Abstract
Implant-related infections are serious complications of trauma and orthopedic surgery and are most difficult to treat. The bacterial biofilms of 34 clinical Staphylococcus sp. isolates (Staphylococcus aureus, n = 14; coagulase-negative staphylococci, n = 19) were incubated with daptomycin (DAP; 5, 25, or 100 mg/liter), vancomycin (VAN; 5, 25, or 100 mg/liter), tigecycline (TGC; 1, 5, or 25 mg/liter), fosfomycin (FOM; 100, 250, or 1,000 mg/liter), and cefamandole (FAM; 50, 100, or 500 mg/liter) for 24 h at three different ambient temperatures: 35°C, 40°C, and 45°C. To quantify the reduction of the biomass, the optical density ratio (ODr) of stained biofilms and the number of growing bacteria were determined. Increasing the temperature to 45°C or to 40°C during incubation with FAM, FOM, TGC, VAN, or DAP led to a significant but differential reduction of the thickness of the staphylococcal biofilms compared to that at 35°C (P < 0.05). Growth reduction was enhanced for DAP at 100 mg/liter at 35°C, 40°C, and 45°C (log count reductions, 4, 3.6, and 3.3, respectively; P < 0.05). A growth reduction by 2 log counts was detected for FAM at a concentration of 500 mg/liter at 40°C and 45°C (P = 0.01). FOM at 1,000 mg/liter reduced the bacterial growth by 1.2 log counts (not significant). The antibacterial activity of antimicrobial agents is significantly but differentially enhanced by increasing the ambient temperature and using high concentrations. Adjuvant hyperthermia may be of value in the treatment of biofilm-associated implant-related infections.
Implant-related infections are severe complications of trauma and orthopedic surgery that frequently require long-term antimicrobial treatment, supportive management, and multiple additional surgical procedures (53). Staphylococcus aureus and coagulase-negative staphylococci (CoNS), primarily Staphylococcus epidermidis, are the most common organisms associated with implant-related infections after trauma and orthopedic surgery (13).
Bacterial biofilms develop on the surfaces of the implants (12, 13, 30, 41). The biofilm consists of a structured community of bacterial cells enclosed in a self-produced polymeric matrix that adheres to an inert or living surface. The bacteria embedded in the biofilm are quasiprotected by this self-made polymeric matrix; thus, resistance to antimicrobials is increased such that the concentrations needed to kill the biofilm bacteria are 500 to 5,000 times higher than the levels needed to kill planktonic bacteria (8, 12, 13). Although removal of the colonized foreign material may be the most effective means to treat biofilm-associated infections (14), the implant must sometimes be retained because of technical or physiological complications. In this case, any hope for cure or at least the stability of the patient lies in antimicrobial treatment alone.
Antimicrobial agents for the treatment of staphylococcal infections include beta-lactam antibiotics and, in the case of methicillin resistance, vancomycin (VAN), fosfomycin (FOM), tigecycline (TGC), or daptomycin (DAP) (32, 38). FOM is a small-molecule antibiotic with a wide antibacterial spectrum and excellent tissue penetration, representing an excellent alternative antimicrobial agent for the treatment of deep-seated infections (18, 27). Both TGC and DAP are highly active against Gram-positive cocci resistant to commonly used antibiotics, including methicillin-resistant staphylococci (18, 43). Antimicrobial agents may reduce biofilm and bacterial growth (35, 39). In a previous study, we demonstrated that the antibiotic concentrations achieved under normal physiological conditions did not decrease the growth of established staphylococcal biofilms (21). Bacterial biofilm growth was significantly reduced by the use of antimicrobials at excessive concentrations or when antimicrobials were used in combination with azithromycin (21, 37).
Because the effects of antibiotics on established biofilms are unsatisfactory, other measures to reduce biofilm thickness and to kill biofilm bacteria may be helpful (37). Adjunctive therapy to enhance the activity of an antimicrobial to save an infected but unremovable implant or to improve the engraftment of a new implant after reimplantation may be a benefit for the patient (13, 16). Experimental measures taken to reduce bacterial biofilms included antibiotic combination therapy, particularly with rifampin; therapy with a combination of antibiotics and chemicals like EDTA or N-acytylcysteine; or therapy with a combination of antibiotics and physical measures, such as ultrasound or an electric current (7, 15, 39, 51). Low-frequency ultrasound was used to prevent uncontrolled heating and associated tissue damage (5, 6). For physical therapy, however, ultrasound of 1 MHz (unpulsed) had been described to have a beneficial effect on osteoarthritis (45). Further, application of heat has traditionally been used in physical therapy with various rates of success and minimal harm (9).
Increasing the temperature induced the formation of thicker biofilms (40). Although the amount of heating was never measured in the ultrasound or electric current experiments, heating may have an influence on the effects of antibiotics on biofilms. Thus, we investigated the effects of stepwise increases in the ambient temperature together with antibiotic treatment on biofilm thickness and bacterial growth. Static biofilms of clinical isolates from orthopedic implant infections or preoperative skin isolates, including S. aureus and CoNS (S. epidermidis, S. lugdunensis, S. hominis, and S. capitis), were incubated at an ambient temperature of 35, 40, or 45°C for 24 h with DAP, VAN, FOM, TGC, or FAM. Three concentrations of each of the antimicrobial agents were investigated.
(Some of the data presented here were presented as poster K2069 at the 48th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy-Infectious Diseases Society of America 46th Annual Meeting, San Francisco, CA, 2009.)
MATERIALS AND METHODS
The University Hospital of Vienna in Vienna, Austria, is a 2,200-bed primary- and tertiary-care teaching hospital. Overall, 35 staphylococci were tested: 19 isolates from implant-related infections (S. aureus, n = 13; S. epidermidis, n = 5; and S. capitis, n = 1) and 15 skin isolates (S. aureus, n = 1; S. epidermidis, n = 9; S. lugdunensis, n = 4; S. hominis, n = 1) taken from the thigh before surgical disinfection and incision for hip arthroplasty after femoral neck fracture. Because of the low numbers of non-S. epidermidis isolates, S. epidermidis, S. lugdunensis, S. capitis, and S. hominis were grouped together as CoNS. Biofilm-producing strains S. aureus ATCC 29213 and S. epidermidis DSM3269 served as reference strains. In addition, a biofilm-producing S. epidermidis isolate (isolate KO8) from the skin of non-hospital-associated healthy volunteer was tested as the internal control. Antibiotic susceptibility was determined by the disk diffusion method on cation-adjusted Mueller-Hinton agar (bioMerieux, Marcy l'Etoile, France). Resistance to oxacillin to detect methicillin resistance was determined by incubating the plates with disks containing oxacillin at 5 μg and at temperatures of 30°C and 37°C.
