Abstract
We have evaluated the efficacy of posaconazole (PSC), voriconazole (VRC), and amphotericin B (AMB) in a murine model of systemic infection by Cryptococcus gattii using immunocompromised animals and three clinical strains of the fungus. AMB was the most effective drug in prolonging the survival of mice and also in reducing tissue burden in all organs tested. To a lesser degree, VRC at 60 mg/kg of body weight in lung tissue and PSC at 40 mg/kg also in spleen demonstrated good efficacy in reducing the fungal load. The PSC and VRC levels in serum and brain tissue, determined by an agar diffusion bioassay method at 4 h after the last dose of the therapy, were above the corresponding MIC values. However, these drugs were not able to reduce the fungal load in brain tissue. Our results demonstrated that PSC and, to a lesser degree, VRC, have fungistatic activity and potential for the treatment of human pulmonary cryptococcosis.
Cryptococcosis is an emerging infection commonly involving the lungs, from which it can disseminate to different tissues, usually the central nervous system (CNS) (20, 23). Cryptococcus neoformans and Cryptococcus gattii are the main agents responsible for this disease, which can affect both immunosuppressed and healthy individuals. Despite antifungal therapies, this infection still has mortality rates near 20% (20).
The first choice in the primary therapy of CNS infections remains fungicidal drugs, with amphotericin B (AMB) alone or in combination with flucytosine being the most widely used (20, 23). Fungistatic drugs like itraconazole and fluconazole, with less toxicity, are also used in the maintenance of the therapy and in pulmonary cryptococcosis, but their use in CNS infections has been less than satisfactory. In addition, the extended duration of the therapy with these azoles increases the risk of developing drug resistance (11, 23, 26). It has been suggested that that C. gattii has a higher pathogenicity than C. neoformans (27), which emphasizes the importance of the correct species identification and makes it necessary to improve and search for alternatives to the current therapy.
On the basis of the promising results obtained with posaconazole (PSC) and voriconazole (VRC) against C. neoformans in animal models (1, 19, 24) and also in a clinical setting (9, 15, 21, 22), we have evaluated in this study the efficacy of PSC, VRC, and AMB in a murine model of disseminated infection by C. gattii.
MATERIALS AND METHODS
Three clinical isolates of C. gattii, FMR 8394, FMR 8396, and FMR 8410, were used in this study. We tested their in vitro antifungal susceptibilities to AMB, PSC, and VRC by using a broth microdilution method following the CLSI guidelines for yeasts (8).
In the in vivo study, male OF1 mice (Charles River, Criffa S.A., Barcelona, Spain) with a mean weight of 30 g were used. The animals were housed in standard boxes with corncob bedding and free access to food and water. All animal care procedures were supervised and approved by the Universitat Rovira i Virgili Animal Welfare Committee.
Mice were immunosuppressed by a single intraperitoneal (i.p.) injection of 200 mg/kg of body weight of cyclophosphamide (Genoxal; Laboratorios Funk S.A., Barcelona, Spain) plus 5-fluorouracil (Fluorouracilo; Ferrer Farma S.A., Barcelona, Spain) at 150 mg/kg intravenously 1 day before the infection. In order to prevent bacterial infections, all mice received 5 mg/day ceftazidime subcutaneously from days 1 to 7 after the infection.
On the day of infection, 1-day cultures on potato dextrose agar (PDA) were suspended in sterile saline and filtered through sterile gauze to remove clumps of cells. The resulting suspension was adjusted to the desired inoculum based on hemocytometer counts. Dilutions of the original suspension were cultured on PDA plates to confirm the hemocytometer counts. Mice were infected with a conidial suspension of 2 × 105 CFU in 0.2 ml of sterile saline solution into the lateral tail vein. This inoculum was chosen in previous studies and was the minimal dose able to produce acute infections, with all the animals dying within 15 days postinfection (data not shown).
