ANBs, including CB1954, that display trypanocidal activity against both parasites.
MATERIALS AND METHODS
Chemicals.
Aziridinyl nitrobenzamide structures are shown in Table 1. Their synthesis is described elsewhere (21).
Cell culturing.
T. brucei brucei BSF (2T1), which constitutively expresses the tetracycline repressor protein and permits targeted integration at a specific ribosomal DNA (rDNA) locus (2), was grown at 37°C under a 5% CO2 atmosphere in modified Iscove's medium containing 1 μg ml−1 phleomycin (24). Transformed 2T1 parasites overexpressing T. brucei NTR (TbNTR) were maintained in this medium containing 2.5 μg ml−1 hygromycin (54).
T. cruzi (clone Cl-Brener) epimastigotes were grown in RPMI-1640 medium supplemented as described previously (27). Epimastigotes constitutively expressing luciferase were maintained in the medium containing 100 μg ml−1 G418. T. cruzi epimastigote cultures in the stationary phase of growth (8- to 10-day-old cultures) were used to infect African green monkey kidney (Vero) cells. The T. cruzi-infected monolayers were incubated overnight at 37°C under a 5% CO2 atmosphere. Cultures were then washed with RPMI 1640 medium to remove residual parasites and incubated at 37°C under a 5% CO2 atmosphere. Every 3 to 4 days, the growth medium was changed. Ten to fourteen days after the initial infection, bloodstream trypomastigotes were microscopically observed. These were collected and used to infect fresh Vero cell monolayers.
The Vero cell line was grown at 37°C under a 5% CO2 atmosphere in RPMI 1640 medium supplemented with 10% fetal calf serum, 20 mM HEPES (pH 7.4), 2 mM sodium glutamate, 2 mM sodium pyruvate, 2.5 U ml−1 penicillin, and 2.5 μg ml−1 streptomycin.
Plasmids.
A DNA fragment from the 3′ region of the ribosomal spacer was amplified from T. cruzi CL-Brener genomic DNA and cloned into the KpnI site of the vector pTEX (26). The resultant plasmid was taken, and the 5′ untranslated region of glycosomal glyceraldehyde-3-phosphate dehydrogenase (gGAPDH) plus the adjacent multiple cloning site (MCS) was replaced with the T. cruzi ribosomal promoter/spacer sequence plus the MCS from pRiboTEX (33). This produced the integrative expression vector pTRIX. The luciferase gene from pGEM-Luc (Promega) was then inserted into the MCS of pTRIX. The construct was linearized prior to electroporation into T. cruzi epimastigotes (53).
Antiproliferative assays.
All assays were performed in a 96-well plate format. T. brucei BSF parasites were seeded at 1 × 103 ml−1 in 200 μl growth medium containing different concentrations of ANB. Where appropriate, protein expression was induced by adding tetracycline (1 μg ml−1). After incubation at 37°C for 3 days, 20 μl Alamar Blue (Invitrogen) was added to each well, and the plates incubated for a further 16 h. The fluorescence of each culture was determined using a Gemini fluorescent plate reader (Molecular Devices) at an excitation wavelength of 530 nm, emission wavelength of 585 nm, and filter cutoff at 550 nm. The change in fluorescence resulting from the reduction of Alamar Blue is proportional to the number of live cells. The 50% inhibitory concentration (IC50) for each compound was then established.
Growth inhibition of T. cruzi amastigotes was monitored as follows. Vero cells were seeded at 1.5 × 104 ml−1 in 100 μl in growth medium and allowed to adhere to the well for 6 h. T. cruzi trypomastigotes (10,000 in 100 μl growth medium) were then added to each well, and infections were performed overnight at 37°C under 5% CO2. The cultures were then washed twice in growth medium to remove noninternalized parasites, and the supernatant was replaced with fresh growth medium containing drug. Drug-treated infections were incubated for a further 3 days at 37°C under a 5% CO2 atmosphere. The growth medium was then removed, and the cells were lysed in 50 μl cell culture lysis reagent (Promega). Activity was then measured using the luciferase assay system (Promega), and light emission was measured on a β-plate counter (Wallac). The luminescence is proportional to the number of live cells. The IC50 for each compound was then established.
To assess mammalian cell cytotoxicity, Vero cells were seeded at 1 × 104 ml−1 in 200 μl growth medium containing different concentrations of compound. After incubation at 37°C for 6 days, 20 μl Alamar Blue (Biosource UK Ltd.) was added to each well, and the plates were incubated for a further 8 h. The cell density of each culture was determined as described above, and the IC50 was established.
Protein purification and enzyme assay.
Recombinant TbNTR was purified from E. coli extracts as previously described (20). Enzyme activity was measured by following the NTR-mediated reduction of ANB. The nitroaromatic substrate (100 μM) was incubated with 200 μM NADH, 3 mM glucose, and 1 U glucose dehydrogenase in 50 mM NaH2PO4 (pH 7.5) at 37°C. The reaction was initiated by the addition of TbNTR (50 μg). At appropriate time intervals (20 to 600 s), aliquots (100 μl) were removed, the reaction was stopped by addition of 400 μl ice-cold methanol, and the mixture was diluted in water (50:50). Samples were immediately transferred to −20°C to precipitate proteins and then upon thawing were clarified. Fractions were examined by reversed-phase high-performance liquid chromatography (HPLC) analysis. Samples (20 μl) were injected onto a Hypersil 5 μm column preequilibrated at 4% acetonitrile. Elution was carried out with a gradient of 4 to 56% acetonitrile over 30 min. Absorbance was monitored at 254, 340, and 450 nm, and the disappearance of the substrate peak over time was quantified by comparison with a standard (50 μM nifurtimox).