Antimicrobial agents.
Commercially available DAP, VAN, FOM, TGC, and FAM were purchased from Novartis (Vienna, Austria), Lilly (Vienna, Austria), Astropharma (Vienna, Austria), Wyeth-Lederle (Vienna, Austria), and Sandoz (Kundl, Austria), respectively.
Determination of MICs.
Before the biofilms were treated with DAP, VAN, FOM, TGC, or FAM, the MICs of each isolate to be tested were determined. The determination of MICs was done according to the protocol of the Clinical and Laboratory Standards Institute using the microtiter plate method (11). According to this protocol, cation-adjusted Mueller-Hinton medium and Mueller-Hinton broth (MHB) containing 50 μg/ml calcium were used for in vitro susceptibility testing of DAP. For the susceptibility testing of FOM, glucose-6-phosphate (25 mg/liter) was added (Table 1).
TABLE 1.
MICs of the isolates investigated
| Clinical isolate | Isolation site | No. of isolates | MIC (mg/liter) |
||||
|---|---|---|---|---|---|---|---|
| FAM | FOM | TGC | VAN | DAP | |||
| S. aureus | Prosthetic joint infection | 13 | 1 (0.5-64)a | 4 (0.25-64) | 0.125 (0.01-0.25) | 0.5 (0.5-1) | 0.125 (0.06-0.5) |
| CoNS | Prosthetic joint infection | 6 | 1 (0.25-2) | 16 (4-64) | 0.06 (0.01-0.25) | 2 (1-2) | 0.125 (0.06-0.25) |
| S. aureus | Preoperative skin | 1 | 0.25 | 64 | 0.06 | 0.5 | 0.125 |
| CoNS | Preoperative skin | 14 | 0.25 (0.06-16) | 32 (0.5-64) | 0.03 (0.01-0.5) | 0.75 (0.5-2) | 0.250 (0.01-0.25) |
| Control strains | |||||||
| SEK8 | Skin of healthy control | 0.25 | 16 | 0.125 | 1 | 0.25 | |
| ATCC 29213 | Reference strain | 0.5 | 4 | 0.06 | 0.5 | 0.125 | |
| DSM3269 | Reference strain | 0.5 | 1 | 0.5 | 2 | 0.25 | |
Values in parentheses are the MIC50 ranges for the clinical isolates.
Thermostability testing.
To test for thermostability, all antimicrobials in powder and in solution, as used for MIC testing, were incubated at 45°C for 1 and 24 h in three independent experiments. For those experiments S. aureus ATCC 29213 and a clinical isolate of S. epidermidis (OR1) were used. The increase in the MICs—if any—was not significant (Table 2).
TABLE 2.
Thermostability of the antimicrobials with incubation for 24 h at 45°C as powders as well as in solution
| Strain | Antibiotic | Median (range) MIC50 (mg/liter) |
||
|---|---|---|---|---|
| Standard | After incubation with antimicrobial agenta |
|||
| As powder | In solution | |||
| S. aureus | Cefamandole | 0.25 (0.25-0.25) | 0.25 (0.25-0.25) | 0.5 (0.25-0.5) |
| ATCC | Fosfomycin | 0.5 (0.25-1) | 1 (0.5-1) | 0.5 (0.5-1) |
| 29213 | Tigecycline | 1 (1-1) | 1 (1-1) | 1 (1-1) |
| Vancomycin | 0.5 (0.5-2) | 0.5 (0.5-1) | 1 (0.5-1) | |
| Daptomycin | 0.25 (0.25-0.5) | 0.5 (0.5-1) | 1 (0.5-1) | |
| S. epidermidis | Cefamandole | 1 (1-2) | 1 (1-2) | 2 (1-2) |
| OR1 | Fosfomycin | 32 (16-32) | 32 (32-32) | 32 (16-32) |
| Tigecycline | 1 (1-2) | 1 (1-1) | 2 (1-2) | |
| Vancomycin | 2 (2-2) | 2 (1-2) | 2 (1-2) | |
| Daptomycin | 0.5 (0.25-1) | 0.5 (0.5-1) | 1 (0.5-1) | |
Incubation was for 24 h at 45°C.
Biofilm model.
Biofilms were studied using the static microtiter plate model established by Christensen et al. (10). The S. aureus and CoNS isolates were prepared in MHB at a concentration of a McFarland 0.5 standard and diluted 1:100 with MHB. Each well of a 96-well polystyrene flat-bottomed microtiter plate was filled with 50 μl of diluted bacteria and 50 μl supplemented MHB (containing calcium), and the plate was incubated for 24 h in ambient air at 35°C. The medium and planktonic cells were removed. The biofilms in the wells were fixed with formalin (37%, diluted 1:10) plus 2% natrium acetate, and each well was stained with 150 to 250 μl 1% crystal violet for 5 min. The stained biofilms were then washed two times with approximately 300 μl distilled water. The wells were then visually checked for the presence or absence of a biofilm, based on the presence of staining at the bottom of the well. The mean optical density (OD) was used for quantification using a routine microtiter plate reader at a 550-nm wavelength. All biofilm experiments were done 4 times for each isolate to minimize variability in the OD measurements. To ascertain the biofilm formation, biofilms were grown on cover slides using a 24-well plate. After 24 h, the biofilms were fixed with 2% glutaraldehyde, and biofilm formation was verified by scanning electron microscopy. Established biofilms grown for 24 h were used for the experiments.
Incubation of the biofilms with DAP, VAN, FOM, TGC, or FAM at ambient temperatures of 35°C, 40°C, and 45°C.
