Abstract
Nemonoxacin, a novel nonfluorinated quinolone, exhibits potent in vitro and in vivo activities against community-acquired pneumonia (CAP) pathogens, including multidrug-resistant Streptococcus pneumoniae. Patients with mild to moderate CAP (n = 265) were randomized to receive oral nemonoxacin (750 mg or 500 mg) or levofloxacin (500 mg) once daily for 7 days. Clinical responses were determined at the test-of-cure visit in intent-to-treat (ITT), clinical per protocol (PPc), evaluable-ITT, and evaluable-PPc populations. The clinical cure rates for 750 mg nemonoxacin, 500 mg nemonoxacin, and levofloxacin were 89.9%, 87.0%, and 91.1%, respectively, in the evaluable-ITT population; 91.7%, 87.7%, and 90.3%, respectively, in the evaluable-PPc population; 82.6%, 75.3%, and 80.0%, respectively, in the ITT population; and 83.5%, 78.0%, and 82.3%, respectively, in the PPc population. Noninferiority to levofloxacin was demonstrated in both the 750-mg and 500-mg nemonoxacin groups for the evaluable-ITT and evaluable-PPc populations, and also in the 750 mg nemonoxacin group for the ITT and PPc populations. Overall bacteriological success rates were high for all treatment groups in the evaluable-bacteriological ITT population (90.2% in the 750 mg nemonoxacin group, 84.8% in the 500 mg nemonoxacin group, and 92.0% in the levofloxacin group). All three treatments were well tolerated, and no drug-related serious adverse events were observed. Overall, oral nemonoxacin (both 750 mg and 500 mg) administered for 7 days resulted in high clinical and bacteriological success rates in CAP patients. Further, good tolerability and excellent activity against common causative pathogens were demonstrated. Nemonoxacin (750 mg and 500 mg) once daily is as effective and safe as levofloxacin (500 mg) once daily for the treatment of CAP.
Community-acquired pneumonia (CAP) is a common infectious disease of the lower respiratory tract. It is the leading cause of death from an infectious disease worldwide (3, 24, 34). Approximately 1 million episodes of CAP have been estimated to occur annually in adults ≧65 years of age in the United States (24). The majority of CAP patients are treated in the outpatient setting (23, 26). However, in patients who were admitted to a hospital, mortality rates were found to be as high as 8% for those admitted to medical wards and 28% for those admitted to intensive-care units (29).
Streptococcus pneumoniae is the organism most frequently isolated from CAP patients. Other common pathogens include Haemophilus influenzae, Moraxella catarrhalis, and Klebsiella pneumoniae (2, 8). Atypical pathogens, such as Mycoplasma pneumoniae, Chlamydia pneumoniae, and Legionella pneumophila, have also been isolated with marked frequencies (4). New emerging pathogens, such as community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA), drug-resistant S. pneumoniae (DRSP), and Acinetobacter baumannii, are increasingly being recognized (9, 12, 35). Factors such as the severity of the disease, presence of comorbidities, and risk of selection for antibiotic resistance should be considered very carefully before selecting an antimicrobial therapy. Current Infectious Diseases Society of America/American Thoracic Society consensus guidelines strongly recommend monotherapy with a respiratory fluoroquinolone as an appropriate empirical treatment for adult CAP inpatients and complicated-CAP outpatients with cardiopulmonary disease or comorbidities (24).
Nemonoxacin, a novel nonfluorinated quinolone, has demonstrated broad-spectrum activities against clinical isolates both in vitro and in vivo (1, 13, 14, 18, 19, 28). It exhibits potent antibacterial activities against Gram-positive, Gram-negative, and atypical pathogens, including antibiotic-resistant pathogens, such as penicillin- and quinolone-resistant S. pneumoniae and methicillin- and vancomycin-resistant S. aureus (1, 6, 28). Nemonoxacin has also demonstrated a reduced propensity for resistance development in vitro. Mutations in two quinolone resistance-determining regions (QRDR) of genes encoding DNA gyrase (gyrA and gyrB) and topoisomerase IV (parC and parE) cause resistance to fluoroquinolones (27). However, bacteria become resistant to nemonoxacin only when three different mutations occur in their QRDR genes (17). Thus, nemonoxacin has a low propensity for selecting resistant pathogens compared to other fluoroquinolones.
In previous phase I studies, nemonoxacin was found to be safe and well tolerated at doses of 75 to 1,000 mg daily (7, 21) for 10 consecutive days. The pharmacokinetic parameters of single and repeated doses are generally dose proportional (7). Nemonoxacin has a good safety profile and excellent antibacterial activity against common pathogens causing CAP (5). Therefore, nemonoxacin is suggested to be effective in treating patients with CAP. The convenient once-daily dosing regimen of nemonoxacin can offer an advantage in terms of improved patient compliance and thereby a reduction in the likelihood of resistance selection. The present study investigated the safety and efficacy of nemonoxacin compared with that of levofloxacin in outpatients with CAP.
(Preliminary data were presented at the 48th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy [ICAAC]-Infectious Diseases Society of America [IDSA] 46th Annual Meeting, abstr. L-678, 2008.)
MATERIALS AND METHODS
Study design.
This randomized, double-blind, multicenter study compared the safety and efficacy of nemonoxacin with those of levofloxacin in adult patients with CAP. Eligible subjects were randomly assigned to one of three treatment groups in a 1:1:1 ratio to receive once-daily oral nemonoxacin at 750 mg, nemonoxacin at 500 mg, or levofloxacin at 500 mg for 7 days. The first dose of the study drug was taken under supervision in the clinic. For all subsequent doses, patients took their study drug with water in the morning after an overnight fast of at least 10 h. Subjects were not allowed to eat for 2 h after study drug administration, but water intake (no more than 8 oz, or 240 ml) was permitted. The primary objective was to demonstrate the noninferiority of nemonoxacin versus levofloxacin with regard to safety and clinical efficacy. The study was conducted in compliance with good clinical practice and the Declaration of Helsinki. The study protocol was approved by the institutional review boards of all participating institutions, and all subjects provided written informed consent before enrollment in the study.
Eligibility criteria.
Subjects ≧18 years of age were eligible for enrollment if they had a clinical diagnosis of mild to moderate CAP characterized by fever (oral temperature, ≧38°C, or equivalent tympanic or rectal temperature) within the previous 24 h and at least one of the following signs and symptoms: chills, shortness of breath, tachypnea (>20 breaths/min), cough, pleuritic chest pain, purulent sputum, crackles on auscultation, rales, rhonchi, egophony, pulmonary consolidation, or dullness to percussion. If fever was absent, the subject needed to have presented with two or more of the above-mentioned clinical signs and symptoms to be eligible. Subjects were also required to have a chest radiograph demonstrating new or persistent/progressive infiltrates; the radiographs were confirmed by a radiologist.
