Abstract
The outcomes for 73 invasive fusariosis patients treated with voriconazole were investigated. Patients with proven (n = 67) or probable (n = 6) infections were identified from the voriconazole clinical database (n = 39) and the French National Reference Center for Mycoses and Antifungals database (n = 34). Investigator-determined success was a complete or partial response. Survival was determined from day 1 of voriconazole therapy to the last day known alive. Patients were 2 to 79 years old (median, 43 years), and 66% were male. Identified Fusarium species (62%) were F. solani, F. moniliforme, F. proliferatum, and F. oxysporum. Underlying conditions analyzed included hematopoietic stem cell transplant (HSCT; 18%), hematologic malignancy (HM; 60%), chronic immunosuppression (CI; 12%), or other condition (OC; 10%). Infection sites were brain (5%), disseminated excluding brain (67%), lungs/sinus (15%), and other (12%). Most patients (64%) were or had recently been neutropenic (<500 cells/mm3). Therapy duration was 1 to 480 days (median, 57 days), with a 47% success rate. Baseline neutropenia impacted success adversely (P ≤ 0.03). Success varied by underlying condition (HSCT, 38%; HM, 45%; CI, 44%; OC, 71%) and infection site (brain, 0%; disseminated, 45%; other, 56%; lung/sinus, 64%) (P > 0.05). Combination therapy (13 patients) was no better than treatment with voriconazole alone. Overall, 59% of the patients died (49% died of fusariosis), and 90-day survival was 42%. Site of infection influenced survival (P = 0.02). Median survival (in days) by species was as follows: F. solani, 213; F. oxysporum, 112; Fusarium spp., 101; F. proliferatum, 84; F. moniliforme, 76. We conclude that voriconazole is a therapeutic option for invasive fusariosis.
Species of the genus Fusarium are major plant pathogens with a global distribution and are responsible for billions of dollars of agricultural losses annually (27). The secondary metabolic products they produce can also cause significant toxicoses in animals and humans, necessitating costly monitoring of plant foodstuffs (10, 19). In addition, Fusarium species may cause allergic reactions in humans, while the major species (notably, F. incarnatum, F. moniliforme, F. oxysporum, and F. solani) are responsible for a range of superficial, subcutaneous, and invasive infections in immunocompetent or immunocompromised individuals (12).
The incidence of Fusarium infections in humans may be stable (11), or possibly rising slightly, particularly in patients with acute myeloid leukemia (15). This has stimulated the production of a number of literature reviews (8, 9, 12, 21, 22, 25). Clearly, Fusarium spp. are problematic human pathogens, with some species showing significant levels of apparent resistance to the common systemic antifungal agents (2, 7, 26). Consequently, in hematology and transplant patients, these organisms can cause invasive infections that are difficult to treat and for which the prognosis is poor (3, 14, 15).
The optimal therapy for invasive fusariosis remains unclear, although a lipid formulation of amphotericin B with or without an azole antifungal is commonly used (3, 5). Voriconazole was approved for the therapy of Fusarium infections in 2002, and although 34 cases reported in the literature were summarized by Stanzani et al. in 2007 (24), published clinical experience remains dispersed and limited. In the review by Stanzani et al. (24), 23 of the voriconazole-treated patients had invasive infections, and a successful response to voriconazole therapy was seen in 69% of these. In four of the patients voriconazole was used in combination with liposomal amphotericin B. In this international analysis we review the outcomes for 73 patients with invasive infection who were treated with voriconazole as initial or salvage therapy; the results for 9 of these patients were in part previously presented (17).
MATERIALS AND METHODS
Data sources.
The Pfizer voriconazole clinical database was queried for invasive Fusarium infections from 1996 until 2002. In addition, invasive Fusarium infections reported to the French National Reference Center for Mycoses and Antifungals (NRCMA), Institute Pasteur, Paris, France, from June 2002 to January 2009 were identified, and data for each patient were collected. Patient data were queried for sociodemographic features, predisposing factors, medical history, and therapy received prior to presentation, as well as the duration of any antifungal therapies and survival.