To test the effects of the antimicrobials, the 24-h biofilms were incubated with DAP, VAN, FOM, TGC, or FAM at three concentrations and at three different temperatures (35°C, 40°C, or 45°C) for 24 h. Three concentrations of each antimicrobial agent were tested: DAP at 5, 25, and 100 mg/liter; VAN at 5, 25, and 100 mg/liter; TGC at 1, 5, and 25 mg/liter; FOM at 100, 250, and 1,000 mg/liter; and FAM at 50, 100, and 500 mg/liter. The lower concentrations of the antimicrobial agents were chosen with regard to their achievable trough or peak levels in serum. To investigate the impact of a very high concentration, those concentrations were investigated.
The OD was used to measure the biofilm thickness. To correct for the individual biofilm formation of each isolate, the OD ratio (ODr), which was the OD of the untreated biofilm/OD of the treated biofilm, was calculated. The baseline ODr of the untreated biofilm is set equal to 1. The explanation for this was that, using the uncorrected ODs of the biofilms, the intra-assay variability was determined from quadruplicate analyses of the treated biofilms. The median coefficient of variance (CV) for all 34 staphylococcal isolates was 20.5% (range, 0.6 to 110.5%). The median CV for intra-assay variability for S. aureus isolates was 15.76% (range, 0.62 to 110.6%), and that for CoNS isolates was 20.78% (range, 3.65 to 6%). The interassay (day-to-day) variability was based on bacterial growth without antibiotic (control condition) on two different days: the median CV was 45.8% (range, 13.44 to 102.1%). The median CV for interassay (day-to-day) variability for the S. aureus isolates was 44.7% (range, 39.7 to 50.7%), and that for CoNS isolates was 48.8% (range, 13.4 to 102.1%). Taking the ODr of all isolates, the median CV for intra-assay variability was 28.5% (range, 5 to 117%). The median CV for intra-assay variability for S. aureus isolates was 26.7% (range, 5 to 117%), and that for CoNS isolates was 28.2% (range, 9.9 to 108.7%). The interday variability was not calculated because by taking the ODr equal to the OD of the treated biofilm/OD of the untreated biofilm, the baseline (untreated) value was equal to 1, thus excluding any variability. Given the intersubject variability, the ODr was used for the calculations.
Efficacies of antimicrobial agents against biofilms in all experiments.
To test for viable bacteria in the biofilms in all experiments, the biofilms were not fixed and dyed but were scraped off and resuspended in MHB, seeded onto Columbia agar, and examined for growth. The numbers of bacteria in suspension were enumerated by serial dilutions, and 0.1 ml of each dilution was inoculated onto blood agar plates. The plates were incubated at 35°C in ambient air and were read after 48 h. Each test was performed in duplicate, and the results were expressed as the mean log10 numbers of CFU per ml (mean log10 bacterial count).
Statistical methods.
The significance of the differences was assessed by means of the chi-square test for categorical variables. For subgroups and nonnormally distributed variables, the Mann-Whitney U test was used. To determine the changes due to the different antibiotic combinations and concentrations of every isolate, a general linear model for repeated measurement was calculated. All tests were performed using SPSS for Windows, release 17 (SPSS). A P value of <0.05 was considered significant for the reduction of the biofilm ODr. For the reduction of growth, a decrease in the colony count of >2 log counts was considered significant.
RESULTS
Biofilm density.
The biofilm density increased at the two higher incubation temperatures. The optical densities of the untreated native biofilms were 0.62 ± 0.34 at 35°C, 1.67 ± 0.79 at 40°C, and 1.65 ± 0.8 at 45°C (mean ± standard deviation [SD]). For the S. aureus isolates, the optical densities of the untreated native biofilms were 0.53 ± 0.25 at 35°C, 1.86 ± 0.71 at 40°C, and 2.19 ± 0.64 at 45°C; and for the CoNS isolates, the optical densities of the untreated native biofilms were 0.68 ± 0.38 at 35°C, 1.54 ± 0.82 at 40°C, and 1.27 ± 0.69 at 45°C. For the isolates from implant infections, the optical densities of the untreated native biofilms were 0.52 ± 0.18 at 35°C, 1.8 ± 0.76 at 40°C, and 1.91 ± 0.79 at 45°C. For the isolates from preoperative skin, the optical densities of the untreated native biofilms were 0.74 ± 0.44 at 35°C, 1.51 ± 0.81 at 40°C, and 1.32 ± 0.69 at 45°C.
Incubation with antimicrobial agents.
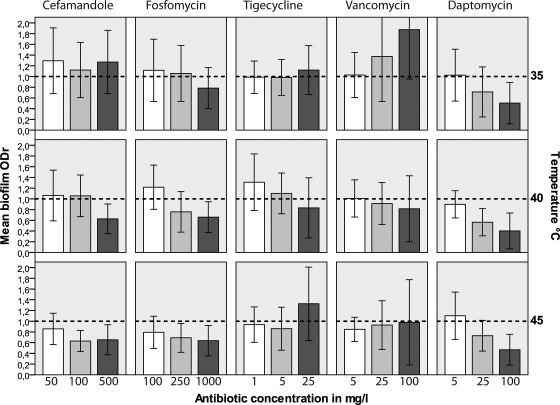
Increasing the temperature to 45°C or to 40°C during incubation with the antimicrobial agents led to significant but differential reductions in the biofilm thickness in comparison to that at 35°C and that for the untreated biofilm (Fig. 1; Table 3).
FIG. 1.
Mean reduction of the biofilm thickness given as the ODr values obtained with cefamandole, fosfomycin, tigecycline, vancomycin, and daptomycin as a function of the incubation temperature. The error bars indicate ± 1 SD. The baseline ODr of the untreated biofilm is 1 (dashed lines).
TABLE 3.
