Abstract
Malaria infection is initiated by Plasmodium sporozoites infecting the liver. Preventing sporozoite infection would block the obligatory first step of the infection and perhaps reduce disease severity. In addition, such an approach would decrease Plasmodium vivax hypnozoite formation and therefore disease relapses. Here we describe the activity of a trisubstituted pyrrole, 4-[2-(4-fluorophenyl)-5-(1-methylpiperidine-4-yl)-1H-pyrrol-3-yl] pyridine, in inhibiting motility, invasion, and consequently infection by P. berghei sporozoites. In tissue culture, the compound was effective within the first 3 h of sporozoite addition to HepG2 cells. In vivo, intraperitoneal administration of the compound significantly inhibited liver-stage parasitemia in P. yoelii sporozoite-infected mice and prevented the appearance of blood-stage parasites. P. berghei sporozoites lacking the parasite cGMP-dependent protein kinase, the primary target of the compound in erythrocyte-stage parasites, remained infectious to HepG2 cells and sensitive to the drug. These results suggest that the drug has an additional target(s) in sporozoites. We propose that drugs that inhibit sporozoite infection offer a feasible approach to malaria prophylaxis.
The malaria parasite, Plasmodium spp., infects 500 million people a year, killing over 1 million globally. Plasmodium parasites are introduced into humans via mosquito bites, in the form of sporozoites that infect the liver to form liver-stage parasites (LS). LS are eventually released into the bloodstream, where they infect erythrocytes and initiate the symptomatic phase of malaria. Although the liver stage of infection by Plasmodium falciparum and P. vivax, the two major human parasites, is asymptomatic, P. vivax infections often result in the formation of dormant LS called “hypnozoites.” When reactivated, hypnozoites cause disease relapses up to 1 year after the initial infection. Therefore, achieving global eradication of malaria will require prevention or treatment of P. vivax hypnozoites in addition to treatment of erythrocyte stages of both species.
The spread of drug-resistant parasites, limited vector control measures, and lack of an effective vaccine make novel drug discovery vital. Drugs that prevent sporozoite infection of the liver would block an obligatory step in the parasite's life cycle. They would also prevent the formation of hypnozoites by P. vivax and the pathology caused by the erythrocyte stages of all human Plasmodium species. Although the liver stages are asymptomatic, reducing the parasite burden in the liver is likely to significantly diminish blood-stage infection and reduce disease severity in a subpopulation of patients (1). Furthermore, the small number of parasites and the limited number of replications during the liver phase of malaria infection compared to those in the erythrocytic phase reduces the potential for the emergence of drug-resistant parasites (13). However, of the currently licensed drugs, only primaquine has been formally demonstrated to act against sporozoites (13, 22).
Here, we investigate the efficacy of a trisubstituted pyrrole, 4-[2-(4-fluorophenyl)-5-(1-methylpiperidine-4-yl)-1H-pyrrol-3-yl] pyridine, against Plasmodium sporozoites. This compound was previously shown to inhibit the in vitro development of several apicomplexan parasites (11). In vitro, the trisubstituted pyrrole blocks the development of late-erythrocyte-stage schizonts of P. falciparum (23). In vivo, it is partially effective against erythrocyte stages, lengthening the survival of mice infected with lethal strains of P. berghei (8).
Here, we tested the effect of the trisubstituted pyrrole on sporozoites from the rodent parasite species P. berghei and P. yoelii. The compound significantly inhibited the infection of hepatocytes by Plasmodium sporozoites. In vitro, this inhibition was dose dependent and was observed within the first 3 h of sporozoite addition to HepG2 cells. Neither the treatment of HepG2 cells with the trisubstituted pyrrole prior to the addition of sporozoites nor the addition of the trisubstituted pyrrole to cell culture after infection by sporozoites decreased infection. While sporozoite motility was partially inhibited by the trisubstituted pyrrole, sporozoite invasion of HepG2 cells could be completely blocked in a dose-dependent manner. In vivo, administration of a single dose of the trisubstituted pyrrole caused a significant reduction in the liver-stage parasitemia of mice infected with P. yoelii sporozoites and led to partial protection against the appearance of blood-stage parasites. A higher dose of the trisubstituted pyrrole completely prevented the appearance of blood-stage parasites in a sporozoite-initiated infection. The compound's inhibition of sporozoite infection was not mediated solely through the drug's effect on P. berghei cGMP-dependent protein kinase (PbPKG). Sporozoites lacking PbPKG remained sensitive to the trisubstituted pyrrole. Our data suggest that the trisubstituted pyrrole might have additional targets in sporozoites.
MATERIALS AND METHODS
Parasite maintenance and sporozoite collection.
