Abstract
Single-dose nevirapine (NVP) is effective in reducing mother-to-child transmission (MTCT) of HIV; however, the subsequent development of drug resistance is problematic. The pharmacokinetic profile of the HIV entry inhibitor maraviroc after a single intrapartum dose in rhesus macaques was studied to determine whether maraviroc could serve as an alternative to NVP in a single-dose strategy. Four pregnant macaques received an oral dose of maraviroc 2 h before delivery, and both infant and maternal plasma maraviroc concentrations and CCR5 receptor occupancy on CD4+ lymphocytes were measured over time. Maximum plasma maraviroc concentrations were found at delivery (2-h-postintrapartum dose) in both the mothers and infants, with median concentrations of 974 ng/ml (range, 86 to 2,830 ng/ml) and 22 ng/ml (range, 4 to 99 ng/ml), respectively. Maraviroc was detected in the plasma of mothers up to 48 h after dosing but only as long as 3.5 h in the infants. The median fetal-maternal area under the concentration-time curve (AUC) ratio was 0.009 (range, 0.000 to 0.015). Maraviroc receptor occupancy data showed evidence of unprotected CCR5 receptors on CD4+ cells in the mothers 24 to 48 h after dosing. Extremely low CCR5 expression on CD4+ cells of newborn macaques prevented determination of receptor occupancy in the infants. In rhesus macaques, maraviroc was poorly transferred across the placenta and was quickly cleared from the infants’ blood. The low concentrations of fetal maraviroc and short pharmacokinetic profile in infants suggest that a single maternal intrapartum dose of maraviroc would not be effective in reducing the risk of MTCT of HIV.
Mother-to-child transmission (MTCT) is the second leading mode of human immunodeficiency virus (HIV) transmission worldwide, after heterosexual transmission (3). The prevalence of MTCT is between 15 and 30% in non-breast-fed infants (10) and between 30 and 45% in breast-fed infants (11, 30, 38). MTCT prevalence is reduced to <2% in developed nations, where women have access to suppressive combination antiretroviral regimens (15, 26). However, in resource-limited settings, such as sub-Saharan Africa, the costs and logistics of using combination antiretroviral therapy present a major challenge.
WHO guidelines recommend an antepartum, intrapartum, and postpartum regimen of antiretroviral drugs to reduce the risk of MTCT (4). However, in areas where care and infrastructure are insufficient to deliver these regimens, the WHO recommends, at minimum, administration of a single dose of nevirapine (NVP) to the mother at the onset of labor and a single dose to the infant after birth. This approach, although suboptimal, has been shown to reduce MTCT by nearly 50% (19). Unfortunately, the finding that up to half of women who receive single-dose NVP develop overt resistance to the nonnucleoside reverse transcriptase inhibitor (NNRTI) class (5, 14) has dampened enthusiasm for this simple and affordable strategy. Subsequent studies have revealed even higher rates of resistance when more sensitive methods of detection are used (22). This outcome could lead to increased rates of transmitted drug resistance and a reduced efficacy of subsequent NNRTI regimens (23-24). While single-dose NVP is still the most feasible option for many resource-poor settings, other single-dose approaches of preventing MTCT, which do not jeopardize future treatment options, are desperately needed.
Maraviroc is a CCR5 receptor antagonist approved to treat HIV infection in adults as part of a combination regimen. Maraviroc prevents HIV infection by blocking the binding of HIV to the CCR5 coreceptor on the surface of the target host cells (12, 37). Although some HIV strains can use alternative coreceptors, transmission and early HIV infection are dominated by CCR5-using strains (27, 32). Preventing infection of the neonate by CCR5-using viruses during delivery could potentially reduce the risk of MTCT. In addition, maraviroc has a relatively high genetic barrier to the selection of resistance (28), and being the only approved entry inhibitor, its intrapartum use would be unlikely to undermine subsequent antiretroviral regimens used to treat the mother or infant.