TbNTR-generated CB1954 metabolites were isolated and analyzed as described previously (22). CB1954 (400 μM) was incubated with 400 μM NADH and 10 μg ml−1 TbNTR in 50 mM NaH2PO4 (pH 7.5) at 37°C for 30 min. Reactions were then examined by using reversed-phase HPLC. Aliquots (100 μl) were injected onto a Kromasil C18 column preequilibrated at 4% acetonitrile. Elutions were then carried out as described above using a 4 to 56% acetonitrile gradient, and absorbance was monitored at 260 nM. The 2- and 4-hydroxylamine peaks, identified by their molecular weights and absorption spectra, were collected. Concentrations of both metabolites were then determined using ɛ260 (molar extinction coefficient at 260 nm) = 7,880 M−1 cm−1 for 4-hydroxylamine and 5,420 M−1 cm−1 for 2-hydroxylamine (45). Trypanocidal activity against T. brucei was carried out as described above using freshly isolated 2- and 4-hydroxylamines (0-1 μM).
RESULTS
Construction and evaluation of luciferase-expressing Trypanosoma cruzi.
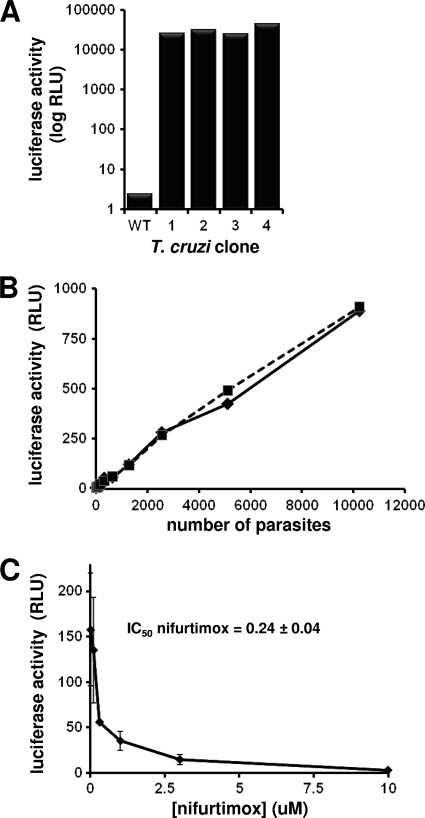
To develop an assay suitable for high-throughput screening of nitroheterocycles, we constructed a T. cruzi line that constitutively expresses luciferase: other systems, such as those based on β-galactosidase or DNA staining, are unsuitable for colored compounds, or their use cannot be readily extended to monitor real-time in vivo mammalian infections (6, 15). The integrative vector pTRIX was generated by sequentially cloning DNA fragments containing the 5′ and 3′ T. cruzi Cl-Brener rRNA promoter/spacer sequences on either side of the expression/neomycin resistance cassettes derived from pTEX (26). The luciferase gene was then cloned into this construct, and the reporter/drug cassette containing the DNA fragment was excised and introduced into T. cruzi CL-Brener epimastigotes by electroporation (53). After selection with G418, the luciferase activity from 4 clones was determined and shown to be >10,000-fold higher than that of the parental line (Fig. 1 A). The effect of luciferase expression on various T. cruzi life cycle stages was then evaluated. This showed that the reporter did not influence the following: (i) growth of epimastigotes parasites, (ii) the ability of epimastigote cells to differentiate into infective metacyclic trypomastigotes, (iii) invasion of mammalian cells by metacyclic trypomastigotes, (iv) growth of intracellular amastigote parasites, (v) differentiation of amastigote cells into infective bloodstream trypomastigotes, or (vi) the ability of bloodstream trypomastigotes to infect mammalian cells. Therefore, it is implicit that luciferase has no effect on trypanosome growth, differentiation, and infectivity.
FIG. 1.
Luciferase expression in insect- and mammalian-stage T. cruzi. (A) The luciferase activity, in relative light units (RLU), of four recombinant T. cruzi Cl-Brener epimastigote clones was determined and compared to that of the parental line. Twenty thousand cells were used in each analysis. (B) Correlation between amastigote load (between 10 and 10,000 cells) and luciferase activity. Two of the clones noted in part A were analyzed in parallel. (C) Vero cells infected with recombinant T. cruzi Cl-Brener amastigotes were grown in the presence of different concentrations of nifurtimox, and the luciferase activity was determined (see Materials and Methods). The concentration of nifurtimox that inhibited parasite growth by 50% (IC50) was established. The data are the means from three experiments ± SDs.
When serially diluted amastigotes from lysed Vero cells were used, the relationship between luciferase activity and the trypanosomal load was shown be linear with use of 10 to 10,000 parasites (Fig. 1B). With this established, we then evaluated whether the intracellular recombinant T. cruzi forms could be used in drug screens. Mammalian cells infected with parasites were treated with either nifurtimox or benznidazole. Initial studies, performed in 24-well plates, demonstrated the validity of the approach, and this was subsequently adapted for use in a 96-well plate format. In the latter growth inhibition experiments, IC50s for nifurtimox and benznidazole were calculated (0.24 ± 0.04 μM and 2.88 ± 0.27 μM, respectively) (Fig. 1C).