Changes in ODr of biofilms incubated with cefamandole, fosfomycin, tigecycline, vancomycin, and daptomycin at ambient temperature of 35°C, 40°C, or 45°C for 24 h
| Antibiotic | Concn (mg/liter) | Mean ODr change ± SD |
||
|---|---|---|---|---|
| 35°C | 40°C | 45°C | ||
| Cefamandole | 50 | 1.29 ± 0.61 | 1.07 ± 0.46 | 0.86 ± 0.29a,b |
| 100 | 1.12 ± 0.52 | 1.06 ± 0.38 | 0.63 ± 0.19a,b,c | |
| 500 | 1.27 ± 0.59 | 0.62 ± 0.27a,b,c | 0.65 ± 0.28a,b,c | |
| Fosfomycin | 100 | 1.07 ± 0.57 | 1.22 ± 0.41 | 0.78 ± 0.29a,b |
| 250 | 1.01 ± 0.52 | 0.75 ± 0.38a,b,c | 0.69 ± 0.26a,b,c | |
| 1,000 | 0.78 ± 0.38a | 0.65 ± 0.28a,c | 0.63 ± 0.28a,b,c | |
| Tigecycline | 1 | 0.98 ± 0.3 | 1.31 ± 0.52 | 0.93 ± 0.33b |
| 5 | 0.99 ± 0.33 | 1.1 ± 0.38 | 0.86 ± 0.40a,b | |
| 25 | 1.12 ± 0.45 | 0.83 ± 0.56a,b | 1.32 ± 0.68 | |
| Vancomycin | 5 | 1.04 ± 0.41 | 1 ± 0.35 | 0.85 ± 0.22a,b |
| 25 | 1.37 ± 0.82 | 0.91 ± 0.39b | 0.93 ± 0.45b | |
| 100 | 1.87 ± 0.92 | 0.81 ± 0.61a,b,c | 0.98 ± 0.79b | |
| Daptomycin | 5 | 1.02 ± 0.48 | 0.9 ± 0.25a | 1.1 ± 0.44 |
| 25 | 0.71 ± 0.46a,c | 0.56 ± 0.25a,b,c | 0.73 ± 0.29a,c | |
| 100 | 0.5 ± 0.39a,c | 0.40 ± 0.33a,b,c | 0.47 ± 0.29a,c | |
Significant reduction of biofilm OD compared to OD of the untreated biofilm. The reference ODr of the untreated biofilm is 1 (P < 0.05).
Significant reduction of biofilm OD compared to OD of biofilm incubated with the respective antibiotic at 35°C (P < 0.05).
Significant reduction of biofilm OD compared to OD of biofilm incubated with the lowest concentration of the respective antibiotic (P < 0.05).
Cefamandole, fosfomycin, and daptomycin.
FAM, FOM, and DAP reduced the biofilm mass with increasing temperature. After incubation at 45°C, FAM and FOM at all three concentrations tested reduced the biofilm ODr significantly compared to the biofilm ODr after incubation at 35°C (for FAM at the three concentrations, ODr values were 0.86 ± 0.29, 0.63 ± 0.19, and 0.65 ± 0.28, respectively, at 45°C versus 1.29 ± 0.61, 1.12 ± 0.52, and 1.27 ± 0.59, respectively, at 35°C; for FOM at the three concentrations, ODr values were 0.78 ± 0.29, 0.69 ± 0.26, and 0.63 ± 0.28, respectively, at 45°C versus 1.07 ± 0.57, 1.01 ± 0.52, and 0.78 ± 0.38 at 35°C; values are means ± SDs; P < 0.05). Compared to the results for the untreated biofilms, FAM (100 mg/liter and 500 mg/liter) and FOM (250 mg/liter and 1,000 mg/liter) achieved significant reductions in biofilm growth at 45°C and 40°C. At 40°C, DAP at all three concentrations reduced the biofilm ODr significantly compared to the ODr at 35°C (ODr values, 0.9 ± 0.25, 0.56 ± 0.25, and 0.40 ± 0.33, respectively, at 40°C versus ODr values of 1.02 ± 0.48, 0.71 ± 0.46, and 0.5 ± 0.39, respectively, at 35°C; P < 0.05). At the concentration of 100 mg, the biofilm ODr was already significantly reduced at 35°C. An increase in the temperature did not bring any further reduction in the ODr (Table 1; Fig. 1A). Antimicrobials administered at very high concentrations were more effective at reducing the biofilm ODr compared to the ODr of the untreated biofilms, particularly at 40°C and 45°C. DAP at concentrations of 25 mg/liter and 100 mg/liter achieved significant reductions in the biofilm ODr values at 35°C, 40°C, and 45°C.
Tigecycline and vancomycin.
TGC and VAN achieved variable effects with increasing temperature. At 45°C, TGC at concentrations of 1 mg/liter and 5 mg/liter achieved significant reductions in the biofilm ODr compared to the ODr of biofilms incubated with TGC at 35°C. TGC at concentrations of 5 mg/liter and 25 mg/liter achieved significant reductions in the biofilm ODr compared to the ODr of the untreated biofilm at 45°C only. VAN at all three concentrations at 45°C and VAN at concentrations of 25 mg/liter and 100 mg/liter at 40°C reduced the biofilm ODr compared to the biofilm ODr at 35°C. However, at 35°C, biofilms incubated with VAN were exceedingly thicker than the untreated biofilms. At 40°C but not at 45°C, VAN (25 mg/liter and 100 mg/liter) reduced the biofilm thickness compared to that of the untreated biofilms.
Antibacterial efficacy.
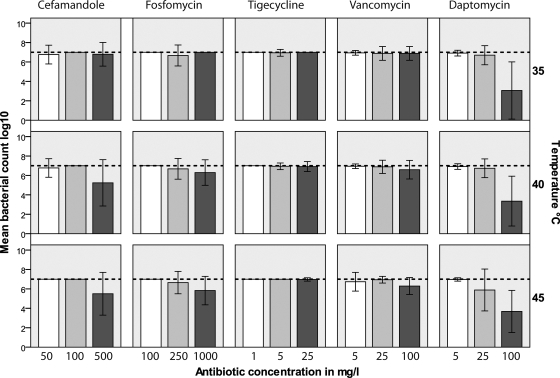
The colony counts of the untreated biofilms at 35°C, 40°C, and 45°C were 1.5 × 108 ± 1.3 × 107, 1.3 × 108 ± 1.1 × 107, and 2.8 × 107 ± 2.7 × 107, respectively (means ± SDs). For DAP and FAM, bacterial growth was significantly reduced but was not eradicated with increases in the incubation temperature and antibiotic concentration. After incubation at 35°C, a significant reduction in the amount of bacterial growth was observed only with DAP at a concentration of 100 mg/liter (log reduction, 2) and not with any other antibiotic tested in the study. The reduction of growth in biofilms was enhanced at 45°C for DAP at 25 mg/liter (log reduction, 1.2; P was not significant) and at 35°C, 40°C, and 45°C for DAP at 100 mg/liter (log count reductions, 4, 3.6, and 3.3, respectively; P < 0.05). Bacterial growth reduction by 2 log counts was detected for FAM at a concentration of 500 mg/liter at 40°C and 45°C (P = 0.01). FOM at 1,000 mg/liter reduced the bacterial growth by 1.2 log counts (P was not significant). No growth reduction was observed for VAN and TGC at any concentration or any incubation temperature (Fig. 2).
