Abstract
The drug-drug interaction between rifabutin (RFB) and darunavir/ritonavir (DRV/r) was examined in a randomized, three-way crossover study of HIV-negative healthy volunteers who received DRV/r 600/100 mg twice a day (BID) (treatment A), RFB 300 mg once a day (QD) (treatment B), and DRV/r 600/100 mg BID plus RFB 150 mg every other day (QOD) (treatment C). The sequence of treatments was randomized, and each treatment period lasted 12 days. Full pharmacokinetic profiles were determined for DRV, ritonavir, and RFB and its active metabolite, 25-O-desacetylrifabutin (desRFB), on day 13. The DRV and ritonavir areas under the plasma concentration-time curve from zero to 12 h (AUC12h) increased by 57% and 66%, respectively, in the presence of RFB. The RFB exposure was comparable between treatment with RFB QD alone (treatment B) and treatment with DRV/r plus RFB QOD (treatment C); however, based on least-square means ratios, the minimum plasma concentration (Cmin) increased by 64% and the maximum plasma concentration (Cmax) decreased by 28%, respectively. The exposure (AUC within the dosage interval and at steady state [AUCτ]) to desRFB was considerably increased (by 881%) following treatment with DRV/r/RFB. The exposure to the parent drug plus the metabolite increased 1.6-fold in the presence of DRV/r. Adverse events (AEs) were more commonly reported during combined treatment (83% versus 44% for RFB and 28% for DRV/r); similarly, grade 3-4 AEs occurred in 17% versus 11% and 0%, respectively, of volunteers. Eighteen of 27 volunteers (66.7%) prematurely discontinued the trial; all volunteers discontinuing for safety reasons (n = 9) did so during RFB treatment phases. These results suggest that DRV/r may be coadministered with RFB with a dose adjustment of RFB to 150 mg QOD and increased monitoring for RFB-related AEs. Based on the overall safety profile of DRV/r, no dose adjustment of DRV/r is considered to be warranted. Given the safety profile seen with the combination of RFB with a boosted protease inhibitor in this and other studies, it is not recommended to conduct further studies with this combination in healthy volunteers.
The protease inhibitor (PI) darunavir (DRV) with low-dose ritonavir (DRV/r) has demonstrated substantial efficacy and safety across the whole treatment spectrum of HIV-1-infected patients (6, 13, 14). DRV/r is approved in the United States (25), Europe (26), and other countries for treatment-experienced (600/100 mg twice a day [BID]) and treatment-naïve (800/100 mg once a day [QD]) HIV-1-infected adult patients.
Rifabutin (RFB) is an antibiotic used in patients with HIV infection to prevent and treat disseminated Mycobacterium avium complex infections and (in combination with other agents) to treat tuberculosis (16). Patients already receiving DRV/r may, therefore, also need to receive RFB.
The predominant metabolite of RFB is 25-O-desacetylrifabutin (desRFB), which is normally present at 10-fold lower plasma concentrations than RFB. DesRFB has similar activity to RFB and contributes up to 10% of the antimicrobial activity (16). DRV, ritonavir, and RFB are metabolized predominantly by the isoenzyme cytochrome P450 3A4 (CYP3A4) (16, 25, 26). Ritonavir and DRV are inhibitors of CYP3A4 metabolism (25, 26), and RFB is reported to be an inducer of CYP3A4 (16). A pharmacokinetic (PK) drug-drug interaction is therefore expected when DRV/r and RFB are coadministered.
This study examines the PK interaction between DRV/r and RFB in HIV-negative healthy volunteers and provides dose recommendations for coadministration in HIV-1-infected patients.
MATERIALS AND METHODS
Study design.
TMC114-C163 was a phase I, open-label, randomized, three-way, crossover trial in HIV-negative, healthy volunteers investigating the PK interaction between DRV/r and RFB and its active metabolite desRFB at steady state. The study population was planned to consist of 18 healthy male or female volunteers aged 18 to 55.
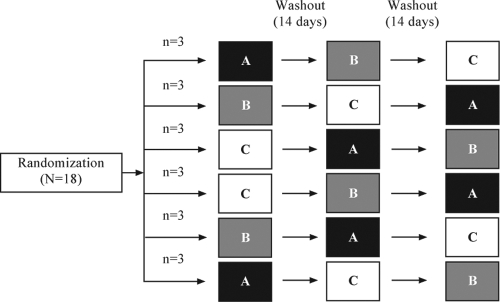
Each volunteer underwent three sessions (A, B, and C) in a sequence determined by randomization: in treatment A, volunteers received DRV/r 600/100 mg BID on days 1 to 12, with an additional morning dose on day 13; during treatment B they received RFB 300 mg QD on days 1 to 13; and during treatment C, they received DRV/r 600/100 mg BID on days 1 to 13 plus RFB 150 mg once every other day (QOD) from day 1 to 13 (Fig. 1). RFB was administered at a dose of 150 mg QOD when coadministered with DRV/r, as this is the commonly used dose regimen when RFB is given in combination with boosted PIs (16). In order to allow all possible treatment sequences to be tested, six randomization groups were set up to receive the three treatments. Subsequent sessions were separated by a washout period of ≥14 days. DRV, ritonavir, and RFB were taken together with water within 10 min after a meal. DRV and ritonavir had to be taken twice daily (per day), between 7 and 9 a.m. and 7 and 9 p.m., respectively. RFB was taken once daily, between 7 and 9 a.m. On days of coadministration (treatment C) the order of intake was RFB, then ritonavir (within 5 min), and then DRV (within 5 min).