Metabolism of aziridinyl nitrobenzamides by the trypanosomal NTR.
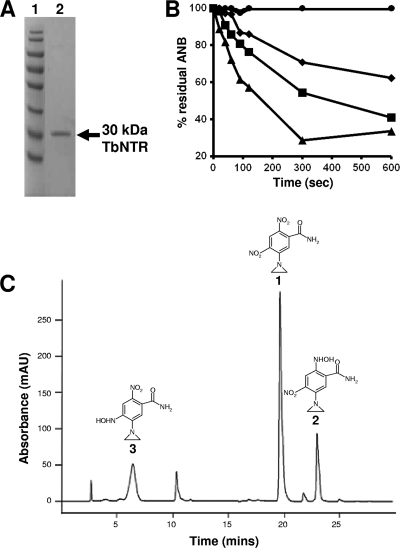
CB1954 is the archetypal ANB, consisting of a 2,4-dinitrobenzylamide ring linked at position 5 to an aziridinyl substituent (Table 1). Using purified recombinant T. brucei type I NTR (Fig. 2 A), CB1954 and eight related ANBs were screened to determine whether they could function as substrates for the parasite enzyme. Initial assays monitored the change in absorbance at 340 nm, corresponding to NADH oxidation. However, many of the nitro compounds themselves undergo a considerable change in absorbance at this particular wavelength. Instead, enzyme activity was assayed by following the disappearance of the nitroaromatic over time, with residual substrate levels monitored using reversed-phase HPLC. Of the nine compounds screened, only CB1954, NH1, and NH2 were metabolized by TbNTR, with the preference being NH2, followed by NH1, followed by CB1954 (Fig. 2B). These three compounds all contain the same common features, namely, an amide or related substituent at position 1, two nitro groups at positions 2 and 4, and an aziridinyl ring at position 5.
FIG. 2.
Activity of trypanosomal NTRs toward different aziridinyl nitrobenzamides. (A) SDS-PAGE gel (10%) stained with Coomassie blue. Lane 1, size standards. Lane 2, recombinant protein eluted from a nickel-nitrilotriacetic acid (Ni-NTA) column using 500 mM imidazole-0.5% Triton X-100. (B) Activity of purified His-tagged TbNTR was followed by monitoring of the reduction of each ANB by HPLC. Each substrate (100 μM) was incubated with enzyme (50 μg) in the presence of NADH (200 μM), glucose (3 mM), and glucose dehydrogenase (1 U). The ANBs shown are CB1954 (diamonds), NH1 (squares), and NH2 (triangles). All other substrates showed no or little reduction by TbNTR, as typified by NH3 (circles). (C) HPLC chromatogram of CB1954 reduction products following TbNTR metabolism. The parental compound CB1954 (peak 1) and the 2- and 4-hydroxyalmine derivatives (peaks 2 and 3, respectively) were detected.
The major CB1954 reduction products generated by TbNTR metabolism were identified by liquid chromatography-mass spectrometry (Fig. 2C) and confirmed as the 2- and 4-hydroxylamine derivatives by the mass spectral and absorbance properties of peaks 2 and 3 in Fig. 2C, respectively. Under the conditions used here, other minor peaks were observed but could not be identified based on published data, and the amine forms were not detected.
Trypanocidal activity of aziridinyl nitrobenzamides.
To evaluate whether the differences in biochemical activity exhibited by ANBs translated into trypanosomal killing, all compounds were screened against T. brucei BSF and luciferase-expressing T. cruzi amastigote parasites. Out of the nine compounds screened, five had no effect on T. brucei growth while six had no effect on the growth of intracellular-form T. cruzi at concentrations up to 10 μM. These were not analyzed further. For the remaining compounds, inhibition assays were performed to determine their IC50s (Table 2). Against both parasites, the most potent trypanocidal compounds corresponded to the structures previously shown to be substrates for TbNTR. In the case of CB1954, the two reduction metabolites identified and isolated by HPLC were screened for parasite killing activity. Intriguingly, only 2-hydroxylamine showed significant levels of cytotoxicity against BSF T. brucei (IC50 of 0.31 ± 0.10 μM): no toxicity for the 4-hydroxylamine derivative was observed in the range tested (up to 1 μM). This is in marked contrast to mammalian systems, where both derivatives display killing properties (22, 52).
TABLE 2.
Susceptibilities of T. brucei bloodstream-form, T. cruzi amastigote, and mammalian cells to aziridinyl nitrobenzamidesa
| Compound | IC50 (μM) ± SD for: |
Therapeutic indexb |
||||
|---|---|---|---|---|---|---|
| T. brucei | T. cruzi | Vero | V79 | V79/T. brucei | V79/T. cruzi | |
| Nifurtimox | 2.06 ± 0.09 | 0.24 ± 0.04 | 64.11 ± 0.57 | 35 | 17 | 146 |
| NH3 to -6, NH8 | >10 | >10 | ||||
| CB1954 | 2.97 ± 0.25 | 0.57 ± 0.05 | >250 | 543 | 183 | 953 |
| NH1 | 0.89 ± 0.04 | 1.59 ± 0.10 | >250 | 624 | 701 | 392 |
| NH2 | 1.45 ± 0.05 | 0.69 ± 0.11 | >250 | 634 | 437 | 919 |
| NH7 | 6.40 ± 0.40 | >10 | ND | ND | ND | ND |
Growth inhibition data for Chinese hamster fibroblast (V79) cells were taken from the work of Helsby et al. (21) (CB1954, NH1, and NH2) or De Conti et al. (12) (nifurtimox). ND, not determined.