Clinical review.
All cases were reviewed by three of us (O. Lortholary, G. Obenga, and P. Troke), and only those classified as proven or probable infections were included (4). The presence of neutropenia was assessed at baseline for all patients and at the end of therapy when available. Efficacy of therapy was based on investigator assessment at the end of therapy, and in line with current practice (23), a complete or partial response was classified as a success, while all other responses were classified as failures. Death caused by invasive fungal infection (IFI) was reviewed by our investigators and defined as follows: (i) any death considered by the investigator to be due to IFI; (ii) any death where fusariosis was microbiologically evolutive or where attribution was uncertain or not specified as clearly due to another cause by the investigator (with some supporting information for this conclusion).
Survival was defined throughout the study as the time from the first day of voriconazole therapy until death or the last day the patient was known to be alive.
Statistical analysis.
For statistical analyses, the distributions of categorical variables were compared using the chi-square or Fisher's exact test, as appropriate. All-cause mortality was assessed using the Kaplan-Meier curve. Subjects with missing death times were censored at their last known date of survival. Survival comparisons across strata were assessed using the log-rank test. Cause-specific mortality, due to IFI or due to other causes, was evaluated by using descriptive methods only. Analyses were conducted by using SAS, version 8.2.
RESULTS
Demography, Fusarium spp., and site of infection.
There were 73 patients in total, including 39 from the Pfizer database (11 from phase II/III protocols and 28 from named patient/compassionate studies) and 34 patients from the NRCMA (i.e., from 2002 onwards). Their median age was 43 years (range, 2 to 79 years), with 19 patients <18 years old (Table 1). Some 66% (48/73) of the patients were male, and 75% (53/73) were Caucasian. There were 67 (92%) proven and 6 probable infections.
TABLE 1.
Demographic, clinical, and microbiological data for 73 patients with invasive fusariosis
| Data type and parameter | No. (%) of patients with trait |
|---|---|
| Demographic data | |
| Total patients | 73 (100) |
| Male | 48 (66) |
| Female | 25 (34) |
| Age (yr) | 43 (range, 2-79) |
| Clinical data | |
| Proven infection | 67 (92) |
| Probable infection | 6 |
| Prior therapy | |
| Yes | 57 (78) |
| No | 16 (22) |
| Neutropeniaa | |
| Yes | 47 (64) |
| No or unknown | 7 or 19 (36 [both categories combined]) |
| Fusarium species | |
| All species | 28 (38) |
| F. dimerum | 4 |
| F. incarnatumb | 1 |
| F. oxysporum complex | 7 (10) |
| F. moniliforme complex | 8 (11) |
| F. proliferatum complex | 8 (11) |
| F. solani complex | 16 (22) |
| F. subglutinansb | 2 |
Neutropenia was defined as a neutrophil count of less than 500/mm3 at or immediately before start of voriconazole therapy.
One dual infection with F. incarnatum and F. subglutinans was reported.
The species of Fusarium was identified in the majority of patients (62%), with isolates from the F. solani complex predominating (Table 1). Most patients (73%, 53/73) had disseminated infections (to the brain in 4 and to more than one body site excluding the brain in 49 patients) or lung/sinus infections (15%, 11/73) (Table 2). Blood culture was positive for Fusarium in 26/73 (36%) overall and in 26/53 (49%) patients with disseminated infection.
TABLE 2.
Outcome and survival of fusariosis patients treated with voriconazole, by site of infection
| Site of infection (no. of patients) | Median (range) duration of voriconazole therapy (days) | No. (%) with clinical response | Median (range) survivala (days) | No. that died (no. that died due to IFI) |
|---|---|---|---|---|
| Brain (4) | 37 (1-75) | 0 (0) | 40 (5-75) | 4 (4) |
| Disseminated (49) | 33 (3-480) | 22 (45) | 84 (4-808) | 30 (16) |
| Lung/sinus (11) | 90 (24-379) | 7 (64) | 329 (29-435) | 5 (1) |
| Otherb (9) | 57 (2-259) | 5 (56) | 202 (19-365) | 4 (1) |
| Total (73) | 57 (1-480) | 34 (47) | 120 (4-808) | 43 (22) |
Significant (P = 0.02) for all survival comparisons, based on the log-rank test.