FIG. 1.
Overview of trial design. Volunteers received three treatments in a sequence allocated randomly. Treatments: A, DRV/r 600/100 mg BID on days 1 to 12, with an additional morning dose on day 13; B, RFB 300 mg QD on days 1 to 13; C, DRV/r 600/100 mg BID on days 1 to 13 plus RFB 150 mg QOD from day 1 to 13.
The study protocol and amendment were approved by the Comité de Protection des Personnes, Ile De France VIII Institutional Review Board, and the Agence Française de Sécurité Sanitaire des Produits de Santé health authority and conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all volunteers.
Pharmacokinetic assessments.
Plasma samples for DRV and ritonavir (treatments A and C) and for RFB and desRFB (treatments B and C) were taken predose in the morning on days 1, 11, 12, and 13 and at 0.5, 1, 1.5, 2, 2.5, 3, 4, 5, 6, 9, and 12 h postdose on day 13.
Plasma concentrations of DRV, ritonavir, RFB, and desRFB were determined using validated liquid chromatography-tandem mass spectrometry methods (LC-MS-MS) (4, 25). Internal and reference standards were provided by Janssen Pharmaceutica (Beerse, Belgium) for the DRV and ritonavir assays and by Syncom (Groningen, Netherlands) and Tibotec (Mechelen, Belgium) for the RFB and desRFB assays. The recovery of DRV and ritonavir was consistent over the evaluated concentration range for both analytes (for DRV, 91.7% at 20.0 ng/ml, 104.9% at 200 ng/ml, and 106.1% at 10,000 ng/ml, and for ritonavir, 104.2% at 20.0 ng/ml, 95.9% at 200 ng/ml, and 102.4% at 10,000 ng/ml). The validated analytical range was 2 to 2,000 ng/ml for RFB and desRFB. The interassay precision was 12.6% for DRV, 8.3% for ritonavir, 18.3% for RFB, and 13.5% for desRFB. The interassay accuracy was 108% for DRV, 104% for ritonavir, and 102% for RFB and desRFB.
The primary PK parameters were area under the concentration-time curve (AUC) from time of administration to 12 h postdosing (AUC0-12h), predose plasma concentration (C0h), and maximum (Cmax) and minimum (Cmin) plasma concentrations. Other parameters included AUC within the dosage interval and at steady state (AUCτ) and the sum of individual AUC values of parent and metabolite (AUCτ,par+met) for RFB.
Safety assessments.
Overall, safety data were available for 18 volunteers in each of the treatment sessions (i.e., 27 volunteers overall). Safety and tolerability were assessed throughout the study. Adverse events (AEs) and laboratory abnormalities were assessed and graded using the Division of AIDS AE table (1).
Statistical methods.
As this was an explorative, crossover trial, no formal sample size calculations were performed. However, a minimum of at least 13 volunteers completing all sessions was considered sufficient to allow for relevant conclusions (5, 12, 17, 20, 22). With this number of volunteers and the expected within-subject variation on the logarithmic scale of 0.035 in AUC from time point zero up to the time point of the last quantifiable concentration (AUClast) for DRV, the range of the 90% confidence interval (CI) of the ratio was estimated to be from −12.3% to +14.0%. To account for possible discontinuations, a total of 18 volunteers (three per treatment sequence) were planned to be included in the current trial. If volunteers were prematurely withdrawn from the trial after starting treatment for reasons other than drug tolerability/safety, additional volunteers were recruited to aim for at least 13 evaluable volunteers (volunteers who had completed all three sessions of the trial).
The statistical analyses compared data from treatment C (test) to data from treatment A (reference) for DRV and ritonavir and data from treatment C (test) to data from treatment B (reference) for RFB and desRFB. All available paired and unpaired observations for compared treatments were included in the analysis. Before calculation of the treatment ratio for AUCτ, individual AUCτ values of the reference treatment were multiplied by 2 to correct for differences in dosage interval between the test (τ = 48 h) and reference (τ = 24 h) treatments. Pharmacokinetic analyses were performed using WinNonlin Professional (version 4.1; Pharsight Corporation, Mountain View, CA). The least square (LS) means of the primary PK parameters were calculated with a linear mixed-effects model, controlling for treatment, period, and sequence as fixed effects and the volunteer as a random effect. Period effects were considered significant at the 5% level, and sequence effects were considered significant at the 10% level.
