Abstract
OqxAB has recently been identified as one of the mechanisms of plasmid-mediated quinolone resistance (PMQR). Compared to what is observed for other PMQR determinants, there is a paucity of data with regard to the prevalence and epidemiology of OqxAB and its contribution to resistance to different antimicrobials. In this study, the prevalence and dissemination of oqxAB and other PMQR genes in Escherichia coli isolates from animals, farmworkers, and the environment in 2002 in China were investigated. Of the 172 E. coli isolates, 39.0% carried oqxA, while only 4.1%, 2.9%, and 0.6% carried qnr (1 qnrB6 isolate, 5 qnrS1 isolates, and 1 qnrD isolate), qepA, and aac(6′)-Ib-cr, respectively. Among the 33 isolates from farmworkers, 10 (30.3%) were positive for oqxA. oqxAB was associated with IS26 and was carried on the 43- to 115-kb IncF transferable plasmid. Transconjugants carrying oqxAB showed 4- to 16-fold increases in the MICs of quinolones, 16- to 64-fold increases in the MICs of quinoxalines, 8- to 32-fold increases in the MICs of chloramphenicol and trimethoprim-sulfamethoxazole, and 4- to 8-fold increases in the MICs of florfenicol compared to the levels for the recipient. The pulsed-field gel electrophoresis (PFGE) analysis showed that the high levels of prevalence and dissemination of oqxAB in E. coli in animal farms were primarily due to the transmission of plasmids carrying oqxAB, although clonal transmission between human and swine E. coli isolates was observed. It is concluded that oqxAB was widespread in animal farms in China, which may be due to the overuse of quinoxalines in animals. This study warrants the prudent use of quinoxalines in food animals.
Fluoroquinolone resistance in animal bacterial isolates became an important public health problem due to the concern regarding the transmission of resistant bacterial pathogens to humans, since fluoroquinolones are the first choice of treatment for some human bacterial infections. The mechanisms of quinolone resistance were initially identified to be mediated by target mutations and overexpression of chromosomally encoded efflux pumps (13). In 1998, a plasmid-mediated quinolone resistance (PMQR) mechanism was firstly described to occur in a Klebsiella pneumoniae isolate from the United States (19). To date, three types of plasmid-mediated-quinolone-resistance determinants, including Qnr peptides (QnrA, QnrB, QnrS, QnrD, and QnrC), AAC(6′)-Ib-cr, and QepA, have been identified in clinical isolates (2, 23, 26, 28, 33, 36). Although the PMQR determinants can confer only low-level resistance to quinolones, their significant role may lie in that the low-level resistance ensures that the bacteria survive and subsequently generate target mutations for high-level fluoroquinolone resistance (25). A RND family pump, OqxAB, which confers resistance to olaquindox [N-(2-hydroxyethyl)-3-methyl-2-quinoxalinecarboxamide-1,4-di-N-oxide], one of the quinoxaline-N,N-dioxides, was discovered in Escherichia coli isolated from swine manure (10, 27). This pump was later identified to be a multidrug efflux pump that confers resistance to multiple agents, including fluoroquinolones (10, 11). OqxAB is encoded by the genes oqxA and oqxB in the same operon. OqxA and OqxB consist of several conserved blocks of amino acids similar to other verified and putative RND family proteins (10). The putative OqxB protein contains 12 transmembrane α helices, the numbers and positions of which are consistent with the crystal structure of the E. coli AcrB and MexF efflux pumps from Xanthomonas axonopodis (10). Similar to other members of the RND family of efflux pumps, the OqxAB efflux system also requires TolC to form the transmembrane channel (10). However, OqxAB was not recognized as a PMQR determinant until recently, and the data on the prevalence and epidemiology of OqxAB are limited compared to those observed for other PMQR determinants (28).
High prevalences of fluoroquinolone resistance in human and animal E. coli isolates have been reported to occur in China, which may be due to the overuse of quinolones as feed additive and therapies in food animals (8, 35, 37). The surveillance of PMQR determinants, in particular OqxAB, in E. coli isolates will provide insights into the understanding of the epidemiology and dissemination of OqxAB as well as the mechanism of the high prevalence of fluoroquinolone resistance in food animal bacterial isolates. In this study, we investigated the E. coli isolates from animals, farmworkers, and the farm environment in four pig farms and a chicken farm to understand the prevalence and dissemination of oqxAB and other PMQR genes and their contribution to bacterial antimicrobial resistance.
MATERIALS AND METHODS
Sampling and bacterial isolates.
Fecal samples or rectal swabs were randomly obtained from sows, piglets, weaners, and boars in four swine farms and chickens in a chicken farm located in different regions of Guangdong Province during 2002. Environmental samples from the farms, including surface soil, sewage, sullage, drinking water, and pond water samples, were randomly collected from different locations in each farm. Rectal swabs were obtained from consenting farmworkers. All samples were cultured on eosin methylene blue (EMB) agar plates and incubated at 37°C for 24 h. One suspicious colony with typical E. coli morphology was selected from each sample for identification.
PMQR gene detection.