Other infection sites were bone (4 patients), gallbladder (1), and superficial (4).
Prior therapy.
Most patients (57/73, 78%) had failed prior antifungal therapy and were receiving voriconazole as salvage therapy (Table 1). Prior therapies included any amphotericin B formulation (21/57, 37%), caspofungin (8/57, 14%), various azoles (fluconazole, 4; itraconazole, 4; posaconazole, 2; 18% combined), and micafungin (2/57, 3%). The specific therapy was unrecorded for 26 patients. In addition, 12/57 (21%) patients were known to have received more than one prior therapy, sometimes in combination.
Underlying condition.
The majority of patients had hematologic malignancy (44 [60%]) or had undergone hematopoietic cell transplantation (13 [18%]) (Table 3). Those defined as having chronic immunosuppression included cases of lymphoma and aplastic anemia (Table 3). Consistent with these severe underlying conditions, 64% (47/73) of all patients were or had recently been neutropenic (Table 1). In the 19 patients for whom neutropenic status at baseline was unrecorded, 15 (79%) were recent transplants or had a hematologic malignancy.
TABLE 3.
Outcome and survival of fusariosis patients treated with voriconazole, by underlying condition
| Underlying condition (no. of patients) | Median (range) duration of voriconazole therapy (days) | No. (%) with clinical response | Median (range) survival (days) | No. that died (no. that died due to IFI) |
|---|---|---|---|---|
| HSCT (13) | 18 (3-182) | 5 (38) | 27 (6-202) | 9 (4) |
| HMa (44) | 61 (1-480) | 20 (45) | 112 (4-808) | 27 (15) |
| Chronicb (9) | 57 (3-267) | 4 (44) | 200 (6-435) | 5 (3) |
| Otherc (7) | 30 (19-259) | 5 (71) | Not reached (19-365) | 2 (0) |
| Total (73) | 57 (1-480) | 34 (47) | 120 (4-808) | 43 (22) |
HM, hematologic malignancy.
Chronic conditions included lymphoma (3 patients), aplastic anemia (3), tumor lysis syndrome (1), neuroblastoma (1), and kidney transplant (1).
Other conditions were immunocompetence (4 patients), diabetes mellitus (2), paralytic ileus (1), and burns (1).
Data detailing the actual durations of neutropenia were not available. However, of the 47 patients known to be neutropenic at baseline, 19 (40%) were still so at the end of therapy (EOT).
Response to therapy.
A complete or partial response to voriconazole therapy was achieved in 47% (34/73) of patients (Table 2), with a median therapy duration of 57 days (range, 1 to 480 days). When the 66 (90%) patients receiving at least 5 days of voriconazole therapy were assessed separately, their response rate was 34/66 (52%). In 13 (18%) patients, caspofungin (2), liposomal amphotericin B (8), terbinafine (1), posaconazole (4), or white blood cell transfusion (1) was administered simultaneously or immediately after voriconazole treatment (Table 4). However, there was no significant difference in response rates between these patients and those receiving voriconazole alone (Table 4).
TABLE 4.
Comparisons of outcomes for invasive fusariosis cases treated with voriconazole
| Comparison (statistical method)a | No. with clinical response/total no. of patients (%) | P value |
|---|---|---|
| Male vs female (C) | 21/48 (44) vs 13/25 (52) | NSb |
| Proven vs probable infection (C) | 31/67 (46) vs 4/6 (67) | NS |
| Primary vs salvage/unknown therapy (C) | 7/16 (44) vs 27/57 (47) | NS |
| Combination vs voriconazole alone/unknown (C) | 6/13 (46) vs 28/60 (47) | NS |
| Voriconazole database vs NRCMA (C) | 20/39 (51) vs 14/34 (41) | NS |
| Neutropenia (F) | ≤0.03c | |
| Recent | 17/47 (36) | |
| None | 5/7 (71) | |
| Status unknown | 12/19 (63) | |
| Fusarium species (F) | NS | |
| F. solani complex | 9/16 (56) | |
| F. moniliforme complex | 2/8 (25) | |
| F. proliferatum complex | 4/8 (50) | |
| F. oxysporum complex | 6/7 (86) | |
| All other Fusarium spp. | 13/34 (38) |
C, chi-square test; F, Fisher's exact test.
