Abstract
Carbapenemase-producing Klebsiella pneumoniae (KPC) bacteria are rapidly becoming one of the most detrimental drug-resistant Gram-negative pathogens. Doripenem is the newest FDA-approved carbapenem that has the greatest in vitro potency against a wide range of Gram-negative organisms, including multidrug-resistant organisms. Previous work in an animal model has shown efficacy against Pseudomonas aeruginosa with MICs above the current breakpoints of susceptibility. The purpose of this study is to evaluate the efficacy of 1-g and 2-g dose prolonged infusions of doripenem against KPC isolates in both an immunocompetent and neutropenic murine thigh model. Seven clinical KPC isolates (broth microdilution [BMD] MIC range, 4 to 32 μg/ml; Etest MIC range, 3 to >32 μg/ml) were used. After infection, groups of mice were administered doripenem doses previously shown to simulate the exposures observed in humans after the administration of 1 or 2 g every 8 h as a 4-h infusion. In immunocompromised mice, 1- and 2-g doses of doripenem achieved bacteriostasis against isolates with MICs up to and including 8 μg/ml and 16 μg/ml, respectively. In immunocompetent animals, statistically significant reductions in the number of CFU were observed with overall decreases of approximately 1 log (P < 0.05). While carbapenemase-producing Klebsiella pneumoniae continues to decrease our meager supply of active agents, the ability of doripenem to produce CFU reductions in the presence of white blood cells (WBCs) using humanized exposures suggests the potential utility of this agent in combination against this increasingly problematic pathogen.
The emergence of multidrug-resistant Gram-negative pathogens during the last few decades has had detrimental effects on the health of patients. Infections caused by these pathogens are associated with increased mortality rates, longer hospital stays, and increased costs compared to infections with susceptible organisms (10). In addition, practitioners have seen declines in the number of antimicrobial agents available for successful treatment of these serious infections.
One group of organisms that has been gaining in frequency is carbapenemase-producing Klebsiella pneumoniae (KPC) (8). Traditionally, carbapenems have been considered the agents of choice for combating multidrug-resistant Gram-negative pathogens, but due to the emergence of these carbapenemase producers, this last line of defense has weakened (8). Recent data from the MYSTIC surveillance study have shown that the serine-based carbapenemases are no longer limited to Klebsiella spp. (16). Increases in carbapenem resistance rates are now seen in other members of the family Enterobacteriaceae, such as Escherichia coli, causing the clinical utility of these antimicrobial agents to seemingly decrease even further.
Despite these ominous data, recent animal work by Craig et al. has shown that in a murine thigh infection model with carbapenemase-producing Klebsiella spp., the ability to achieve the requisite 40% free time above the MIC (fT>MIC), which demonstrates therapeutic efficacy with a carbapenem, is not affected by the presence of this enzyme (6). Additionally, other studies have shown that the use of high-dose, prolonged infusions of carbapenems, specifically meropenem, show efficacy against resistant Pseudomonas aeruginosa, Acinetobacter spp., and Burkholderia cepacia (9, 12). Recent in vitro work by our group has also demonstrated that high-dose, prolonged infusions of meropenem were able to demonstrate efficacy against Klebsiella pneumoniae with MICs of 2 μg/ml, despite the presence of a carbapenemase (2).
Doripenem, the newest FDA-approved carbapenem (500 mg every 8 h [q8h]), displays enhanced in vitro potency against Gram-negative organisms compared to the other carbapenems. Work by our group has shown that by increasing the dose and extending the infusion times of doripenem, we are able to achieve efficacy against Pseudomonas aeruginosa with MICs well above the currently defined FDA breakpoints (7). Based on these data, it is reasonable to assume that doripenem may achieve efficacy against Klebsiella pneumoniae despite the activity of the carbapenemase present. Herein, we describe the efficacy of doripenem, human simulated regimens of 1 and 2 g every 8 h as a 4-h infusion, in both an immunocompromised and immunocompetent murine thigh infection model.
MATERIALS AND METHODS
Antimicrobial test agent.