Uncloned lines of P. berghei NK65, P. yoelii 17XNL, and P. berghei ANKA parasites were grown in Anopheles stephensi mosquitoes. Sporozoites were obtained by dissection of salivary glands in Dulbecco's modified Eagle's medium (DMEM). P. yoelii and P. berghei ANKA sporozoites were used for the in vivo assays. P. berghei NK65 sporozoites were used for all other assays.
In vivo assay for sporozoite infection by determining prepatent period.
Female Swiss-Webster mice (6 to 8 weeks old) were treated intraperitoneally either with 50 mg/kg of body weight of the trisubstituted pyrrole diluted in phosphate-buffered saline (PBS) or with PBS alone. In the first experiment, mice (n = 3) were administered the trisubstituted pyrrole or PBS once prior to an intravenous injection of P. yoelii sporozoites (1 × 104/mouse). The appearance of blood-stage parasites was monitored daily by microscopic examination of Giemsa-stained blood smears. In the second experiment, mice (n = 10) were administered three doses of either the trisubstituted pyrrole or PBS. The first dose of the trisubstituted pyrrole was given 15 min prior to an intravenous infection with P. yoelii sporozoites (5 × 103/mouse). The second and third doses were given 6 h and 12 h postinfection (p.i.), respectively. The prepatent period of infection was determined as described above. In the third experiment, female C57BL/6 mice (4 to 6 weeks old) (n = 3) were infected through bite of mosquitoes infected with P. berghei ANKA (approximately 10 mosquitoes/animal). They were administered three doses of either the trisubstituted pyrrole or PBS as described for the second experiment.
Assay for liver infection by sporozoites, carried out using quantitative real-time PCR.
Female Swiss-Webster mice (6 to 8 weeks old) (n = 4) were treated intraperitoneally with a single dose of either 50 mg/kg of the trisubstituted pyrrole or PBS. They were infected intravenously with P. yoelii sporozoites (1 × 104/mouse). Livers were dissected 40 h p.i. and homogenized in TRIzol reagent (Invitrogen), and total liver RNA was obtained following the manufacturer's protocol. First-strand cDNA synthesis was carried out and parasite load in the liver was quantified as previously described (3), with duplicate samples for each mouse, using P. yoelii 18S rRNA-specific primers (5′ GGGGATTGGTTTTGACGTTTTTGCG and 5′ AAGCATTAAATAAAGCGAATACATCCTTAT). The quantitative real-time PCR conditions used were as follows: 95°C for 15 min and 50 cycles of 95°C for 20 s, 60°C for 30 s, and 72°C for 30 s, followed by 72°C for 30 s. Results were analyzed using the Mann-Whitney two-tailed test.
Cell culture of HepG2 cells.
HepG2 cells were grown in DMEM containing 10% fetal calf serum and 1% penicillin-streptomycin. Cells (1.8 × 105/well) were seeded in 8-chamber Lab-Tek slides 1 day prior to the addition of sporozoites.
In vitro assay for sporozoite infection.
Sporozoites (5 × 104/well) were added to HepG2 cells in the presence of 0.5 μM, 2 μM, or 10 μM trisubstituted pyrrole, in a final volume of 200 μl. The trisubstituted-pyrrole-containing medium was replaced with compound-free medium 12 h p.i. To detect LS at 40 h p.i., cells were treated with cold methanol, washed with PBS, blocked for 1 h at 37°C with PBS-1% bovine serum albumin (BSA), stained with 10 μg/ml monoclonal antibody (MAb) 2E6 (24) for 1 h at 37°C, and washed with PBS, followed by staining with a 1:100 dilution of anti-mouse IgG conjugated with fluorescein isothiocyanate (FITC) for 1 h at 37°C. LS were counted using a fluorescence microscope with a 40× objective.
Assay for sporozoite gliding motility.
An 8-well Lab-Tek slide was coated with 10 μg/ml of monoclonal antibody (MAb) 3D11 directed against the repeat region of the P. berghei circumsporozoite protein (25), incubated overnight at 4°C, and washed thrice with PBS. Sporozoites (1 × 104/well) were added to each well in the presence of 0.5 μM, 2 μM, or 10 μM trisubstituted pyrrole, in a final volume of 200 μl DMEM containing 3% BSA. The slide was incubated at 37°C for 2 h. At the end of the incubation, the medium was removed, and wells were fixed with 4% paraformaldehyde for 30 min, washed with PBS, and blocked with PBS-1% BSA for 1 h at 37°C in a final volume of 200 μl. Trails deposited by motile sporozoites were visualized by staining with biotinylated 3D11 (5) for 1 h at 37°C, followed by washes with PBS and staining with streptavidin conjugated to FITC for 1 h at 37°C.
Assay for cell invasion by sporozoites.