All isolates were screened for oqxA and other PMQR genes [qnrA, qnrB, qnrC, qnrD, qnrS, aac(6′)-Ib-cr, and qepA] by PCR using specific primers as previously described (2, 12, 15, 18). The whole coding region of qnrD was amplified using the primers qnrD-F (5′-TTTTCGCTAACTAACTCGC-3′) and qnrD-R (5′-GAAAGGATAAACAGGCAAAT-3′). All oqxA-positive isolates were also screened for the oqxB gene (16). As qepA was always associated with the 16S rRNA methylase gene rmtB (17), qepA-positive isolates were also screened for rmtB as previously described (4). The association of IS26 with oqxA, as reported previously (16, 22), was investigated by PCR using forward primer IS26-F (5′-GCTGTTACGACGGGAGGAG-3′) located in IS26 and reverse primer oqxA-R (5′-GGAGACGAGGTTGGTATGGA-3′) located in oqxA. All PCR products were sequenced and underwent BLAST searches to confirm the correct amplifications.
After PCR confirmation, the whole coding region of the oqxAB gene was amplified using the primers oqxAB-F (5′-CCCTGGACCGCACATAAAG-3′) and oqxAB-R (5′-AAAGAACAAGATTCACCGCAAC-3′). The resultant 5,140-bp PCR product of the oqxAB gene was then cloned into the pMD18-T vector (TaKaRa Biotechnology, Dalian, China) to construct pMD18-T::oqxAB and sequenced.
gyrA and parC mutations.
The quinolone resistance-determining regions (QRDRs) of the gyrA and parC genes in PMQR-positive isolates were sequenced to confirm the mutations as previously described (21).
Conjugation experiments and plasmid analysis.
The transferability of oqxAB genes was studied by conjugation experiments using streptomycin-resistant E. coli C600 as the recipient strain as previously described (4). Briefly, 5 to 10 oqxAB-positive E. coli isolates from each farm with distinct pulsed-field gel electrophoresis (PFGE) patterns or sources (animals, workers, or the environment) were selected for conjugation experiments. A donor bacterium and recipient were grown in tryptic soy broth (TSB) to logarithmic phase, mixed at a 1:4 ratio (vol/vol), collected in a filter, and incubated at 37°C for 20 h. Transconjugants were selected on MacConkey agar plates containing olaquindox (64 μg/ml) and streptomycin (1,000 μg/ml). Restriction fragment length polymorphism (RFLP) analysis was performed on plasmids from transconjugants. Briefly, plasmids from transconjugants were extracted using a rapid alkaline lysis procedure (29) and digested with the endonuclease EcoRI (TaKaRa Biotechnology, Dalian, China) to analyze the RFLP profile and estimate the sizes of the plasmids.
PCR-based replicon typing was performed on conjugative plasmids as described by Carattoli et al. (1). Eighteen primer pairs, targeting the FIA, FIB, FIC, HI1, HI2, I1-Iγ, L/M, N, P, W, T, A/C, K, B/O, X, Y, F, and FII replicons, were used.
Antimicrobial susceptibility testing.
Susceptibilities to ampicillin, cefazolin, streptomycin, kanamycin, gentamicin, amikacin, tetracycline, and trimethoprim-sulfamethoxazole (SXT) were determined by the antimicrobial disk diffusion test according to the Clinical and Laboratory Standards Institute (CLSI) guidelines (6). In addition, the MICs for ciprofloxacin, mequindox, olaquindox, chloramphenicol, and enrofloxacin were determined by the agar dilution method. The breakpoints for each antimicrobial were recommended by the CLSI (6, 7). Resistance rates were calculated by dividing the number of intermediate-resistant and resistant strains by the total number of strains.
The MICs of 13 antimicrobial agents for donors, their corresponding oqxAB-positive transconjugants, and E. coli DH5α carrying pMD18-T::oqxAB were determined using the agar dilution method.
Epidemiological typing.
PFGE analysis of XbaI-digested genomic DNA of all PMQR-positive isolates was performed using a CHEF Mapper system (Bio-Rad Laboratories, Hercules, CA) as described by Gautom (9). PFGE patterns were interpreted according to the criteria of Tenover et al. (30). The isolates that had PFGE patterns with no more than four band differences were considered clonally related. The phylogenetic group of the PMQR-positive isolates was determined by the multiplex PCR-based method as previously described by Clermont et al. (5).
Multilocus sequence typing (MLST) of some representative strains from different sources and farms was performed according to the previously described protocol (http://www.shigatox.net/mlst). The seven housekeeping genes (aspC, clpX, fadD, icdA, lysP, mdh, and uidA) were amplified and sequenced. The allelic profile of the seven gene sequences and the sequence types (STs) were obtained via the electronic database at the E. coli MLST website.
Nucleotide sequence accession numbers.
The oqxAB and qnrD sequences were deposited into the GenBank database under the assigned accession numbers GQ497565, GU453932, and GU477622.
RESULTS
Prevalence of oqxAB and other PMQR genes.