RESULTS
Initially, 18 HIV-negative healthy volunteers were randomized. However, due to nine volunteers discontinuing early for reasons other than safety (withdrawal of consent [n = 8] and noncompliance [n = 1]), an additional nine volunteers were recruited in the same sequence as for those withdrawn (i.e., three in the A/B/C treatment sequence, two in the C/B/A sequence, and four in the B/A/C sequence). Therefore, in total, 27 volunteers were randomized. The median age was 30 years (range, 18 to 46). Nine volunteers completed the trial, and 18 (67%) discontinued the study, 8 during coadministration of DRV/r and RFB and 5 each during DRV/r or RFB monotherapy. Discontinuations were due to withdrawal of consent (n = 8), AEs (n = 3), serious AEs (SAEs, n = 4), laboratory abnormalities (n = 2; a grade 3 and a grade 4 decreased lymphocyte count, not reported as an AE), and noncompliance (n = 1). Full PK profiles of DRV and ritonavir were available for 16 volunteers in treatment A and 11 volunteers in treatment C. For treatments B and C, full PK profiles of RFB and desRFB were available for 15 and 11 volunteers, respectively. Thus, despite the large number of dropouts, sufficient PK information was obtained from the trial to draw conclusions.
DRV pharmacokinetics.
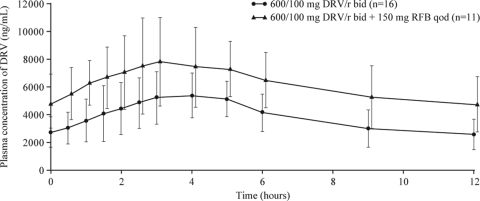
The mean DRV plasma concentrations were higher when DRV/r was coadministered with RFB than alone (Fig. 2). The PK parameters of DRV at steady state when given as DRV/r or as DRV/r plus RFB are shown in Table 1. Based on the ratios of the LS means, the DRV AUC12h at steady state was 57% higher in the presence of RFB 150 mg QOD (90% CI, 28 to 93%). The Cmin and Cmax of DRV at steady state increased by 75% (28 to 137%) and 42% (21 to 67%), respectively, in the presence of RFB.
FIG. 2.
Mean plasma concentration-time curves (including standard deviations [SD]) of DRV after administration of DRV/r alone (treatment A; day 13) and in combination with RFB 150 mg QOD (treatment C; day 13).
TABLE 1.
Pharmacokinetics of DRV and of ritonavir after administration of DRV/r alone (treatment A) and in combination with RFB (treatment C)
| Drug, parameter | Pharmacokinetics [mean ± SD or Tmaxa median (range)] with treatment: |
LS means ratio (90% CI) | |
|---|---|---|---|
| A (DRV/r) | C (DRV/r + RFB) | ||
| DRV | |||
| No. of volunteers | 16b | 11 | |
| Tmax (h) | 4.0 (1.0-9.0) | 3.0 (1.5-5.0) | |
| C0h (ng/ml) | 2,768 ± 1,077 | 4,825 ± 2,140 | 1.648 (0.975-2.783) |
| Cmin (ng/ml) | 2,349 ± 1,006 | 4,322 ± 1,955 | 1.745 (1.283-2.374) |
| Cmax (ng/ml) | 5,874 ± 1,637 | 8,719 ± 2,942 | 1.421 (1.213-1.665) |
| AUC12h (ng·h/ml) | 46,720 ± 15,430 | 74,590 ± 25,630 | 1.571 (1.281-1.926) |
| Ritonavir | |||
| No. of volunteers | 16b | 11 | |
| Tmax (h) | 4.5 (1.5-5.0) | 4.0 (1.0-6.0) | |
| C0h (ng/ml) | 293.6 ± 154.7 | 330.1 ± 252.1 | 1.114 (0.544-2.281) |
| Cmin (ng/ml) | 176.4 ± 87.56 | 233.2 ± 131.1 | 1.307 (0.877-1.946) |
| Cmax (ng/ml) | 918.0 ± 404.1 | 1,379 ± 640.5 | 1.682 (1.236-2.289) |
| AUC12h (ng·h/ml) | 5,394 ± 2,281 | 8,335 ± 3,772 | 1.657 (1.271-2.159) |
Tmax, time to maximum plasma concentration.
n = 15 volunteers for C0h.
Ritonavir pharmacokinetics.