The therapeutic index of a compound was calculated as a ratio of the IC50 against V79 cells to the IC50 against the parasite.
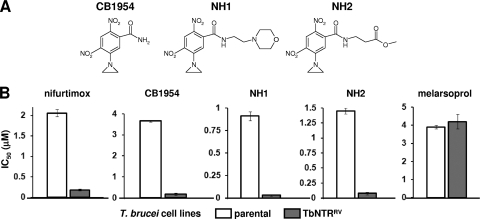
To conclusively show that the most potent ANBs are activated by the trypanosomal NTR in the parasite itself, the susceptibility of BSF T. brucei induced to overexpress the enzyme was investigated (20, 54). For CB1954, NH1, and NH2 (Fig. 3 A), cells with elevated levels of TbNTR were >10 fold more sensitive to the aziridinyl compounds than controls (Fig. 3B). This phenotype was limited to nitroaromatic prodrugs: parasites overexpressing TbNTR had the same susceptibility to melarsoprol, a nonnitroheterocylic drug, as controls. Additionally, the TbNTR-mediated activation was shown to be specific to this enzyme, since parasites induced to overexpress other trypanosomal proteins postulated to interact with nitroheterocyclic prodrugs, namely, prostaglandin F2α synthase and two cytochrome P450 reductases, exhibited the same susceptibility profiles for CB1954, NH1, and NH2 as the parental cells (data not shown).
FIG. 3.
Susceptibility of bloodstream-form T. brucei overexpressing TbNTR to aziridinyl nitrobenzamides. (A) Structures of the ANBs with highest trypanocidal activity (Table 2). (B) Growth-inhibitory effect (IC50s in μM) of CB1954, NH1, and NH2 on T. brucei cells overexpressing TbNTR (TbNTRRV). Data are means from 4 experiments ± SD, and the differences in susceptibility were statistically significant (P < 0.01), as assessed by Student's t test. Melarsoprol and nifurtimox were used as drug controls.
Cytotoxicity of aziridinyl nitrobenzamides to mammalian cells.
The three compounds shown to have trypanocidal activity against both T. brucei BSFs and T. cruzi amastigotes were assayed for cytotoxicity against mammalian cells (Table 2). In all cases, CB1954, NH1, and NH2 had a no growth-inhibitory effect on Vero cells at concentrations up to 250 μM, whereas nifurtimox had an IC50 of 64.11 ± 0.57 μM. Previous cytotoxicity studies of these compounds against other mammalian cells have been carried out (Table 2) (21). Comparison of these data with the parasitic killing activities reported here clearly indicates that the three ANBs display selectivity in vitro toward the parasite.
DISCUSSION
To facilitate high-throughput drug discovery against the medically relevant, intracellular stage of T. cruzi, we have generated parasite lines expressing the luciferase reporter. Initial constructs designed to target and replace one of the gGAPDH loci produced luminescent epimastigotes. However, these cells failed to develop into invasive parasites, consistent with a previous report suggesting gGAPDH levels are critical for T. cruzi differentiation (56). To circumvent this problem, we integrated the luciferase construct into the parasite's ribosomal array. Characterization of the resultant lines established that expression of luciferase at this genomic site had no effect on trypanosome growth, differentiation, and infectivity. The system has now been standardized in a 96-well plate format, where we can reproducibly detect as few as 10 parasites in a mammalian cell infection. Although the experiments conducted here assessed only small number of compounds, the system can be readily scaled up to permit high-throughput compound library screening.
Nitrobenzamide-based compounds containing substituent groups, such as an aziridinyl ring or a mustard chain, are being evaluated as treatments for hypoxic cancers (25, 40). A key step in their activity involves reduction of the nitro group(s) to its hydroxylamine derivatives, a reaction mediated by type I NTRs (10, 13). This conversion results in a rearrangement of electrons around the aromatic ring that facilitates presentation of cytotoxic moieties to the cell. Since trypanosomes are one of a few eukaryotic organisms to express a type I NTR (54), it is envisaged that such nitroheterocyclic anticancer compounds may have potential in treating African sleeping sickness and Chagas' disease.
Several classes of ANBs have been developed, differing in the number and positioning of nitro groups on the benzyl ring (Table 1) (21). CB1954, the archetypal ANB, has two nitro groups located at the 2 and 4 positions of a benzamide ring with an aziridinyl ring at position 5 (designated group Ia). This compound was readily metabolized by TbNTR (Fig. 2B) and displayed considerable trypanocidal activity against T. brucei BSF and T. cruzi amastigote parasites, the two stages that replicate in the mammalian host (Table 2). Modification of the amide group generated the derivatives NH1 and NH2, which behaved similarly to CB1954 in both screens. To categorically link trypanocidal activity with TbNTR metabolism, the sensitivity of parasites overexpressing the enzyme to group Ia ANBs was examined. For all three compounds, trypanosomes with elevated levels of TbNTR were >10-fold more susceptible to the agents than controls (Fig. 3), mirroring observations made for other trypanocidal, NTR-activated nitroaromatic prodrugs (20, 54).