HepG2 cells were infected with sporozoites (5 × 104 sporozoites/well) in the presence of 0.5 μM, 2 μM, or 10 μM trisubstituted pyrrole for 1 h at 37°C. Cells were fixed with 4% paraformaldehyde and stained as previously described (21) to distinguish between intracellular and extracellular sporozoites.
RESULTS
Inhibition of in vitro infection of hepatocytes by P. berghei sporozoites.
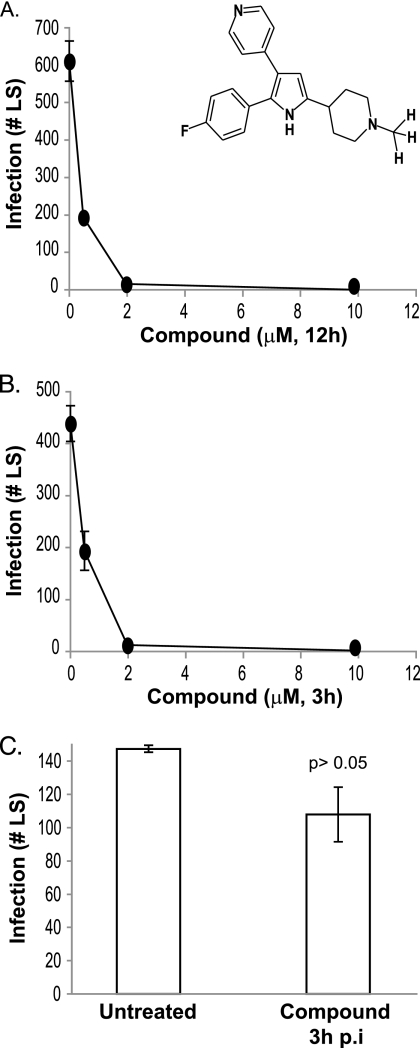
To test whether the trisubstituted pyrrole affects sporozoite infection of hepatocytes, P. berghei sporozoites were allowed to infect the human hepatoma cell line HepG2 in the presence of different concentrations of the compound. The compound was removed 12 h postinfection (p.i.). Sporozoite infectivity was measured by quantifying the numbers of intracellular LS that developed in cell cultures 40 h p.i. The trisubstituted pyrrole inhibited the transformation of sporozoites into LS in a dose-dependent manner, with a concentration of 2 μM decreasing LS numbers to below detection (Fig. 1A).
FIG. 1.
A trisubstituted pyrrole (inset) inhibits the development of P. berghei sporozoites into LS in HepG2 cells. (A) Dose-dependent effect of the trisubstituted pyrrole on P. berghei sporozoite development into LS (40 h p.i.) in HepG2 cells, when present for the first 12 h of infection. (B) Dose-dependent effect of the trisubstituted pyrrole on P. berghei sporozoite development into LS (40 h p.i.) in HepG2 cells, when present for the first 3 h of infection. (C) Effect on sporozoites of addition of 10 μM trisubstituted pyrrole to HepG2 cells 3 h p.i. Each experiment was done at least thrice, each time in duplicate. Results of a representative experiment are shown. Error bars represent standard deviations (P > 0.05 relative to untreated group, t test).
In our model, the observed reduction in sporozoite infection could result from the inhibition of several steps that constitute the infection process—sporozoite motility, host cell invasion, or intravacuolar development of the parasite. To determine which of these steps the trisubstituted pyrrole inhibits, we determined the timing of the compound's action. We infected cells with sporozoites in the presence of 2 μM compound and then removed the compound-containing medium 3 h postinfection. The trisubstituted pyrrole inhibited LS numbers to similar extents when present for 3 h or 12 h (Fig. 1B). Since sporozoite invasion of host cells is generally complete within 2 to 3 h, these results suggest that the trisubstituted pyrrole inhibits sporozoite motility and/or invasion of host cells rather than the intravacuolar development of the parasite. To directly test the effect of the compound on the intravacuolar development of the parasite, we added 10 μM compound to cells 3 h after the addition of sporozoites. This concentration of the trisubstituted pyrrole, when present at the time of sporozoite addition to cells, completely blocked the transformation of sporozoites into LS (Fig. 1B). However, when added to cells 3 h after the addition of sporozoites, 10 μM compound did not affect the number of LS present 40 h postinfection (Fig. 1C). Taken together, these results suggest that the trisubstituted pyrrole inhibits sporozoite motility or sporozoite invasion of host cells or very early steps of LS formation rather than the later intracellular steps of LS development.