Coadministration with RFB generally resulted in higher mean plasma concentrations of ritonavir than DRV/r alone. The PK parameters of ritonavir at steady state when given as DRV/r or as DRV/r plus RFB are shown in Table 1. Based on the ratios of the LS means, the Cmin, Cmax, and AUC12h values of ritonavir at steady state increased by 31% (−12 to 95%), 68% (24 to 129%), and 66% (27 to 116%), respectively, when DRV/r was coadministered with RFB.
RFB and desRFB pharmacokinetics.
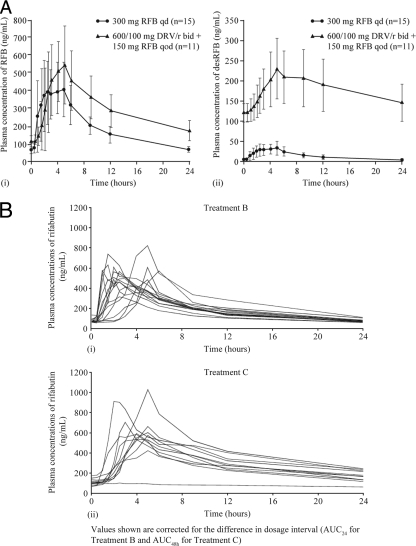
The presence of DRV/r increased the mean plasma concentrations of both RFB and desRFB (Fig. 3A). A similar increase was observed across different individuals, demonstrating reasonable interindividual consistency (Fig. 3B). Similar consistency of results across different individuals was found with desRFB, DRV, and ritonavir (data not shown).
FIG. 3.
(A) Mean plasma concentration-time curves (including SDs) of RFB (i) and desRFB (ii) after administration of RFB alone (treatment B, day 13) and in combination with DRV/r (treatment C; day 13). (B) Individual plasma concentration-time curves of RFB for each volunteer after administration of RFB alone (treatment B; day 13) and in combination with DRV/r (treatment C; day 13).
The PK parameters of RFB when given as RFB or as RFB plus DRV/r are shown in Table 2 . Based on the ratios of the LS means, the Cmin of RFB at steady state was increased by 64% (48 to 81%) when coadministered with DRV/r versus RFB alone; however, the Cmax decreased by 28% (−45 to −7%). The mean AUCτ in treatment C (corrected for dosing interval in treatment B) was comparable to 2 times the mean AUCτ in treatment B.
TABLE 2.
Pharmacokinetics of RFB and of desRFB after administration of RFB alone (treatment B) and in combination with DRV/r (treatment C)
| Drug, parameter | Pharmacokinetics [mean ± SD or Tmaxa median (range)] with treatment: |
LS means ratio (90% CI) | |
|---|---|---|---|
| B (RFB) | C (DRV/r + RFB) | ||
| RFB | |||
| No. of volunteers | 15 | 11 | |
| Tmax (h) | 2.5 (1.0-6.0) | 4.0 (2.0-5.0) | |
| C0h (ng/ml) | 67.54 ± 19.82 | 116.0 ± 31.92 | 1.730 (1.536-1.948) |
| Cmin (ng/ml) | 61.99 ± 14.23 | 111.8 ± 34.62 | 1.637 (1.481-1.808) |
| Cmax (ng/ml) | 565.2 ± 132.5 | 619.7 ± 242.3 | 0.717 (0.550-0.935) |
| AUCτb,c (ng·h/ml) | 4,659 ± 968.9 | 10,790 ± 3,171 | 0.933 (0.796-1.093) |
| desRFB | |||
| No. of volunteers | 15 | 11 | |
| Tmax (h) | 2.5 (1.5-6.0) | 5.0 (2.5-12.0) | |
| C0h (ng/ml) | 4.147 ± 1.133 | 121.0 ± 23.93 | 28.97 (23.90-35.11) |
| Cmin (ng/ml) | 3.684 ± 1.110 | 112.4 ± 26.62 | 27.10 (22.20-33.20) |
| Cmax (ng/ml) | 40.98 ± 15.83 | 236.5 ± 74.53 | 4.767 (4.040-5.625) |
| AUCτb,c (ng·h/ml) | 339.1 ± 116.7 | 7,497 ± 2,044 | 9.812 (8.087-11.90) |
| AUCτ,par+met (ng·h/ml)d | 5,016 ± 1,043 | 18,670 ± 4,623 | 1.55 (1.326-1.811) |
| Percentage ratio (AUCτ,par+met) | 7.315 ± 2.060 | 72.96 ± 19.51 | |
Tmax, time to maximum plasma concentration.
AUCτ is AUC24h for treatment B and AUC48h for treatment C.
τ (dosing interval) is 24 h for treatment B and 48 h for treatment C, before statistical analysis. AUCτ was corrected for the difference in dosage interval. All other PK parameters shown are absolute values.
n = 9 volunteers for both test and reference arms.