The goal of this pilot study was to determine whether maraviroc could efficiently cross the placenta in rhesus macaques and have a suitable pharmacokinetic (PK) profile in neonates to serve as a potential candidate for a single-dose intrapartum strategy to prevent MTCT of HIV.
MATERIALS AND METHODS
Animals.
Four pregnant macaques were selected from the type D retrovirus-free and simian immunodeficiency virus (SIV)-free primate colony at the California Primate Center, University of California, Davis. Multiparous mothers were selected for this study to decrease the risk of neglect of the infants postoperatively. Animals were housed in accordance with the American Association for Accreditation of Laboratory Animal Care standards and handled according to the Guide for Care and Use of Laboratory Animals (20). When necessary, animals were immobilized with intramuscular injection of 10 mg/kg of body weight ketamine HCl (Parke-Davis, Morris Plains, NJ).
Intervention.
Two hours prior to cesarean section, mothers received a single oral dose of maraviroc (anhydrous; provided by Pfizer Inc., Groton, CT) dissolved in 15 ml 0.05 M citric acid buffer (pH 2.0) containing 10% ethanol by intubation. Two mothers received 60 mg/kg, and 2 received 100 mg/kg. These doses were near the highest that produced no observable adverse effects in cynomolgus monkeys in preclinical toxicity studies (17). Infants were delivered by cesarean section performed using inhalation anesthesia by a staff veterinarian according to standard operating procedures. Blood was collected from the mothers at 0, 1, 2, 4, 8, 24, and 48 h postdosing and from the infants at 4, 8, 24, and 48 h. Cord blood was used for the 2-h (at delivery) time point on the infants. Follow-up blood samples were collected 1 and 2 weeks postdelivery.
Laboratory procedures.
Plasma for PK analysis was isolated from EDTA-anticoagulated whole blood by centrifugation at 800 × g for 10 min and then stored at −70°C. Amniotic fluid was also collected and stored at −70°C. Maraviroc concentrations in plasma and amniotic fluid were determined by high-performance liquid chromatography-mass spectrometry (HPLC-MS) as previously described (7), with a lower limit of detection of 1 ng/ml and intra- and interday variability of <10%. The peak and last serum concentrations of maraviroc (Cmax and Clast, respectively) and time required to reach Cmax and Clast (Tmax and Tlast, respectively) were obtained directly by inspection of concentration-time profiles. The area under the concentration-time curve over 12 h (AUC0-12) and total exposure [AUC(total)] were determined using the log-linear trapezoidal rule (WinNonlin Professional, version 5.2.1; Pharsight Corp., Mountain View, CA). CCR5 receptor occupancy on CD4+ lymphocytes was determined by flow cytometry from whole blood using a MIP-1β ex vivo challenge assay (16). Results were reported as the percentages of CCR5 receptors protected from internalization by MIP-1β.
RESULTS
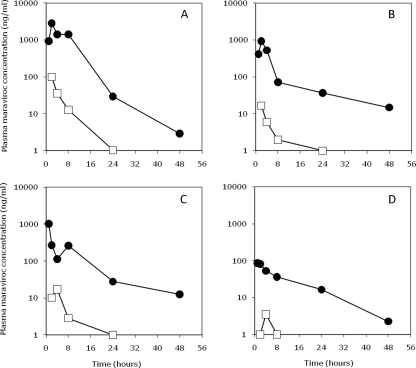
Plasma maraviroc concentrations over time in the 4 mother-infant pairs are shown in Fig. 1, and the calculated PK values are shown in Table 1. Maternal maraviroc plasma concentrations were similar between the 60-mg/kg and 100-mg/kg doses, except for a 10-fold-lower Cmax value for one animal that received the higher 100-mg/kg dose. Overall, maternal Cmaxs (median, 975 ng/ml; range, 86 to 2,830 ng/ml) were found at or near delivery (median, 2 h postdose; range, 1 to 2 h). The median half-life of maraviroc in the mothers was 14.1 h (range, 7.2 to 21.1 h). Maraviroc was still detectable in maternal plasma 48 h after dosing but not in plasma collected after 1 and 2 weeks.