NS, not significant.
P value for outcome in patients with neutropenia versus patients without neutropenia or with neutropenia status unknown.
There was also no significant difference in response rates by gender between patients receiving primary or salvage therapy, between those with proven or probable infections, or between those in the voriconazole database or NRCMA (Table 4). In contrast, patients with current or recent baseline neutropenia exhibited a significantly worse response to voriconazole therapy than nonneutropenic patients (P ≤ 0. 03). However, 17/47 (36%) patients who were neutropenic at baseline responded to therapy (Table 4).
The neutropenia status of patients at EOT, their voriconazole median therapy duration, and clinical response rate were as follows: for neutropenic patients (19/73 [26%]), median duration of therapy was 7 days and response rate was 5%; for nonneutropenic patients (24/73 [33%]), median duration of therapy was 101 days and response rate was 63%; and for patients with unknown neutropenia status (30/73 [41%]), median duration of therapy was 57 days and response rate was 60%.
Although response rates by Fusarium species varied from 38% to 86%, these differences were not statistically significantly different (Table 4). Clinical response by site of infection (Table 2) varied from 0% (brain) to 64% (lung/sinus) and by underlying condition (Table 3) from 38% (HSCT) to 71% (other), but none of the differences in responses between these groups was statistically significant.
Survival.
A total of 43 (59%) patients died, and a further 30 (41%) had their survival censored on the last day they were known to be alive (range, >18 days to >808 days). When the 66 patients receiving at least 5 days of voriconazole therapy were assessed separately, 36/66 (55%) had died. Only 12% of patients were known to have survived for >365 days. Of those who died, 51% (22/43) had progressive invasive fungal infection and 13/22 (59%) were still neutropenic (Table 2). For patients who were neutropenic at baseline, all-cause mortality was 68% (32/47), compared with 42% (11/26) for all other patients. However, 19/43 (44%) patients who were neutropenic at baseline were still neutropenic at EOT and had an all-cause mortality of 100%.
More patients with hematologic malignancy and hematopoietic cell transplant died (36/57 [63%], with 53% experiencing progressive fungal infection), compared with all other patients (7/16 [44%], with 43% experiencing progressive fungal infection). More patients with disseminated infection and central nervous system (CNS) disease died (34/53, 64%) than all others (9/20, 45%).
Overall median survival was 120 days (range, 4 to 808 days). Median failure time for death due to invasive fungal infection was not reached (range, 4 to 135 days), while the median failure time for death due to other causes was 431 days (range, 11 to 461 days).
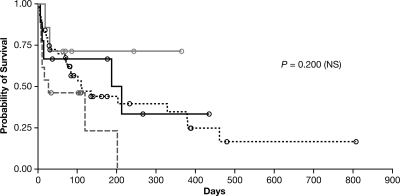
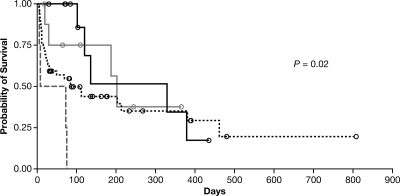
Hematopoietic cell transplant patients had the shortest median survival time (27 days; range, 6 to 202 days), and patients without notable immune suppression (“other”) survived the longest (range, 19 to 365 days), although a median survival time was not reached for this group (Fig. 1). However, none of these survival differences was statistically significant. There was also no significant difference in survival times by Fusarium spp. However, differences in survival times by site of infection were significant (P = 0.02), with those patients with CNS or disseminated infection exhibiting the worst survival (Table 2 and Fig. 2).