The PK parameters of desRFB when given as RFB or as RFB plus DRV/r are shown in Table 2. At steady state, the mean values for desRFB Cmin, Cmax, and AUCτ (corrected for dosing interval in treatment B) were increased by 2,610% (2,120 to 3,220%), 377% (304 to 463%), and 881% (709 to 1,090%), respectively, following concomitant DRV/r and RFB. AUCτ,par+met was increased 1.6-fold in the presence of DRV/r.
Safety and tolerability.
AEs were more commonly reported during combined treatment with DRV/r and RFB (treatment C) (15/18 [83%]) than with DRV/r (treatment A) (5/18 [28%]) or RFB (treatment B) (8/18 [44%]) alone (Table 3). The most common AEs (in ≥4 volunteers) were headache, diarrhea, back pain, pyrexia, and dizziness, of which headache, diarrhea, and back pain were considered at least possibly treatment related.
TABLE 3.
Safety summary
| Event | Incidence, n (%) (n = 18 volunteers/treatment) |
||
|---|---|---|---|
| A (DRV/r) | B (RFB) | C (DRV/r + RFB) | |
| Any adverse event | 5 (28) | 8 (44) | 15 (83) |
| ≥1 serious adverse event | 0 | 2 (11) | 2 (11) |
| ≥1 grade 3 or 4 adverse event | 0 | 2 (11) | 3 (17) |
| ≥1 adverse event leading to permanent discontinuation of trial medication | 0 | 2 (11) | 5 (28) |
| Laboratory abnormalities | |||
| ≥1 worst grade 3 or 4 laboratory abnormality | 1 (6) | 9 (53) | 3 (17) |
| ≥1 laboratory abnormality leading to permanent discontinuation | 0 | 2 (11) | 0 |
During DRV/r treatment alone, all AEs were grade 1 or 2. With RFB alone, two volunteers had a grade 4 SAE (both neutropenia), which led to discontinuation. During combined treatment with DRV/r and RFB, three volunteers reported grade 3 AEs (abdominal pain, maculopapular rash, and pyrexia), which led to discontinuation. The abdominal pain and maculopapular rash were considered by the investigator to be SAEs and doubtfully related and very likely related to the medication, respectively. Two volunteers with grade 2 AEs (hypersensitivity and maculopapular rash) also discontinued during combined treatment. All volunteers who discontinued the trial as a result of an SAE or AE recovered fully, and the condition resolved (albeit with some sequelae for the individual with abdominal pain). AEs of interest were reported in three volunteers, all of whom experienced maculopapular rash with onset at 2, 8, or 10 days after beginning combined DRV/r and RFB.
Most laboratory abnormalities were grade 1 or 2. The worst grade 3 or 4 abnormalities were observed during trial medication administration (1 in treatment A, 9 in treatment B, and 3 in treatment C) (Table 3), and these led to discontinuation in two patients (a grade 3 and a grade 4 decreased absolute lymphocyte count under treatment B). All treatment-emergent liver function abnormalities were grade 1. Increased alanine aminotransferase was reported for one volunteer during treatment A and for two volunteers each during treatments B and C.
In all nine patients who discontinued for either an AE or laboratory abnormality, the discontinuation occurred between 5 and 13 days after starting a treatment session containing RFB (monotherapy or combination).
DISCUSSION
Coadministration of DRV/r and RFB resulted in increased systemic exposure to both DRV and ritonavir; however, a dose adjustment for DRV/r is not considered to be warranted. This conclusion is based on the known safety profile of DRV/r, the lack of correlation between DRV/r pharmacokinetics and safety/tolerability in the phase IIb/III trials, and the fact that exposures of both compounds were generally comparable to those observed in previous drug interaction studies with DRV/r and other comedications (10, 18, 19, 21, 23, 24). The mechanism for the observed increase in exposure is unknown but may result from a transporter-based interaction (e.g., with SLCO1B1 or P-glycoprotein) or a complex interaction due to concomitant ritonavir, which may diminish the inductive capacity of RFB.
Systemic exposure to RFB was comparable between treatment with RFB alone (300 mg QD) and DRV/r plus RFB (150 mg QOD), while desRFB exposure was considerably increased following treatment with concomitant DRV/r and RFB. One possible explanation for the large extent of the increase in desRFB exposure is that higher concentrations of desRFB (caused by reduction of desRFB clearance through CYP3A4 inhibition by DRV and ritonavir) may inhibit the enzyme responsible for rifabutin deacylation (9).