FIG. 2.
Mean reduction of bacterial growth after incubation with cefamandole, fosfomycin, tigecycline, vancomycin, and daptomycin as a function of the incubation temperature. The error bars indicate ± 1 SD. The colony count of the untreated biofilm is 107 CFU/ml (dashed lines).
DISCUSSION
Temperature is a basic requirement for bacterial growth. Most clinically significant microorganisms are mesophiles that grow optimally at temperatures of between 25°C and 40°C (33). Microbial biofilms present ubiquitous strategies to survive in hostile environments and are influenced by many factors. These factors include the species, number, and physiology of the microorganisms themselves; the presence and concentrations of nutrients or toxins; as well as environmental conditions, such as shear forces, pH, and temperature (20, 40). Ambient temperature has a remarkable influence on microbial biofilms. Increased ambient temperature decreases the biofilm formation of Streptococcus intermedius (1) and the rate of recovery of sonicated cultures (31) and induces bacterial stress and increased vulnerability in Pseudomonas putida (48). Increasing the temperature to 42°C led to an increase in the biofilm density in an S. epidermidis mutant derived from a clinical isolate (40). This effect is confirmed by the findings of the present study, because untreated biofilms incubated at 40°C or 45°C exhibited greater densities than biofilms incubated at 35°C. However, the activity of disinfectants was described to be increased at increased temperatures (44). In the present study, we investigated the influence of increases in the incubation temperature to 40°C and 45°C on the effects of antimicrobial agents on established biofilms.
Incubation with the antibiotics at 40°C or 45°C achieved significant reductions in the biofilm thickness and bacterial growth (Table 3; Fig. 1). Similar to the findings of a study of electric current and the activities of antimicrobial agents against Pseudomonas aeruginosa, S. aureus, and S. epidermidis biofilms by del Pozo et al. (15), the effects of increases in temperature were different for the different antibiotics in the present study. For VAN and TGC, TGC incubated at 45°C achieved a significant reduction in the biofilm ODr compared to the ODr of biofilms treated with TGC at 35°C but not compared to the ODr of untreated biofilms at 35°C. At 45°C, VAN reduced the biofilm ODr compared to the biofilm ODr at 35°C, but at 35°C, biofilms that were treated with VAN were exceedingly thicker than the untreated biofilms. Subinhibitory concentrations of tetracycline were reported to enhance the expression of the icaABCD gene cluster, which is responsible, although not exclusively, for biofilm production in staphylococci. This may explain the effects of TGC, for TGC shares part of its chemical structure with tetracycline, but for VAN, enhancement of icaABCD operon expression has not yet been observed (40).
For FOM, the increase in temperature led to a significant reduction in biofilm thickness but not of bacterial growth. There was a decrease in the biofilm thickness, as shown for DAP and FAM (Fig. 1 and 2). For DAP, the concentration was pivotal. DAP at a concentration of 25 mg/liter or 100 mg/liter reduced the biofilm density at 35°C, 40°C, and 45°C compared to that achieved with DAP at 5 mg/liter and that of the untreated biofilm (Table 3). The reduction in the biofilm thickness due to DAP at a concentration of 25 mg/liter or 100 mg/liter is already significant, so an increase in the temperature does not have any further effect on the biofilm thickness. However, with regard to bactericidal activity, an increase in the temperature to 45°C decreases the bacterial growth for DAP at 25 mg/liter (Table 3; Fig. 1 and 2).
A moderate increase in the ambient temperature increased the activities of the antibiotics DAP, FAM, and FOM, yet eradication of the bacteria was not observed for any antibiotic even at the very high concentrations tested. As the lower concentrations used were chosen according to the serum levels reached in clinical practice, these levels might be reached after application of the dosages used. However, when plasma protein binding is taken into account, the TGC or DAP dosages would rise to very high concentrations (Table 4), yet these calculations are based on the results of studies with a great variety of populations whose pharmacokinetics might be different from those of patients with implant infections. Additionally, the very high concentrations used in the present study may not be attainable in synovial fluid after systemic administration, although doses of DAP higher than the recommended dose of 6 mg/kg of body weight, for example, have already been investigated for the treatment of implant infections (46). However, topical application of highly concentrated antibiotics through the use of either intra-articular administration, antibiotic-impregnated polymethylmethacrylate, or bonding of the antimicrobials on implant surfaces may be an option. The VAN included in cement spacers used in two-stage revisions of infected hip implants was tolerated at concentrations of >1,500 μg/ml (23). However, the safety and efficacy of at least DAP, TGC, FAM, and FOM for topical use are not well studied. Rifampin, which is probably the single most important drug currently used against biofilm infections of arthroplasties (49), was not investigated in the present study because of the existence of many experimental and clinical data (50). Although rifampin is very active against bacterial and staphylococcal biofilms in particular, resistance to rifampin is increasing in many organisms and not only staphylococci (22, 28).
TABLE 4.