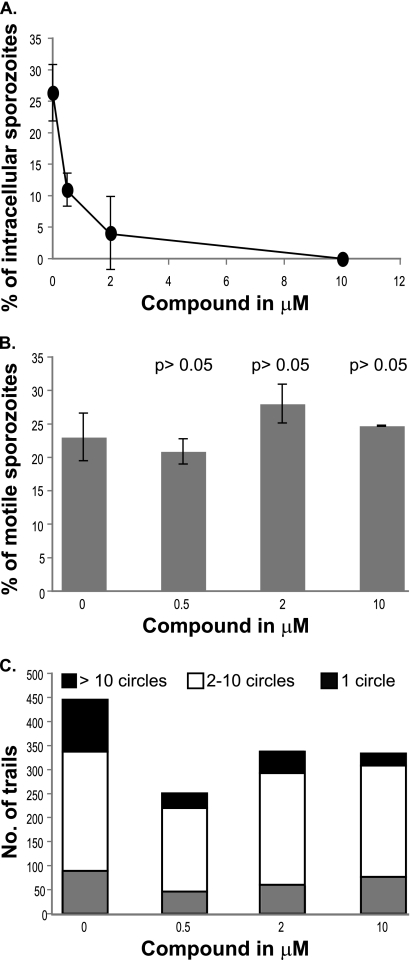
The trisubstituted pyrrole inhibits host cell invasion by P. berghei sporozoites.
We assayed the effect of the trisubstituted pyrrole on sporozoite invasion of host cells by quantifying the number of sporozoites that were intracellular 1 h after the compound was added to host cells. The compound inhibited sporozoite invasion in a dose-dependent manner similarly to its inhibition of sporozoite infection, with a 50% inhibitory concentration (IC50) below 1 μM (Fig. 2A). Since the trisubstituted pyrrole might block sporozoite invasion by blocking sporozoite motility, we assayed the number of sporozoites that deposited proteinaceous trails in the presence of different doses of the compound. The trisubstituted pyrrole did not affect the percentage of sporozoites that were motile (Fig. 2B). Next, we determined the quality of trails deposited by the motile sporozoites by determining the complexity of each deposited trail. The trisubstituted pyrrole caused a 50% decrease in the number of complex trails, defined as trails with over 10 circles (P < 0.05 relative to untreated group, t test). However, this decrease was not dose dependent (Fig. 2C). Therefore, we conclude that the trisubstituted pyrrole has a partial effect on sporozoite motility.
FIG. 2.
A trisubstituted pyrrole inhibits invasion of host cells by P. berghei sporozoites. (A) Dose-dependent inhibition of P. berghei sporozoite invasion of HepG2 cells by the trisubstituted pyrrole. (B) Effect of the trisubstituted pyrrole on the motility of P. berghei sporozoites (P > 0.05 relative to untreated group, t test). (C) Effect of the trisubstituted pyrrole on the quality of trails deposited by P. berghei sporozoites. Each experiment was done at least thrice, each time in duplicate. Results from a representative experiment are shown. Error bars represent standard deviations.
The trisubstituted pyrrole inhibits murine infection by P. yoelii and P. berghei sporozoites.
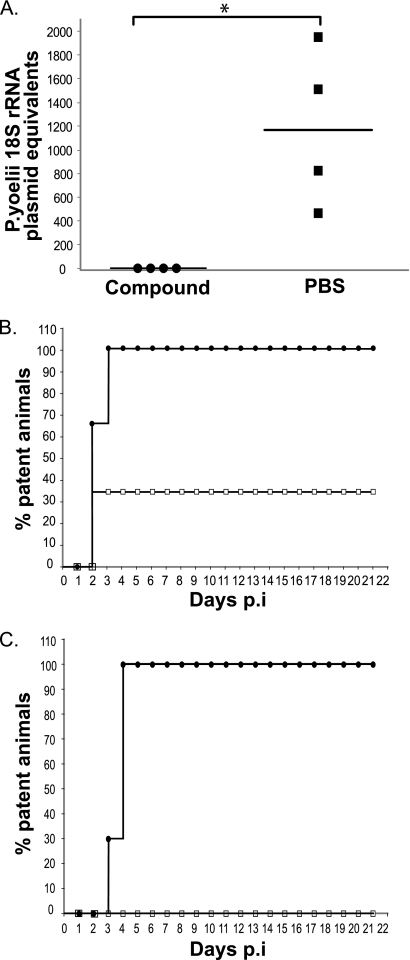
To determine whether the trisubstituted pyrrole is effective in blocking sporozoite infection in vivo, we quantified the parasite load in the livers of mice treated with either a single 50 mg/kg dose of the compound or PBS before infection with 1 × 104 P. yoelii sporozoites. We used P. yoelii for these experiments since P. yoelii sporozoites are more infectious to mice than P. berghei. In the compound-treated mice, the parasite burden in the liver was reduced by more than 1,000-fold compared to that for PBS-treated mice (P < 0.05) (Fig. 3A).
FIG. 3.