As shown in Table 1, 172 E. coli isolates were randomly isolated from 172 samples of animals, farmworkers, and the environment from five farms (18 to 40 isolates per farm). The oqxA gene was present in 67 (39.0%) E. coli isolates. About 39.8% (39/98) E. coli isolates from animals, 43.9% (18/41) from the farm environment, and 30.3% (10/33) from farmworkers were positive for oqxA. About 46.3% of E. coli isolates from pig farms were positive for oqxA, while only ∼13% from the chicken farm were positive, significantly lower than the percentage of isolates from pig farms (P < 0.01). All except one oqxA-positive isolate were also positive for oqxB. The qnr, qepA, and aac(6′)-Ib-cr genes were detected in 7 (1 qnrB6 isolate, 5 qnrS1 isolates, and 1 qnrD isolate) (4.1%), 5 (2.9%), and 1 (0.6%) of the total 172 E. coli isolates, respectively. All 7 qnr-positive isolates were also positive for oqxAB, and all 5 qepA-positive isolates were also positive for rmtB. No qnrA and qnrC genes were detected in any of the E. coli isolates (Table 1). In addition, 49 of the 67 oqxA-positive isolates were positive for IS26. The whole coding region of the oqxAB genes was entirely sequenced for one isolate and was found to be nearly identical to that previously reported, with only two silent mutations in the oqxB gene (9, 16).
TABLE 1.
Prevalence and diversity of PMQR determinants in E. coli
| Sample source | Total no. of isolates | No. (%) of isolates positive for: |
No. of isolates with any PMQR gene (%) | No. of PFGE subtypes of PMQR-positive isolatesa | |||||
|---|---|---|---|---|---|---|---|---|---|
| oqxA | qepA | qnrB6 | qnrS1 | qnrD | aac(6′)-Ib-cr | ||||
| Farms 1-4 | |||||||||
| Pigs | 73 | 36 | 4 | 4 | 1 | 40 | |||
| Workers | 27 | 8 | 1 | 11 | |||||
| Environment | 34 | 16 | 1 | 1 | 16 | ||||
| Total | 134 | 62 (46.3) | 5 | 1 | 4 | 1 | 1 | 67 (50.0) | 53 (6) |
| Farm 5 | |||||||||
| Chickens | 25 | 3 | 1 | 3 | |||||
| Workers | 6 | ||||||||
| Environment | 7 | 2 | 2 | ||||||
| Total | 38 | 5 (13.2) | 1 | 5 (13.2) | 3 | ||||
| Total farms | |||||||||
| Animals | 98 | 39 (39.8) | 4 | 5 | 1 | 43 (43.9) | |||
| Workers | 33 | 10 (30.3) | 1 | 11 (33.3) | |||||
| Environment | 41 | 18 (43.9) | 1 | 1 | 18 (43.9) | ||||
| Total | 172 | 67 (39.0) | 5 | 1 | 5 | 1 | 1 | 72 (41.9) | 56 (6) |
The number of nontypeable isolates is indicated in parentheses.
Antimicrobial susceptibility analysis.
The MICs of quinoxalines, chloramphenicol, and fluoroquinolones were determined for all E. coli isolates. The MIC50s of mequindox, olaquindox, chloramphenicol, enrofloxacin, and ciprofloxacin were 8- to 32-fold higher in oqxAB-positive isolates than in oqxAB-negative isolates. The MICs of mequindox and olaquindox were higher than 32 μg/ml in all oqxA-positive isolates except for one isolate (16 μg/ml) that was negative for oqxB, while the MICs of mequindox and olaquindox were much lower (≤32 μg/ml) in oqxAB-negative isolates. The MICs of chloramphenicol, enrofloxacin, and ciprofloxacin differed among oqxAB-positive isolates. However, the rates of resistance to chloramphenicol and ciprofloxacin were higher in oqxAB-positive isolates (85.5% and 47.8%, respectively) than in oqxAB-negative isolates (38.8% and 21.4%, respectively) (P < 0.01). More than 75% of the oqxAB-positive isolates were resistant to ampicillin, kanamycin, tetracycline, and trimethoprim-sulfamethoxazole. No significant differences in resistance to gentamicin and amikacin were found between oqxAB-positive and oqxAB-negative E. coli isolates. All isolates were susceptible to cefazolin.
gyrA and parC mutations.
Of the 60 E. coli isolates carrying only oqxAB, 25 (41.7%) isolates had wild-type (WT) gyrA and parC genes, with ciprofloxacin MICs ranging from 0.008 to 0.5 μg/ml; 4 had a single mutation in gyrA at codon 83 to the L codon, with ciprofloxacin MICs ranging from 0.25 to 1 μg/ml; and 5 had one point mutation in both gyrA and parC, with ciprofloxacin MICs ranging from 1 to 4 μg/ml (Table 2). Mutations at both codon 83 to the L codon and codon 87 to the N codon in gyrA were found in 26 (43.3%) isolates with ciprofloxacin MICs of ≥16 μg/ml. Among these isolates, 23 had a single point mutation at codon 80 or 84 in parC, and 2 had both mutations in parC. These target mutations explained the difference in MICs of fluoroquinolones for oqxAB-positive isolates.
TABLE 2.
Distribution of QRDR mutations of gyrA and parC in the PMQR-positive E. coli isolates
| PMQR gene(s) | QRDR mutation(s)a |
No. of isolates | MIC range for ciprofloxacin (μg/ml) | |
|---|---|---|---|---|
| gyrA | parC | |||
| oqxAB(n = 60) | None | None | 25 | 0.008-0.5 |
| L83 | None | 4 | 0.25-1 | |
| L83 | I80 | 3 | 4 | |
| L83 | G84 | 1 | 4 | |
| L83 | R84 | 1 | 1 | |
| L83, N87 | None | 1 | >32 | |
| L83, N87 | R80 | 1 | 32 | |
| L83, N87 | I80 | 20 | 16-32 | |
| L83, N87 | K84 | 2 | 32->32 | |
| L83, N87 | I80, A84 | 2 | >32 | |
| qepA | L83, N87 | I80 | 4 | 32->32 |
| L83, N87 | K84 | 1 | >32 | |
| oqxAB, qnrD | L83 | R80 | 1 | 2 |
| oqxAB, qnrS1 | None | None | 5 | 1-2 |
| oqxAB, qnrB6, aac(6′)-Ib-cr | None | None | 1 | 2 |
“L83” represents a mutation at codon 83 to the L codon, etc. “None” indicates the wild type.