The drugs assayed were AMB (Fungizone; Squibb Industrial Farmacéutica S. A., Barcelona, Spain), PSC (Noxafil; Schering-Plough Ltd., Hertfordshire, United Kingdom), and VRC (Vfend; Pfizer S.A., Madrid, Spain), administered as follows: AMB at doses of 1.5 mg/kg of body weight, i.p. once daily; PSC at doses of 10, 20, or 40 mg/kg orally once daily; or VRC at doses of 10, 40, or 60 mg/kg orally once daily. From 3 days before infection, the mice that received VRC were given grapefruit juice instead of water (28). Control animals received no treatment. All treatments began 1 day after challenge, and the therapy lasted for 10 days. The efficacy of the different drugs was evaluated by prolongation of survival and reduction of fungal tissue burden.
Groups of 30 mice were randomly established for each strain and treatment. Ten mice were used for survival studies, and 20 mice were used for tissue burden studies. For each strain and study, groups of 10 mice were also established as controls. Mice in the survival group were checked daily for 30 days. At the end of the experiment, survivors were sacrificed by carbon dioxide inhalation. The mice in one tissue burden group were sacrificed on day 5, and the other mice were sacrificed on day 11 postinfection; their lungs, brain, and spleen were aseptically removed and homogenized in 1 ml of sterile saline. Serial 10-fold dilutions of the homogenates were plated on PDA, incubated at 30°C, and examined daily for 3 days. The number of CFU/g of tissue was calculated for each sample.
An additional group of five mice was similarly infected with the strain FMR 8396 and treated with the same antifungals and doses used in the treatment study. These mice were used to determine antifungal drug levels in the brain and serum 4 h after the final dose on day 10 of therapy. Levels were determined by bioassay following described methods for quantification of azoles and AMB in serum and tissues (3, 7, 10).
Mean survival time was estimated by a Kaplan-Meier method and compared among groups by using a log rank test. Colony counts in tissue burden studies were analyzed by a Kruskal-Wallis test. When this test was significant, we used a Mann-Whitney U test to compare pairs of strains. The Bonferroni correction was used to avoid an increase of the type I error due to multiple comparisons. Differences were considered statistically significant at a P value of <0.05.
RESULTS
The in vitro susceptibility test showed AMB and VRC MICs of 0.5 mg/liter and a PSC MIC of 0.25 mg/liter for strains FMR 8394 and FMR 8396 while for strain FMR 8410 MICs were 0.12 mg/liter, 0.12 mg/liter, and 0.25 mg/liter, respectively.
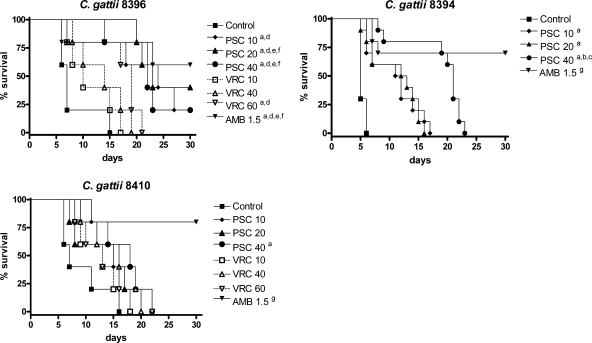
Results of survival studies are shown in Fig. 1. AMB and PSC significantly prolonged survival with respect to survival of the control group for all the strains, with the exception of PSC at 10 and 20 mg/kg against strain FMR 8410. In addition, AMB was able to significantly prolong survival relative to survival with the rest of the therapies for strains FMR 8410 and FMR 8394 and relative to survival with VRC against strain FMR 8396. None of the VRC doses prolonged survival, with the exception of VRC at 60 mg/kg against strain FMR 8396.
FIG. 1.
The cumulative mortality of mice infected with 2 × 105 CFU of C. gattii. Significance is indicated on the figure as follows: a, P < 0.05 versus control; b, P < 0.05 versus PSC at 10 mg/kg; c, P < 0.05 versus PSC at 20 mg/kg; d, P < 0.05 versus VRC at 10 mg/kg; e, P < 0.05 versus VRC at 40 mg/kg; f, P < 0.05 versus VRC at 60 mg/kg; and g, P < 0.05 versus all the therapies.