Hypothetical i.v. dosage, corrected for plasma protein binding, of antibiotics needed to reach the in vitro concentrations tested in the present study
| Antibiotic | Dose administered i.v. | Mean concn (mg/liter) | % protein binding | Hypothetical i.v. dose | Reference(s) |
|---|---|---|---|---|---|
| Cefamandole | 1,000 g | Serum, 77.7; blister, 22.1 (peak) | 74 | Serum, 8 g for 50 mg/liter, 16 g for 100 mg/liter, 80 g for 500 mg/liter | Armstrong et al. (2), Fong et al. (17) |
| Fosfomycin | 8 g (100 mg/kg) | Serum, 394.7 (peak); bone tissue, 96.4 | Negligible | Serum, 2 g for 100 mg/liter, 6 g for 250 mg/liter, 24 g for 1,000 mg/liter; bone, 8 g for 100 mg/liter | Ode et al. (34), Schintler et al. (47) |
| Tigecycline | 100 mg | Serum, 0.198; bone, 0.077; synovial fluid, 0.116 (after 4 h) | 71-97 | Serum, >7-10 g for 1 mg/liter, >35-50 g for 5 mg/liter; bone, >70-100 g for 1 mg/liter | Rodvold et al. (43) |
| Vancomycin | 1 g | Plasma, 12.9; cancellous bone, 2.66; cortical bone, 11.53 (variable interval after dosing) | 10-50 | Serum, 0.5-1 g for 5 mg/liter, 12.5-10 g for 25 mg/liter; cancellous bone, 2-4 g for 5 mg/liter, 10-20 g for 25 mg/liter | Garazzino et al. (19) |
| Daptomycin | 12 mg/kg | Serum, 164.8 (peak) and ca. 20 (trough) | 90-93 | Serum, 8 mg/kg for 5 mg/liter; 40 mg/kg for 25 mg/kg | Benvenuto et al. (3) |
The question is how heat can be applied topically and used for a long period without risking thermal burns. Hyperthermia treatment is used in cancer therapy, and application of heat has been described to be helpful in preventing postoperative wound infections (25, 29). Shortwave ultrasound and electric current are used for acceleration of infected wound healing and the treatment of septic nonunions (4, 36, 52). Ultrasound and electric current modulate bacterial biofilms and the effects of antibiotics on biofilm bacteria. Both electric current and ultrasound produce heat either by resistivity or by reflection on the surface. Evidently, treatment with ultrasound influences the formation and/or the thickness of the biofilms (7). In the present study, the application of low-frequency (28.48-kHz) ultrasound plus antibiotics was shown to reduce the colony counts of Escherichia coli but not those of Pseudomonas aeruginosa. In another study with an animal model of staphylococcal implant infection, the same group used low-frequency (<500-kHz) ultrasound because it did not produce heating to the same levels as the higher-frequency (>1-MHz) ultrasound used for medical imaging and physical therapy (6). Heating of implants was carefully avoided—although the level was not measured—because tissue damage was reported even with low-frequency ultrasound, although there was no significant heating of the skin (42). Heating by the use of ultrasound will work only if the frequency used is appropriate for the respective implant surface. Electric current was shown to enhance the activities of antimicrobial agents (15). However, to create heat, an alternating current might be applied to the implant at an appropriate frequency. Other physical measures that could be used to create heating at the implant surface could be microwaves or lasers, both at appropriate frequencies, or, as described recently, magnetic resonance scanning (26). However, as evidence of clinical safety is not yet available, the increase in the temperature to 45°C, particularly for a prolonged period, may be associated with thermal burn injuries. Thus, topical thermal treatment of the implant surface might be possible only with implants with specifically modified surface properties (embedded antibacterial nanoparticles, ferromagnetic particles, or beads) or, e.g., with implants made of improved alloys, accompanied by careful monitoring of the surface temperature via sensors or magnetic resonance scanning computing algorithms. Future research and experiments will be needed to build a solid foundation for safe clinical applications.
In conclusion, the moderate increase in the incubation temperature to 45°C resulted in a decrease in biofilm thickness with treatment with DAP, VAN, TGC, FOM, and FAM and significant reductions in bacterial growth with DAP at 25 mg/liter and 100 mg/liter and FAM at 500 mg/liter. For all antibiotics and for DAP in particular, high antimicrobial concentrations were the most effective. To find a feasible approach to the treatment of implant-related infections in trauma and orthopedic surgery, experimental studies are needed to investigate the most effective physical measures to be applied; the most effective means of controlling the delivery of heat to the infected implant surface by the use of either ultrasound, microwaves, electric current, or magnetic resonance scanning; and the effects of local heat in combination with antibiotics.
Acknowledgments
None of us has any conflict of interest to declare.
Footnotes
Published ahead of print on 2 August 2010.
REFERENCES
- 1.Ahmed, N. A., F. C. Petersen, and A. A. Scheie. 2008. Biofilm formation and autoinducer-2 signaling in Streptococcus intermedius: role of thermal and pH factors. Oral Microbiol. Immunol. 23:492-497. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong, G. C., R. Wise, R. M. Brown, and J. Hancox. 1981. Comparison of ceftazidime and cefamandole pharmacokinetics and blister fluid concentrations. Antimicrob. Agents Chemother. 20:356-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benvenuto, M., D. P. Benziger, S. Yankelev, and G. Vigliani. 2006. Pharmacokinetics and tolerability of daptomycin at doses up to 12 milligrams per kilogram of body weight once daily in healthy volunteers. Antimicrob. Agents Chemother. 50:3245-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brighton, C. T., J. Black, Z. B. Friedenberg, J. L. Esterhai, L. J. Day, and J. F. Connolly. 1981. A multicenter study of the treatment of non-union with constant direct current. J. Bone Joint Surg. Am. 63:2-13. [PubMed] [Google Scholar]
- 5.Carmen, J. C., J. L. Nelson, B. L. Beckstead, C. M. Runyan, R. A. Robison, G. B. Schaalje, and W. G. Pitt. 2004. Ultrasonic-enhanced gentamicin transport through colony biofilms of Pseudomonas aeruginosa and Escherichia coli. J. Infect. Chemother. 10:193-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carmen, J. C., B. L. Roeder, J. L. Nelson, B. L. Beckstead, C. M. Runyan, G. B. Schaalje, R. A. Robison, and W. G. Pitt. 2004. Ultrasonically enhanced vancomycin activity against Staphylococcus epidermidis biofilms in vivo. J. Biomater. Appl. 18:237-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carmen, J. C., B. L. Roeder, J. L. Nelson, R. L. Ogilvie, R. A. Robison, G. B. Schaalje, and W. G. Pitt. 2005. Treatment of biofilm infections on implants with low-frequency ultrasound and antibiotics. Am. J. Infect. Control 33:78-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ceri, H., M. Olson, D. Morck, D. Storey, R. Read, A. Buret, and B. Olson. 2001. The MBEC assay system: multiple equivalent biofilms for antibiotic and biocide susceptibility testing. Methods Enzymol. 337:377-385. [DOI] [PubMed] [Google Scholar]
- 9.Chou, R., and L. H. Huffman. 2007. Nonpharmacologic therapies for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann. Intern. Med. 147:492-504. [DOI] [PubMed] [Google Scholar]
- 10.Christensen, G. D., W. A. Simpson, J. J. Younger, L. M. Baddour, F. F. Barrett, D. M. Melton, and E. H. Beachey. 1985. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J. Clin. Microbiol. 22:996-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, M7-A7, 6th ed. Clinical and Laboratory Standards Institute, Wayne, PA.