FIG. 1.
Plasma maraviroc concentrations of macaque mothers (closed circles) or their infants (open squares) over 48 h following a single intrapartum dose of maraviroc (mothers for panels A and B received 60 mg/kg; mothers for panels C and D received 100 mg/kg). Mothers were administered maraviroc at 0 h, and the infants were delivered at 2 h. The lower limit of detection of maraviroc was 1 ng/ml.
TABLE 1.
Pharmacokinetic parameters of mother-infant pairs following a single intrapartum dose of maraviroca
| Animal | Dose (mg/kg) | Cmax (ng/ml) | Tmax (h) | Clast (ng/ml) | Tlast (h) | T1/2 (h) | AUC0-12 (ng·h/ml) | AUCT (ng·h/ml) | Cmax ratio (I/M) | AUCT ratio (I/M) |
|---|---|---|---|---|---|---|---|---|---|---|
| Mother A | 60 | 2,830 | 2 | 2.9 | 48 | 7.2 | 15,696 | 18,275 | ||
| Infant A | 99 | 2 | 1.0 | 24 | NC | 197 | 235 | 0.03 | 0.013 | |
| Mother B | 60 | 927 | 2 | 14.8 | 50 | 18.3 | 3,479 | 4,718 | ||
| Infant B | 17 | 2 | 2.0 | 8 | NC | NC | 26 | 0.02 | 0.006 | |
| Mother C | 100 | 1,022 | 1.3 | 12.7 | 48 | 21.1 | 2,970 | 4,301 | ||
| Infant C | 28 | 2 | 2.8 | 8 | NC | NC | 66 | 0.03 | 0.015 | |
| Mother D | 100 | 86 | 1 | 2.3 | 48 | 9.8 | 577 | 1,020 | ||
| Infant D | 4 | 4 | 3.5 | 4 | NC | NC | NC | 0.04 | 0.000 | |
| Median, mothers | 975 | 2 | 7.8 | 48 | 14.1 | 3,225 | 4,510 | |||
| Median, infants | 22 | 2 | 2.4 | 8 | NC | 197 | 66 | 0.03 | 0.009 |
NC, not calculable; AUCT, total area under the time-concentration curve; I/M, infant/mother ratio.
In the infants, maximal plasma maraviroc concentrations (median, 22 ng/ml) were also found at delivery. However, infant Cmaxs were a median of 44-fold lower (range, 21- to 55-fold) than the median mothers’ Cmax, and maraviroc was undetectable in all infants by 48 h. The maraviroc half-life for the infants could not be accurately calculated due to an insufficient number of time points with detectable drug. There was a 68-fold-lower total AUC for the infants than for the mothers. Amniotic fluid concentrations were 2- to 4-fold lower than that of the corresponding cord blood for each infant.
CCR5 receptor occupancy data showed a profile similar to the PK data for the mothers. Complete protection from internalization of CCR5 receptors on the surface of maternal CD4+ lymphocytes following ex vivo MIP-1β challenge was found during the first 8 h after maraviroc dosing, indicating complete receptor occupancy. Partial protection was observed over 24 to 48 h, when a median of 65% of the maternal CCR5 receptors (range, 54 to 77%) were protected from internalization, indicating approximately 35% of receptors no longer had bound maraviroc. There was a significant correlation between CCR5 receptor occupancy and plasma maraviroc concentrations (r2 = 0.546; P < 0.0001). However, by 1 week postdosing, there was no evidence of protection of CCR5 receptors in the mothers.
In the infants, attempts to measure CCR5 receptor occupancy were complicated by the inherently extremely low expression of CCR5 receptors on newborn macaque CD4+ lymphocytes. CCR5 expression ranged from 0 to 1% on infant CD4+ lymphocytes; the low number of analyzable events and limitations on the amount of blood that could be collected prevented accurate assessment of receptor internalization in infant blood samples in the ex vivo assay.