The tissue burden results are shown in Tables 1 and 2. On day 5, for all strains all treatments, except VRC at 10 mg/kg, significantly reduced the fungal load in lung and spleen relative to the load in the control group. In addition, AMB significantly reduced the fungal load in these organs in comparison to VRC at any dose for strains FMR 8396 and 8410 and to PSC at 10 and 20 mg/kg for all strains, with the exception of the lung against strain FMR 8396. AMB showed better efficacy than PSC at 40 mg/kg in reducing the fungal load only in the spleen against strain FMR 8396 and in lungs against strain FMR 8410. Overall, PSC showed significantly better efficacy than VRC in reducing the fungal recovery in spleen, and both drugs had similar activities in lungs. None of the treatments reduced the fungal load in brain against the strain FMR 8396. Against strains FMR 8394 and FMR 8410, AMB and PSC at 20 and 40 mg/kg reduced the tissue burden in brain in comparison to the burden in the control group. Against strain FMR 8394, PSC at 40 mg/kg significantly reduced the brain fungal load relative to the load with AMB.
TABLE 1.
The effect of antifungal treatment on colony counts in lung, spleen, and brain on day 5 of therapy
| Isolate | Drug (dose [mg/kg/day]) | Mean log10 CFU/g (95% CI)h |
||
|---|---|---|---|---|
| Lung | Spleen | Brain | ||
| FMR 8396 | None | 4.32 (3.76-4.88) | 4.23 (4.04-4.41) | 2.80 (2.53-3.08) |
| PSC (10) | 2.50 (0.75-4.25)a,b,c | 2.74 (2.04-3.44)a,b,c,d | 2.93 (2.38-3.47) | |
| PSC (20) | 3.65 (2.75-4.55)a,b,c | 2.95 (2.74-3.17)a,b,c,d | 2.54 (2.27-2.81) | |
| PSC (40) | 2.76 (1.23-4.32)a,b,c | 2.89 (2.04-3.74)a,b,c | 2.63 (2.56-2.70) | |
| VRC (10) | 4.29 (4.04-4.53) | 3.81 (3.63-3.99)a | 2.71 (2.41-3.00) | |
| VRC (40) | 4.06 (3.79-4.33)a | 3.74 (3.53-3.95)a | 2.69 (2.35-3.03) | |
| VRC (60) | 3.37 (2.67-4.07)a,b,c | 3.50 (3.13-3.87)a | 2.44 (2.12-2.77) | |
| AMB (1.5) | 1.39 (0.90-1.88)a,b,c,d | 1.58 (0.45-2.70)e | 2.41 (1.91-2.41) | |
| FMR 8394 | None | 4.93 (3.95-5.91) | 6.14 (5.56-6.71) | 5.02 (4.63-5.42) |
| PSC (10) | 3.12 (1.49-4.76)a | 3.09 (2.69-3.49)a | 4.53 (3.92-5.15) | |
| PSC (20) | 2.93 (1.41-4.45)a | 2.61 (2.13-3.08)a | 4.14 (3.73-4.56)a | |
| PSC (40) | 0.20 (0-0.52)a,f,g | 0.94 (0.34-1.53)a,f,g | 2.66 (2.38-2.94)e | |
| AMB (1.5) | NDa,f,g | 0.40 (0.37-0.99)a,f,g | 3.97 (3.72-4.22)a | |
| FMR 8410 | None | 5.68 (5.31-6.05) | 6.37 (6.22-6.52) | 5.46 (5.12-5.80) |
| PSC (10) | 5.15 (5.07-5.22)a | 4.10 (3.73-4.47)a,b | 5.35 (5.19-5.51) | |
| PSC (20) | 4.64 (4.26-5.03)a,f | 3.58 (3.48-3.68)a,b,c,d | 4.43 (3.84-5.02)a,f | |
| PSC (40) | 3.97 (3.85-4.10)a,b,f | 3.33 (3.03-3.63)a,b,c,d | 4.33 (3.90-4.76)a,f | |
| VRC (10) | 4.47 (4.10-4.84)a,f | 4.80 (4.63-4.97)a | 4.97 (4.85-5.08) | |
| VRC (40) | 4.23 (4.04-4.42)a,f | 4.55 (4.34-4.76)a,b | 4.89 (4.65-5.13) | |
| VRC (60) | 4.02 (3.73-4.30)a,f | 4.27 (3.99-4.55)a,b | 4.60 (4.31-4.89)a,f | |
| AMB (1.5) | 1.52 (0.74-2.30)e | 2.85 (2.57-3.12)a,b,c,d,f,g | 3.95 (3.71-4.19)a,b,c,d,f | |
P < 0.005 versus control.