Pregnant or lactating subjects were excluded from the study. Other exclusion criteria were as follows: history of hypersensitivity or allergic reactions to any quinolone; fluoroquinolone tendinopathy; history of chronic renal failure or a calculated creatinine clearance of less than 50 ml/min; clinically significant hepatic disease, hematological malignancy, or immunodeficiency, such as neutropenia; history of prolonged electrocardiogram QT corrected (QTc) interval or requirement for concomitant medication associated with increased QTc interval; clinically significant conduction or other abnormality on a 12-lead electrocardiogram (ECG) at screening or a QTc interval greater than 430 ms in males and greater than 450 ms in females at screening; known or suspected severe bronchiectasis, cystic fibrosis, active tuberculosis, bronchial obstruction, postobstructive pneumonia, pulmonary malignancies, lung abscess, or empyema; alcohol or drug abuse; treatment with chemotherapeutic agents or oncolytics during 6 months before randomization or an anticipated requirement for such agents during the course of the study; inability to take the drug orally; treatment with any antibiotic within the 7 days before enrollment in the study; and administration of any other investigational drug within 1 month before randomization.
Subjects could be withdrawn from the study at any time or if their symptoms worsened or failed to improve after at least 3 days of treatment. The investigator could then switch the subject to a nonquinolone antibacterial agent if continued CAP therapy was required.
Clinical assessment.
Clinical response at the test of cure (TOC) or early termination (ET) visit was the primary efficacy endpoint of the study. Subjects were assessed at the following visits: pretreatment (day −1), on treatment (days 5 ± 1), end of treatment (days 7 ± 2), and test of cure (days 14 to 21).
Evaluation of clinical response was based on signs and symptoms of pneumonia. Subjects were categorized as follows: cured (complete resolution of all signs and symptoms of pneumonia with improvements in the chest radiograph), failure (persistence or worsening of signs and symptoms after 3 to 5 days of therapy or failure to show improvement in at least three clinical findings after 3 days of therapy), and unevaluable (missing posttreatment information or early discontinuation of treatment). In addition, the subjects had to be compliant with the protocol procedures to be assessed for clinical response.
Bacteriological assessment.
Bacteriological response was the secondary efficacy variable of this study. Sputum samples were collected by expectoration after deep coughing at the pretreatment visit (day −1). If a productive cough persisted after the first visit, the sputum samples were collected during the subsequent visits. Fresh specimens were first tested using Gram stain and cultured in a local laboratory certified to perform testing on human specimens. Cultures were performed only if the Gram stain revealed ≦10 squamous epithelial cells and ≧25 leukocytes per low-power field. Isolates from such specimens were sent to a central laboratory for reidentification, and antimicrobial susceptibility testing was performed according to the procedures of the Clinical and Laboratory Standards Institute (formerly National Committee for Clinical Laboratory Standards). MICs of nemonoxacin and levofloxacin were determined for all isolated pathogens. In addition, M. catarrhalis and Haemophilus sp. isolates were tested for production of β-lactamase, and S. pneumoniae isolates were tested for susceptibility to penicillin. Serology tests for C. pneumoniae, M. pneumoniae, and L. pneumophila were performed at the pretreatment and TOC visits. Urine samples were also collected for identifying L. pneumophila by antigen testing.
Major bacteriological responses were categorized as follows: eradicated (original pathogen[s] was absent at the TOC visit), presumed eradicated (subject was considered clinically cured and positive for atypical pathogen infection, or a repeat sputum/blood culture was absent), and persisted (original pathogen[s] persisted at the TOC visit).
Safety assessment.
The safety population included all subjects who received at least one whole dose of the study drug. Both clinical and laboratory adverse events (AEs) were monitored for all subjects in the study.
Statistical analysis.
Four subject populations were planned for the analysis of clinical and bacteriological efficacy in this study. The intent-to-treat (ITT) population consisted of all subjects who took at least one whole dose of the study drug. The bacteriological ITT (bITT) population comprised all ITT subjects who had at least one pathogen identified at any visit or a diagnosis with positive atypical-pathogen infection. The clinical per-protocol (PPc) population and the bacteriological per-protocol (PPb) population included subjects from a subset of the ITT and bITT populations, respectively. The subjects in the PPc and PPb populations were those who adhered to the protocol without any major protocol violations. To achieve a power of 90% and assuming a clinical response of 89% in one of the nemonoxacin treatment arms, 90% in the other nemonoxacin treatment arm, and 90% in the levofloxacin treatment arm at the TOC visit with a noninferiority margin of 15%, and assuming that 20% of the subjects would not be evaluable, the sample size was determined to be 264 subjects.
The primary efficacy variable was the clinical cure rate at the TOC visit in the ITT population. Secondary efficacy variables were the clinical cure rate in the PPc population and bacteriological success rates in the bITT and PPb populations. The Cochran-Mantel-Haenszel point estimate and two-sided 97.5% confidence interval (CI) were used to compare the differences in clinical and bacteriological success rates of the treatment populations. Noninferiority was defined as the lower limit of the 97.5% CI for the difference between groups being greater than −15%.
Four other subject populations were defined in a posthoc analysis. The evaluable ITT (Eval-ITT) and evaluable PPc (Eval-PPc) populations excluded all subjects who had an unevaluable or missing clinical outcome. The evaluable bITT (Eval-bITT) and evaluable PPb (Eval-PPb) populations excluded all subjects who had an unevaluable bacteriological outcome. Noninferiority was determined by a step-down procedure. Comparison with levofloxacin at 500 mg was started with the high-dose group (nemonoxacin at 750 mg). If the definition of noninferiority (the lower limit of the 95% CI for the difference between groups was greater than −15%) was met, the comparison proceeded to the low-dose group (nemonoxacin at 500 mg). If the definition of noninferiority was not met for the high-dose group compared with levofloxacin at 500 mg, the statistical analysis was stopped and the low-dose group was not compared with levofloxacin at 500 mg.
RESULTS
Subject demographics and baseline characteristics.
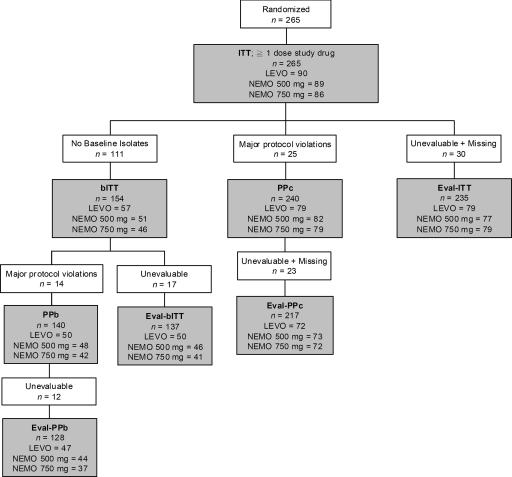
A total of 265 subjects were randomized and received at least one dose of the study drug (ITT population): 86 in the nemonoxacin at 750 mg group, 89 in the nemonoxacin at 500 mg group, and 90 in the levofloxacin at 500 mg group (Fig. 1). Of these subjects, 9.3% (8 of 86) in the nemonoxacin at 750 mg group, 14.6% (13 of 89) in the nemonoxacin at 500 mg group, and 7.8% (7 of 90) in the levofloxacin at 500 mg group prematurely discontinued the study. The most common reasons for discontinuation included loss to follow-up (2.6%), occurrence of AEs (1.9%), and noncompliance (1.5%). The individual reasons for discontinuation were not statistically significant between the three treatment groups.