Alteration of the group Ia structure by removal of the nitro group found at the para position relative to the aziridinyl ring, replacement of this nitro group with a SO2Me substituent, or changing of the position of the amide group on the benzyl ring generated compounds that were not metabolized by TbNTR and, in most cases, did not display trypanocidal activity. NH7 did kill T. brucei, but because it failed to have an effect on T. cruzi and was not metabolized by TbNTR, it was not analyzed further. These findings are consistent with observations made using mammalian cells expressing the E. coli type I NTR, NfsB, and highlight the important contribution 2-nitro reduction products make in mediating cytotoxicity (21, 22).
For CB1954, both nitro groups can be reduced by bacterial type I NTR to produce 2- or 4-hydroxylamine metabolites (30): a 2,4-dihydroxylamine derivative can never be formed because of an unfavorable electronic configuration. In mammalian cells, both hydroxylamine forms are cytotoxic either directly or through formation of downstream amine or acetoxy products (22). Controversy surrounds which of these is the major killing factor. Following studies using DNA cross-link repair-defective cells, it had been proposed that 4-hydroxylamine and its acetoxy derivative were the most cytotoxic, acting as DNA-DNA cross-linking agents (28, 29). However, this has been questioned given the superior bystander effects displayed by the 2-amine form coupled with it showing potency similar to that of the 4-hydroxylamine derivative in DNA cross-link repair-competent cells (22). Metabolism of CB1954 by TbNTR produced both the 2- and 4-hydroxylamine forms (Fig. 2C), but we could not detect either of the amine derivatives. When HPLC fractions were screened for trypanocidal activity against T. brucei BSF cells, only samples containing the 2-hydroxylamine displayed any growth-inhibitory effect. The reason for this is unclear, but it may reflect differences in uptake of the two forms, inability of the parasite to further process the 4-derivative to cytotoxic metabolites, variation in repair mechanisms displayed by the pathogen compared to the host, or differential instability in the two hydroxylamine forms.
We have now identified three new NTR-activated trypanocidal agents based on CB1954 with the most potent showing IC50s of <1 μM. Comparative toxicity studies have shown that these compounds display selectivity in vitro toward the parasites (Table 2) having therapeutic indices of >700-fold when targeting BSF T. brucei and >900-fold against T. cruzi amastigotes. The basis for this difference relies on the presence of a type I NTR activity in parasites, a property that can be exploited in the development of new antiparasitic agents. Since nitroheterocyclic compound-resistant parasites can be readily selected for in the laboratory (50, 54), it is envisaged that such compounds would be best suited for combinational therapies.
Acknowledgments
This study was supported by The Wellcome Trust.
Footnotes
Published ahead of print on 2 August 2010.
REFERENCES
- 1.Adagu, I. S., D. Nolder, D. C. Warhurst, and J. F. Rossignol. 2002. In vitro activity of nitazoxanide and related compounds against isolates of Giardia intestinalis, Entamoeba histolytica and Trichomonas vaginalis. J. Antimicrob. Chemother. 49:103-111. [DOI] [PubMed] [Google Scholar]
- 2.Alsford, S., T. Kawahara, L. Glover, and D. Horn. 2005. Tagging a T. brucei RRNA locus improves stable transfection efficiency and circumvents inducible expression position effects. Mol. Biochem. Parasitol. 144:142-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrett, M. P., R. J. Burchmore, A. Stich, J. O. Lazzari, A. C. Frasch, J. J. Cazzulo, and S. Krishna. 2003. The trypanosomiases. Lancet 362:1469-1480. [DOI] [PubMed] [Google Scholar]
- 4.Bern, C., and S. P. Montgomery. 2009. An estimate of the burden of Chagas disease in the United States. Clin. Infect. Dis. 49:e52-e54. [DOI] [PubMed] [Google Scholar]
- 5.Brun, R., J. Blum, F. Chappuis, and C. Burri. 2010. Human African trypanosomiasis. Lancet 375:148-159. [DOI] [PubMed] [Google Scholar]
- 6.Buckner, F. S., C. L. Verlinde, A. C. La Flamme, and W. C. Van Voorhis. 1996. Efficient technique for screening drugs for activity against Trypanosoma cruzi using parasites expressing beta-galactosidase. Antimicrob. Agents Chemother. 40:2592-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chandor, A., S. Dijols, B. Ramassamy, Y. Frapart, D. Mansuy, D. Stuehr, N. Helsby, and J. L. Boucher. 2008. Metabolic activation of the antitumor drug 5-(aziridin-1-yl)-2,4-dinitrobenzamide (CB1954) by NO synthases. Chem. Res. Toxicol. 21:836-843. [DOI] [PubMed] [Google Scholar]
