Abstract
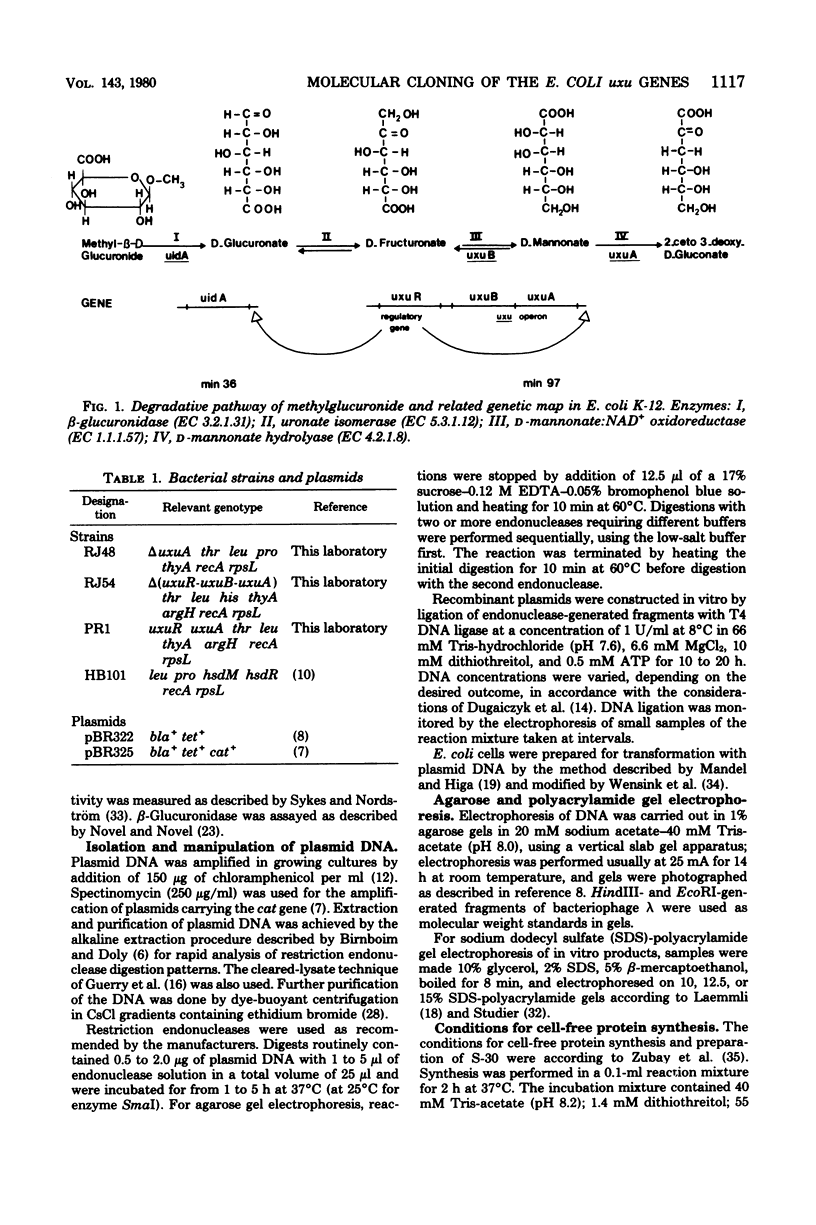
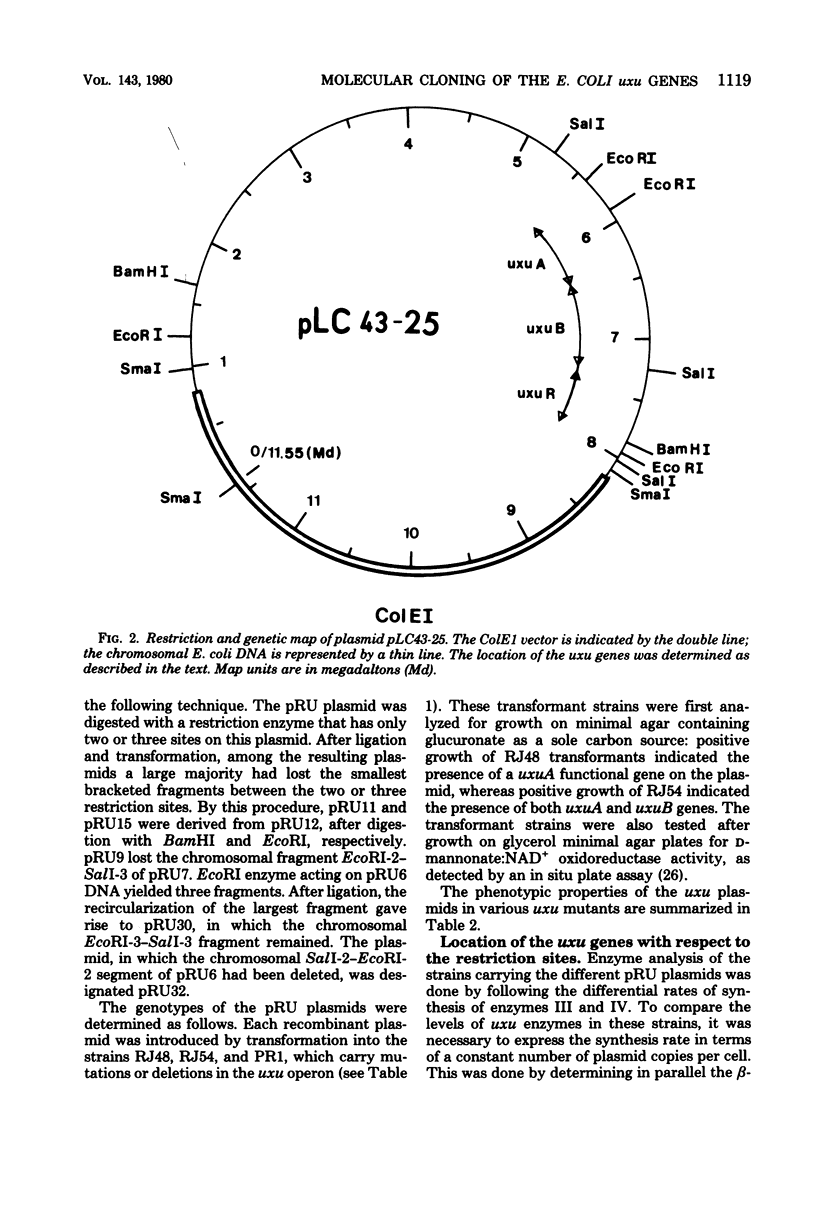
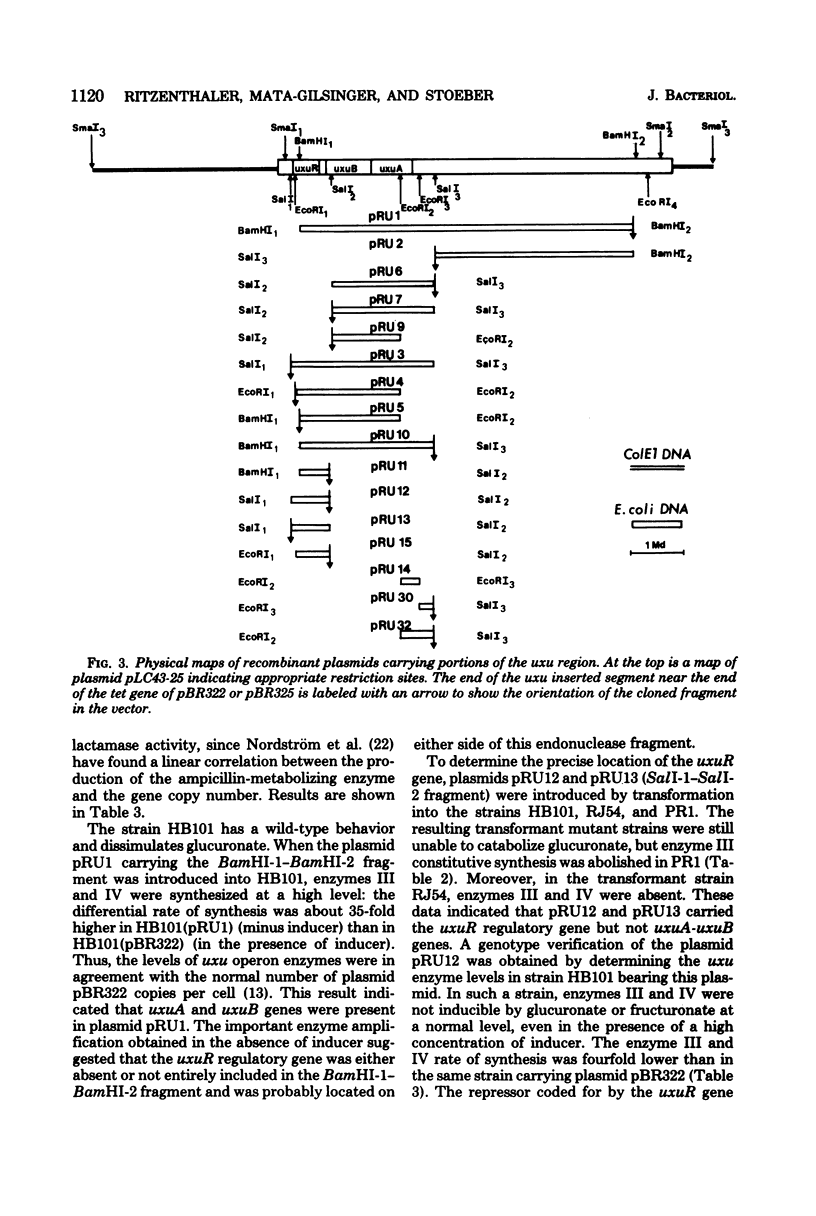
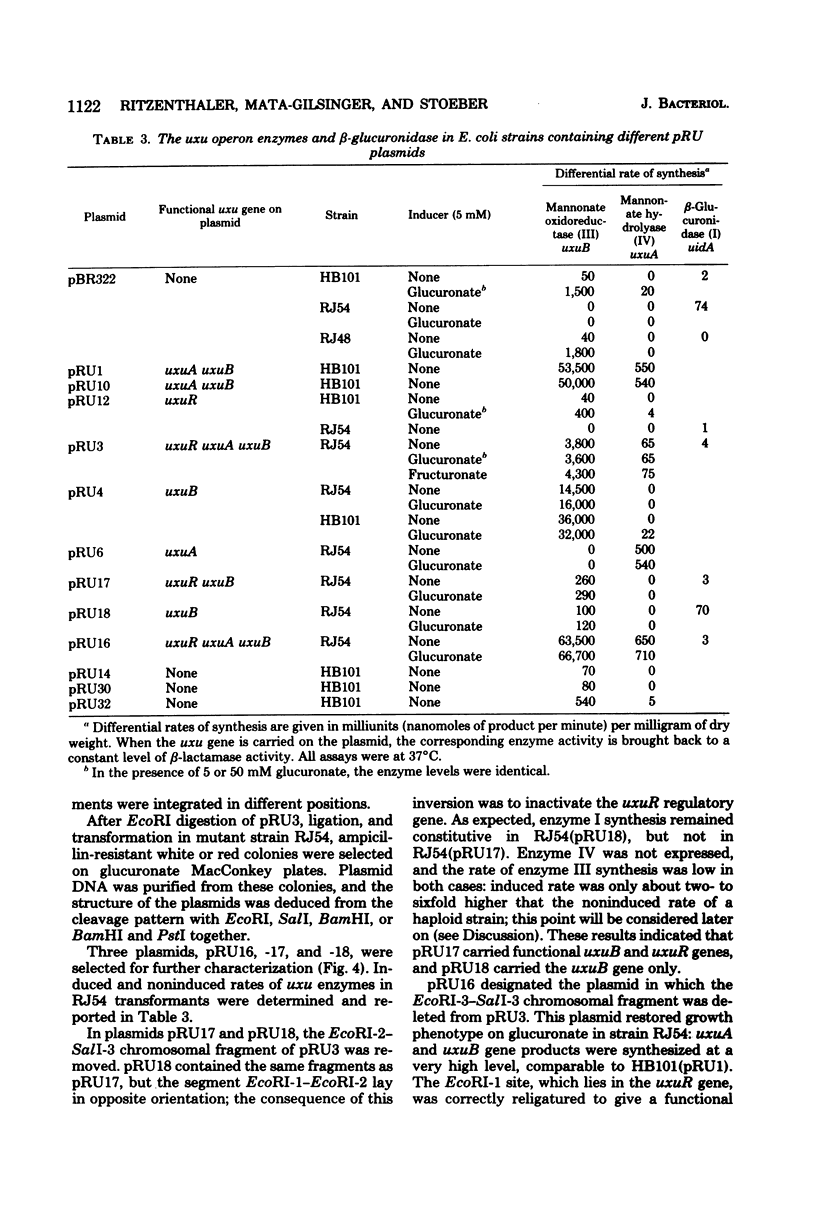
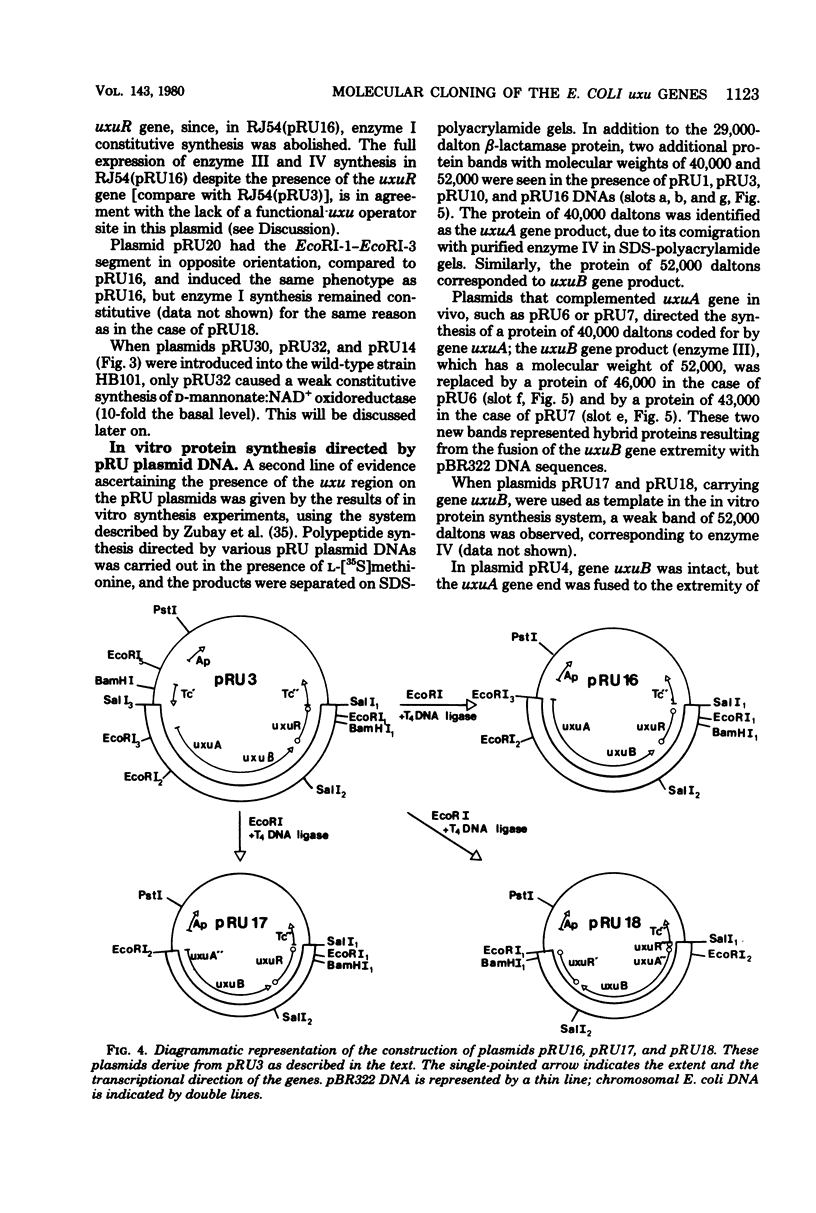
The three genes of the Escherichia coli K-12 uxu region (uxu operon and regulatory gene) were isolated on a ColE1-uxu hybrid plasmid from the bank of Clarke and Carbon, and a restriction map of this region was established. In vitro recombination techniques were used to subclone the uxu restriction fragments into the plasmid vector pBR322 or pBR325. The various chimeric plasmids obtained were analyzed by restriction mapping and characterized genetically by introducing them in uxu mutant or wild-type strains. Differential rates of synthesis of the enzymes coded for by the uxu region were measured in the plasmid-containing strains; amplification of the products of the cloned genes was up to 40-fold the level found in haploid strains. The enzymes coded for by uxuA and uxuB were synthesized in vitro in a coupled transcription-translation system, confirming the results of the cloning experiments. The