FIG. 1.
Patient population flow chart. LEVO, levofloxacin; NEMO, nemonoxacin.
Demographic parameters of gender, age, body mass index (BMI), race, medical history (including hypertension, chronic airway disease, and diabetes mellitus), history of cigarette smoking, and pneumonia severity index (PSI) class were comparable across the treatment groups (Table 1). As defined by the PSI class, the majority of subjects had mild to moderate severity of pneumonia, and the distribution of PSI classes was similar between treatment groups. Patients categorized as PSI classes I to III comprised 95.3% (nemonoxacin at 750 mg), 95.5% (nemonoxacin at 500 mg), and 92.2% (levofloxacin at 500 mg). No significant differences were observed between the treatment groups with regard to baseline clinical signs and symptoms. The most common clinical signs and symptoms were fever (83%), crackles on auscultation (82%), pleuritic chest pain (77%), mucopurulent sputum production (76%), chills (75%), tachypnea (72%), and moderate cough (63%).
TABLE 1.
Demographic and baseline characteristics of the ITT population
| Characteristic | Value |
||
|---|---|---|---|
| Nemonoxacin |
Levofloxacin 500 mg (n = 90) | ||
| 750 mg (n = 86) | 500 mg (n = 89) | ||
| Gender; no. (%) | |||
| Male | 41 (47.7) | 47 (52.8) | 55 (61.1) |
| Female | 45 (52.3) | 42 (47.2) | 35 (38.9) |
| Age (yr); mean (SD) | 45.0 (16.0) | 41.1 (13.5) | 44.5 (16.4) |
| BMI (kg/m2); mean (SD) | 24.6 (6.7) | 23.3 (5.8) | 23.4 (4.9) |
| Race; no. (%) | |||
| Asian | 12 (14.0) | 14 (15.7) | 13 (14.4) |
| African | 53 (61.6) | 55 (61.8) | 56 (62.2) |
| White | 20 (23.3) | 18 (20.2) | 17 (18.9) |
| Other | 1 (1.2) | 2 (2.2) | 4 (4.4) |
| Medical history; no. (%) | |||
| Hypertension | 19 (22.1) | 9 (10.1) | 13 (14.4) |
| Chronic airway diseasea | 8 (9.3) | 6 (6.7) | 11 (12.2) |
| Diabetes mellitus | 2 (2.3) | 4 (4.5) | 3 (3.3) |
| History of cigarette smoking; no. (%) | |||
| Never smoked | 53 (61.6) | 63 (70.8) | 46 (51.1) |
| Current smoker | 20 (23.3) | 20 (22.5) | 30 (33.3) |
| Ex-smoker | 13 (15.1) | 6 (6.7) | 14 (15.6) |
| PSI class; no. (%) | |||
| I | 41 (47.7) | 50 (56.2) | 38 (42.2) |
| II | 30 (34.9) | 29 (32.6) | 35 (38.9) |
| III | 11 (12.8) | 6 (6.7) | 10 (11.1) |
| IV | 4 (4.7) | 4 (4.5) | 7 (7.8) |
Includes chronic obstructive pulmonary disease, chronic bronchitis, and tuberculosis.
Clinical response.
The clinical responses at the TOC or ET visit for the three treatment groups are outlined in Table 2. The clinical-cure rates for the clinically evaluable populations were 89.9% (nemonoxacin at 750 mg), 87.0% (nemonoxacin at 500 mg), and 91.1% (levofloxacin at 500 mg) in the Eval-ITT population and 91.7% (nemonoxacin at 750 mg), 87.7% (nemonoxacin at 500 mg), and 90.3% (levofloxacin at 500 mg) in the Eval-PPc population. The 95% CI for the treatment differences between nemonoxacin at 750 mg and levofloxacin at 500 mg were −10.4% to 7.9% in the Eval-ITT population and −8.0% to 10.8% in the Eval-PPc population. Thus, in both the Eval-ITT and Eval-PPc populations, nemonoxacin at 750 mg was found to be noninferior to levofloxacin at 500 mg because the lower limit of the 95% CI of the treatment difference was greater than −15%. Noninferiority of nemonoxacin at 750 mg to levofloxacin at 500 mg was demonstrated; therefore, the comparison of nemonoxacin at 500 mg to levofloxacin at 500 mg was subsequently evaluated. The 95% CI of the difference in the clinical cure rates between nemonoxacin at 500 mg and levofloxacin at 500 mg was −13.9% to 5.7% in the Eval-ITT population and −12.8% to 7.6% in the Eval-PPc population. Noninferiority of nemonoxacin at 500 mg to levofloxacin at 500 mg was also demonstrated in both clinically evaluable populations.
TABLE 2.
Clinical response at TOC or ET visit
| Population | Clinical response | No. of patients (%) |
Difference in clinical-cure rates between treatment groupsb,c |
|||
|---|---|---|---|---|---|---|
| Nemonoxacin |
Levofloxacin 500 mg | Nemonoxacin 750 mg-levofloxacin 500 mg | Nemonoxacin 500 mg-levofloxacin 500 mg | |||
| 750 mg | 500 mg | |||||
| Eval-ITT | n | 79 | 77 | 79 | −10.4 to 7.9 | −13.9 to 5.7 |
| Clinical cure | 71 (89.9) | 67 (87.0) | 72 (91.1) | |||
| Clinical failurea | 8 (10.1) | 10 (13.0) | 7 (8.9) | |||
| Eval-PPc | n | 72 | 73 | 72 | −8.0 to 10.8 | −12.8 to 7.6 |
| Clinical cure | 66 (91.7) | 64 (87.7) | 65 (90.3) | |||
| Clinical failurea | 6 (8.3) | 9 (12.3) | 7 (9.7) | |||
| ITT | n | 86 | 89 | 90 | −10.5 to 15.7 | −18.6 to 9.1 |
| Clinical cure | 71 (82.6) | 67 (75.3) | 72 (80.0) | |||
| Clinical failure | 15 (17.4) | 22 (24.7) | 18 (20.0) | |||
| PPc | n | 79 | 82 | 79 | −12.1 to 14.6 | −18.7 to 9.1 |
| Clinical cure | 66 (83.5) | 64 (78.0) | 65 (82.3) | |||
| Clinical failure | 13 (16.5) | 18 (22.0) | 14 (17.7) | |||
Unevaluable and missing responses were excluded.
The 95% CI is shown for Eval-ITT and Eval-PPc groups. A step-down procedure was usedtocompare each dose group of nemonoxacin with levofloxacin for the determination of noninferiority. If the lower limit of the 95% CI was greater than −15%, nemonoxacin was considered noninferior to levofloxacin.
The 97.5% CI is shown for ITT and PPc groups. If the lower limit of the 97.5% CI was greater than −15% in either comparison, nemonoxacin was considered noninferior to levofloxacin.