Of the 5 qepA-positive isolates, all had two mutations in gyrA and one mutation in parC and had ciprofloxacin MICs of ≥32 μg/ml. In contrast, of the 7 isolates carrying both qnr and oqxAB, 6 had wild-type gyrA and parC genes, with ciprofloxacin MICs of 1 to 2 μg/ml (Table 2).
Transferability of the oqxAB gene.
Thirteen transconjugants were successfully obtained from 41 OqxAB-producing isolates by conjugation experiments. The conjugative transfer frequencies ranged from 10−9 to 10−5 transconjugants per recipient. The qnrB6 and aac(6′)-Ib-cr genes were also cotransferred with oqxAB from an ST2 donor that was isolated from a soil sample from farm 4 (Table 3).
TABLE 3.
MICs for transconjugants and characterization of plasmids carrying oqxABa
| Strain | Donor |
Plasmid |
MIC (μg/ml)b |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Origin | MLST | Size (kb) | RFLP pattern | Replicon | OLA | MEQ | NAL | NOR | CIP | ENR | CHL | FFC | SXT | TET | AMP | GEN | STR | |
| E. coli C600 | 8 | 4 | 2 | 0.03 | 0.008 | 0.016 | 4 | 2 | 0.5 | 1 | 4 | 0.5 | >512 | |||||
| W281-T | F1 sow | ST921 | ND | ND | ND | 128 | 64 | 16 | 0.125 | 0.06 | 0.06 | 128 | 16 | 16 | 2 | >128 | 0.5 | >512 |
| W191-T | F1 sullage | ST172 | 115 | I | FII | 256 | 128 | 16 | 0.125 | 0.06 | 0.125 | 128 | 16 | 16 | 64 | >128 | 1 | >512 |
| W322-T | F1 worker | ND | 43 | II | UT | 512 | 256 | 64 | 0.25 | 0.06 | 0.125 | 64 | 16 | 4 | 1 | >128 | 0.125 | >512 |
| W245-T | F1 boar | ST920 | 58 | III | UT | 512 | 128 | 16 | 0.125 | 0.06 | 0.06 | 64 | 16 | 8 | 1 | >128 | 0.25 | >512 |
| G262-T | F2 soil | ST922 | 100 | IV | FII | 256 | 128 | 16 | 0.25 | 0.06 | 0.125 | 128 | 16 | 16 | 32 | 8 | 0.5 | >512 |
| G062-T | F2 piglet | ND | 57 | V | FII | 256 | 128 | 32 | 0.125 | 0.06 | 0.125 | 32 | 16 | 8 | 4 | >128 | 0.5 | >512 |
| G375-T | F2 worker | ND | 53 | VI | UT | 256 | 256 | 64 | 0.25 | 0.06 | 0.125 | 32 | 16 | 8 | 4 | 16 | 0.25 | >512 |
| X1B1-T | F3 weaner | ST134 | ND | ND | ND | 256 | 64 | 16 | 0.125 | 0.06 | 0.125 | 128 | 16 | 16 | 32 | 16 | 0.5 | >512 |
| XT11-T | F3 soil | ST928 | 115 | VII | FIVC | 256 | 128 | 16 | 0.25 | 0.06 | 0.06 | 128 | 16 | 16 | 64 | >128 | 0.5 | >512 |
| SW8-T | F4 pond water | ST926 | 115 | VII | FII | 512 | 256 | 64 | 0.5 | 0.06 | 0.125 | 128 | 16 | 16 | 1 | >128 | 32 | >512 |
| ST2-Tc | F4 soil | ST925 | 81 | VIII | UT | 512 | 128 | 256 | 4 | 2 | 2 | 32 | 4 | 16 | 2 | 4 | 0.25 | >512 |
| SP8-T | F4 pig | ND | 91 | IX | UT | 256 | 256 | 32 | 0.125 | 0.06 | 0.06 | 64 | 16 | 8 | 2 | >128 | 0.25 | >512 |
| D83-T | F5 chicken | ND | ND | ND | ND | 256 | 128 | 8 | 0.125 | 0.03 | 0.06 | 32 | 8 | 8 | 4 | 16 | 0.25 | >512 |
| E. coli DH5α/ pMD18-T::oqxAB | 128 | 64 | 32 | 0.25 | 0.125 | 0.125 | 64 | 16 | 8 | 4 | >128 | 0.125 | 2 | |||||
| E. coli DH5α | 8 | 4 | 2 | 0.03 | 0.008 | 0.016 | 4 | 4 | 0.5 | 2 | >128 | 0.5 | 4 | |||||
ND, not determined; UT, untypeable; F1 to F5, farm 1 to farm 5, respectively.