restriction analysis also suggests that the transcriptional direction of the uxu operon is from uxuA to uxuB and that the order of the loci in this region is: uxuR (regulatory gene), uxuB, uxuA, uxuAp (promoter), uxuAo (operator).
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alton N. K., Vapnek D. Nucleotide sequence analysis of the chloramphenicol resistance transposon Tn9. Nature. 1979 Dec 20;282(5741):864–869. doi: 10.1038/282864a0. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backman K., Ptashne M., Gilbert W. Construction of plasmids carrying the cI gene of bacteriophage lambda. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4174–4178. doi: 10.1073/pnas.73.11.4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry G., Squires C. L., Squires C. Control features within the rplJL-rpoBC transcription unit of Escherichia coli. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4922–4926. doi: 10.1073/pnas.76.10.4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F. Construction and characterization of new cloning vehicles. III. Derivatives of plasmid pBR322 carrying unique Eco RI sites for selection of Eco RI generated recombinant DNA molecules. Gene. 1978 Oct;4(2):121–136. doi: 10.1016/0378-1119(78)90025-2. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Effect of growth conditions on the formation of the relaxation complex of supercoiled ColE1 deoxyribonucleic acid and protein in Escherichia coli. J Bacteriol. 1972 Jun;110(3):1135–1146. doi: 10.1128/jb.110.3.1135-1146.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B. Nature of Col E 1 plasmid replication in Escherichia coli in the presence of the chloramphenicol. J Bacteriol. 1972 May;110(2):667–676. doi: 10.1128/jb.110.2.667-676.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugaiczyk A., Boyer H. W., Goodman H. M. Ligation of EcoRI endonuclease-generated DNA fragments into linear and circular structures. J Mol Biol. 1975 Jul 25;96(1):171–184. doi: 10.1016/0022-2836(75)90189-8. [DOI] [PubMed] [Google Scholar]
- Ebina Y., Kishi F., Nakazawa T., Nakazawa A. Gene expression in vitro of colicin El plasmid. Nucleic Acids Res. 1979 Oct 10;7(3):639–649. doi: 10.1093/nar/7.