P < 0.005 versus VRC at 10 mg/kg.
P < 0.005 versus VRC 40 at mg/kg.
P < 0.005 versus VRC at 60 mg/kg.
P < 0.005 versus all the therapies.
P < 0.005 versus PSC at 10 mg/kg.
P < 0.005 versus PSC at 20 mg/kg.
CI, confidence interval; ND, not detected.
TABLE 2.
The effect of antifungal treatment on colony counts in lung, spleen, and brain on day 11 after challenge
| Isolate | Drug (dose [mg/kg/day]) | Mean log10 CFU/g (95% CI)c |
||
|---|---|---|---|---|
| Lung | Spleen | Brain | ||
| FMR 8396 | None | — | — | — |
| PSC (10) | 3.55 (2.26-4.83) | 1.60 (0.43-2.76)a | 4.52 (3.89-5.15)b | |
| PSC (20) | 4.22 (3.75-4.90) | 0.87 (−0.10 to 1.84)a | 4.38 (3.99-4.77)b | |
| PSC (40) | 1.67 (0.08-3.25) | NDa | 4.35 (3.70-5.34)b | |
| VRC (10) | 4.62 (3.90-5.34) | 4.38 (3.94-4.81)b | 4.64 (4.13-5.14)b | |
| VRC (40) | 4.52 (3.99-5.05) | 4.15 (3.86-4.45)b | 4.05 (3.64-4.46)b | |
| VRC (60) | 3.89 (3.05-4.73) | 4.20 (2.37-4.49)b | 4.13 (3.74-4.52)b | |
| AMB (1.5) | NDa | NDb | 1.04 (0.56-1.52)a | |
| FMR 8394 | None | — | — | — |
| PSC (10) | NDa | NDa | 6.61 (6.47-6.75)b | |
| PSC (20) | NDa | NDa | 6.12 (5.33-6.92)b | |
| PSC (40) | ND | NDa | 3.55 (3.06-4.03)b | |
| AMB (1.5) | ND | ND | 3.05 (2.50-3.61)a | |
P < 0.05 versus fungal load on day 5 of therapy (significantly lower).
P < 0.05 versus fungal load on day 5 of therapy (significantly higher).
CI, confidence interval; —, control mice did not survive to day 11; ND, not detected.
On day 11, AMB cleared the fungal load in spleen and lungs and significantly reduced the tissue burden in brain in comparison to the results of day 5. Against strain FMR 8394, all PSC doses cleared fungal loads in spleen and lungs, and PSC at 40 mg/kg cleared the fungal load in spleen against strain FMR 8396. In the brain, all groups treated with VRC and PSC surprisingly showed higher fungal loads than at day 5.
Bioassay results are shown in Table 3. At day 10 of therapy, for all treatments administered, antifungal levels in serum and brain were above the corresponding MICs. PSC and VRC levels in serum and brain increased with dose escalation.
TABLE 3.