FIG. 1.
Kaplan-Meier curves of patient survival, by underlying condition. Differences between groups were not significant (P = 0.2). Hematopoietic stem cell transplant, gray dashed line; hematologic condition, black dotted line; chronic immune suppression, solid black line; other underlying condition, solid gray line; censored patients, black or gray circles.
FIG. 2.
Kaplan-Meier curves of patient survival, by site of infection. Differences between groups were significant (P = 0.02). Brain, gray dashed line; disseminated (excluding brain), black dotted line; lung/sinus, solid black line; other, solid gray line; censored patients, black or gray open circles.
DISCUSSION
We have not presented the susceptibility data for the Fusarium isolates in this analysis, although voriconazole MICs for isolates from 15 patients in the Pfizer database have been published and range from 1.0 μg/ml to 16.0 μg/ml (6). However, the results of antifungal susceptibility testing of Fusarium spp. reveal a wide susceptibility range, with F. solani apparently resistant to most antifungals, thus making such testing of low clinical value for any therapeutic decision (1, 2, 18). In addition, it should be noted that no in vitro/in vivo correlation has yet been demonstrated for the antifungal management of fusariosis.
Consistent with observations in the literature (3, 12, 15), most patients in this analysis had hematologic malignancy or a hematopoietic cell transplant as their underlying condition. Consequently, the majority had disseminated fusariosis and presented with recent or current neutropenia (3, 12, 15). Fusarium infections in such patients result in a poor prognosis, with death rates of up to 75%, as opposed to 36% in patients where infection is not disseminated (3, 12). In the current study, despite 73% of patients having disseminated/CNS disease and 78% experiencing hematologic malignancy or a recent hematopoietic cell transplant, the overall response rate was 47% and the 3-month survival was 42%. Survival rates at 3 months, specifically in hematologic malignancy or hematopoietic cell transplant patients, were 38% and 39%, respectively, compared to 21% and 13%, respectively, in recent studies of patients not treated with voriconazole (13, 14). However, it is clear that a subset of patients did not recover from their neutropenia, rapidly failed therapy, and died. Others have also shown that neutropenia status impacts survival significantly (3).
There have been no formal clinical trials for fusariosis. Consequently, estimates of efficacy for any antifungal agent depend on case studies or small retrospective analyses. A combination of a lipid amphotericin derivative with voriconazole or another azole is currently considered the best therapy (3, 5). In our study, the outcome of the 13 patients receiving combination therapy was no better than with voriconazole alone, despite 8/13 patients having received liposomal amphotericin B with their voriconazole. Clearly, the value of combination versus monotherapy for fusariosis requires further exploration with a much larger sample of patients (3).
For amphotericin derivatives in general, published efficacy rates range from 32% for amphotericin B to 46% for amphotericin B lipid complex (ABLC) (total cumulative dose, 5 g) and other lipid formulations (13, 14, 16). Preliminary data obtained with voriconazole in nine patients with intolerance to therapy or invasive infections refractory to primary therapy showed a 44% response rate with a 3-month survival of 71% (17). Posaconazole has also been studied as salvage therapy in 21 patients, including almost one-third who were neutropenic, with an overall success rate of 48% (20). However, in all these publications, the response rates were heavily dependent on persistence of underlying immunosuppression and dissemination of infection. The presence of neutropenia has a critical role in the outcome (3, 12). Consequently, the results obtained here with voriconazole in a high-risk population (64% were confirmed to have recent or current neutropenia) are encouraging. However, 40% of the patients who were neutropenic at baseline still succumbed rapidly and died.
Finally, voriconazole given as primary therapy (in 22% of patients, with 75% of these having a hematologic malignancy or a hematopoietic cell transplant) or in patients failing initial therapy was associated with a similar outcome. In conclusion, the results of this large, retrospective international study show the potential efficacy of voriconazole in the management of disseminated fusariosis in heavily immunocompromised hosts.