Clinical failure occurred in 8 (10.1%), 10 (13.0%), and 7 (8.9%) subjects in the nemonoxacin at 750 mg, nemonoxacin at 500 mg, and levofloxacin at 500 mg groups, respectively. Among them, five subjects were terminated early from the study (two in the nemonoxacin at 750 mg group and three in the nemonoxacin at 500 mg group). Clinical failure was caused primarily by the persistence or progression of chest radiographic abnormalities (two subjects for each group), as well as incomplete resolution of signs and symptoms (one in the nemonoxacin at 750 mg, four in the nemonoxacin at 500 mg, and one in the levofloxacin at 500 mg groups), persistence or worsening of signs and symptoms (one in the nemonoxacin at 750 mg, two in the nemonoxacin at 500 mg, and two in the levofloxacin at 500 mg groups), and relapse (one in the nemonoxacin at 750 mg and two in the nemonoxacin at 500 mg groups).
The clinical-cure rates for the total populations were 82.6% (nemonoxacin at 750 mg), 75.3% (nemonoxacin at 500 mg), and 80.0% (levofloxacin at 500 mg) in the ITT population and 83.5% (nemonoxacin at 750 mg), 78.0% (nemonoxacin at 500 mg), and 82.3% (levofloxacin at 500 mg) in the PPc population. In both the ITT and PPc populations, nemonoxacin at 750 mg was also found to be noninferior to levofloxacin at 500 mg. The 97.5% CI of the difference in the clinical-cure rates between nemonoxacin at 750 mg and levofloxacin at 500 mg was −10.5% to 15.7% in the ITT population and −12.1% to 14.6% in the PPc population. Descriptive results were also expressed according to the PSI classification at study entry (Table 3). Clinical-cure rates for patients categorized in PSI classes I to III were similar between the three treatment groups. For patients categorized in PSI class IV, nemonoxacin treatment groups had slightly lower cure rates than with levofloxacin. However, this difference was not statistically significant due to the limited number of patients in PSI class IV.
TABLE 3.
Descriptive clinical cure rates according to the PSI
| Population | PSI class | No. of patients with clinical cure/PSI class (%) |
||
|---|---|---|---|---|
| Nemonoxacin |
Levofloxacin 500 mg | |||
| 750 mg | 500 mg | |||
| Eval-ITT | n | 79 | 77 | 79 |
| I | 34/40 (85.0) | 37/43 (86.0) | 31/34 (91.2) | |
| II | 26/27 (96.3) | 23/26 (88.5) | 27/31 (87.1) | |
| III | 9/9 (100.0) | 5/5 (100.0) | 9/9 (100.0) | |
| IV | 2/3 (66.7) | 2/3 (66.7) | 5/5 (100.0) | |
| Eval-PPc | n | 72 | 73 | 72 |
| I | 32/37 (86.5) | 35/40 (87.5) | 30/33 (90.9) | |
| II | 24/25 (96.0) | 22/25 (88.0) | 25/29 (86.2) | |
| III | 8/8 (100.0) | 5/5 (100.0) | 6/6 (100.0) | |
| IV | 2/2 (100.0) | 2/3 (66.7) | 4/4 (100.0) | |
| ITT | n | 86 | 89 | 90 |
| I | 34/41 (82.9) | 37/50 (74.0) | 31/38 (81.6) | |
| II | 26/30 (86.7) | 23/29 (79.3) | 27/35 (77.1) | |
| III | 9/11 (81.8) | 5/6 (83.3) | 9/10 (90.0) | |
| IV | 2/4 (50.0) | 2/4 (50.0) | 5/7 (71.4) | |
| PPc | n | 79 | 82 | 79 |
| I | 32/38 (84.2) | 35/45 (77.8) | 30/35 (85.7) | |
| II | 24/28 (85.7) | 22/27 (81.5) | 25/32 (78.1) | |
| III | 8/10 (80.0) | 5/6 (83.3) | 6/7 (85.7) | |
| IV | 2/3 (66.7) | 2/4 (50.0) | 4/5 (80.0) | |
Clinical signs and symptoms.
No clinically significant treatment group differences were noted for any of the pretreatment (baseline) clinical signs and symptoms. In more than 70% of patients, the most common pretreatment clinical signs and symptoms were chills, cough, crackles on auscultation, fever, pleuritic chest pain, mucopurulent sputum production, and tachypnea. Resolution of fever and other clinical symptoms has been proposed to be a potential morbidity endpoint in CAP trials (15). At the on-treatment visit (days 5 ± 1), fever resolved in 95.7%, 93.4%, and 92.1% of the patients in the nemonoxacin at 750 mg, nemonoxacin at 500 mg, and levofloxacin groups, respectively. Overall, all clinical signs and symptoms of lower respiratory tract infection showed continued improvement from baseline to the end of study treatment in all three treatment groups.
Bacteriological response.
The bacteriological responses at the TOC or ET visit in bacteriologically evaluable populations for the three treatment groups are outlined in Table 4. The bacteriological success rates for the bacteriologically evaluable populations were 90.2% (nemonoxacin at 750 mg), 84.8% (nemonoxacin at 500 mg), and 92.0% (levofloxacin at 500 mg) in the Eval-bITT population and 91.9% (nemonoxacin at 750 mg), 84.1% (nemonoxacin at 500 mg), and 93.6% (levofloxacin at 500 mg) in the Eval-PPb population. The 95% CI for the treatment difference between nemonoxacin at 750 mg and levofloxacin at 500 mg was −13.6% to 10.0% in the Eval-bITT population and −13.0% to 9.5% in the Eval-PPb population. Thus, in both the Eval-bITT and Eval-PPb populations, nemonoxacin at 750 mg was found to be noninferior to levofloxacin at 500 mg.
TABLE 4.
Bacteriological response at TOC or ET visit for bacteriologically evaluable populations
| Population | Bacteriological response | No. of patients (%) |
Difference in bacteriological success rates between treatment groups (95% CI)c |
|||
|---|---|---|---|---|---|---|
| Nemonoxacin |
Levofloxacin 500 mg | Nemonoxacin 750 mg-levofloxacin 500 mg | Nemonoxacin 500 mg-levofloxacin 500 mg | |||
| 750 mg | 500 mg | |||||
| Eval-bITT | n | 41 | 46 | 50 | −13.6 to 10.0 | −20.0 to 5.6 |
| Bacteriological successa | 37 (90.2) | 39 (84.8) | 46 (92.0) | |||
| Bacteriological failureb | 4 (9.8) | 7 (15.2) | 4 (8.0) | |||
| Eval-PPb | n | 37 | 44 | 47 | −13.0 to 9.5 | −22.4 to 3.3 |
| Bacteriological successa | 34 (91.9) | 37 (84.1) | 44 (93.6) | |||
| Bacteriological failureb | 3 (8.1) | 7 (15.9) | 3 (6.4) | |||
The success response included eradication and presumed eradication.
The failure response included persistence, presumed persistence, recurrence, colonization, superinfection, and new infection. Unevaluable response was excluded.
A step-down procedure was used to compare each dose group of nemonoxacin with levofloxacin for the determination of noninferiority. If the lower limit of the 95% CI was greater than −15%, nemonoxacin was considered noninferior to levofloxacin.