- 12.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 13.Darouiche, R. O. 2004. Treatment of infections associated with surgical implants. N. Engl. J. Med. 350:1422-1429. [DOI] [PubMed] [Google Scholar]
- 14.Darouiche, R. O., M. D. Mansouri, P. V. Gawande, and S. Madhyastha. 2009. Antimicrobial and antibiofilm efficacy of triclosan and dispersin B combination. J. Antimicrob. Chemother. 64:88-93. [DOI] [PubMed] [Google Scholar]
- 15.Del Pozo, J. L., M. S. Rouse, J. N. Mandrekar, M. F. Sampedro, J. M. Steckelberg, and R. Patel. 2009. Effect of electrical current on the activities of antimicrobial agents against Pseudomonas aeruginosa, Staphylococcus aureus, and Staphylococcus epidermidis biofilms. Antimicrob. Agents Chemother. 53:35-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Falagas, M. E., A. M. Kapaskelis, V. D. Kouranos, O. K. Kakisi, Z. Athanassa, and D. E. Karageorgopoulos. 2009. Outcome of antimicrobial therapy in documented biofilm-associated infections: a review of the available clinical evidence. Drugs 69:1351-1361. [DOI] [PubMed] [Google Scholar]
- 17.Fong, I. W., E. D. Ralph, E. R. Engelking, and W. M. Kirby. 1976. Clinical pharmacology of cefamandole as compared with cephalothin. Antimicrob. Agents Chemother. 9:65-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frossard, M., C. Joukhadar, B. M. Erovic, P. Dittrich, P. E. Mrass, M. Van Houte, H. Burgmann, A. Georgopoulos, and M. Muller. 2000. Distribution and antimicrobial activity of fosfomycin in the interstitial fluid of human soft tissues. Antimicrob. Agents Chemother. 44:2728-2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garazzino, S., A. Aprato, L. Baietto, A. D'Avolio, A. Maiello, F. G. De Rosa, D. Aloj, M. Siccardi, A. Biasibetti, A. Masse, and P. G. Di. 2008. Glycopeptide bone penetration in patients with septic pseudoarthrosis of the tibia. Clin. Pharmacokinet. 47:793-805. [DOI] [PubMed] [Google Scholar]
- 20.Goller, C. C., and T. Romeo. 2008. Environmental influences on biofilm development. Curr. Top. Microbiol. Immunol. 322:37-66. [DOI] [PubMed] [Google Scholar]
- 21.Hajdu, S., A. Lassnigg, W. Graninger, A. M. Hirschl, and E. Presterl. 2009. Effects of vancomycin, daptomycin, fosfomycin, tigecycline and ceftriaxone on Staphylococcus epidermidis biofilms. J. Orthop. Res. 27:1361-1365. [DOI] [PubMed] [Google Scholar]
- 22.Hellmark, B., M. Unemo, A. Nilsdotter-Augustinsson, and B. Soderquist. 2009. Antibiotic susceptibility among Staphylococcus epidermidis isolated from prosthetic joint infections with special focus on rifampicin and variability of the rpoB gene. Clin. Microbiol. Infect. 15:238-244. [DOI] [PubMed] [Google Scholar]
- 23.Hsieh, P. H., Y. H. Chang, S. H. Chen, S. W. Ueng, and C. H. Shih. 2006. High concentration and bioactivity of vancomycin and aztreonam eluted from Simplex cement spacers in two-stage revision of infected hip implants: a study of 46 patients at an average follow-up of 107 days. J. Orthop. Res. 24:1615-1621. [DOI] [PubMed] [Google Scholar]
- 24.Huang, Y. T., C. H. Liao, L. J. Teng, and P. R. Hsueh. 2007. Daptomycin susceptibility of unusual gram-positive bacteria: comparison of results obtained by the Etest and the broth microdilution method. Antimicrob. Agents Chemother. 51:1570-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hynynen, K. 2010. MRI-guided focused ultrasound treatments. Ultrasonics 50:221-229. [DOI] [PubMed] [Google Scholar]
- 26.James, J. R., Y. Gao, V. C. Soon, S. M. Topper, A. Babsky, and N. Bansal. 2010. Controlled radio-frequency hyperthermia using an MR scanner and simultaneous monitoring of temperature and therapy response by (1)H, (23)Na and (31)P magnetic resonance spectroscopy in subcutaneously implanted 9L-gliosarcoma. Int. J. Hyperthermia 26:79-90. [DOI] [PubMed] [Google Scholar]
- 27.Joukhadar, C., N. Klein, P. Dittrich, M. Zeitlinger, A. Geppert, K. Skhirtladze, M. Frossard, G. Heinz, and M. Muller. 2003. Target site penetration of fosfomycin in critically ill patients. J. Antimicrob. Chemother. 51:1247-1252. [DOI] [PubMed] [Google Scholar]
- 28.Martin, A., S. Panaiotov, F. Portaels, S. Hoffner, J. C. Palomino, and K. Angeby. 2008. The nitrate reductase assay for the rapid detection of isoniazid and rifampicin resistance in Mycobacterium tuberculosis: a systematic review and meta-analysis. J. Antimicrob. Chemother. 62:56-64. [DOI] [PubMed] [Google Scholar]
- 29.Melling, A. C., B. Ali, E. M. Scott, and D. J. Leaper. 2001. Effects of preoperative warming on the incidence of wound infection after clean surgery: a randomised controlled trial. Lancet 358:876-880. [DOI] [PubMed] [Google Scholar]
- 30.Mermel, L. A., B. M. Farr, R. J. Sherertz, I. I. Raad, N. O'Grady, J. S. Harris, and D. E. Craven. 2001. Guidelines for the management of intravascular catheter-related infections. J. Intraven. Nurs. 24:180-205. [PubMed] [Google Scholar]
- 31.Monsen, T., E. Lovgren, M. Widerstrom, and L. Wallinder. 2009. In vitro effect of ultrasound on bacteria and suggested protocol for sonication and diagnosis of prosthetic infections. J. Clin. Microbiol. 47:2496-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monzon, M., C. Oteiza, J. Leiva, M. Lamata, and B. Amorena. 2002. Biofilm testing of Staphylococcus epidermidis clinical isolates: low performance of vancomycin in relation to other antibiotics. Diagn. Microbiol. Infect. Dis. 44:319-324. [DOI] [PubMed] [Google Scholar]
- 33.Murray, P. R., E. J. Baron, J. H. Jorgensen, M. A. Pfaller, and R. H. Yolken (ed.). 2003. Manual of Clinical Microbiology, 8th ed. ASM Press, Washington, DC.