- 8.Checchi, F., P. Piola, H. Ayikoru, F. Thomas, D. Legros, and G. Priotto. 2007. Nifurtimox plus eflornithine for late-stage sleeping sickness in Uganda: a case series. PLoS Negl. Trop. Dis. 1:e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, S., R. Knox, K. Wu, P. S. Deng, D. Zhou, M. A. Bianchet, and L. M. Amzel. 1997. Molecular basis of the catalytic differences among DT-diaphorase of human, rat, and mouse. J. Biol. Chem. 272:1437-1439. [DOI] [PubMed] [Google Scholar]
- 10.Chen, Y., and L. Hu. 2009. Design of anticancer prodrugs for reductive activation. Med. Res. Rev. 29:29-64. [DOI] [PubMed] [Google Scholar]
- 11.Chung-Faye, G., D. Palmer, D. Anderson, J. Clark, M. Downes, J. Baddeley, S. Hussain, P. I. Murray, P. Searle, L. Seymour, P. A. Harris, D. Ferry, and D. J. Kerr. 2001. Virus-directed, enzyme prodrug therapy with nitroimidazole reductase: a phase I and pharmacokinetic study of its prodrug, CB1954. Clin. Cancer Res. 7:2662-2668. [PubMed] [Google Scholar]
- 12.De Conti, R., D. A. Oliveira, A. M. A. P. Fernandes, P. S. Melo, J. A. Rodriguez, M. Haun, S. L. De Castro, A. R. M. Souza-Brito, and N. Duran. 1998. Application of a multi-endpoint cytotoxicity assay to the trypanocidal compounds 2-propen-1-amine derivatives and determination of their acute toxicity. In Vitro Mol. Toxicol. 11:153-160. [Google Scholar]
- 13.Denny, W. A. 2003. Prodrugs for gene-directed enzyme-prodrug therapy (suicide gene therapy). J. Biomed. Biotechnol. 2003:48-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Docampo, R., R. P. Mason, C. Mottley, and R. P. Muniz. 1981. Generation of free radicals induced by nifurtimox in mammalian tissues. J. Biol. Chem. 256:10930-10933. [PubMed] [Google Scholar]
- 15.Engel, J. C., K. K. Ang, S. Chen, M. R. Arkin, J. H. McKerrow, and P. S. Doyle. 2010. Image-based high-throughput drug screening targeting the intracellular stage of Trypanosoma cruzi, the agent of Chagas' disease. Antimicrob. Agents Chemother. 54:3326-3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferreira, R. C., and L. C. Ferreira. 1986. Mutagenicity of nifurtimox and benznidazole in the Salmonella/microsome assay. Braz. J. Med. Biol. Res. 19:19-25. [PubMed] [Google Scholar]
- 17.Filardi, L. S., and Z. Brener. 1987. Susceptibility and natural resistance of Trypanosoma cruzi strains to drugs used clinically in Chagas disease. Trans. R. Soc. Trop. Med. Hyg. 81:755-759. [DOI] [PubMed] [Google Scholar]
- 18.Gascon, J., C. Bern, and M. J. Pinazo. 2010. Chagas disease in Spain, the United States and other non-endemic countries. Acta Trop. 115:22-27. [DOI] [PubMed] [Google Scholar]
- 19.Grunberg, E., and E. H. Titsworth. 1973. Chemotherapeutic properties of heterocyclic compounds: monocyclic compounds with five-membered rings. Annu. Rev. Microbiol. 27:317-346. [DOI] [PubMed] [Google Scholar]
- 20.Hall, B. S., X. Wu, L. Hu, and S. R. Wilkinson. 2010. Exploiting the drug-activating properties of a novel trypanosomal nitroreductase. Antimicrob. Agents Chemother. 54:1193-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Helsby, N. A., G. J. Atwell, S. Yang, B. D. Palmer, R. F. Anderson, S. M. Pullen, D. M. Ferry, A. Hogg, W. R. Wilson, and W. A. Denny. 2004. Aziridinyldinitrobenzamides: synthesis and structure-activity relationships for activation by E. coli nitroreductase. J. Med. Chem. 47:3295-3307. [DOI] [PubMed] [Google Scholar]
- 22.Helsby, N. A., D. M. Ferry, A. V. Patterson, S. M. Pullen, and W. R. Wilson. 2004. 2-Amino metabolites are key mediators of CB 1954 and SN 23862 bystander effects in nitroreductase GDEPT. Br. J. Cancer 90:1084-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Helsby, N. A., S. J. Wheeler, F. B. Pruijn, B. D. Palmer, S. Yang, W. A. Denny, and W. R. Wilson. 2003. Effect of nitroreduction on the alkylating reactivity and cytotoxicity of the 2,4-dinitrobenzamide-5-aziridine CB 1954 and the corresponding nitrogen mustard SN 23862: distinct mechanisms of bioreductive activation. Chem. Res. Toxicol. 16:469-478. [DOI] [PubMed] [Google Scholar]
- 24.Hirumi, H., and K. Hirumi. 1989. Continuous cultivation of Trypanosoma brucei blood stream forms in a medium containing a low concentration of serum protein without feeder cell layers. J. Parasitol. 75:985-989. [PubMed] [Google Scholar]