The bacteriological success rates of the total populations were 80.4% (nemonoxacin at 750 mg), 76.5% (nemonoxacin at 500 mg), and 80.7% (levofloxacin at 500 mg) in the bITT population and 81.0% (nemonoxacin at 750 mg), 77.1% (nemonoxacin at 500 mg), and 88.0% (levofloxacin at 500 mg) in the PPb population. Noninferiority of nemonoxacin (750 mg and 500 mg) to levofloxacin (500 mg) was not demonstrated in both the bITT and PPb populations. However, these results should be interpreted with caution due to the limited number of subjects included in the bITT and PPb populations and, correspondingly, the reduced power of this analysis.
Bacteriological responses to individual pathogens.
The bacteriological success rates for the most common typical and atypical pathogens isolated at baseline are presented in Table 5. Within the Eval-bITT population, in 36.6% (15 of 41; nemonoxacin at 750 mg), 23.9% (11 of 46; nemonoxacin at 500 mg), and 48.0% (24 of 50; levofloxacin at 500 mg) of patients, typical bacterial pathogens were isolated at baseline. Of these pathogens, H. influenzae and S. pneumoniae were the most common. The number of patients with specific typical pathogens was quite low; therefore, it was difficult to draw any firm conclusions. The bacteriological success rates for S. pneumoniae were 100% (nemonoxacin at 750 mg), 75% (nemonoxacin at 500 mg), and 100% (levofloxacin at 500 mg), and for H. influenzae they were 83% (nemonoxacin at 750 mg), 100% (nemonoxacin at 500 mg), and 100% (levofloxacin at 500 mg).
TABLE 5.
Bacteriological success rates for the most common typical and atypical pathogens isolated at baseline and their in vitro susceptibilities (Eval-bITT population)
| Baseline pathogen (n)a | Bacteriological success/organisms isolated at baseline (%) |
MIC range baseline (μg/ml) |
|||
|---|---|---|---|---|---|
| Nemonoxacin |
Levofloxacin 500 mg | Nemonoxacin | Levofloxacin | ||
| 750 mg | 500 mg | ||||
| Typical pathogens | |||||
| H. influenzae (17) | 5/6 (83.3) | 4/4 (100.0) | 7/7 (100.0) | ≤0.008-0.06 | ≤0.008-0.03 |
| S. pneumoniae (14) | 5/5 (100.0) | 3/4 (75.0) | 5/5 (100.0) | 0.06-0.12 | 0.5-1.0 |
| S. aureus (4) | 1/1 (100.0) | 2/2 (100.0) | 1/1 (100.0) | 0.03-0.06 | 0.12-0.5 |
| Atypical pathogens | |||||
| M. pneumoniae (93) | 26/29 (89.7) | 25/31 (80.6) | 31/33 (93.9) | ||
| C. pneumoniae (23) | 8/8 (100.0) | 8/8 (100.0) | 6/7 (85.7) | ||
| L. pneumophila (8) | 3/3 (100.0) | 2/2 (100.0) | 3/3 (100.0) | ||
n, number of isolates.
A relatively high number of atypical-pathogen infections were identified serologically in 34 patients treated with nemonoxacin at 750 mg, 38 patients treated with nemonoxacin at 500 mg, and 39 patients treated with levofloxacin at 500 mg. The most common atypical pathogen identified serologically was M. pneumoniae. The success rates were as follows: M. pneumoniae, 89.7% (nemonoxacin at 750 mg), 80.6% (nemonoxacin at 500 mg), and 93.9% (levofloxacin at 500 mg); C. pneumoniae, 100% (nemonoxacin at 750 mg), 100% (nemonoxacin at 500 mg), and 85.7% (levofloxacin at 500 mg); and L. pneumophila, 100% in all three treatment groups.
Six patients yielded positive blood cultures for S. pneumoniae (two in the nemonoxacin at 750 mg group, one in the nemonoxacin at 500 mg group, and three in the levofloxacin at 500 mg group) at baseline in the Eval-bITT population. All five patients in the nemonoxacin at 750 mg group and levofloxacin at 500 mg group showed bacteriological success at the TOC or ET visit. One failure occurred in the nemonoxacin at 500 mg group.
Bacteriological failure at the TOC or ET visit for the Eval-bITT population was recorded in four patients treated with nemonoxacin at 750 mg (one persistence and three presumed persistence), seven patients treated with nemonoxacin at 500 mg (seven presumed persistence), and four patients treated with levofloxacin at 500 mg (one persistence and three presumed persistence). One patient in the nemonoxacin at 750 mg group had a bacteriological response of persistence with Pseudomonas aeruginosa, and one patient in the levofloxacin at 500 mg group had persistence with K. pneumoniae at the TOC or ET visit. The other 13 patients had a bacteriological response of presumed persistence, corresponding to the clinical response of failure.
The pretherapy MIC range of nemonoxacin was lower than that of levofloxacin against the common typical pathogens. The MIC range of S. pneumoniae was 0.06 to 0.12 μg/ml for nemonoxacin and 0.5 to 1.0 μg/ml for levofloxacin, and that of S. aureus was 0.03 to 0.06 μg/ml for nemonoxacin and 0.12 to 0.5 μg/ml for levofloxacin. One patient in the nemonoxacin at 750 mg group experienced bacteriological failure with P. aeruginosa, despite a low nemonoxacin MIC (1.0 μg/ml) observation at the TOC visit.
Safety.
Subjects who experienced at least one treatment-emergent adverse event (TEAE) comprised 55.8%, 44.9%, and 48.9% of the treatment groups of nemonoxacin at 750 mg, nemonoxacin at 500 mg, and levofloxacin at 500 mg, respectively (Table 6). Higher rates of AEs occurred in the system organ class of gastrointestinal and nervous system disorders for the nemonoxacin groups. These AEs included diarrhea (nemonoxacin at 750 mg, 17.4%; nemonoxacin at 500 mg, 11.2%; levofloxacin at 500 mg, 8.9%) and dizziness and headache (nemonoxacin at 750 mg, 9.3%; nemonoxacin at 500 mg, 6.7%; levofloxacin at 500 mg, 4.4%). However, similar rates of drug-related TEAEs were noted across the treatment groups of nemonoxacin at 750 mg, nemonoxacin at 500 mg, and levofloxacin: 31.4%, 30.3%, and 30.0%, respectively, with no clinically significant treatment group differences (Table 7).
TABLE 6.