The drug levels in serum and brain tissue measured by bioassay on day 10 of the therapy and 4 h after final dosinga
| Drug and dose (mg/kg) | Serum (μg/ml) | Brain (μg/g) |
|---|---|---|
| PSC | ||
| 10 | 5.75 ± 1.86 | 3.08 ± 0.36 |
| 20 | 6.73 ± 1.29 | 3.64 ± 0.91 |
| 40 | 8.83 ± 0.79 | 7.41 ± 1.12 |
| VRC | ||
| 10 | 4.09 ± 0.98 | 2.62 ± 0.42 |
| 40 | 5.54 ± 1.55 | 4.12 ± 0.97 |
| 60 | 7.16 ± 2.13 | 6.47 ± 1.03 |
| AMB | ||
| 1.5 | 6.52 ± 1.47 | 4.46 ± 0.73 |
Results are expressed as the means ± standard deviations.
DISCUSSION
We have studied the efficacy of PSC, VRC, and AMB in the treatment of a disseminated infection by C. gattii in immunosuppressed mice. Although C. gattii seems to have a predilection for immunocompetent hosts, it is also known to infect immunocompromised patients such as those with AIDS (2, 16, 17, 25). It has been suggested that the differences in host incidence between Cryptococcus species could be related to a lesser exposure of AIDS patients to C. gattii since this species is less common in the urban areas where these patients typically reside (6).
PSC and VRC have shown efficacy in human infections by C. neoformans (9, 15, 21, 22), but little data exist on their efficacy against C. gattii infections. To our knowledge, this is the first study that explores the efficacy of these drugs in a murine C. gattii infection. In contrast to the high in vitro activities of PSC and VRC, which agree with the findings of other authors (13, 29), these drugs showed lower efficacy than AMB in vivo against C. gattii. In this study, the high mortality of mice treated with VRC or PSC seems to be associated with the inability of these drugs to reduce the fungal load in the brain despite increased drug levels in the organ. In contrast, these drugs, especially PSC, showed efficacy in reducing the fungal load in lung. These poor results obtained with PSC and VRC in brain tissue could be related to their fungistatic activity and to the inhibition of the lymphocyte function provoked by cyclophosphamide administration (12, 18). It has been suggested that a T-cell-independent defense mechanism in pulmonary C. neoformans infections (14) together with the antifungal administration could explain the fungal clearance in that organ in our model. It is likely that such a defensive response would not be expressed in brain (14). T cells are necessary to reduce the cryptococcal burden, and their depletion would provoke an increase in the brain fungal load (4, 30).
In summary, the poor efficacy of PSC and VRC in reducing the fungal load in brain and in prolonging mouse survival in an acute disseminated cryptococcosis caused by C. gattii suggests that, as other azoles, these drugs should not be administered as primary therapies. PSC and VRC could be alternatives to itraconazole and fluconazole in the maintenance of therapy and, as was observed here, in the treatment of pulmonary cryptococcosis.
Footnotes
Published ahead of print on 12 July 2010.
REFERENCES
- 1.Barchiesi, F., A. M. Schimizzi, F. Caselli, D. Giannini, V. Camiletti, B. Fileni, A. Giacometti, L. F. Di Francesco, and G. Scalise. 2001. Activity of the new antifungal triazole, posaconazole, against Cryptococcus neoformans. J. Antimicrob. Chemother. 48:769-773. [DOI] [PubMed] [Google Scholar]
- 2.Bodasing, N., R. A. Seaton, G. S. Shankland, and D. Kennedy. 2004. Cryptococcus neoformans var. gattii meningitis in an HIV-positive patient: first observation in the United Kingdom. J. Infect. 49:253-255. [DOI] [PubMed] [Google Scholar]
- 3.Bodet C. A., III, J. H. Jorgensen, and D. J. Drutz. 1985. Simplified bioassay method for measurement of flucytosine or ketoconazole. J. Clin. Microbiol. 22:157-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchanan, K. L., and H. A. Doyle. 2000. Requirement for CD4+ T lymphocytes in host resistance against Cryptococcus neoformans in the central nervous system of immunized mice. Infect. Immun. 68:456-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reference deleted.