Analytical-grade doripenem provided by Johnson & Johnson Pharmaceutical Research & Development, LLC, Raritan, NJ, was utilized for all in vivo studies. Based on the potency, doripenem powder was weighed in a quantity sufficient to achieve the required concentration and reconstituted immediately with normal saline (NS) prior to use. Doripenem solutions were stored at room temperature, protected from light, and discarded after 8 h.
Bacterial isolates.
A total of 7 clinical K. pneumoniae isolates with KPC genotypes, all Hodge test positive and blaKPC positive (courtesy of Steve Jenkins, Mount Sinai Medical Center), were used in this analysis. The MIC of each isolate was determined, in triplicate, by broth microdilution (BMD) using methods outlined by the Clinical and Laboratory Standards Institute (3), and the modal MIC was reported. MICs were also conducted in triplicate by Etest (bioMérieux Inc., Hazelwood, MO) and interpreted according to the manufacturer's procedures, and the modal MIC was reported. The quality control isolate PSA 27853 was used for all MIC testing. Isolates were maintained in double-strength skim milk (BD Biosciences, Sparks, MD) at −80°C. Each isolate was subcultured twice on Trypticase soy agar with 5% sheep blood (BD Biosciences) and grown at 35°C prior to use in the experiments.
Animal infection model.
Pathogen-free, female ICR mice weighing approximately 25 g were acquired from Harlan Sprague Dawley, Inc. (Indianapolis, IN) and utilized throughout all experiments. The study was reviewed and approved by the Hartford Hospital Institutional Animal Care and Use Committee (IACUC). Animals were maintained and utilized per the guidelines of the Hartford Hospital IACUC and provided food and water ad libitum. For immunocompromised murine trials, the mice were rendered neutropenic when they were given 100- and 150-mg doses of cyclophosphamide (Cytoxan; Bristol-Myers Squibb, Princeton, NJ) per kg of body weight. Specifically, the mice were given 100- and 150-mg/kg doses of cyclophosphamide by intraperitoneal (i.p.) injections 1 and 4 days prior to inoculation, respectively. Three days prior to inoculation, all mice were given a single 5-mg/kg i.p. injection of uranyl nitrate. This produces a predictable degree of renal impairment to slow drug clearance necessary to simulate human dosing regimens (1). Two hours prior to the initiation of antimicrobial therapy, each thigh was inoculated intramuscularly with a 0.1-ml NS solution containing approximately 106 CFU of the test isolate per ml.
Two hours after inoculation, the mice were randomly divided into cohorts to receive subcutaneous injections at a volume of 0.2 ml containing either doripenem (treatment group) or normal saline (control group). The treatment groups received dosing regimens that were previously determined by our group through pharmacokinetic and pharmacodynamic experimentation using Pseudomonas aeruginosa in a murine thigh model (7, 11). The free doripenem concentration profile simulated a free time above the MIC (fT>MIC) observed in humans given 1 g and 2 g every 8 h as a 4-h infusion (11). Pharmacokinetic studies were not undertaken in this study for humane reasons, as these dosing regimens have been previously determined and rigorously validated by our group in a previous study (11). Each 24-h dosing regimen consisted of three 8-h dosing intervals. To serve as control animals, an additional group of mice were administered normal saline at the same volume, route, and frequency as the mice in the treatment regimens. All animals were harvested 24 h after the initiation of therapy. The harvesting procedure for all mice began with euthanization by CO2 exposure followed by cervical dislocation. After sacrifice, their thighs were removed and individually homogenized in 5 ml of normal saline. Serial dilutions of the thigh homogenate were plated on Trypticase soy agar with 5% sheep blood for CFU determination. In addition to the above mentioned treatment and control groups, another group of three infected, untreated mice were harvested at the initiation of dosing (i.e., 0-h control). Efficacy, designated as the change in bacterial density, was calculated as the change in log10 bacterial CFU obtained for doripenem-treated mice after 24 h from the 0-h control animals. Bacteriostasis was defined as a <1-log-unit change in CFU from the value for the 0-h control animals.
Statistical analysis.