A trisubstituted pyrrole inhibits the development of P. yoelii sporozoites into LS in vivo and the appearance of blood-stage parasites. (A) Parasite burden in the livers of mice infected with P. yoelii sporozoites (1 × 104/mouse) and treated with a single dose of either the trisubstituted pyrrole or PBS (n = 4 for each group). Results at 40 h p.i. were determined by parasite-specific quantitative PCR. Infection is expressed as the numbers of copies of P. yoelii 18S rRNA plasmid (*, P = 0.0294 relative to PBS-treated group; Mann-Whitney test). (B) Treatment with a single dose of the trisubstituted pyrrole partially protects from sporozoite infection. Animals were infected with P. yoelii sporozoites (1 × 104/mouse) after receiving a single dose of either the trisubstituted pyrrole (open squares) (n = 3) or PBS (filled circles) (n = 3). (C) Percentages of animals that were positive for blood-stage infection after infection with P. yoelii sporozoites (5 × 103/mouse) and treatment with three doses of either the trisubstituted pyrrole (open squares) (n = 10) or PBS (filled circles) (n = 10).
Next, we determined whether the trisubstituted-pyrrole-mediated reduction in liver parasitemia delayed or eliminated blood-stage infection. In the first experiment, we measured the prepatent period of infection in mice that were treated with a single dose of the trisubstituted pyrrole (50 mg/kg) or PBS and infected intravenously with 1 × 104 P. yoelii sporozoites. While all three PBS-treated mice became positive (average [±standard deviation] prepatent period, 2.33 ± 0.57 days), only one of three compound-treated mice became patent (prepatent period, 4 days) during the course of the experiment (Fig. 3B). A delay in patency of 1 day correlated with a 10-fold decrease in liver infection by sporozoites (10). Therefore, the single animal that became patent after treatment with the trisubstituted pyrrole experienced a significant decrease in liver-stage infection. In the second experiment, 10 infected mice (5 × 103 sporozoites/mouse) were administered three doses of the trisubstituted pyrrole (50 mg/kg). At this higher dose, the compound prevented the appearance of blood-stage parasites in all 10 mice for at least 3 weeks, at which time the experiment was terminated (Fig. 3C). In contrast, all 10 control mice became infected, with an average prepatent period of 3.7 ± 0.48 days. These results were further confirmed with P. berghei. Mice were infected with P. berghei through mosquito bite, the natural route of sporozoite transmission. Treatment with the trisubstituted pyrrole resulted in a significantly longer average prepatent period (5.33 ± 1.52 days) than that for the mice treated with PBS (2.66 ± 0.57 days). These results strongly suggest that the trisubstituted pyrrole effectively blocks P. yoelii and P. berghei sporozoite infection in vivo, which results in a significant delay or abrogation of the ensuing blood-stage infection.
Sporozoites lacking PbPKG remain sensitive to the trisubstituted pyrrole.
In P. falciparum gametocytes and erythrocytic-stage parasites (14, 23), the primary target of the trisubstituted pyrrole is the parasite cGMP-dependent protein kinase (PKG). Substitution of a key threonine residue (T618Q) in the putative trisubstituted-pyrrole-binding pocket of P. falciparum PKG (PfPKG) renders the mutant kinase insensitive to the compound (14). Transgenic P. falciparum erythrocytic-stage parasites or gametocytes expressing the mutant PKG protein were 10- to 20-fold less sensitive to the trisubstituted pyrrole than the wild type (23).
In order to test whether the trisubstituted-pyrrole-mediated inhibition of sporozoite infectivity results from inhibition of P. berghei PKG (PbPKG), we attempted to generate transgenic P. berghei parasites in which the cognate threonine residue (T617) required for binding to the trisubstituted pyrrole was replaced with either methionine or glutamine. Despite extensive efforts, we recovered only parasites in which the mutation was corrected, i.e., the targeting vector had undergone the desired integration event but the PbPKG sequence was wild type (data not shown). The failure to recover the desired PbPKG mutants and our success in manipulating the PbPKG locus in other ways (9) strongly suggest that, unlike in P. falciparum, these mutations are lethal in P. berghei asexual stages. It is likely that the difference between P. falciparum and P. berghei is that PKG mutations that can be tolerated by parasites in vitro are lethal in parasites growing in animals.
As an alternative approach, we used conditional mutagenesis to test whether PbPKG is the primary target of the trisubstituted pyrrole in sporozoites. Using an Flp/FRT (flippase/flippase recognition target sequence)-based system, we generated PbPKG mutants (PbPKG cKO parasites) in which the PbPKG locus is normal in erythrocyte stages but is deleted in sporozoites (9). As a control, we generated parasites in which PKG is normally expressed at all stages (CON parasites) (9).