- 6.Chen, Y. C., S. C. Chang, C. C. Shih, C. C. Hung, K. T. Luhbd, Y. S. Pan, and W. C. Hsieh. 2000. Clinical features and in vitro susceptibilities of the two varieties of Cryptococcus neoformans in Taiwan. Diagn. Microbiol. Infect. Dis. 36:175-183. [DOI] [PubMed] [Google Scholar]
- 7.Christiansen, K. J., E. M. Bernard, J. W. Gold, and D. Armstrong. 1985. Distribution and activity of amphotericin B in humans. J. Infect. Dis. 152:1037-1043. [DOI] [PubMed] [Google Scholar]
- 8.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility of yeasts. Approved standard M27-A3, 3rd ed. Clinical and Laboratory Standards Institute, Wayne, PA.
- 9.Esposito, V., R. Viglietti, M. Gargiulo, R. Parrella, M. Onofrio, V. Sangiovanni, D. Ambrosino, and A. Chirianni. 2009. Successful treatment of cryptococcal meningitis with a combination of liposomal amphotericin B, flucytosine and posaconazole: two cases reports. In Vivo 23:465-468. [PubMed] [Google Scholar]
- 10.Fittler, A., B. Kocsis, I. Gerlinger, and L. Botz. 2010. Optimization of bioassay method for the quantitative microbiological determination of amphotericin B. Mycoses 53:57-61. [DOI] [PubMed] [Google Scholar]
- 11.Friese, G., T. Discher, R. Füssle, A. Schmalreck, and J. Lohmeyer. 2001. Development of azole resistance during fluconazole maintenance therapy for AIDS-associated cryptococcal disease. AIDS 15:2344-2345. [DOI] [PubMed] [Google Scholar]
- 12.Glück, T., B. Kiefmann, M. Grohmann, W. Falk, R. H. Straub, and J. Schölmerich. 2005. Immune status and risk for infection in patients receiving chronic immunosuppressive therapy. J. Rheumatol. 32:1473-1480. [PubMed] [Google Scholar]
- 13.Gómez-López, A., O. Zaragoza, M. Dos Anjos Martins, M. C. Melhem, J. L. Rodríguez-Tudela, and M. Cuenca-Estrella. 2008. In vitro susceptibility of Cryptococcus gattii clinical isolates. Clin. Microbiol. Infect. 14:727-730. [DOI] [PubMed] [Google Scholar]
- 14.Hill, J. O., and P. L. Dunn. 1993. A T cell-independent protective host response against Cryptococcus neoformans expressed at the primary site of infection in the lung. Infect. Immun. 61:5302-5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Izumikawa, K., Y. Zhao, K. Motoshima, T. Takazono, T. Saijo, S. Kurihara, S. Nakamura, T. Miyazaki, M. Seki, H. Kakeya, Y. Yamamoto, K. Yanagihara, Y. Miyazaki, T. Hayashi, and S. Kohno. 2008. A case of pulmonary cryptococcosis followed by pleuritis in an apparently immunocompetent patient during fluconazole treatment. Med. Mycol. 46:596-599. [DOI] [PubMed] [Google Scholar]
- 16.Lindenberg Ade, S., M. R. Chang, A. M. Paniago, Mdos S. Lázera, P. M. Moncada, G. F. Bonfim, S. A. Nogueira, and B. Wanke. 2008. Clinical and epidemiological features of 123 cases of cryptococcosis in Mato Grosso do Sul, Brazil. Rev. Inst. Med. Trop. Sao Paulo 50:75-78. [DOI] [PubMed] [Google Scholar]
- 17.Litvintseva, A. P., R. Thakur, L. B. Reller, and T. G. Mitchell. 2005. Prevalence of clinical isolates of Cryptococcus gattii serotype C among patients with AIDS in sub-Saharan Africa. J. Infect. Dis. 192:888-892. [DOI] [PubMed] [Google Scholar]
- 18.Lutsiak, M. E., R. T. Semnani, R. De Pascalis, S. V. Kashmiri, J. Schlom, and H. Sabzevari. 2005. Inhibition of CD4+ 25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood 105:2862-2868. [DOI] [PubMed] [Google Scholar]