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerry P., LeBlanc D. J., Falkow S. General method for the isolation of plasmid deoxyribonucleic acid. J Bacteriol. 1973 Nov;116(2):1064–1066. doi: 10.1128/jb.116.2.1064-1066.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson E. N., Yanofsky C. Internal promoter of the tryptophan operon of Escherichia coli is located in a structural gene. J Mol Biol. 1972 Aug 21;69(2):307–313. doi: 10.1016/0022-2836(72)90232-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Nordström K., Ingram L. C., Lundbäck A. Mutations in R factors of Escherichia coli causing an increased number of R-factor copies per chromosome. J Bacteriol. 1972 May;110(2):562–569. doi: 10.1128/jb.110.2.562-569.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novel M., Novel G. Regulation of beta-glucuronidase synthesis in Escherichia coli K-12: pleiotropic constitutive mutations affecting uxu and uidA expression. J Bacteriol. 1976 Jul;127(1):418–432. doi: 10.1128/jb.127.1.418-432.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portalier R. C., Robert-Baudouy J. M., Némoz G. M. Etudes de mutations affectant les gènes de structure de l'isomerase uronique et de l'oxydoreductase altronique chez Escherichia coli K 12. Mol Gen Genet. 1974;128(4):301–319. doi: 10.1007/BF00268518. [DOI] [PubMed] [Google Scholar]
- Portalier R. C., Robert-Baudouy J. M., Stoeber F. R. Localisation génétique et caractérisation biochimique de mutations affectant le gène de structure de l'hydrolyase altronique chez Escherichia coli K 12. Mol Gen Genet. 1972;118(4):335–350. doi: 10.1007/BF00333569. [DOI] [PubMed] [Google Scholar]
- Portalier R. C., Stoeber F. R. Dosages colorimétriques des oxydoréductases aldoniques d'Escherichia coli K 12: applications. Biochim Biophys Acta. 1972 Nov 10;289(1):19–27. doi: 10.1016/0005-2744(72)90103-9. [DOI] [PubMed] [Google Scholar]
- Portalier R. C., Stoeber F. R. La D-mannonate: NAD-oxydoréductase d'Escherichia coli K12. Purification, propriétés et individualité. Eur J Biochem. 1972 Mar 27;26(2):290–300. doi: 10.1111/j.1432-1033.1972.tb01767.x. [DOI] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert-Baudouy J. M., Portalier R. C., Stoeber F. R. Régulation due métabolisme des hexuronates chez Escherichia coli K12. Modalités de l'induction des enzymes du système hexuronate. Eur J Biochem. 1974 Mar 15;43(1):1–15. doi: 10.1111/j.1432-1033.1974.tb03378.x. [DOI] [PubMed] [Google Scholar]
- Robert-Baudouy J. M., Stoeber F. R. Purification et propriétés de la D-mannonate hydrolyase d'Escherichia coli. Biochim Biophys Acta. 1973 Jun 6;309(2):473–485. doi: 10.1016/0005-2744(73)90045-4. [DOI] [PubMed] [Google Scholar]
- SISTROM W. R. On the physical state of the intracellularly accumulates substrates of beta-galactoside-permease in Escherichia coli. Biochim Biophys Acta. 1958 Sep;29(3):579–587. doi: 10.1016/0006-3002(58)90015-5. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Sykes R. B., Nordström K. Microiodometric determination of beta-lactamase activity. Antimicrob Agents Chemother. 1972 Feb;1(2):94–99. doi: 10.1128/aac.1.2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wensink P. C., Finnegan D. J., Donelson J. E., Hogness D. S. A system for mapping DNA sequences in the chromosomes of Drosophila melanogaster. Cell. 1974 Dec;3(4):315–325. doi: 10.1016/0092-8674(74)90045-2. [DOI] [PubMed] [Google Scholar]