Comparisons of efficacy between 1- and 2-g doses against each isolate, in addition to comparisons between immunocompetent and immunocompromised animals for each dose/isolate were made using a Student t test or Mann-Whitney U test if the data were not normally distributed. A P value of <0.05 was defined a priori as statistically significant.
Immunocompetent mouse thigh infection model.
Groups of ICR mice underwent the same procedure as the neutropenic mice but without the use of cyclophosphamide prior to infection with an inoculum of ∼108 CFU. The three isolates used for this study were treated with either the 1-g simulated doripenem regimen (KP 354 and KP 356) or the 2-g simulated doripenem regimen (KP 354, KP 356, and KP 359).
RESULTS
Bacterial isolates.
The phenotypic profiles for the 7 KPC isolates utilized in this study are listed in Table 1. Doripenem BMD MICs ranged between 4 and 32 μg/ml, while Etest MICs ranged between 3 and >32 μg/ml.
TABLE 1.
In vitro susceptibilities and predicted pharmacodynamic exposures of carbapenemase-producing Klebsiella pneumoniae isolates to doripenema
| Isolate | MIC (μg/ml) by: |
Predicted % fT>MIC for the following dose of doripenem: |
||
|---|---|---|---|---|
| BMD | Etest | 1 g | 2 g | |
| KPC 353 | 4 | 3 | 70 | 82.5 |
| KPC 354 | 4 | 4 | 70 | 82.5 |
| KPC 356 | 8 | 12 | 52.5 | 70 |
| KPC 357 | 8 | 6 | 52.5 | 70 |
| KPC 359 | 16 | >32 | 0 | 52.5 |
| KPC 360 | 16 | 16 | 0 | 52.5 |
| KPC 361 | 32 | >32 | 0 | 0 |
In vitro susceptibilities are shown by the MICs found by broth microdilution (BMD) and by Etest, and the predicted pharmacodynamic exposure of the isolates is shown by the predicted percent free time above the MIC (%fT>MIC) for a 4-h infusion.
In vivo efficacy. (i) Immunocompromised murine trials.
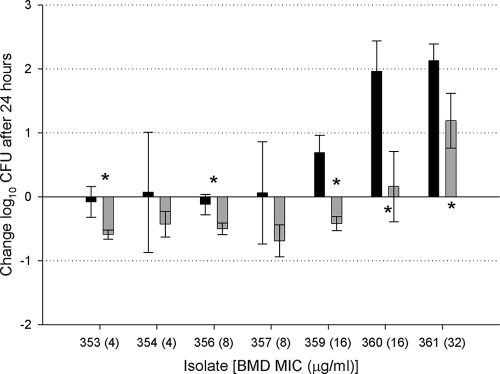
At the start of dosing, 0-h control mice displayed mean bacterial burdens of 5.7 ± 0.18 log10 CFU per thigh. The bacterial load in untreated mice increased by an average of 2.36 ± 0.51 log10 CFU after 24 h. All doripenem-treated and control mice survived to the 24-h sampling point. The results of the efficacy studies for the immunocompromised murine trials are shown in Fig. 1. Despite a predicted fT>MIC of >40%, 1-g doripenem simulations produced a static response with an approximate 0.02 ± 0.1 log10 CFU decrease for isolates with MICs of 4 to 8 μg/ml. Consistent with a predicted fT>MIC of 0%, isolates with MICs of 16 and 32 μg/ml produced an approximately 1.59 ± 0.78 log increase in bacterial density. By increasing the doripenem dose to 2 g, a static response was observed up to and including MICs of 16 μg/ml. The bacterial density of the isolate with an MIC of 32 μg/ml increased approximately 1.19 ± 0.43 log unit, consistent with a predicted fT>MIC of 0%.
FIG. 1.
Comparison of the efficacies of two different doses of doripenem against carbapenemase-producing Klebsiella pneumoniae isolates in immunocompromised animals. Mice were given 1-g (black bars) and 2-g (gray bars) doses of doripenem. The broth microdilution (BMD) MICs for the seven KPC isolates (KPC 353 to KPC 361) are shown on the x axis. Data are presented as the means ± standard deviations. Values that are statistically significantly different (P < 0.05) are indicated by an asterisk.