Summary of common (>2%) treatment-emergent adverse events by system organ class and preferred term
| System organ class (preferred term) | No. of subjects (%) |
|||
|---|---|---|---|---|
| Nemonoxacin |
Levofloxacin 500 mg (n = 90) | Total (n = 265) | ||
| 750 mg (n = 86) | 500 mg (n = 89) | |||
| Subjects with any TEAEs | 48 (55.8) | 40 (44.9) | 44 (48.9) | 132 (49.8) |
| Blood and lymphatic system disorders | 15 (17.4) | 15 (16.9) | 15 (16.7) | 45 (17.0) |
| Neutropenia | 9 (10.5) | 9 (10.1) | 10 (11.1) | 28 (10.6) |
| Thrombocythemia | 4 (4.7) | 2 (2.2) | 2 (2.2) | 8 (3.0) |
| Anemia | 2 (2.3) | 2 (2.2) | 2 (2.2) | 6 (2.3) |
| Lymphopenia | 0 | 2 (2.2) | 1 (1.1) | 3 (1.1) |
| Gastrointestinal disorders | 15 (17.4) | 10 (11.2) | 8 (8.9) | 33 (12.5) |
| Diarrhea | 4 (4.7) | 7 (7.9) | 2 (2.2) | 13 (4.9) |
| Nausea | 7 (8.1) | 1 (1.1) | 3 (3.3) | 11 (4.2) |
| Nervous system disorders | 8 (9.3) | 6 (6.7) | 4 (4.4) | 18 (6.8) |
| Dizziness | 5 (5.8) | 5 (5.6) | 2 (2.2) | 12 (4.5) |
| Headache | 4 (4.7) | 2 (2.2) | 1 (1.1) | 7 (2.6) |
TABLE 7.
Summary of drug-related TEAEs (>2%)
| System organ class (preferred term)a | No. of subjects (%) |
|||
|---|---|---|---|---|
| Nemonoxacin |
Levofloxacin 500 mg (n = 90) | Total (n = 265) | ||
| 750 mg (n = 86) | 500 mg (n = 89) | |||
| Subjects with any drug-related TEAE | 27 (31.4) | 27 (30.3) | 27 (30.0) | 81 (30.6) |
| Neutropenia | 8 (9.3) | 8 (9.0) | 10 (11.1) | 26 (9.8) |
| Dizziness | 3 (3.5) | 4 (4.5) | 2 (2.2) | 9 (3.4) |
| Nausea | 5 (5.8) | 1 (1.1) | 3 (3.3) | 9 (3.4) |
| Diarrhea | 1 (1.2) | 5 (5.6) | 1 (1.1) | 7 (2.6) |
| Thrombocythemia | 4 (4.7) | 2 (2.2) | 1 (1.1) | 7 (2.6) |
| ECG QTc interval prolonged | 2 (2.3) | 0 | 3 (3.3) | 5 (1.9) |
| Blood amylase increased | 1 (1.2) | 1 (1.1) | 2 (2.2) | 4 (1.5) |
| Headache | 1 (1.2) | 2 (2.2) | 0 | 3 (1.1) |
| ALT/SGPT | 0 | 2 (2.2) | 1 (1.1) | 3 (1.1) |
| AST/SGOT | 0 | 2 (2.2) | 0 | 2 (0.8) |
ALT/SGPT, alanine aminotransferase/serum glutamic-pyruvic transaminase; AST/SGOT, aspartate aminotransferase/serum glutamic-oxaloacetic transaminase.
Two subjects in the nemonoxacin groups died due to events that were either considered unlikely to be related to the study drug (one subject due to sepsis in the 750-mg group) or unrelated to the study drug (one subject due to pulmonary tuberculosis in the 500-mg group). The percentages of subjects who experienced serious TEAEs in the nemonoxacin at 750 mg, nemonoxacin at 500 mg, and levofloxacin groups were 4.7%, 5.6%, and 1.1%, respectively. All the serious TEAEs were considered to be non-drug related. Serious TEAEs included the following: nemonoxacin at 750 mg group (3 subjects), 1 subject with intracardiac thrombus, mitral valve stenosis, and rheumatic heart disease, 1 with pulmonary tuberculosis, and 1 with sepsis; nemonoxacin at 500 mg group (5 subjects), 1 subject with anemia, 1 with pulmonary tuberculosis, 1 with lung abscess, 1 with diabetes mellitus and hyperglycemia, and 1 with pneumonia; and levofloxacin at 500 mg group (one subject), pulmonary tuberculosis and epilepsy. No clinically significant differences among the treatment groups were observed with regard to laboratory data, vital signs, and ECG.
DISCUSSION
This study demonstrated that oral administration of nemonoxacin at 750 mg once daily for 7 days achieved noninferiority in its primary endpoint of clinical cure rate in the ITT population compared with levofloxacin at 500 mg for the treatment of adult patients with CAP, and similarly, in its secondary endpoint of clinical cure rate in the PPc population. For the clinically evaluable populations, the clinical success rates for nemonoxacin at 750 mg, nemonoxacin at 500 mg, and levofloxacin at 500 mg were 89.9%, 87.0%, and 91.1%, respectively, in the Eval-ITT population and 91.7%, 87.7%, and 90.3%, respectively, in the Eval-PPc population. Therefore, nemonoxacin in both the 750-mg and 500-mg groups met the statistical criteria for noninferiority compared with the levofloxacin at 500 mg group in the clinically evaluable populations.
Although a relatively small proportion of this study population had an identifiable pretherapy typical bacterial pathogen, overall bacteriological success rates were comparable in subjects administered nemonoxacin (90.2% in the 750-mg group and 84.8% in the 500-mg group) and those administered the comparator regimen (92.0%) in the Eval-bITT population. All three treatment regimens were highly effective in the eradication or presumed eradication of the common typical pathogens of CAP. High success rates were achieved against atypical pathogens in all regimens, with 89.7% (nemonoxacin at 750 mg), 80.6% (nemonoxacin at 500 mg), and 93.9% (levofloxacin at 500 mg) for M. pneumoniae. On the other hand, the success rates were 100% (both nemonoxacin groups) and 85.7% (levofloxacin) for C. pneumoniae. Good success rates were also achieved for L. pneumoniae (100%) in all three regimens. One of the reasons for the higher detection rates of atypical pathogens in the current study is the age factor—the average age of our enrolled patients was lower than that in previously reported CAP trials. An earlier study found that CAP patients aged <60 years were at risk for an atypical bacterial etiology, particularly M. pneumoniae, with an odds ratio of 2.3 (30).
Although the number of patients with specific typical pathogens was lower, excellent clinical and bacteriological success rates were achieved with nemonoxacin against the most common typical and atypical pathogens. These findings supported in vitro and in vivo observations that nemonoxacin has potent and broad-spectrum activity against different isolates.
Nemonoxacin and levofloxacin were generally well tolerated in the current trial. A higher TEAE rate was reported in the nemonoxacin at 750 mg group (55.8%) than in the nemonoxacin at 500 mg (44.9%) and levofloxacin at 500 mg (48.9%) groups. However, no significant difference was noted in drug-related TEAEs among these three groups. Among the AEs, diarrhea, dizziness, and headache were most frequently reported in the nemonoxacin-treated groups; this was similar to the AEs reported in the previous multiple-dosing study on healthy subjects (7). Higher percentages of subjects experienced serious TEAEs in the nemonoxacin groups (750-mg group, 4.7%, and 500-mg group, 5.6%) than with levofloxacin (1.1%). As assessed by the investigators, all serious TEAEs were unrelated to the study drug. Six TEAEs led to discontinuation from the study—three from the nemonoxacin at 750 mg group (two tuberculosis and one pregnancy, reported as an AE by an investigator) and three from the nemonoxacin at 500 mg group (two tuberculosis and one lung abscess). An earlier CAP study in adult patients found that 3.3% of etiologies were tuberculosis (31). In this study, tuberculosis was a major reason for discontinuation in nemonoxacin treatment groups, but not with levofloxacin. The observation that Mycobacterium tuberculosis was less susceptible to nemonoxacin in the groups in this study was consistent with the findings of a previous in vitro study (32). In that study, the MIC90 values of levofloxacin and moxifloxacin were much lower than that of nemonoxacin, which implied that they may be suitable as alternative anti-tuberculosis agents. However, levofloxacin and moxifloxacin may mask or delay the diagnosis of tuberculosis, because tuberculosis can sometimes be mistaken for CAP. Moreover, some concerns were recently raised about the duration of exposure of M. tuberculosis to fluoroquinolones, which may be a risk factor for the development of resistance (22). Nemonoxacin might be less of a concern in this regard if it is used as the primary treatment for CAP.