The available PK interaction data for RFB combined with other boosted PIs generally show a slight increase in the exposure to the PI, an increase in RFB exposure and, consistent with the results of this study, a much larger increase in exposure to desRFB. Coadministration of atazanavir/r (ATV/r) and RFB 150 mg QOD resulted in substantial increases in the RFB Cmax (149%) and the desRFB Cmax (780%) (atazanavir concentrations were not assessed in this study) (28). When lopinavir/r (LPV/r) was coadministered with RFB, a slight increase in LPV concentration was observed; the concentrations of RFB and desRFB increased 3.0- and 47.5-fold, respectively, relative to the results for RFB 300 mg QD (15). When coadministered with RFB, fosamprenavir plasma concentration increased by approximately 35%. The RFB AUC0-48h remained unchanged, and the desRFB AUC0-48h increased 11-fold (7). A dose reduction of RFB of ≥75% (i.e., 150 mg every other day) is recommended when RFB is coadministered with ATV/r, LPV/r, or fosamprenavir/r (3). Some clinical studies of PIs, such as LPV/r, have found, however, that insufficient plasma drug levels of RFB are achieved with this reduced RFB dosage, with the concomitant risk of RFB therapeutic failure (5, 11). Nonetheless, in the present study, RFB exposure during DRV/r and RFB 150 mg QOD coadministration was comparable to that with RFB 300 mg QD alone. Hence, it is considered that the risk of RFB underdosing with 150 mg QOD during DRV/r coadministration is not significant.
Although a number of volunteers discontinued this trial, sufficient data were available for the pairwise PK comparisons of treatment A versus C and treatment B versus C, and therefore, it was not necessary to continue recruitment to ensure that a minimum of 13 volunteers completed all three treatment periods (as described in Materials and Methods). These data are therefore considered adequate for interpretation of the PK findings from this study.
The overall AE incidence was higher during coadministration of DRV/r and RFB than during DRV/r or RFB treatment alone. All volunteers who discontinued the trial for safety reasons did so during phases which included RFB administration. Furthermore, all AEs leading to discontinuation have been reported as AEs in clinical trials of patients treated with RFB (16). The relative contribution of RFB and its desRFB metabolite to observed AEs is unknown (8, 27).
Other studies have also reported an increased AE profile when RFB and boosted PIs are coadministered in healthy volunteers (7, 15, 28). Similar findings were observed in another study of healthy volunteers receiving a boosted PI and rifampin (3), where the incidence of liver-related AEs was higher than seen in HIV-1-infected patients in routine clinical management. While data suggest that rifabutin may be less well tolerated in healthy volunteers than in HIV-infected patients (2), a risk of similar toxicities in HIV-infected patients receiving rifabutin for Mycobacterium avium complex or tuberculosis cannot be ruled out.
In conclusion, while no data are available on concomitant use of DRV/r and RFB in HIV-infected patients, based on these results, DRV/r may be coadministered with RFB with a dose adjustment of RFB to 150 mg once every other day, consistent with recommendations for other boosted PIs. However, increased monitoring for RFB-related AEs is warranted in patients receiving the combination. Based on the overall safety profile for DRV/r, no dose adjustment of DRV is considered necessary. Given the safety profile seen with concomitant RFB (150 mg QOD) and a boosted PI in healthy volunteers in this and other studies (7, 15, 28), it is not recommended to conduct further studies with this combination in healthy volunteers.
Acknowledgments
We wish to acknowledge Catherine Elliott and Catherine McCarthy Bragg (Medical Writers, Gardiner-Caldwell Communications) for assistance in drafting the manuscript and collating author contributions.
This support, as well as the study, was funded by Tibotec Pharmaceuticals Ltd.; all authors were employees of Tibotec at the time of writing.
Footnotes
Published ahead of print on 26 July 2010.
REFERENCES
- 1.AIDS Clinical Trials Group. 2004. Division of AIDS table for grading the severity of adult and pediatric events. http://www.ucdmc.ucdavis.edu/clinicaltrials/documents/DAIDS_AE_GradingTable_FinalDec2004.pdf.
- 2.Apseloff, G. 2003. Severe neutropenia among healthy volunteers given rifabutin in clinical trials. Clin. Pharmacol. Ther. 74:591-592. [DOI] [PubMed] [Google Scholar]
- 3.Baciewicz, A. M., C. R. Chrisman, C. K. Finch, and T. H. Self. 2008. Update on rifampin and rifabutin drug interactions. Am. J. Med. Sci. 335:126-136. [DOI] [PubMed] [Google Scholar]
- 4.Bouche, M. P., L. Michielsen, M. Piot, and P. Timmerman. 2006. Swift and simultaneous determination of darunavir and ritonavir in human plasma using LC-MS/MS, abstr. TuP-042. 17th Int. Mass Spectrom. Conf., Prague, Czech Republic, 27 August to 1 September 2006.