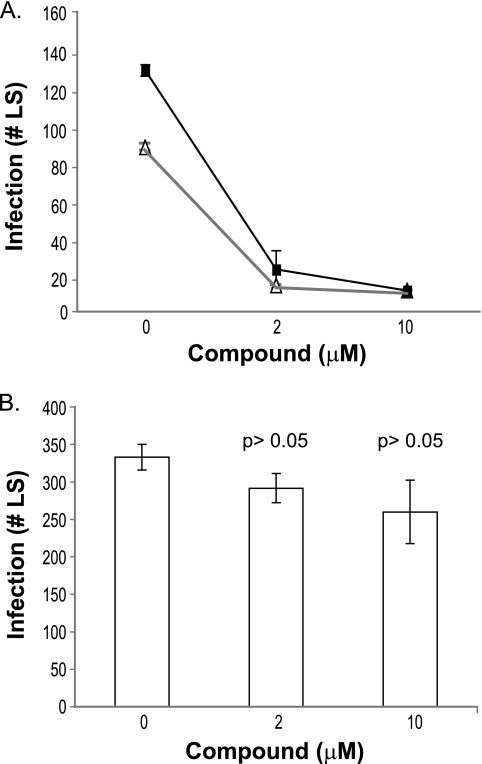
PbPKG cKO parasites produced viable sporozoites in numbers comparable to those of CON parasites (9). When used to infect HepG2 cells, the cKO and CON sporozoites developed into similar numbers of LS (Fig. 4A) (9). Further, the PbPKG cKO and control sporozoites were sensitive to the trisubstituted pyrrole (Fig. 4A). These results raise the possibility that the trisubstituted pyrrole's inhibition of sporozoite infectivity is not mediated solely via its effect on PbPKG and that the compound has additional targets in either host cells or sporozoites. To test whether the trisubstituted pyrrole inhibits a host protein, we treated HepG2 cells with different concentrations of the compound. After 3 h, the cells were washed with plain medium prior to addition of sporozoites. Pretreatment of cells with the trisubstituted pyrrole did not decrease the number of LS that developed 40 h postinfection (Fig. 4B). This result is consistent with the interpretation that the trisubstituted pyrrole inhibits sporozoite infection by interfering with another sporozoite protein rather than host proteins.
FIG. 4.
P. berghei sporozoites lacking PbPKG remain sensitive to the trisubstituted pyrrole. (A) PbPKG cKO (filled squares) and CON (open triangles) sporozoites were used to infect HepG2 cells in the presence of the trisubstituted pyrrole. The experiment was repeated twice, each time in duplicate. The results of a representative experiment are shown. Error bars represent standard deviations. (B) Effect on sporozoites of pretreatment of HepG2 cells with the trisubstituted pyrrole (P > 0.05 relative to untreated group, t test).
DISCUSSION
In the work described here, we show that the trisubstituted pyrrole inhibits sporozoite infection both in vitro and in vivo. In vitro results indicate that the inhibition is a result of blocking sporozoite attachment to and invasion of host cells. In vivo, treatment of sporozoite-infected mice with the trisubstituted pyrrole prevented the appearance of blood-stage parasites. The dose used in these experiments was previously shown to be nontoxic to animals (11, 17). Inhibition of cell invasion might be due in part to the compound's effect on sporozoite motility. Indeed, the trisubstituted pyrrole is a potent inhibitor of P. berghei ookinete motility, with an IC50 of less than 100 nM (16). In contrast, in our in vitro assays, the percentage of motile sporozoites remained unchanged even in the presence of 10 μM trisubstituted pyrrole, although the formation of complicated trails was reduced. One explanation of these results is that the residual motility seen in our assays may not be biologically relevant and that the partial reduction seen in vitro translates into an effective block in vivo. Another explanation is that the trisubstituted pyrrole's block of sporozoite invasion results from the inhibition of other processes, such as protein secretion or junction formation between the invading sporozoite and the host cell. Current work is focused on distinguishing these possibilities.
An intriguing question raised by our results is whether or not the trisubstituted pyrrole's effect is mediated solely through PbPKG, its primary target in erythrocyte and gametocyte stages. Since the phenotype of PbPKG cKO sporozoites is not the same as that of the trisubstituted pyrrole-treated sporozoites, the trisubstituted pyrrole may inhibit an additional sporozoite kinase with functional overlap with the PbPKG pathway. It is also possible that PbPKG cKO sporozoites retain PbPKG activity, due to the presence of residual amounts of protein, which allows them to carry out normal invasion and infection in the absence of the trisubstituted pyrrole. Another explanation is that the trisubstituted pyrrole's additional target is a host protein. The trisubstituted pyrrole inhibits the growth and development of some Toxoplasma gondii strains by acting directly on the host cell (20). If the trisubstituted pyrrole's effect on the host protein is reversible, then the pretreatment of HepG2 cells with the compound may not decrease sporozoite infectivity.