- 25.Jameson, M. B., D. Rischin, M. Pegram, J. Gutheil, A. V. Patterson, W. A. Denny, and W. R. Wilson. 2010. A phase I trial of PR-104, a nitrogen mustard prodrug activated by both hypoxia and aldo-keto reductase 1C3, in patients with solid tumors. Cancer Chemother. Pharmacol. 65:791-801. [DOI] [PubMed] [Google Scholar]
- 26.Kelly, J. M., H. M. Ward, M. A. Miles, and G. Kendall. 1992. A shuttle vector which facilitates the expression of transfected genes in Trypanosoma cruzi and Leishmania. Nucleic Acids Res. 20:3963-3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kendall, G., A. F. Wilderspin, F. Ashall, M. A. Miles, and J. M. Kelly. 1990. Trypanosoma cruzi glycosomal glyceraldehyde-3-phosphate dehydrogenase does not conform to the ‘hotspot’ topogenic signal model. EMBO J. 9:2751-2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knox, R. J., F. Friedlos, P. J. Biggs, W. D. Flitter, M. Gaskell, P. Goddard, L. Davies, and M. Jarman. 1993. Identification, synthesis and properties of 5-(aziridin-1-yl)-2-nitro-4-nitrosobenzamide, a novel DNA crosslinking agent derived from CB1954. Biochem. Pharmacol. 46:797-803. [DOI] [PubMed] [Google Scholar]
- 29.Knox, R. J., F. Friedlos, T. Marchbank, and J. J. Roberts. 1991. Bioactivation of CB 1954: reaction of the active 4-hydroxylamino derivative with thioesters to form the ultimate DNA-DNA interstrand crosslinking species. Biochem. Pharmacol. 42:1691-1697. [DOI] [PubMed] [Google Scholar]
- 30.Knox, R. J., F. Friedlos, R. F. Sherwood, R. G. Melton, and G. M. Anlezark. 1992. The bioactivation of 5-(aziridin-1-yl)-2,4-dinitrobenzamide (CB1954). II. A comparison of an Escherichia coli nitroreductase and Walker DT diaphorase. Biochem. Pharmacol. 44:2297-2301. [DOI] [PubMed] [Google Scholar]
- 31.Knox, R. J., T. C. Jenkins, S. M. Hobbs, S. Chen, R. G. Melton, and P. J. Burke. 2000. Bioactivation of 5-(aziridin-1-yl)-2,4-dinitrobenzamide (CB 1954) by human NAD(P)H quinone oxidoreductase 2: a novel co-substrate-mediated antitumor prodrug therapy. Cancer Res. 60:4179-4186. [PubMed] [Google Scholar]
- 32.Korba, B. E., A. B. Montero, K. Farrar, K. Gaye, S. Mukerjee, M. S. Ayers, and J. F. Rossignol. 2008. Nitazoxanide, tizoxanide and other thiazolides are potent inhibitors of hepatitis B virus and hepatitis C virus replication. Antiviral Res. 77:56-63. [DOI] [PubMed] [Google Scholar]
- 33.Martinez-Calvillo, S., I. Lopez, and R. Hernandez. 1997. pRIBOTEX expression vector: a pTEX derivative for a rapid selection of Trypanosoma cruzi transfectants. Gene 199:71-76. [DOI] [PubMed] [Google Scholar]
- 34.Moraga, A. A., and U. Graf. 1989. Genotoxicity testing of antiparasitic nitrofurans in the Drosophila wing somatic mutation and recombination test. Mutagenesis 4:105-110. [DOI] [PubMed] [Google Scholar]
- 35.Moreno, S. N., R. P. Mason, and R. Docampo. 1984. Reduction of nifurtimox and nitrofurantoin to free radical metabolites by rat liver mitochondria. Evidence of an outer membrane-located nitroreductase. J. Biol. Chem. 259:6298-6305. [PubMed] [Google Scholar]
- 36.Muller, J., J. Wastling, S. Sanderson, N. Muller, and A. Hemphill. 2007. A novel Giardia lamblia nitroreductase, GlNR1, interacts with nitazoxanide and other thiazolides. Antimicrob. Agents Chemother. 51:1979-1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murta, S. M., R. T. Gazzinelli, Z. Brener, and A. J. Romanha. 1998. Molecular characterization of susceptible and naturally resistant strains of Trypanosoma cruzi to benznidazole and nifurtimox. Mol. Biochem. Parasitol. 93:203-214. [DOI] [PubMed] [Google Scholar]
- 38.Nixon, J. E., A. Wang, J. Field, H. G. Morrison, A. G. McArthur, M. L. Sogin, B. J. Loftus, and J. Samuelson. 2002. Evidence for lateral transfer of genes encoding ferredoxins, nitroreductases, NADH oxidase, and alcohol dehydrogenase 3 from anaerobic prokaryotes to Giardia lamblia and Entamoeba histolytica. Eukaryot. Cell 1:181-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pal, D., S. Banerjee, J. Cui, A. Schwartz, S. K. Ghosh, and J. Samuelson. 2009. Giardia, Entamoeba, and Trichomonas enzymes activate metronidazole (nitroreductases) and inactivate metronidazole (nitroimidazole reductases). Antimicrob. Agents Chemother. 53:458-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patel, P., J. G. Young, V. Mautner, D. Ashdown, S. Bonney, R. G. Pineda, S. I. Collins, P. F. Searle, D. Hull, E. Peers, J. Chester, D. M. Wallace, A. Doherty, H. Leung, L. S. Young, and N. D. James. 2009. A phase I/II clinical trial in localized prostate cancer of an adenovirus expressing nitroreductase with CB1984. Mol. Ther. 17:1292-1299. (Author's correction, 17:1302.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peterson, F. J., R. P. Mason, J. Hovsepian, and J. L. Holtzman. 1979. Oxygen-sensitive and -insensitive nitroreduction by Escherichia coli and rat hepatic microsomes. J. Biol. Chem. 254:4009-4014. [PubMed] [Google Scholar]