DISCUSSION
The results of this study demonstrated that in rhesus macaques, a single intrapartum maternal dose of maraviroc was poorly transferred to the fetus. Plasma maraviroc concentrations in the newborn macaques were less than 1% of the mothers’ plasma concentrations. While the single intrapartum doses used in this study (60 to 100 mg/kg) were 15- to 25-fold higher than the standard human dose (300 mg, or 5 mg/kg), the Cmax and AUC for the macaque mothers were similar to those found for humans (1-2, 13). However, Cmax and AUC values plateau above doses of 300 mg in humans (1), and thus, the similar plasma concentrations found in the macaques were not unexpected. The high maraviroc doses were designed to achieve maximal concentrations in maternal blood, suitable for assessing placental transfer into fetal compartments; however, only low fetal levels were found.
MTCT of HIV is associated with high maternal viral loads (8, 18, 26, 34, 36). Potent antiretroviral therapy that reduces or suppresses maternal circulating HIV levels in advance of delivery has been shown to reduce the rate of MTCT to less than 1% (15, 21). In resource-poor settings, where access to HIV diagnostic testing or potent ARV therapy is limited, administration of a single intrapartum dose of NVP at delivery has been shown to substantially reduce MTCT and is recommended as a minimum intervention (4). However, previous studies showed single-dose NVP (29) or AZT (34) administered during labor did not significantly affect the maternal viral load by the time of delivery, although reductions were measured at later time points. Thus, protection from intrapartum/early-postpartum MTCT is likely due to a combination of reduction in the maternal breast milk viral load and provision of prophylactic NVP concentrations to the newborn (9, 29, 31, 34). Pharmacologically, NVP reaches concentrations in newborns that are approximately 90% of maternal concentrations, and it is detectable in newborn plasma for up to 1 week (25). NVP has a low molecular mass (267 Da) and low protein binding (<30%), which are favorable characteristics of drugs that efficiently cross the placenta (35). In contrast, maraviroc has a high molecular mass (513 Da) and is approximately 76% protein bound (17), and in the present study, it was no longer detectable in the plasma of newborn macaques within 48 h of birth.
Studies in humans have shown that maraviroc may have extended antiviral activity despite being discontinued or undetectable in the blood. A delayed rebound in the viral load in humans following cessation of treatment was hypothesized to be due to prolonged occupancy of CCR5 receptors (16). In results presented here, receptor occupancy on maternal CD4 lymphocytes waned in a temporal relationship with plasma maraviroc levels. By 48 h after dosing, a proportion of CCR5 receptors were unoccupied when plasma maraviroc concentrations were only slightly above the limit of detection. Evidence of unoccupied CCR5 receptors suggests CCR5-using HIV could infect these CD4+ lymphocytes. In the infant macaques, our attempts to measure receptor occupancy in the ex vivo assay were compromised by extremely low CCR5 expression on CD4+ lymphocytes, which is consistent with extremely low CCR5 expression (<1%) on CD4+ lymphocytes in human neonates (6, 33). Indeed, subsequently analyzed data from other newborn macaques showed that 0.5 to 2.4% of lymphocytes (12 to 51 cells per μl of blood) express CCR5 receptors in 1- to 3-day-old macaques (K. K. A. Van Rompay, unpublished data). Nevertheless, given that CCR5 receptors became unoccupied within 48 h after dosing in mothers who had plasma maraviroc concentrations that were 44-fold higher than those of the infants, it seems unlikely that CCR5 receptors on infant CD4+ cells remained occupied for any longer period of time.
The work presented here included only a maternal dose of maraviroc. The current minimum recommended intervention to reduce MTCT of HIV is a single maternal NVP dose at delivery plus a single NVP dose to the infant following delivery. The goal of this study was to determine whether maraviroc possessed maternal-fetal pharmacokinetic parameters that were similar to those of NVP (good placental transfer and a long half-life in the infant). It is possible that alternative maraviroc-based pre- and postpartum protocols, such as infant dosing with maraviroc or inclusion of additional drugs, such as ritonavir, to reduce maraviroc clearance, may change the PK profile and improve the potential efficacy of single-dose maraviroc to reduce MTCT.