Acknowledgments
The French Mycoses Study Group included the following investigators: Pierre Berger, Institut Paoli Calmettes, Marseille; Alain Bonnin, Hôpital du Bocage de Dijon; Marie-Elisabeth Bougnoux, Hôpital Necker-Enfants Malades, Paris; Benoit Brethon, Hôpital Saint Louis, Paris; Anne Breton, Hôpital des Enfants, Purpan Toulouse; Giovanna Cannas, Hôpital Edouard Herriot, Lyon; Aurelien Dinh, CHU Raymond-Poincaré, Garches; Catherine Kauffmann-Lacroix, Hôpital de la Miletrie, Poitiers; Faezeh Legrand, Hôpital Jean Minjoz, Besançon; Arnaud Petit, Hôpital Armand-Trousseau, Paris; Jean-Louis Poirot, Hôpital Saint-Antoine, Paris; Denis Pons, CHU Clermont-Ferrand; Emmanuel Raffoux, Hôpital Saint Louis, Paris; Stéphane Ranque, CHU LaTimone, Marseille; Patricia Ribaux, Hôpital Saint Louis, Paris; Anne Vekhoff, Hôtel-Dieu, Paris; and Benjamin Wyplosz, Hôpital Paul Brousse, Villejuif.
O.L. and G.O. received a grant from Pfizer to aid collection of the French Mycoses Group data for the manuscript. P.T. received an honorarium from Pfizer in connection with the data finalization, analysis, and writing of the manuscript.
We also thank Koldo Aguirrebengoa (Spain), Barbara Johnson (Australia), and Michael Whitby (Australia) for providing additional antifungal therapy and survival data on three patients included in the analysis.
Regarding potential conflicts of interest, O.L. is a member of the speaker bureaus of Pfizer, MSD, Astellas, and Gilead Sciences; P.B. is a statistician employed by Pfizer; R.H. received a research grant from Pfizer for another study, has been a consultant for Astellas, Basilea, Gilead, MSD, Pfizer, and Schering-Plough, and is a member of speaker bureaus of Gilead, MSD, Pfizer, and Schering-Plough; I.R. has previously received grants from Pfizer, and he is also a member of the speaker bureaus for Pfizer and Schering-Plough; and P.T. was previously an employee of and is currently a consultant to Pfizer. G.O., D.C., E.C., A.-L.B., J.G., C.L., and F.G. have no conflicts of interest.
Footnotes
Published ahead of print on 12 July 2010.
REFERENCES
- 1.Azor, M., J. Cano, J. Gene, and J. Guarro. 2009. High genetic diversity and poor in vitro response to antifungals of clinical strains of Fusarium oxysporum. J. Antimicrob. Chemother. 63:1152-1155. [DOI] [PubMed] [Google Scholar]
- 2.Azor, M., J. Gene, J. Cano, and J. Guarro. 2007. Universal in vitro antifungal resistance of genetic clades of the Fusarium solani species complex. Antimicrob. Agents Chemother. 51:1500-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campo, M., R. E. Lewis, and D. P. Kontoyiannis. 2010. Invasive fusariosis in patients with hematologic malignancies at a cancer center. J. Infect. 60:331-337. [DOI] [PubMed] [Google Scholar]
- 4.De Pauw, B. E., T. J. Walsh, J. P. Donnelly, D. A. Stevens, J. E. Edwards, T. Calandra, P. G. Pappas, J. Maertens, O. Lortholary, C. A. Kauffman, D. W. Denning, T. F. Patterson, G. Maschmeyer, J. Bille, W. E. Dismukes, R. Herbrecht, W. W. Hope, C. C. Kibbler, B. J. Kullberg, K. A. Marr, P. Muñoz, F. C. Odds, J. R. Perfect, A. Restrepo, M. Ruhnke, B. H. Segal, J. D. Sobel, T. C. Sorrell, C. Viscoli, J. R. Wingard, T. Zaoutis, and J. E. Bennett. 2008. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 46:1813-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dignani, M. C., and E. Anaissie. 2004. Human fusariosis. Clin. Microbiol. Infect. 10(Suppl. 1):67-75. [DOI] [PubMed] [Google Scholar]