Of the currently used antimalarials, only atovaquone and primaquine are effective against Plasmodium preerythrocyte stages, and most have not been formally tested against sporozoites (7, 13). Given that blocking sporozoite infection can prevent the ensuing blood infection, there is an urgent need to fill this gap. Several groups have recently explored inhibition of preerythrocyte stages, through inhibition of either sporozoite infection or liver-stage parasite development, as a form of malaria prophylaxis (4-6, 12, 18). Such prophylaxis could be combined with a therapeutic step, such as inhibiting erythrocyte-stage parasites, to provide multipronged chemotherapy against malaria. Such an approach has several advantages over an exclusively therapeutic strategy. At the time of infection by mosquito bite, the parasite load is lower—an average mosquito bite is thought to introduce fewer than 100 sporozoites (2, 15, 19)—than that during the liver and erythrocyte stages of the disease. The lower parasite burden associated with sporozoites should make their eradication easier. The limited replication of the parasite at the liver stage, as opposed to repeated rounds of replication in erythrocytes, decreases the potential for emergence of drug-resistant parasites. Even if the prophylaxis does not completely abolish liver-stage parasite development, the reduction in infection will lead to a decrease in erythrocyte-stage parasitemia and therefore disease severity (1).
Acknowledgments
We thank K. Drlica and P. Sinnis for helpful comments on the manuscript, and we thank K. Gopinatham for technical assistance.
This work was supported by the American Heart Association (grant 0735036N to P.B.).
Footnotes
Published ahead of print on 19 July 2010.
REFERENCES
- 1.Alonso, P. L., J. Sacarlal, J. J. Aponte, A. Leach, E. Macete, P. Aide, B. Sigauque, J. Milman, I. Mandomando, Q. Bassat, C. Guinovart, M. Espasa, S. Corachan, M. Lievens, M. M. Navia, M. C. Dubois, C. Menendez, F. Dubovsky, J. Cohen, R. Thompson, and W. R. Ballou. 2005. Duration of protection with RTS,S/AS02A malaria vaccine in prevention of Plasmodium falciparum disease in Mozambican children: single-blind extended follow-up of a randomised controlled trial. Lancet 366:2012-2018. [DOI] [PubMed] [Google Scholar]
- 2.Beier, J. C., M. S. Beier, J. A. Vaughan, C. B. Pumpuni, J. R. Davis, and B. H. Noden. 1992. Sporozoite transmission by Anopheles freeborni and Anopheles gambiae experimentally infected with Plasmodium falciparum. J. Am. Mosq. Control Assoc. 8:404-408. [PubMed] [Google Scholar]
- 3.Bruna-Romero, O., J. C. Hafalla, G. Gonzalez-Aseguinolaza, G. Sano, M. Tsuji, and F. Zavala. 2001. Detection of malaria liver-stages in mice infected through the bite of a single Anopheles mosquito using a highly sensitive real-time PCR. Int. J. Parasitol. 31:1499-1502. [DOI] [PubMed] [Google Scholar]
- 4.Carraz, M., A. Jossang, J. F. Franetich, A. Siau, L. Ciceron, L. Hannoun, R. Sauerwein, F. Frappier, P. Rasoanaivo, G. Snounou, and D. Mazier. 2006. A plant-derived morphinan as a novel lead compound active against malaria liver stages. PLoS Med. 3:e513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coppi, A., M. Cabinian, D. Mirelman, and P. Sinnis. 2006. Antimalarial activity of allicin, a biologically active compound from garlic cloves. Antimicrob. Agents Chemother. 50:1731-1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cunha-Rodrigues, M., S. Portugal, M. Prudencio, L. A. Goncalves, C. Casalou, D. Buger, R. Sauerwein, W. Haas, and M. M. Mota. 2008. Genistein-supplemented diet decreases malaria liver infection in mice and constitutes a potential prophylactic strategy. PLoS One 3:e2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies, C. S., M. Pudney, J. C. Nicholas, and R. E. Sinden. 1993. The novel hydroxynaphthoquinone 566C80 inhibits the development of liver stages of Plasmodium berghei cultured in vitro. Parasitology 106(Pt. 1):1-6. [DOI] [PubMed] [Google Scholar]
- 8.Diaz, C. A., J. Allocco, M. A. Powles, L. Yeung, R. G. Donald, J. W. Anderson, and P. A. Liberator. 2006. Characterization of Plasmodium falciparum cGMP-dependent protein kinase (PfPKG): antiparasitic activity of a PKG inhibitor. Mol. Biochem. Parasitol. 146:78-88. [DOI] [PubMed] [Google Scholar]