The results presented here show that a single maternal intrapartum dose of maraviroc resulted in low fetal maraviroc concentrations. While this study included a small number of macaques, the overall findings were consistent among all animals. Overall, poor transplacental transfer and a short pharmacokinetic profile of maraviroc in newborn macaques in this study suggest that as a sole treatment intervention, a single intrapartum dose of maraviroc would not be effective in reducing MTCT of HIV.
Acknowledgments
This work was supported by a grant from the National Institute for Allergy and Infectious Diseases (5 R03 AI074465-02 to N.S.S.), in part by the Department of Veterans Affairs (VA) (to M.H.), and by a grant from the National Center for Research Resources (NCRR) (a component of the NIH) to the California National Primate Research Center.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the VA, NCRR, or NIH.
We thank Zachary D. Abbott, Yongzhi Geng, the Colony Services, Veterinary, and Clinical Laboratory staff of the California National Primate Research Center, and Stephanie Malone from the UNC Center for AIDS Research Clinical Pharmacology and Analytical Chemistry Core for technical and analytical expertise (AI50410).
Footnotes
Published ahead of print on 9 August 2010.
REFERENCES
- 1.Abel, S., D. Russell, L. A. Whitlock, C. E. Ridgway, A. N. Nedderman, and D. K. Walker. 2008. Assessment of the absorption, metabolism and absolute bioavailability of maraviroc in healthy male subjects. Br. J. Clin. Pharmacol. 65(Suppl. 1):60-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abel, S., E. van der Ryst, M. C. Rosario, C. E. Ridgway, C. G. Medhurst, R. J. Taylor-Worth, and G. J. Muirhead. 2008. Assessment of the pharmacokinetics, safety and tolerability of maraviroc, a novel CCR5 antagonist, in healthy volunteers. Br. J. Clin. Pharmacol. 65(Suppl. 1):5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anonymous. 2009. AIDS epidemic update. UNAIDS/WHO, Geneva, Switzerland.
- 4.Anonymous. 2006. Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants: towards universal access: recommendations for a public health approach. HIV/AIDS Programme, WHO, Geneva, Switzerland.
- 5.Arrive, E., M. L. Newell, D. K. Ekouevi, M. L. Chaix, R. Thiebaut, B. Masquelier, V. Leroy, P. V. Perre, C. Rouzioux, and F. Dabis. 2007. Prevalence of resistance to nevirapine in mothers and children after single-dose exposure to prevent vertical transmission of HIV-1: a meta-analysis. Int. J. Epidemiol. 36:1009-1021. [DOI] [PubMed] [Google Scholar]
- 6.Auewarakul, P., K. Sangsiriwut, K. Pattanapanyasat, C. Wasi, and T. H. Lee. 2000. Age-dependent expression of the HIV-1 coreceptor CCR5 on CD4+ lymphocytes in children. J. Acquir. Immune Defic. Syndr. 24:285-287. [DOI] [PubMed] [Google Scholar]
- 7.Brown, K., K. Patterson, S. Malone, N. Shaheen, H. Prince, J. Dumond, M. Spacek, P. Heidt, M. Cohen, and A. Kashuba. 2010. Antiretrovirals (ARV) for prevention: maraviroc (MVC) exposure in the semen (SE) and rectal tissue (RT) of healthy male volunteers after single and multiple dosing, abstr. 85. Prog. Abstr. 17th Conf. Retrovir. Opportun. Infect., San Francisco, CA.