- 42.Priotto, G., C. Fogg, M. Balasegaram, O. Erphas, A. Louga, F. Checchi, S. Ghabri, and P. Piola. 2006. Three drug combinations for late-stage Trypanosoma brucei gambiense sleeping sickness: a randomized clinical trial in Uganda. PLoS Clin. Trials 1:e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Priotto, G., S. Kasparian, W. Mutombo, D. Ngouama, S. Ghorashian, U. Arnold, S. Ghabri, E. Baudin, V. Buard, S. Kazadi-Kyanza, M. Ilunga, W. Mutangala, G. Pohlig, C. Schmid, U. Karunakara, E. Torreele, and V. Kande. 2009. Nifurtimox-eflornithine combination therapy for second-stage African Trypanosoma brucei gambiense trypanosomiasis: a multicentre, randomised, phase III, non-inferiority trial. Lancet 374:56-64. [DOI] [PubMed] [Google Scholar]
- 44.Priotto, G., S. Kasparian, D. Ngouama, S. Ghorashian, U. Arnold, S. Ghabri, and U. Karunakara. 2007. Nifurtimox-eflornithine combination therapy for second-stage Trypanosoma brucei gambiense sleeping sickness: a randomized clinical trial in Congo. Clin. Infect. Dis. 45:1435-1442. [DOI] [PubMed] [Google Scholar]
- 45.Race, P. R., A. L. Lovering, S. A. White, J. I. Grove, P. F. Searle, C. W. Wrighton, and E. I. Hyde. 2007. Kinetic and structural characterisation of Escherichia coli nitroreductase mutants showing improved efficacy for the prodrug substrate CB1954. J. Mol. Biol. 368:481-492. [DOI] [PubMed] [Google Scholar]
- 46.Rodriques Coura, J., and S. L. de Castro. 2002. A critical review on Chagas disease chemotherapy. Mem. Inst. Oswaldo Cruz 97:3-24. [DOI] [PubMed] [Google Scholar]
- 47.Saulnier Sholler, G. L., L. Brard, J. A. Straub, L. Dorf, S. Illeyne, K. Koto, S. Kalkunte, M. Bosenberg, T. Ashikaga, and R. Nishi. 2009. Nifurtimox induces apoptosis of neuroblastoma cells in vitro and in vivo. J. Pediatr. Hematol. Oncol. 31:187-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saulnier Sholler, G. L., S. Kalkunte, C. Greenlaw, K. McCarten, and E. Forman. 2006. Antitumor activity of nifurtimox observed in a patient with neuroblastoma. J. Pediatr. Hematol. Oncol. 28:693-695. [DOI] [PubMed] [Google Scholar]
- 49.Schofield, C. J., J. Jannin, and R. Salvatella. 2006. The future of Chagas disease control. Trends Parasitol. 22:583-588. [DOI] [PubMed] [Google Scholar]
- 50.Sokolova, A. Y., S. Wyllie, S. Patterson, S. L. Oza, K. D. Read, and A. H. Fairlamb. 2010. Cross-resistance to nitro-drugs and implications for the treatment of human African trypanosomiasis. Antimicrob. Agents Chemother. 54:2893-2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stover, C. K., P. Warrener, D. R. VanDevanter, D. R. Sherman, T. M. Arain, M. H. Langhorne, S. W. Anderson, J. A. Towell, Y. Yuan, D. N. McMurray, B. N. Kreiswirth, C. E. Barry, and W. R. Baker. 2000. A small-molecule nitroimidazopyran drug candidate for the treatment of tuberculosis. Nature 405:962-966. [DOI] [PubMed] [Google Scholar]
- 52.Tang, M. H., N. A. Helsby, W. R. Wilson, and M. D. Tingle. 2005. Aerobic 2- and 4-nitroreduction of CB 1954 by human liver. Toxicology 216:129-139. [DOI] [PubMed] [Google Scholar]
- 53.Taylor, M. C., and J. M. Kelly. 2006. pTcINDEX: a stable tetracycline-regulated expression vector for Trypanosoma cruzi. BMC Biotechnol. 6:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilkinson, S. R., M. C. Taylor, D. Horn, J. M. Kelly, and I. Cheeseman. 2008. A mechanism for cross-resistance to nifurtimox and benznidazole in trypanosomes. Proc. Natl. Acad. Sci. U. S. A. 105:5022-5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu, K., R. Knox, X. Z. Sun, P. Joseph, A. K. Jaiswal, D. Zhang, P. S. Deng, and S. Chen. 1997. Catalytic properties of NAD(P)H:quinone oxidoreductase-2 (NQO2), a dihydronicotinamide riboside dependent oxidoreductase. Arch. Biochem. Biophys. 347:221-228. [DOI] [PubMed] [Google Scholar]
- 56.Zacks, M. A. 2007. Impairment of cell division of Trypanosoma cruzi epimastigotes. Mem. Inst. Oswaldo Cruz 102:111-115. [DOI] [PubMed] [Google Scholar]