OLA, olaquindox; MEQ, mequindox; NAL, nalidixic acid; NOR, norfloxacin; CIP, ciprofloxacin; ENR, enrofloxacin; CHL, chloramphenicol; FFC, florfenicol; SXT, trimethoprim-sulfamethoxazole; TET, tetracycline; AMP, ampicillin; GEN, gentamicin; STR, streptomycin.
ST2-T contained oqxAB, qnrB6, and aac(6′)-Ib-cr.
The MICs of mequindox and olaquindox for all oqxAB-positive transconjugants were similar to those observed for the donor isolates but were about 16- to 64-fold higher than those observed for the recipient (Table 3). The 12 transconjugants carrying only oqxAB showed about 4- to 8-fold increases in the MICs of ciprofloxacin, 8- to 16-fold increases in the MICs of nalidixic acid, and 4- to 16-fold increases in the MICs of enrofloxacin and norfloxacin in comparison to the levels for the recipient, suggesting that oqxAB contributed to the decreased susceptibility to quinolones in E. coli. However, the MICs of nalidixic acid, norfloxacin, enrofloxacin, and ciprofloxacin in the transconjugants carrying both oqxAB and qnrB6/aac(6′)-Ib-cr were 32- to 128-fold higher than those observed for the recipient, approaching levels similar to those observed for the donor isolates, suggesting that the combination of different PMQR determinants can also confer intermediate resistance to quinolone. The MICs of chloramphenicol and SXT for the oqxAB transconjugants ranged from 32 to 128 μg/ml and 4 to 16 μg/ml, respectively, about 8- to 32-fold higher than those observed for the recipient. All oqxAB-positive transconjugants also showed 4- to 8-fold increases in the MICs of florfenicol. In addition, the cotransfer of resistance to ampicillin, tetracycline, and gentamicin was also observed in 8, 4, and 1 of the 13 transconjugants, respectively (Table 3). The E. coli DH5α strain carrying pMD18-T::oqxAB also showed increased MICs of quinoxalines, quinolones, chloramphenicol, florfenicol, and SXT compared to those observed for WT E. coli DH5α (Table 3).
Plasmid analysis.
Plasmid DNA was extracted from 12 transconjugants. Two transconjugants carried two plasmids, and the other 10 carried only one plasmid. The RFLP patterns were determined for the 10 transconjugants carrying only one plasmid. The sizes of the plasmids ranged from ∼43 to 115 kb. Only two plasmids, one from strain X1B1 isolated from a soil sample from farm 3 and one from strain SW8 recovered from a pond water sample from farm 4, showed identical RFLP patterns. PCR-based inc replicon typing showed that plasmids from 4 of the 10 transconjugants carrying only one plasmid belonged to the FII type, 1 belonged to the FIVC type, and the other 5 were untypeable (Table 3).
PFGE and phylogenetic analysis.
Sixty-six out of the 72 PMQR-positive isolates underwent PFGE analysis, and 56 different XbaI-pulsed-field gel electrophoresis patterns were observed (Table 1). It is suggested that the dissemination of oqxAB was not due to the clonal dissemination of oqxAB-positive E. coli. However, E. coli isolates with indistinguishable PFGE patterns were found in sows and farmworkers from farm 2 as well as sows and farmworkers from farm 3 (Fig. 1).
FIG. 1.
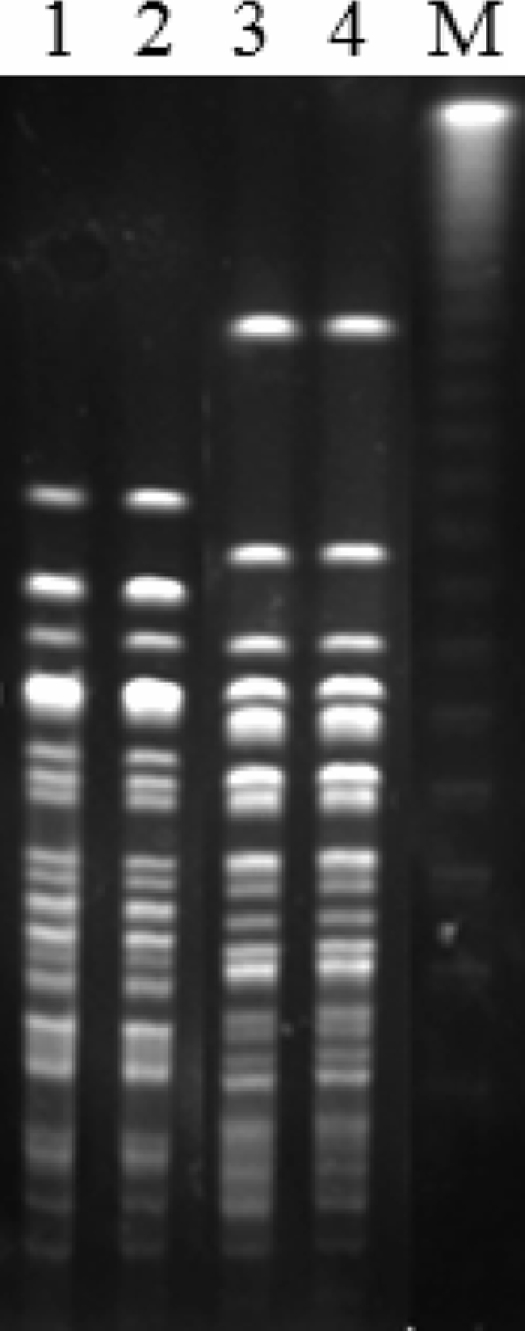
PFGE fingerprinting patterns of XbaI-digested total DNA preparations from E. coli isolates. Lanes: M, lambda ladder PFGE marker used as a molecular size marker; 1, E. coli strain from one worker from farm 3, ST923; 2, E. coli strain from one sow from farm 3, ST923; 3, E. coli strain from one worker from farm 2, ST924; 4, E. coli strain from one sow from farm 2, ST924.