- 8.Chi, B. H., L. Wang, J. S. Read, M. Sheriff, S. Fiscus, E. R. Brown, T. E. Taha, M. Valentine, and R. Goldenberg. 2005. Timing of maternal and neonatal dosing of nevirapine and the risk of mother-to-child transmission of HIV-1: HIVNET 024. AIDS 19:1857-1864. [DOI] [PubMed] [Google Scholar]
- 9.Chung, M. H., J. N. Kiarie, B. A. Richardson, D. A. Lehman, J. Overbaugh, F. Njiri, and G. C. John-Stewart. 2007. Independent effects of nevirapine prophylaxis and HIV-1 RNA suppression in breast milk on early perinatal HIV-1 transmission. J. Acquir. Immune Defic. Syndr. 46:472-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connor, E. M., R. S. Sperling, R. Gelber, P. Kiselev, G. Scott, M. J. O'Sullivan, R. VanDyke, M. Bey, W. Shearer, R. L. Jacobson, et al. 1994. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N. Engl. J. Med. 331:1173-1180. [DOI] [PubMed] [Google Scholar]
- 11.Dabis, F., P. Msellati, N. Meda, C. Welffens-Ekra, B. You, O. Manigart, V. Leroy, A. Simonon, M. Cartoux, P. Combe, A. Ouangre, R. Ramon, O. Ky-Zerbo, C. Montcho, R. Salamon, C. Rouzioux, P. Van de Perre, and L. Mandelbrot. 1999. Six-month efficacy, tolerance, and acceptability of a short regimen of oral zidovudine to reduce vertical transmission of HIV in breastfed children in Cote d'Ivoire and Burkina Faso: a double-blind placebo-controlled multicentre trial. DITRAME Study Group. DIminution de la Transmission Mere-Enfant. Lancet 353:786-792. [DOI] [PubMed] [Google Scholar]
- 12.Dorr, P., M. Westby, S. Dobbs, P. Griffin, B. Irvine, M. Macartney, J. Mori, G. Rickett, C. Smith-Burchnell, C. Napier, R. Webster, D. Armour, D. Price, B. Stammen, A. Wood, and M. Perros. 2005. Maraviroc (UK-427,857), a potent, orally bioavailable, and selective small-molecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type 1 activity. Antimicrob. Agents Chemother. 49:4721-4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dumond, J. B., K. B. Patterson, A. L. Pecha, R. E. Werner, E. Andrews, B. Damle, R. Tressler, J. Worsley, and A. D. Kashuba. 2009. Maraviroc concentrates in the cervicovaginal fluid and vaginal tissue of HIV-negative women. J. Acquir. Immune Defic. Syndr. 51:546-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eshleman, S. H., and J. B. Jackson. 2002. Nevirapine resistance after single dose prophylaxis. AIDS Rev. 4:59-63. [PubMed] [Google Scholar]
- 15.European Collaborative Study. 2005. Mother-to-child transmission of HIV infection in the era of highly active antiretroviral therapy. Clin. Infect. Dis. 40:458-465. [DOI] [PubMed] [Google Scholar]
- 16.Fatkenheuer, G., A. L. Pozniak, M. A. Johnson, A. Plettenberg, S. Staszewski, A. I. Hoepelman, M. S. Saag, F. D. Goebel, J. K. Rockstroh, B. J. Dezube, T. M. Jenkins, C. Medhurst, J. F. Sullivan, C. Ridgway, S. Abel, I. T. James, M. Youle, and E. van der Ryst. 2005. Efficacy of short-term monotherapy with maraviroc, a new CCR5 antagonist, in patients infected with HIV-1. Nat. Med. 11:1170-1172. [DOI] [PubMed] [Google Scholar]
- 17.FDA Antiviral Drugs Advisory Committee. 2007. Maraviroc NDA 22-128. FDA, Silver Spring, MD. http://www.fda.gov/ohrms/dockets/ac/07/briefing/2007-4283b1-01-Pfizer.pdf.