- 5.Boulanger, C., E. Hollender, K. Farrell, J. J. Stambaugh, D. Maasen, D. Ashkin, S. Symes, L. A. Espinoza, R. O. Rivero, J. J. Graham, and C. A. Peloquin. 2009. Pharmacokinetic evaluation of rifabutin in combination with lopinavir-ritonavir in patients with HIV infection and active tuberculosis. Clin. Infect. Dis. 49:1305-1311. [DOI] [PubMed] [Google Scholar]
- 6.Clotet, B., N. Bellos, J. M. Molina, D. Cooper, J. C. Goffard, A. Lazzarin, A. Wöhrmann, C. Katlama, T. Wilkin, R. Haubrich, C. Cohen, C. Farthing, D. Jayaweera, M. Markowitz, P. Ruane, S. Spinosa-Guzman, and E. Lefebvre for the POWER 1 and 2 study groups. 2007. Efficacy and safety of darunavir-ritonavir at week 48 in treatment-experienced patients with HIV-1 infection in POWER 1 and 2: a pooled subgroup analysis of data from two randomised trials. Lancet 369:1169-1178. [DOI] [PubMed] [Google Scholar]
- 7.Ford, S. L., Y. C. Chen, Y. Lou, J. Borland, S. S. Min, G. J. Yuen, and M. J. Shelton. 2008. Pharmacokinetic interaction between fosamprenavir-ritonavir and rifabutin in healthy subjects. Antimicrob. Agents Chemother. 52:534-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hafner, R., J. Bethel, M. Power, B. Landry, M. Banach, L. Mole, H. C. Standiford, S. Follansbee, P. Kumar, R. Raasch, D. Cohn, D. Mushatt, and G. Drusano. 1998. Tolerance and pharmacokinetic interactions of rifabutin and clarithromycin in human immunodeficiency virus-infected volunteers. Antimicrob. Agents Chemother. 42:631-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamzeh, F. M., C. Benson, J. Gerber, J. Currier, J. McCrea, P. Deutsch, P. Ruan, H. Wu, J. Lee, and C. Flexner for the AIDS Clinical Trials Group 365 Study Team. 2003. Steady-state pharmacokinetic interaction of modified-dose indinavir and rifabutin. Clin. Pharmacol. Ther. 73:159-169. [DOI] [PubMed] [Google Scholar]
- 10.Hoetelmans, R. M., K. Mariën, M. De Pauw, A. Hill, M. Peeters, V. J. Sekar, P. De Doncker, B. Woodfall, and E. Lefebvre. 2007. Pharmacokinetic interaction between TMC114/ritonavir and tenofovir disoproxil fumarate in healthy volunteers. Br. J. Clin. Pharmacol. 64:655-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khachi, H., R. O'Connell, D. Ladenheim, and C. Orkin. 2009. Pharmacokinetic interactions between rifabutin and lopinavir/ritonavir in HIV-infected patients with mycobacterial co-infection. J. Antimicrob. Chemother. 64:871-873. [DOI] [PubMed] [Google Scholar]
- 12.Kraft, W. K., J. B. McCrea, G. A. Winchell, A. Carides, R. Lowry, E. J. Woolf, S. E. Kusma, P. J. Deutsch, H. E. Greenberg, and S. A. Waldman. 2004. Indinavir and rifabutin drug interactions in healthy volunteers. J. Clin. Pharmacol. 44:305-313. [DOI] [PubMed] [Google Scholar]
- 13.Madruga, J. V., D. Berger, M. McMurchie, F. Suter, D. Banhegyi, K. Ruxrungtham, D. Norris, E. Lefebvre, M. P. de Béthune, F. Tomaka, M. De Pauw, T. Vangeneugden, and S. Spinosa-Guzman for the TITAN study group. 2007. Efficacy and safety of darunavir-ritonavir compared with that of lopinavir-ritonavir at 48 weeks in treatment-experienced, HIV-infected patients in TITAN: a randomised controlled phase III trial. Lancet 370:49-58. [DOI] [PubMed] [Google Scholar]
- 14.Mills, A. M., M. Nelson, D. Jayaweera, K. Ruxrungtham, I. Cassetti, P. M. Girard, C. Workman, I. Dierynck, V. Sekar, C. Vanden Abeele, and L. Lavreys. 2009. Once-daily darunavir/ritonavir vs. lopinavir/ritonavir in treatment-naive, HIV-1-infected patients: 96-week analysis. AIDS 23:1679-1688. [DOI] [PubMed] [Google Scholar]
- 15.Ng, J., A. Nada, S. Freeman, Y. L. Chiu, D. Cohen, B. Bernstein, W. Awni, and C. Klein. 2009. Pharmacokinetics of rifabutin 150 mg TIW plus lopinavir/ritonavir 400/100 mg BID administered in healthy adult subjects, abstr. O21. 10th Int. Workshop Clin. Pharmacol. HIV Ther. Amsterdam, Netherlands, 15 to 17 April 2009.