Phylogenetic analysis showed that the 72 PMQR-positive isolates mainly belonged to phylogenetic group A (52.8%), followed by groups B1 (36.1%), D (8.3%) and B2 (2.8%). MLST analysis of 17 OqxAB-producing E. coli isolates of different sources identified 15 different sequence types (8 STs are listed in Table 3). Nine novel sequence types (ST920 to ST928) were detected in these E. coli isolates. The E. coli isolates from farms 2 and 3 that showed identical PFGE patterns also showed identical sequence types (Fig. 1).
DISCUSSION
The prevalence and dissemination of oqxAB in E. coli isolated from animals, farmworkers, and the environment were investigated in this study. A surprisingly high prevalence (39.0%) of oqxAB was detected in E. coli isolates, significantly higher than previously reported for Denmark, Sweden (1.8%) (12), and South Korea (0.4%) (16). Olaquindox was commonly used as a therapeutic and preventive antibiotic in swine in China. However, it has been forbidden in poultry since 2000 due to its toxic side effects, which may explain the relatively low prevalence of oqxAB in the chicken farm. A new synthetic quinoxaline 1,4-dioxide (QdNO) derivative, mequindox (3-methyl-2-acetyl-N-1,4-dioxyquinoxaline; C11H10N2O3), which was developed in China, has also been widely used as an antibacterial and animal feed additive in China since the 1990s (14). Antimicrobial usage in food animals is considered the most important factor in the selection of resistant bacteria (34). Therefore, the high levels of prevalence and dissemination of oqxAB in E. coli isolates in animals in China may be due to the overuse of olaquindox and mequindox in food animals.
In addition to animal E. coli isolates, oqxAB was also detected in 30.3% of human commensal E. coli isolates from farmworkers without previous antimicrobial treatment or hospital admission, suggesting the transmission of oqxAB to human isolates. The diverse PFGE patterns within oqxAB-positive E. coli isolates suggest the possible horizontal transmission of the oqxAB determinant instead of the direct clonal dissemination between animals, farmworkers, and the environment. However, the same PFGE pattern was occasionally observed in E. coli isolates from animals and farmworkers, suggesting the transmission of oqxAB-positive E. coli between humans and animals as described in other studies (20, 32). Further studies are needed to investigate the prevalence of oqxAB in human clinical isolates in China and the possible transmission of plasmids carrying oqxAB through the food chain.
In contrast to the previous report of Kim et al. (16), the oqxAB gene was proven by conjugation experiments to be located in transferable plasmid. Five of the 13 transconjugants carried a broad-host-range IncF-type plasmid, which is different from the previous report of oqxAB located in the IncX incompatible group of plasmids (pOLA52) (22). However, oqxAB may be located in a different (IncF) group of plasmids, which is evidenced by various RFLP patterns within the plasmids carrying oqxAB. Consistent with previous report that the oqxAB cassette flanked by IS26 was identical to the chromosome segment (composite transposon Tn6010) of K. pneumoniae MGH 78578 (16, 22), the oqxA gene in this study was also flanked by IS26, which suggests that the dissemination of oqxAB among different E. coli strains may be mediated by the mobile element.
The oqxAB genotype is very consistent with the olaquindox and mequindox resistance phenotype in E. coli isolates, suggesting the role of oqxAB in olaquindox resistance, which was also supported by the conjugation experiments and other studies (10, 11). In contrast, oqxAB contributes only to low-level decreases in susceptibility to quinolones, which was evidenced by the increase of quinolone MICs by 4- to 16-fold in transconjugants, a lower extent of contribution than was observed for the first reported pOLA52-mediated oqxAB gene (11, 28). The various levels of MICs of quinolones in oqxAB-positive E. coli isolates were due to the presence of the target mutations. The oqxAB-positive E. coli isolates without target mutations showed only low-level decreases in susceptibility to ciprofloxacin and other quinolones.
Though oqxAB conferred only low-level quinolone resistance, the significant role of this PMQR may lie in its ability to enable E. coli to survive at a low concentration of fluoroquinolones, which is the prerequisite for subsequent generation of target mutations for resistance to higher-level fluoroquinolone (24). Cesaro et al. found that topoisomerase mutations were rarely selected by ciprofloxacin from strains containing qnr (3). It is not clear whether the first step of quinolone resistance is the acquisition of the QRDR gene(s) or the occurrence of topoisomerase mutation. However, the relatively high frequencies of topoisomerase mutations in oqxAB- and qepA-positive isolates compared to those observed in Qnr-producing isolates suggested that the quinolone efflux pump (OqxAB and QepA) might favor the selection of high-level quinolone resistance compared to Qnr proteins which protect QRDR domains from quinolone attacks (31).