- 19.Perfect, J. R., G. M. Cox, R. K. Dodge, and W. A. Schell. 1996. In vitro and in vivo efficacies of the azole SCH56592 against Cryptococcus neoformans. Antimicrob. Agents Chemother. 40:1910-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perfect, J. R., W. E. Dismukes, F. Dromer, D. L. Goldman, J. R. Graybill, R. J. Hamill, T. S. Harrison, R. A. Larsen, O. Lorthoraly, M. H. Nguyen, P. G. Pappas, W. G. Powderly, N. Singh, J. D. Sobel, and T. C. Sorrell. 2010. Practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of America. Clin. Infect. Dis. 50:291-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perfect, J. R., K. A. Marr, T. J. Walsh, R. N. Greenberg, B. DuPont, J. de la Torre-Cisneros, G. Just-Nübling, H. T. Schlamm, I. Lutsar, A. Espinel-Ingroff, and E. Johnson. 2003. Voriconazole treatment for less-common, emerging, or refractory fungal infections. Clin. Infect. Dis. 36:1122-1131. [DOI] [PubMed] [Google Scholar]
- 22.Pitisuttithum, P., R. Negroni, J. R. Graybill, B. Bustamante, P. Pappas, S. Chapman, R. S. Hare, and C. J. Hardalo. 2005. Activity of posaconazole in the treatment of central nervous system fungal infections. J. Antimicrob. Chemother. 56:745-755. [DOI] [PubMed] [Google Scholar]
- 23.Saag, M. S., J. R. Graybill, R. A. Larsen, P. G. Pappas, J. R. Perfect, W. G. Powderly, J. D. Sobel, and W. E. Dismukes. 2000. Practice guidelines for the management of cryptococcal disease. Clin. Infect. Dis. 30:710-718. [DOI] [PubMed] [Google Scholar]
- 24.Serena, C., F. J. Pastor, M. Mariné, M. Mar Rodríguez, and Josep Guarro. 2007. Efficacy of voriconazole in a murine model of cryptococcal central nervous system infection. J. Antimicrob. Chemother. 60:162-165. [DOI] [PubMed] [Google Scholar]
- 25.Severo, L. C., F. de Mattos Oliveira, and A. T. Londero. 1999. Cryptococcosis due to Cryptococcus neoformans var. gattii in brazilian patients with AIDS. Report of three cases. Rev. Iberoam. Micol. 16:152-154. [PubMed] [Google Scholar]
- 26.Soares, B. M., D. A. Santos, L. M. Kohler, G. da Costa César, I. R. de Carvalho, M. dos Anjos Martins, and P. S. Cisalpino. 2008. Cerebral infection caused by Cryptococcus gattii: a case report and antifungal susceptibility testing. Rev. Iberoam. Micol. 31:242-245. [PubMed] [Google Scholar]
- 27.Sorrell, T. C. 2001. Cryptococcus neoformans variety gattii. Med. Mycol. 39:155-158. [PubMed] [Google Scholar]
- 28.Sugar, A. M., and X. Liu. 2001. Efficacy of voriconazole in treatment of murine pulmonary blastomycosis. Antimicrob. Agents Chemother. 45:601-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson, G. R., III, N. P. Wiederhold, A. W. Fothergill, A. C. Vallor, B. L. Wickes, and T. F. Patterson. 2009. Antifungal susceptibilities among different serotypes of Cryptococcus gattii and Cryptococcus neoformans. Antimicrob. Agents Chemother. 53:309-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uicker, W. C., J. P. McCracken, and K. L. Buchanan. 2006. Role of Cd4+ T cells in a protective immune response against Cryptococcus neoformans in the central nervous system. Med. Mycol. 44:1-11. [DOI] [PubMed] [Google Scholar]