- 34.Ode, B., S. Haidl, B. Hoffstedt, M. Walder, and J. Ursing. 1988. Fosfomycin versus ampicillin in the treatment of acute pyelonephritis. Chemioterapia 7:96-100. [PubMed] [Google Scholar]
- 35.Olson, M. E., H. Ceri, D. W. Morck, A. G. Buret, and R. R. Read. 2002. Biofilm bacteria: formation and comparative susceptibility to antibiotics. Can. J. Vet. Res. 66:86-92. [PMC free article] [PubMed] [Google Scholar]
- 36.Peters, E. J., L. A. Lavery, D. G. Armstrong, and J. G. Fleischli. 2001. Electric stimulation as an adjunct to heal diabetic foot ulcers: a randomized clinical trial. Arch. Phys. Med. Rehabil. 82:721-725. [DOI] [PubMed] [Google Scholar]
- 37.Presterl, E., S. Hajdu, A. M. Lassnigg, A. M. Hirschl, J. Holinka, and W. Graninger. 2009. Effects of azithromycin in combination with vancomycin, daptomycin, fosfomycin, tigecycline, and ceftriaxone on Staphylococcus epidermidis biofilms. Antimicrob. Agents Chemother. 53:3205-3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Presterl, E., A. Lassnigg, B. Parschalk, F. Yassin, H. Adametz, and W. Graninger. 2005. Clinical behavior of implant infections due to Staphylococcus epidermidis. Int. J. Artif. Organs 28:1110-1118. [DOI] [PubMed] [Google Scholar]
- 39.Raad, I., H. Hanna, Y. Jiang, T. Dvorak, R. Reitzel, G. Chaiban, R. Sherertz, and R. Hachem. 2007. Comparative activities of daptomycin, linezolid, and tigecycline against catheter-related methicillin-resistant Staphylococcus bacteremic isolates embedded in biofilm. Antimicrob. Agents Chemother. 51:1656-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rachid, S., K. Ohlsen, W. Witte, J. Hacker, and W. Ziebuhr. 2000. Effect of subinhibitory antibiotic concentrations on polysaccharide intercellular adhesin expression in biofilm-forming Staphylococcus epidermidis. Antimicrob. Agents Chemother. 44:3357-3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramage, G., M. M. Tunney, S. Patrick, S. P. Gorman, and J. R. Nixon. 2003. Formation of Propionibacterium acnes biofilms on orthopaedic biomaterials and their susceptibility to antimicrobials. Biomaterials 24:3221-3227. [DOI] [PubMed] [Google Scholar]
- 42.Rediske, A. M., B. L. Roeder, M. K. Brown, J. L. Nelson, R. L. Robison, D. O. Draper, G. B. Schaalje, R. A. Robison, and W. G. Pitt. 1999. Ultrasonic enhancement of antibiotic action on Escherichia coli biofilms: an in vivo model. Antimicrob. Agents Chemother. 43:1211-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rodvold, K. A., M. H. Gotfried, M. Cwik, J. M. Korth-Bradley, G. Dukart, and E. J. Ellis-Grosse. 2006. Serum, tissue and body fluid concentrations of tigecycline after a single 100 mg dose. J. Antimicrob. Chemother. 58:1221-1229. [DOI] [PubMed] [Google Scholar]
- 44.Russell, A. D. 2003. Biocide use and antibiotic resistance: the relevance of laboratory findings to clinical and environmental situations. Lancet Infect. Dis. 3:794-803. [DOI] [PubMed] [Google Scholar]
- 45.Rutjes, A. W., E. Nuesch, R. Sterchi, and P. Juni. 2010. Therapeutic ultrasound for osteoarthritis of the knee or hip. Cochrane Database Syst. Rev. CD003132. [DOI] [PubMed]
- 46.Schaad, H. J., M. Bento, D. P. Lew, and P. Vaudaux. 2006. Evaluation of high-dose daptomycin for therapy of experimental Staphylococcus aureus foreign body infection. BMC Infect. Dis. 6:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schintler, M. V., F. Traunmuller, J. Metzler, G. Kreuzwirt, S. Spendel, O. Mauric, M. Popovic, E. Scharnagl, and C. Joukhadar. 2009. High fosfomycin concentrations in bone and peripheral soft tissue in diabetic patients presenting with bacterial foot infection. J. Antimicrob. Chemother. 64:574-578. [DOI] [PubMed] [Google Scholar]
- 48.Srivastava, S., A. Yadav, K. Seem, S. Mishra, V. Chaudhary, and C. S. Nautiyal. 2008. Effect of high temperature on Pseudomonas putida NBRI0987 biofilm formation and expression of stress sigma factor RpoS. Curr. Microbiol. 56:453-457. [DOI] [PubMed] [Google Scholar]
- 49.Trampuz, A., and W. Zimmerli. 2005. New strategies for the treatment of infections associated with prosthetic joints. Curr. Opin. Invest. Drugs 6:185-190. [PubMed] [Google Scholar]
- 50.Uckay, I., D. Pittet, P. Vaudaux, H. Sax, D. Lew, and F. Waldvogel. 2009. Foreign body infections due to Staphylococcus epidermidis. Ann. Med. 41:109-119. [DOI] [PubMed] [Google Scholar]
- 51.Venkatesh, M., L. Rong, I. Raad, and J. Versalovic. 2009. Novel synergistic antibiofilm combinations for salvage of infected catheters. J. Med. Microbiol. 58:936-944. [DOI] [PubMed] [Google Scholar]
- 52.Zichner, L. 1981. Repair of nonunions by electrically pulsed current stimulation. Clin. Orthop. Relat. Res. Nov.-Dec.:115-121. [PubMed] [Google Scholar]
- 53.Zimmerli, W., A. Trampuz, and P. E. Ochsner. 2004. Prosthetic-joint infections. N. Engl. J. Med. 351:1645-1654. [DOI] [PubMed] [Google Scholar]