CAP, along with influenza, is the sixth leading cause of death in the United States; therefore, improving the care of patients with CAP has been the focus of many organizations (24, 25). According to one estimate, 915,900 episodes of CAP occur in adults ≧65 years of age every year in the United States (16, 24). Despite advances in antimicrobial therapy, the overall mortality remains relatively high (10). Initial antimicrobial treatment for patients with CAP should provide appropriate coverage against the most common causative organisms, including atypical and resistant strains (33). In addition, many currently available antimicrobials need to be given more than once a day, which can lead to compliance issues (11, 20). Thus, it is apparent that a convenient, shorter-course, once-daily agent with broad-spectrum activity that covers not only the typical respiratory pathogens, but also the atypical and resistant pathogens, remains highly necessary.
In conclusion, oral nemonoxacin (750 mg and 500 mg) administered for 7 days showed high clinical and bacteriological success rates in patients with CAP. Further, it was well tolerated and demonstrated excellent activity against the common causative pathogens compared with levofloxacin. In our era of growing antibacterial resistance, oral nemonoxacin represents a promising alternative for the management of such infections in community clinics. Larger confirmatory studies are required to investigate the efficacy of nemonoxacin in patients with bacterial lung infections.
Acknowledgments
This study was partially supported by a MOEA grant from the Government of Taiwan.
We are grateful to Judy Yuan for critical review of the manuscript. We thank all the subjects and the following investigators for their participation in this study: Kai-Ming Chang (Taichung Veterans General Hospital, Taiwan), Yin-Ching Chuang (Chi-Mei Foundation Hospital, Taiwan), Christiaan De Villiers (De Villiers Clinical Trials, South Africa), M. Fulat (Eastmed Medical Centre, South Africa), Mashra Gani (Mercantile Hospital, South Africa), J. C. J. Jurgens (DJW Navorsing, South Africa), Horng-Chyuan Lin (Chang Gung Memorial Hospital, Linko, Taiwan), Hsin-Hsun Lin (E-Da Hospital, Taiwan), Grant Nieuwoudt (Langeberg Medical Centre, South Africa), Wann-Cherng Perng (Tri-Service General Hospital, South Africa), and C. van Rensburg. (South Africa).
L.-W. C., D. T. C., Y.-T. C., C.-H. R. K., and M.-C. H. are employees of TaiGen Biotechnology Co. Ltd.
Footnotes
Published ahead of print on 26 July 2010.
REFERENCES
- 1.Adam, H. J., N. M. Laing, C. R. King, B. Lulashnyk, D. J. Hoban, and G. G. Zhanel. 2009. In vitro activity of nemonoxacin, a novel nonfluorinated quinolone, against 2,440 clinical isolates. Antimicrob. Agents Chemother. 53:4915-4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bochud, P. Y., F. Moser, P. Erard, F. Verdon, J. P. Studer, G. Villard, A. Cosendai, M. Cotting, F. Heim, J. Tissot, Y. Strub, M. Pazeller, L. Saghafi, A. Wenger, D. Germann, L. Matter, J. Bille, L. Pfister, and P. Francioli. 2001. Community-acquired pneumonia. A prospective outpatient study. Medicine (Baltimore) 80:75-87. [DOI] [PubMed] [Google Scholar]
- 3.British Thoracic Society Standards of Care Committee. 2001. BTS guidelines for the management of community acquired pneumonia in adults. Thorax 56(Suppl. 4):iv1-iv64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carbonara, S., L. Monno, B. Longo, and G. Angarano. 2009. Community-acquired pneumonia. Curr. Opin. Pulm. Med. 15:261-273. [DOI] [PubMed] [Google Scholar]
- 5.Chen, S. J., L. Lin, and L. W. Chang. 2007. Analysis of antibacterial response of nemonoxacin (TG-873870) against major pathogens from respiratory tract and skin infections, abstr. 441. Abstr. 45th Infect. Dis. Soc. Am.
- 6.Chen, Y. H., C. Y. Liu, J. J. Lu, C. H. King, and P. R. Hsueh. 2009. In vitro activity of nemonoxacin (TG-873870), a novel non-fluorinated quinolone, against clinical isolates of Staphylococcus aureus, enterococci and Streptococcus pneumoniae with various resistance phenotypes in Taiwan. J. Antimicrob. Chemother. 64:1226-1229. [DOI] [PubMed] [Google Scholar]
- 7.Chung, D. T., C. Y. Tsai, S. J. Chen, L. W. Chang, C. H. King, C. H. Hsu, K. M. Chiu, H. C. Tan, Y. T. Chang, and M. C. Hsu. 2010. Multiple-dose safety, tolerability, and pharmacokinetics of oral nemonoxacin (TG-873870) in healthy volunteers. Antimicrob. Agents Chemother. 54:411-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falguera, M., O. Sacristán, A. Nogués, A. Ruiz-González, M. Garcia, A. Manonelles, and M. Rubio-Caballero. 2001. Nonsevere community-acquired pneumonia: correlation between cause and severity or comorbidity. Arch. Intern. Med. 161:1866-1872. [DOI] [PubMed] [Google Scholar]
- 9.File, T. M., Jr. 2006. Clinical implications and treatment of multiresistant Streptococcus pneumoniae pneumonia. Clin. Microbiol. Infect. 12(Suppl. 3):31-41. [DOI] [PubMed] [Google Scholar]
- 10.Fine, M. J., M. A. Smith, C. A. Carson, S. S. Mutha, S. S. Sankey, L. A. Weissfeld, and W. N. Kapoor. 1996. Prognosis and outcomes of patients with community-acquired pneumonia. A meta-analysis. JAMA 275:134-141. [PubMed] [Google Scholar]
- 11.Greenberg, R. N. 1984. Overview of patient compliance with medication dosing: a literature review. Clin. Ther. 6:592-599. [PubMed] [Google Scholar]
- 12.Ho, P. L., V. C. Cheng, and C. M. Chu. 2009. Antibiotic resistance in community-acquired pneumonia caused by Streptococcus pneumoniae, methicillin-resistant Staphylococcus aureus, and Acinetobacter baumannii. Chest 136:1119-1127. [DOI] [PubMed] [Google Scholar]
- 13.Hsu, C. H., L. Lin, R. Leunk, and D. Reichart. 2008. In vivo efficacy of nemonoxacin in a mouse protection model, abstr. B-1005. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (IDSA) 46th Annu. Meet. American Society for Microbiology and Infectious Diseases Society of America, Washington, DC.