- 6.Espinel-Ingroff, A., E. Johnson, H. Hockey, and P. Troke. 2008. Activities of voriconazole, itraconazole and amphotericin B in vitro against 590 moulds from 323 patients in the voriconazole phase III clinical studies. J. Antimicrob. Chemother. 61:616-620. [DOI] [PubMed] [Google Scholar]
- 7.Iqbal, N. J., A. Boey, B. J. Park, and M. E. Brandt. 2008. Determination of in vitro susceptibility of ocular Fusarium spp. isolates from keratitis cases and comparison of Clinical and Laboratory Standards Institute M38-A2 and E test methods. Diagn. Microbiol. Infect. Dis. 62:348-350. [DOI] [PubMed] [Google Scholar]
- 8.Iyer, S. A., S. S. Tuli, and R. C. Wagoner. 2006. Fungal keratitis: emerging trends and treatment outcomes. Eye Contact Lens 32:267-271. [DOI] [PubMed] [Google Scholar]
- 9.Latenser, B. A. 2003. Fusarium infections in burn patients: a case report and review of the literature. J. Burn Care Rehab. 24:285-288. [DOI] [PubMed] [Google Scholar]
- 10.Morgavi, D., and R. T. Riley. 2007. Fusarium and their toxins: mycology, occurrence, toxicity, control and economic impact. Anim. Feed Sci. Technol. 137:199-200. [Google Scholar]
- 11.Neofytos, D., D. Horn, E. Anaissie, W. Steinbach, A. Olyaei, J. Fishman, M. Pfaller, C. Chang, K. Webster, and K. Marr. 2009. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: analysis of Multicenter Prospective Antifungal Therapy (PATH) Alliance registry. Clin. Infect. Dis. 48:265-273. [DOI] [PubMed] [Google Scholar]
- 12.Nucci, M., and E. Anaissie. 2007. Fusarium infections in immunocompromised patients. Clin. Microbiol. Rev. 20:695-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nucci, M., E. J. Anaissie, F. Queiroz-Telles, C. A. Martins, P. Trabasso, C. Solza, C. Mangini, B. P. Simoes, A. L. Colombo, J. Vaz, C. E. Levy, S. Costa, V. A. Moreira, J. S. Oliveira, N. Paraguay, G. Duboc, J. C. Voltarelli, A. Maiolino, R. Pasquini, and C. A. Souza. 2003. Outcome predictors of 84 patients with hematologic malignancies and Fusarium infection. Cancer 98:315-319. [DOI] [PubMed] [Google Scholar]
- 14.Nucci, M., K. A. Marr, F. Queiroz-Telles, C. A. Martins, P. Trabasso, S. Costa, J. C. Voltarelli, A. L. Colombo, A. Imhof, R. Pasquini, A. Maiolino, C. A. Souza, and E. Anaissie. 2004. Fusarium infection in hematopoietic stem cell transplant recipients. Clin. Infect. Dis. 38:1237-1242. [DOI] [PubMed] [Google Scholar]
- 15.Pagano, L., M. Caira, A. Candoni, M. Offidani, L. Fianchi, B. Martino, D. Pastore, M. Picardi, A. Bonini, A. Chierichini, R. Fanci, C. Caramatti, R. Invernizzi, D. Mattei, M. E. Mitra, L. Melillo, F. Aversa, M. T. Van Lint, P. Falcucci, C. G. Valentini, C. Girmenia, and A. Nosari. 2006. The epidemiology of fungal infections in patients with hematologic malignancies: the SEIFEM-2004 study. Haematologica 91:1068-1075. [PubMed] [Google Scholar]
- 16.Perfect, J. R. 2005. Treatment of non-Aspergillus moulds in immunocompromised patients, with amphotericin B lipid complex. Clin. Infect. Dis. 40(Suppl. 6):S401-S408. [DOI] [PubMed] [Google Scholar]