- 16.Pfizer, Inc. 2007. Mycobutin (rifabutin) prescribing information. Pfizer, Inc., New York, NY. http://www.pfizer.com/files/products/uspi_mycobutin.pdf.
- 17.Polk, R. E., D. F. Brophy, D. S. Israel, R. Patron, B. M. Sadler, G. E. Chittick, W. T. Symonds, Y. Lou, D. Kristoff, and D. S. Stein. 2001. Pharmacokinetic interaction between amprenavir and rifabutin or rifampin in healthy males. Antimicrob. Agents Chemother. 45:502-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sekar, V. J., S. De Meyer, T. Vangeneugden, E. Lefebvre, M. De Pauw, B. Van Baelen, E. De Paepe, M. P. De Bethune, R. M. Hoetelmans, and W. Parys. 2006. Pharmacokinetic/pharmacodynamic (PK/PD) analyses of TMC114 in the POWER 1 and POWER 2 trials in treatment-experienced HIV-infected patients, abstr. J-121. 13th Conf. Retroviruses Opportunistic Infect., Denver, CO, 5 to 8 February 2006.
- 19.Sekar, V. J., E. De Paepe, B. Van Baelen, P. Vis, F. Tomaka, M. De Pauw, T. Vangeneugden, S. Spinosa-Guzman, and R. M. Hoetelmans. 2007. Pharmacokinetic/pharmacodynamic (PK/PD) analyses of darunavir in the TITAN study, abstr. P4.1/10. 11th Eur. AIDS Conf., Madrid, Spain, 24 to 27 October 2007.
- 20.Sekar, V., E. Lefebvre, T. De Marez, M. De Pauw, E. De Paepe, T. Vangeneugden, and R. M Hoetelmans. 2008. Effect of repeated doses of darunavir plus low-dose ritonavir on the pharmacokinetics of sildenafil in healthy male subjects: phase I randomized, open-label, two-way crossover study. Clin. Drug Invest. 28:479-485. [DOI] [PubMed] [Google Scholar]
- 21.Sekar, V. J., E. Lefebvre, T. De Marez, M. De Pauw, E. De Paepe, T. Vangeneugden, and R. M. Hoetelmans. 2010. Pharmacokinetic interaction between indinavir and darunavir with low-dose ritonavir in healthy volunteers. Intervirology 53:176-182. [DOI] [PubMed] [Google Scholar]
- 22.Sekar, V. J., E. Lefebvre, M. De Pauw, T. Vangeneugden, and R. Hoetelmans. 2008. Pharmacokinetics of darunavir/ritonavir and ketoconazole following co-administration in HIV-healthy volunteers. Br. J. Clin. Pharm. 66:215-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sekar, V. J., E. Lefebvre, K. Marien, M. De Pauw, T. Vangeneugden, A. Pozniak, and R. M. Hoetelmans. 2009. Pharmacokinetic interaction between nevirapine and darunavir with low-dose ritonavir in HIV-1 infected patients. Br. J. Clin. Pharmacol. 68:116-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sekar, V. J., C. Vanden Abeele, B. Van Baelen, P. Vis, L. Lavreys, M. De Pauw, S. Dincq, T. Vangeneugden, S. Spinosa-Guzman, and R. M. Hoetelmans. 2008. Pharmacokinetic-pharmacodynamic analyses of once-daily darunavir in the ARTEMIS study, abstr. 769. 15th Conf. Retroviruses Opportunistic Infect., Boston, MA, 3 to 6 February 2008.
- 25.Tibotec. 2010. Prezista (darunavir) full prescribing information. Tibotec, Raritan, NJ. http://www.prezista.com/prezista/documents/us_package_insert.pdf.
- 26.Tibotec. 2009. Prezista (darunavir) summary of product characteristics. Janssen-Cilag International NV, Beerse, Belgium. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000707/human_med_000988.jsp&murl=menus/medicines/medicines.jsp.
- 27.Trapnell, C. B., C. Jamis-Dow, R. W. Klecker, and J. M. Collins. 1997. Metabolism of rifabutin and its 25-desacetyl metabolite, LM565, by human liver microsomes and recombinant human cytochrome P-450 3A4: relevance to clinical interaction with fluconazole. Antimicrob. Agents Chemother. 41:924-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu, L., L. Mahnke, Y. Wu, M. Stonier, B. He, J. Coumbis, X. Xu, R. Bertz, and J. Zhang. 2009. Pharmacokinetic interactions between atazanavir/ritonavir and intermittent doses of rifabutin in healthy subjects, abstr. O22. 10th Int. Workshop Clin. Pharmacol. HIV Ther. Amsterdam, Netherlands, 15 to 17 April 2009.