- 18.Garcia, P. M., L. A. Kalish, J. Pitt, H. Minkoff, T. C. Quinn, S. K. Burchett, J. Kornegay, B. Jackson, J. Moye, C. Hanson, C. Zorrilla, and J. F. Lew. 1999. Maternal levels of plasma human immunodeficiency virus type 1 RNA and the risk of perinatal transmission. Women and Infants Transmission Study Group. N. Engl. J. Med. 341:394-402. [DOI] [PubMed] [Google Scholar]
- 19.Guay, L. A., P. Musoke, T. Fleming, D. Bagenda, M. Allen, C. Nakabiito, J. Sherman, P. Bakaki, C. Ducar, M. Deseyve, L. Emel, M. Mirochnick, M. G. Fowler, L. Mofenson, P. Miotti, K. Dransfield, D. Bray, F. Mmiro, and J. B. Jackson. 1999. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet 354:795-802. [DOI] [PubMed] [Google Scholar]
- 20.Institute for Laboratory Animal Research, Commission on Life Sciences, and National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC.
- 21.Ioannidis, J. P., E. J. Abrams, A. Ammann, M. Bulterys, J. J. Goedert, L. Gray, B. T. Korber, M. J. Mayaux, L. M. Mofenson, M. L. Newell, D. E. Shapiro, J. P. Teglas, and C. M. Wilfert. 2001. Perinatal transmission of human immunodeficiency virus type 1 by pregnant women with RNA virus loads <1,000 copies/ml. J. Infect. Dis. 183:539-545. [DOI] [PubMed] [Google Scholar]
- 22.Johnson, J. A., J. F. Li, L. Morris, N. Martinson, G. Gray, J. McIntyre, and W. Heneine. 2005. Emergence of drug-resistant HIV-1 after intrapartum administration of single-dose nevirapine is substantially underestimated. J. Infect. Dis. 192:16-23. [DOI] [PubMed] [Google Scholar]
- 23.Jourdain, G., N. Ngo-Giang-Huong, S. Le Coeur, C. Bowonwatanuwong, P. Kantipong, P. Leechanachai, S. Ariyadej, P. Leenasirimakul, S. Hammer, and M. Lallemant. 2004. Intrapartum exposure to nevirapine and subsequent maternal responses to nevirapine-based antiretroviral therapy. N. Engl. J. Med. 351:229-240. [DOI] [PubMed] [Google Scholar]
- 24.Lockman, S., R. L. Shapiro, L. M. Smeaton, C. Wester, I. Thior, L. Stevens, F. Chand, J. Makhema, C. Moffat, A. Asmelash, P. Ndase, P. Arimi, E. van Widenfelt, L. Mazhani, V. Novitsky, S. Lagakos, and M. Essex. 2007. Response to antiretroviral therapy after a single, peripartum dose of nevirapine. N. Engl. J. Med. 356:135-147. [DOI] [PubMed] [Google Scholar]
- 25.Mirochnick, M., T. Fenton, P. Gagnier, J. Pav, M. Gwynne, S. Siminski, R. S. Sperling, K. Beckerman, E. Jimenez, R. Yogev, S. A. Spector, and J. L. Sullivan. 1998. Pharmacokinetics of nevirapine in human immunodeficiency virus type 1-infected pregnant women and their neonates. Pediatric AIDS Clinical Trials Group Protocol 250 Team. J. Infect. Dis. 178:368-374. [DOI] [PubMed] [Google Scholar]
- 26.Mofenson, L. M. 2004. Successes and challenges in the perinatal HIV-1 epidemic in the United States as illustrated by the HIV-1 serosurvey of childbearing women. Arch. Pediatr. Adolesc. Med. 158:422-425. [DOI] [PubMed] [Google Scholar]
- 27.Moore, J. P., S. G. Kitchen, P. Pugach, and J. A. Zack. 2004. The CCR5 and CXCR4 coreceptors—central to understanding the transmission and pathogenesis of human immunodeficiency virus type 1 infection. AIDS Res. Hum. Retroviruses 20:111-126. [DOI] [PubMed] [Google Scholar]