- 14.Hsu, C. H., L. Lin, R. Leunk, and D. Reichart. 2008. In vivo efficacy of nemonoxacin in a mouse pulmonary infection model, abstr. B-056. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (IDSA) 46th Annu. Meet. American Society for Microbiology and Infectious Diseases Society of America, Washington, DC.
- 15.Infectious Diseases Society of America. 2008. Position paper: recommended design features of future clinical trials of antibacterial agents for community-acquired pneumonia. Clin. Infect. Dis. 47(Suppl. 3):S249-S265. [PMC free article] [PubMed] [Google Scholar]
- 16.Jackson, M. L., K. M. Neuzil, W. W. Thompson, D. K. Shay, O. Yu, C. A. Hanson, and L. A. Jackson. 2004. The burden of community-acquired pneumonia in seniors: results of a population-based study. Clin. Infect. Dis. 39:1642-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.King, C. H. R., L. Lin, and R. Leunk. 2008. In vitro resistance development to nemonoxacin for Streptococcus pneumoniae, abstr. C1-1971. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (IDSA) 46th Annu. Meet. American Society for Microbiology and Infectious Diseases Society of America, Washington, DC.
- 18.Lai, C. C., C. K. Tan, S. H. Lin, C. H. Liao, C. H. Chou, H. L. Hsu, Y. T. Huang, and P. R. Hsueh. 2009. Comparative in vitro activities of nemonoxacin, doripenem, tigecycline and 16 other antimicrobials against Nocardia brasiliensis, Nocardia asteroides and unusual Nocardia species. J. Antimicrob. Chemother. 64:73-78. [DOI] [PubMed] [Google Scholar]
- 19.Lauderdale, T. L., Y. R. Shiau, J. F. Lai, H. C. Chen, and C. H. King. 2007. In vitro antibacterial activity of nemonoxacin (TG-873870), a new nonfluorinated quinolone, against clinical isolates, abstr. E-1635. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother., Chicago, IL. American Society for Microbiology, Washington, DC.
- 20.Leophonte, P., T. File, and C. Feldman. 2004. Gemifloxacin once daily for 7 days compared to amoxicillin/clavulanic acid thrice daily for 10 days for the treatment of community-acquired pneumonia of suspected pneumococcal origin. Respir. Med. 98:708-720. [DOI] [PubMed] [Google Scholar]
- 21.Lin, L., L.-W. Chang, C.-Y. Tsai, C.-H. Hsu, D. T. Chung, W. S. Aronstein, F. Ajayi, B. Kuzmak, and R. A. Lyon. 2010. Dose escalation study of the safety, tolerability, and pharmacokinetics of nemonoxacin (TG-873870), a novel potent broad-spectrum nonfluorinated quinolone, in healthy volunteers. Antimicrob. Agents Chemother. 54:405-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Low, D. E. 2009. Fluoroquinolones for treatment of community-acquired pneumonia and tuberculosis: putting the risk of resistance into perspective. Clin. Infect. Dis. 48:1361-1363. [DOI] [PubMed] [Google Scholar]
- 23.Mandell, L. A. 2008. Spectrum of microbial etiology of community-acquired pneumonia in hospitalized patients: implications for selection of the population for enrollment in clinical trials. Clin. Infect. Dis. 47(Suppl. 3):S189-S192. [DOI] [PubMed] [Google Scholar]
- 24.Mandell, L. A., R. G. Wunderink, A. Anzueto, J. G. Bartlett, G. D. Campbell, N. C. Dean, S. F. Dowell, T. M. File, Jr., D. M. Musher, M. S. Niederman, A. Torres, and C. G. Whitney. 2007. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin. Infect. Dis. 44(Suppl. 2):S27-S72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Center for Health Statistics. 2008. Health, United States, with special feature on the health of young adults. http://www.cdc.gov/nchs/data/hus/hus08.pdf. [PubMed]
- 26.Niederman, M. S. 2007. Recent advances in community-acquired pneumonia: inpatient and outpatient. Chest 131:1205-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan, X. S., J. Ambler, S. Mehtar, and L. M. Fisher. 1996. Involvement of topoisomerase IV and DNA gyrase as ciprofloxacin targets in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 40:2321-2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pankuch, G. A., K. Kosowska-Shick, P. McGhee, C. H. R. King, and P. C. Appelbaum. 2008. Comparative antistaphylococcal activity of nemonoxacin, a novel broad-spectrum quinolone, abstr. C1-189. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (IDSA) 46th Annu. Meet. American Society for Microbiology and Infectious Diseases Society of America, Washington, DC.
- 29.Restrepo, M. I., E. M. Mortensen, J. A. Velez, C. Frei, and A. Anzueto. 2008. A comparative study of community-acquired pneumonia patients admitted to the ward and the ICU. Chest 133:610-617. [DOI] [PubMed] [Google Scholar]
- 30.Ruiz, M., S. Ewig, M. A. Marcos, J. A. Martinez, F. Arancibia, J. Mensa, and A. Torres. 1999. Etiology of community-acquired pneumonia: impact of age, comorbidity, and severity. Am. J. Respir. Crit. Care Med. 160:397-405. [DOI] [PubMed] [Google Scholar]
- 31.Song, J. H., W. S. Oh, C. I. Kang, D. R. Chung, K. R. Peck, K. S. Ko, J. S. Yeom, C. K. Kim, S. W. Kim, H. H. Chang, Y. S. Kim, S. I. Jung, Z. Tong, Q. Wang, S. G. Huang, J. W. Liu, M. K. Lalitha, B. H. Tan, P. H. Van, C. C. Carlos, T. So, and the Asian Network for Surveillance of Resistant Pathogens Study Group. 2008. Epidemiology and clinical outcomes of community-acquired pneumonia in adult patients in Asian countries: a prospective study by the Asian network for surveillance of resistant pathogens. Int. J. Antimicrob. Agents. 31:107-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tan, C.-K., C.-C. Lai, C.-H. Liao, C.-H. Chou, H.-L. Hsu, Y.-T. Huang, and P.-R. Hsueh. 2009. Comparative in vitro activities of the new quinolone nemonoxacin (TG-873870), gemifloxacin and other quinolones against clinical isolates of Mycobacterium tuberculosis. J. Antimicrob. Chemother. 64:428-429. [DOI] [PubMed] [Google Scholar]
- 33.Tanaseanu, C., S. Milutinovic, P. I. Calistru, J. Strausz, M. Zolubas, V. Chernyak, N. Dartois, N. Castaing, H. Gandjini, and C. A. Cooper. 2009. Efficacy and safety of tigecycline versus levofloxacin for community-acquired pneumonia. BMC Pulm. Med. 9:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsang, K. W., and T. M. File, Jr. 2008. Respiratory infections unique to Asia. Respirology 13:937-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woodhead, M. 2002. Community-acquired pneumonia in Europe: causative pathogens and resistance patterns. Eur. Respir. J. Suppl. 36:20s-27s. [DOI] [PubMed] [Google Scholar]