- 17.Perfect, J. R., K. A. Marr, T. J. Walsh, R. N. Greenberg, B. DuPont, J. de la Torre-Cisneros, G. Just-Nubling, H. T. Schlamm, I. Lutsar, A. Espinel-Ingroff, and E. Johnson. 2003. Voriconazole treatment for less-common, emerging, or refractory fungal infections. Clin. Infect. Dis. 36:1122-1131. [DOI] [PubMed] [Google Scholar]
- 18.Pfaller, M. A., S. A. Messer, R. J. Hollis, and R. N. Jones. 2002. Antifungal activities of posaconazole, ravuconazole, and voriconazole compared to those of itraconazole and amphotericin B against 239 clinical isolates of Aspergillus spp. and other filamentous fungi: report from SENTRY Antimicrobial Surveillance Program, 2000. Antimicrob. Agents Chemother. 46:1032-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pitt, J. 2000. Toxigenic fungi: which are important? Med. Mycol. 38:17-22. [PubMed] [Google Scholar]
- 20.Raad, I. I., R. Y. Hachem, R. Herbrecht, J. R. Graybill, R. Hare, G. Corcoran, and D. P. Kontoyiannis. 2006. Posaconazole as salvage treatment for invasive fusariosis in patients with underlying hematologic malignancy and other conditions. Clin. Infect. Dis. 42:1398-1403. [DOI] [PubMed] [Google Scholar]
- 21.Raad, I., J. Tarrand, H. Hanna, M. Albitar, E. Janssen, M. Boktour, G. Bodey, M. Mardani, R. Hachem, D. Kontoyiannis, E. Whimbey, and K. Rolston. 2002. Epidemiology, molecular mycology, and environmental sources of Fusarium infection in patients with cancer. Infect. Control Hosp. Epidemiol. 23:532-537. [DOI] [PubMed] [Google Scholar]
- 22.Sampathkumar, P., and C. V. Paya. 2001. Fusarium infection after solid-organ transplantation. Clin. Infect. Dis. 32:1237-1240. [DOI] [PubMed] [Google Scholar]
- 23.Segal, B. H., R. Herbrecht, D. A. Stevens, L. Ostrosky-Zeichner, J. Sobel, C. Viscoli, T. J. Walsh, J. Maertens, T. F. Patterson, J. R. Perfect, B. Dupont, J. R. Wingard, T. Calandra, C. A. Kauffman, J. R. Graybill, L. R. Baden, P. G. Pappas, J. E. Bennett, D. P. Kontoyiannis, C. Cordonnier, M. A. Viviani, J. Bille, N. G. Almyroudis, L. J. Wheat, W. Graninger, E. J. Bow, S. M. Holland, B. J. Kullberg, W. E. Dismukes, and B. E. De Pauw. 2008. Defining responses to therapy and study outcomes in clinical trials of invasive fungal diseases. Mycoses Study Group and European Organization for Research and Treatment of Cancer Consensus Criteria. Clin. Infect. Dis. 47:674-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stanzani, M., F. Tumietto, N. Vianelli, and M. Baccarani. 2007. Update on the treatment of disseminated fusariosis: focus on voriconazole. Ther. Clin. Risk Manag. 3:1165-1173. [PMC free article] [PubMed] [Google Scholar]
- 25.Torres, H. A., I. I. Raad, and D. P. Kontoyiannis. 2003. Infections caused by Fusarium species. J. Chemother. 15:28-35. [DOI] [PubMed] [Google Scholar]
- 26.Tortorano, A. M., A. Prigitano, G. Dho, E. Biraghi, D. A. Stevens, M. Ghannoum, N. Nolard, and M. A. Viviani. 2008. In vitro activity of amphotericin B against Aspergillus terreus isolates from different countries and regions. J. Chemother. 20:756-757. [DOI] [PubMed] [Google Scholar]
- 27.Windels, C. 2000. Economic and social impacts of Fusarium head blight: changing farms and rural communities in the Northern Great Plains. Phytopathology 90:17-21. [DOI] [PubMed] [Google Scholar]