- 9.Falae, A., A. Combe, A. Anburaj, T. G. Carvalho, R. Menard, and P. Bhanot. 2009. The role of Plasmodium berghei cGMP dependent protein kinase in late liver stage development. J. Biol. Chem. 285:3282-3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gantt, S. M., J. M. Myung, M. R. Briones, W. D. Li, E. J. Corey, S. Omura, V. Nussenzweig, and P. Sinnis. 1998. Proteasome inhibitors block development of Plasmodium spp. Antimicrob. Agents Chemother. 42:2731-2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gurnett, A. M., P. A. Liberator, P. M. Dulski, S. P. Salowe, R. G. Donald, J. W. Anderson, J. Wiltsie, C. A. Diaz, G. Harris, B. Chang, S. J. Darkin-Rattray, B. Nare, T. Crumley, P. S. Blum, A. S. Misura, T. Tamas, M. K. Sardana, J. Yuan, T. Biftu, and D. M. Schmatz. 2002. Purification and molecular characterization of cGMP-dependent protein kinase from apicomplexan parasites. A novel chemotherapeutic target. J. Biol. Chem. 277:15913-15922. [DOI] [PubMed] [Google Scholar]
- 12.Mahmoudi, N., R. Garcia-Domenech, J. Galvez, K. Farhati, J. F. Franetich, R. Sauerwein, L. Hannoun, F. Derouin, M. Danis, and D. Mazier. 2008. New active drugs against liver stages of Plasmodium predicted by molecular topology. Antimicrob. Agents Chemother. 52:1215-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mazier, D., L. Renia, and G. Snounou. 2009. A pre-emptive strike against malaria's stealthy hepatic forms. Nat. Rev. Drug Discov. 8:854-864. [DOI] [PubMed] [Google Scholar]
- 14.McRobert, L., C. J. Taylor, W. Deng, Q. L. Fivelman, R. M. Cummings, S. D. Polley, O. Billker, and D. A. Baker. 2008. Gametogenesis in malaria parasites is mediated by the cGMP-dependent protein kinase. PLoS Biol. 6:e139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Medica, D. L., and P. Sinnis. 2005. Quantitative dynamics of Plasmodium yoelii sporozoite transmission by infected anopheline mosquitoes. Infect. Immun. 73:4363-4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moon, R. W., C. J. Taylor, C. Bex, R. Schepers, D. Goulding, C. J. Janse, A. P. Waters, D. A. Baker, and O. Billker. 2009. A cyclic GMP signalling module that regulates gliding motility in a malaria parasite. PLoS Pathog. 5:e1000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nare, B., J. J. Allocco, P. A. Liberator, and R. G. Donald. 2002. Evaluation of a cyclic GMP-dependent protein kinase inhibitor in treatment of murine toxoplasmosis: gamma interferon is required for efficacy. Antimicrob. Agents Chemother. 46:300-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parvanova, I., S. Epiphanio, A. Fauq, T. E. Golde, M. Prudencio, and M. M. Mota. 2009. A small molecule inhibitor of signal peptide peptidase inhibits Plasmodium development in the liver and decreases malaria severity. PLoS One 4:e5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ponnudurai, T., A. H. Lensen, G. J. van Gemert, M. G. Bolmer, and J. H. Meuwissen. 1991. Feeding behaviour and sporozoite ejection by infected Anopheles stephensi. Trans. R. Soc. Trop. Med. Hyg. 85:175-180. [DOI] [PubMed] [Google Scholar]
- 20.Radke, J. R., R. G. Donald, A. Eibs, M. E. Jerome, M. S. Behnke, P. Liberator, and M. W. White. 2006. Changes in the expression of human cell division autoantigen-1 influence Toxoplasma gondii growth and development. PLoS Pathog. 2:e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Renia, L., F. Miltgen, Y. Charoenvit, T. Ponnudurai, J. P. Verhave, W. E. Collins, and D. Mazier. 1988. Malaria sporozoite penetration. A new approach by double staining. J. Immunol. Methods 112:201-205. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz, A. L., and M. R. Hollingdale. 1985. Primaquine and lysosomotropic amines inhibit malaria sporozoite entry into human liver cells. Mol. Biochem. Parasitol. 14:305-311. [DOI] [PubMed] [Google Scholar]
- 23.Taylor, H. M., L. McRobert, M. Grainger, A. Sicard, A. R. Dluzewski, C. S. Hopp, A. A. Holder, and D. A. Baker. 2010. The malaria parasite cyclic GMP-dependent protein kinase plays a central role in blood-stage schizogony. Eukaryot. Cell 9:37-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsuji, M., D. Mattei, R. S. Nussenzweig, D. Eichinger, and F. Zavala. 1994. Demonstration of heat-shock protein 70 in the sporozoite stage of malaria parasites. Parasitol. Res. 80:16-21. [DOI] [PubMed] [Google Scholar]
- 25.Yoshida, N., P. Potocnjak, V. Nussenzweig, and R. S. Nussenzweig. 1981. Biosynthesis of Pb44, the protective antigen of sporozoites of Plasmodium berghei. J. Exp. Med. 154:1225-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]