- 28.Mori, J., M. Mosley, M. Lewis, P. Simpson, J. Torna, W. Huang, J. Whitcomb, G. Ciaramella, and M. Westby. 2007. Characterization of maraviroc resistance in patients failing treatment with CCR5-tropic HIV-1 in MOTIVATE 1 and MOTIVATE 2. Antivir. Ther. 12:S12. [Google Scholar]
- 29.Musoke, P., L. A. Guay, D. Bagenda, M. Mirochnick, C. Nakabiito, T. Fleming, T. Elliott, S. Horton, K. Dransfield, J. W. Pav, A. Murarka, M. Allen, M. G. Fowler, L. Mofenson, D. Hom, F. Mmiro, and J. B. Jackson. 1999. A phase I/II study of the safety and pharmacokinetics of nevirapine in HIV-1-infected pregnant Ugandan women and their neonates (HIVNET 006). AIDS 13:479-486. [DOI] [PubMed] [Google Scholar]
- 30.Petra Study Team. 2002. Efficacy of three short-course regimens of zidovudine and lamivudine in preventing early and late transmission of HIV-1 from mother to child in Tanzania, South Africa, and Uganda (Petra study): a randomised, double-blind, placebo-controlled trial. Lancet 359:1178-1186. [DOI] [PubMed] [Google Scholar]
- 31.Rossenkhan, R., T. Ndung'u, T. K. Sebunya, J. E. Hagan, R. Shapiro, V. Novitsky, S. M. Moyo, I. Thior, S. Lockman, R. Mitchell, S. Kim, R. Musonda, E. van Widenfelt, J. Makhema, and M. Essex. 2009. Temporal reduction of HIV type 1 viral load in breast milk by single-dose nevirapine during prevention of MTCT. AIDS Res. Hum. Retroviruses 25:1261-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scarlatti, G. 2004. Mother-to-child transmission of HIV-1: advances and controversies of the twentieth centuries. AIDS Rev. 6:67-78. [PubMed] [Google Scholar]
- 33.Shalekoff, S., G. E. Gray, and C. T. Tiemessen. 2004. Age-related changes in expression of CXCR4 and CCR5 on peripheral blood leukocytes from uninfected infants born to human immunodeficiency virus type 1-infected mothers. Clin. Diagn. Lab. Immunol. 11:229-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sperling, R. S., D. E. Shapiro, R. W. Coombs, J. A. Todd, S. A. Herman, G. D. McSherry, M. J. O'Sullivan, R. B. Van Dyke, E. Jimenez, C. Rouzioux, P. M. Flynn, and J. L. Sullivan. 1996. Maternal viral load, zidovudine treatment, and the risk of transmission of human immunodeficiency virus type 1 from mother to infant. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N. Engl. J. Med. 335:1621-1629. [DOI] [PubMed] [Google Scholar]
- 35.Syme, M. R., J. W. Paxton, and J. A. Keelan. 2004. Drug transfer and metabolism by the human placenta. Clin. Pharmacokinet. 43:487-514. [DOI] [PubMed] [Google Scholar]
- 36.Taha, T. E., N. I. Kumwenda, D. R. Hoover, S. A. Fiscus, G. Kafulafula, C. Nkhoma, S. Nour, S. Chen, G. Liomba, P. G. Miotti, and R. L. Broadhead. 2004. Nevirapine and zidovudine at birth to reduce perinatal transmission of HIV in an African setting: a randomized controlled trial. JAMA 292:202-209. [DOI] [PubMed] [Google Scholar]
- 37.Westby, M., and E. van der Ryst. 2005. CCR5 antagonists: host-targeted antivirals for the treatment of HIV infection. Antivir. Chem. Chemother. 16:339-354. [DOI] [PubMed] [Google Scholar]
- 38.Wiktor, S. Z., E. Ekpini, J. M. Karon, J. Nkengasong, C. Maurice, S. T. Severin, T. H. Roels, M. K. Kouassi, E. M. Lackritz, I. M. Coulibaly, and A. E. Greenberg. 1999. Short-course oral zidovudine for prevention of mother-to-child transmission of HIV-1 in Abidjan, Cote d'Ivoire: a randomised trial. Lancet 353:781-785. [DOI] [PubMed] [Google Scholar]