In conclusion, the transferable plasmid-mediated multidrug efflux pump gene oqxAB was widespread in animal farms. The overuse of quinoxaline as a feed additive in food animals might contribute to the development and dissemination of oqxAB, which subsequently promotes the development of high-level fluoroquinolone resistance in bacteria. The data in this study warrant the prudent use of mequindox and olaquindox in farm animals in China.
Acknowledgments
We are grateful to Minggui Wang and Xiaogang Xu, Institute of Antibiotics, Huashan Hospital, for kindly sending the qnrC-positive strain. We thank Minggui Wang for revision and helpful comments on the manuscript.
This work was supported by grants 30972218 and U0631006 from the National Natural Science Foundation of China.
Footnotes
Published ahead of print on 9 August 2010.
REFERENCES
- 1.Carattoli, A., A. Bertini, L. Villa, V. Falbo, K. L. Hopkins, and E. J. Threlfall. 2005. Identification of plasmids by PCR-based replicon typing. J. Microbiol. Methods 63:219-228. [DOI] [PubMed] [Google Scholar]
- 2.Cavaco, L. M., H. Hasman, S. Xia, and F. M. Aarestrup. 2009. qnrD, a novel gene conferring transferable quinolone resistance in Salmonella enterica serovars Kentucky and Bovismorbificans of human origin. Antimicrob. Agents Chemother. 53:603-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cesaro, A., R. R. Bettoni, C. Lascols, A. Mérens, C. J. Soussy, and E. Cambau. 2008. Low selection of topoisomerase mutants from strains of Escherichia coli harbouring plasmid-borne qnr genes. J. Antimicrob. Chemother. 61:1007-1015. [DOI] [PubMed] [Google Scholar]
- 4.Chen, L., Z. L. Chen, J. H. Liu, Z. L. Zeng, J. Y. Ma, and H. X. Jiang. 2007. Emergence of RmtB methylase-producing Escherichia coli and Enterobacter cloacae isolates from pigs in China. J. Antimicrob. Chemother. 59:880-885. [DOI] [PubMed] [Google Scholar]
- 5.Clermont, O., S. Bonacorsi, and E. Bingen. 2000. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl. Environ. Microbiol. 66:4555-4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clinical and Laboratory Standards Institute. 2008. Performance standards for antimicrobial disk susceptibility tests for bacteria isolated from animals; approved standard, 3rd ed. Document M31-A3. CLSI, Wayne, PA.
- 7.Clinical and Laboratory Standards Institute. 2008. Performance standards for antimicrobial susceptibility testing; 18th informational supplement. M100-S18. CLSI, Wayne, PA.
- 8.Dai, L., L. M. Lu, C. M. Wu, B. B. Li, S. Y. Huang, S. C. Wang, Y. H. Qi, and J. Z. Shen. 2008. Characterization of antimicrobial resistance among Escherichia coli isolates from chickens in China between 2001 and 2006. FEMS Microbiol. Lett. 286:178-183. [DOI] [PubMed] [Google Scholar]
- 9.Gautom, R. K. 1997. Rapid pulsed-field gel electrophoresis protocol for typing of Escherichia coli O157:H7 and other gram-negative organisms in 1 day. J. Clin. Microbiol. 35:2977-2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansen, L. H., E. Johannesen, M. Burmolle, A. H. Sørensen, and S. J. Sørensen. 2004. Plasmid-encoded multidrug efflux pump conferring resistance to olaquindox in Escherichia coli. Antimicrob. Agents Chemother. 48:3332-3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansen, L. H., L. B. Jensen, H. I. Sørensen, and S. J. Sørensen. 2007. Substrate specificity of the OqxAB multidrug resistance pump in Escherichia coli and selected enteric bacteria. J. Antimicrob. Chemother. 60:145-147. [DOI] [PubMed] [Google Scholar]
- 12.Hansen, L. H., S. J. Sørensen, H. S. Jorgensen, and L. B. Jensen. 2005. The prevalence of the OqxAB multidrug efflux pump amongst olaquindox-resistant Escherichia coli in pigs. Microb. Drug Resist. 11:378-382. [DOI] [PubMed] [Google Scholar]
- 13.Hooper, D. C. 1999. Mechanisms of quinolone resistance. Drug Resist. Updat. 2:38-55. [DOI] [PubMed] [Google Scholar]
- 14.Huang, X. J., A. Ihsan, X. Wang, M. H. Dai, Y. L. Wang, S. J. Su, X. J. Xue, and Z. H. Yuan. 2009. Long-term dose-dependent response of Mequindox on aldosterone, corticosterone and five steroidogenic enzyme mRNAs in the adrenal of male rats. Toxicol. Lett. 91:167-173. [DOI] [PubMed] [Google Scholar]
- 15.Kim, H. B., C. H. Park, C. J. Kim, E. C. Kim, G. A. Jacoby, and D. C. Hooper. 2009. Prevalence of plasmid-mediated quinolone resistance determinants over a 9-year period. Antimicrob. Agents Chemother. 53:639-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim, H. B., M. Wang, C. H. Park, E. C. Kim, G. A. Jacoby, and D. C. Hooper. 2009. oqxAB encoding a multidrug efflux pump in human clinical isolates of Enterobacteriaceae. Antimicrob. Agents Chemother. 53:3582-3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu, J. H., Y. T. Deng, Z. L. Zeng, J. H. Gao, L. Chen, Y. Arakawa, and Z. L. Chen. 2008. Coprevalence of plasmid-mediated quinolone resistance determinants QepA, Qnr, and AAC(6′)-Ib-cr among 16S rRNA methylase RmtB-producing Escherichia coli isolates from pigs. Antimicrob. Agents Chemother. 52:2992-2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma, J., Z. Zeng, Z. Chen, X. Xu, X. Wang, Y. Deng, D. Lü, L. Huang, Y. Zhang, J. Liu, and M. Wang. 2009. High prevalence of plasmid-mediated quinolone resistance determinants qnr, aac(6′)-Ib-cr, and qepA among ceftiofur-resistant Enterobacteriaceae isolates from companion and food-producing animals. Antimicrob. Agents Chemother. 53:519-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martínez-Martínez, L., A. Pascual, and G. A. Jacoby. 1998. Quinolone resistance from a transferable plasmid. Lancet 351:797-799. [DOI] [PubMed] [Google Scholar]
- 20.Moodley, A., and L. Guardabassi. 2009. Transmission of IncN plasmids carrying blaCTX-M-1 between commensal Escherichia coli in pigs and farm workers. Antimicrob. Agents Chemother. 53:1709-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morgan-Linnell, S. K., L. Becnel Boyd, D. Steffen, and L. Zechiedrich. 2009. Mechanisms accounting for fluoroquinolone resistance in Escherichia coli clinical isolates. Antimicrob. Agents Chemother. 53:235-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Norman, A., L. H. Hansen, Q. She, and S. J. Sørensen. 2008. Nucleotide sequence of pOLA52: a conjugative IncX1 plasmid from Escherichia coli which enables biofilm formation and multidrug efflux. Plasmid 60:59-74. [DOI] [PubMed] [Google Scholar]
- 23.Pe'richon, B., P. Courvalin, and M. Galimand. 2007. Transferable resistance to aminoglycosides by methylation of G1405 in 16S rRNA and to hydrophilic fluoroquinolones by QepA-mediated efflux in Escherichia coli. Antimicrob. Agents Chemother. 51:2464-2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piddock, L. J. 2006. Clinically relevant chromosomally encoded multidrug resistance efflux pumps in bacteria. Clin. Microbiol. Rev. 19:382-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poirel, L., V. Cattoir, and P. Nordmann. 2008. Is plasmid-mediated quinolone resistance a clinically significant problem? Clin. Microbiol. Infect. 14:295-297. [DOI] [PubMed] [Google Scholar]
- 26.Robicsek, A., J. Strahilevitz, G. A. Jacoby, M. Macielag, D. Abbanat, K. Bush, and D. C. Hooper. 2006. Fluoroquinolone modifying enzyme: a novel adaptation of a common aminoglycoside acetyltransferase. Nat. Med. 12:83-88. [DOI] [PubMed] [Google Scholar]
- 27.Sørensen, A. H., L. H. Hansen, E. Johannesen, and S. J. Sørensen. 2003. Conjugative plasmid conferring resistance to olaquindox. Antimicrob. Agents Chemother. 47:798-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strahilevitz, J., G. A. Jacoby, D. C. Hooper, and A. Robicsek. 2009. Plasmid-mediated quinolone resistance: a multifaceted threat. Clin. Microbiol. Rev. 22:664-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takahashi, S., and Y. Nagano. 1984. Rapid procedure for isolation of plasmid DNA and application to epidemiological analysis. J. Clin. Microbiol. 20:608-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tran, J. H., G. A. Jacoby, and D. C. Hooper. 2005. Interaction of the plasmid-encoded quinolone resistance protein Qnr with Escherichia coli DNA gyrase. Antimicrob. Agents Chemother. 49:118-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van den Bogaard, A. E., N. London, C. Driessen, and E. E. Stobberingh. 2001. Antibiotic resistance of faecal Escherichia coli in poultry, poultry farmers and poultry slaughterers. J. Antimicrob. Chemother. 47:763-771. [DOI] [PubMed] [Google Scholar]
- 33.Wang, M., Q. Guo, X. Xu, X. Wang, X. Ye, S. Wu, D. C. Hooper, and M. Wang. 2009. New plasmid-mediated quinolone resistance gene, qnrC, found in a clinical isolate of Proteus mirabilis. Antimicrob. Agents Chemother. 53:1892-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Witte, W. 1998. Medical consequences of antibiotic use in agriculture. Science 279:996-997. [DOI] [PubMed] [Google Scholar]
- 35.Xiao, Y. H., J. Wang, Y. Li, et al. 2008. Bacterial resistance surveillance in China: a report from Mohnarin 2004-2005. Eur. J. Clin. Microbiol. Infect. Dis. 27:697-708. [DOI] [PubMed] [Google Scholar]
- 36.Yamane, K., J. I. Wachino, S. Suzuki, K. Kimura, N. Shibata, H. Kato, K. Shibayama, T. Konda, and Y. Arakawa. 2007. New plasmid-mediated fluoroquinolone efflux pump, QepA, found in an Escherichia coli clinical isolate. Antimicrob. Agents Chemother. 51:3354-3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang, H., S. Chen, D. G. White, S. Zhao, P. McDermott, R. Walker, and J. Meng. 2004. Characterization of multiple-antimicrobial-resistant Escherichia coli isolates from diseased chickens and swine in China. J. Clin. Microbiol. 42:3483-3489. [DOI] [PMC free article] [PubMed] [Google Scholar]