(ii) Immunocompetent murine trial.
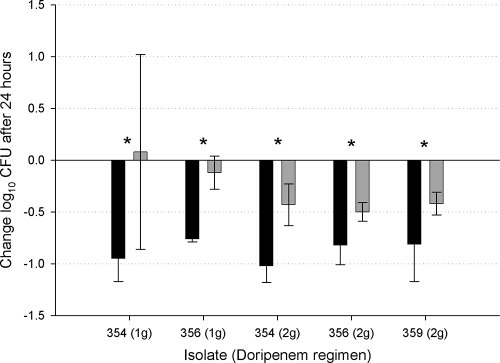
At the beginning of dosing, 0-h control mice displayed mean bacterial burdens of 6.95 ± 0.21 log10 CFU per thigh. The bacterial load in untreated mice remained an average bacterial burden of 6.85 ± 0.45 log units after 24 h. All doripenem-treated and control mice survived to the 24-h sampling point. The results of the efficacy studies with 1 g of doripenem given every 8 h and 2 g of doripenem given every 8 h to the immunocompetent animals are shown in Fig. 2. The mean observed reduction in bacterial density after 24 h of treatment in the presence of white blood cells (WBCs) was approximately 1 log CFU for all isolates using either the 1- or 2-g dose. Relative to the neutropenic animals, the immunocompetent animals displayed statistically significant reductions in CFU when given the same dose (P < 0.05).
FIG. 2.
Comparison of the efficacies of two different doses of doripenem against carbapenemase-producing K. pneumoniae isolates in immunocompromised and immunocompetent animals. Immunocompetent (black bars) and immunocompromised (gray bars) mice were given 1-g and 2-g doses of doripenem. Five KPC isolates (KPC 354 to KPC 359) and the doripenem dose are shown on the x axis. Data are presented as the means ± standard deviations. Values that are statistically significantly different (P < 0.05) are indicated by an asterisk.
DISCUSSION
Carbapenems have long been considered the drugs of choice when combating an infection caused by multidrug-resistant Gram-negative pathogens. When the MICs of these pathogens have risen, the doses and/or infusion times of the carbapenems have also increased in order to achieve the required 40% fT>MIC, and in turn clinical efficacy (7). In this analysis, we sought to determine whether the use of human simulated, high-dose, prolonged infusions of doripenem, the newest and potentially most potent carbapenem, would prove efficacious against carbapenemase-producing Klebsiella pneumoniae at various MICs. To our knowledge, this is the first study in the current literature in which human simulated, high-dose, prolonged infusions of doripenem have been tested against KPC bacteria.
Within our data there is a clear distinction between the results of the 1-g dose simulation and the 2-g dose simulation in the immunocompromised murine model. The 1-g dose was able to achieve and maintain bacteriostasis for the KPC isolates with MICs up to and including 8 μg/ml, while the 2-g dose maintained a similar effect for MICs up to and including 16 μg/ml. When comparing these results to the recent work done by Crandon et al. using these same dosing regimens against Pseudomonas aeruginosa, the 1- and 2-g doses were able to achieve ≥2-log decreases in bacterial density against isolates with MICs up to and including 8 μg/ml and 16 μg/ml, respectively (7). The observed differences in CFU reductions and the corresponding percentages in the fT>MICs between the current study and that done with P. aeruginosa may have to do with interspecies difference, mechanisms of resistance, or a combination of both. While some may argue that the optimal endpoint of these in vivo studies is bactericidal activity, the ability of doripenem to achieve and maintain either stasis or slight reductions in bacterial density against an active carbapenemase producer is not a negative outcome. By having the ability to achieve a 0.5-log reduction in bacterial density, the dosing regimen of 2-g doripenem maintains stability in the face of carbapenemase production.
Recent animal work done by Craig et al. using the same neutropenic murine model demonstrated that the presence of a carbapenemase in Enterobacteriaceae has no effect on the ability of the carbapenems to achieve the required exposures for this class of compounds (6). In that study, the investigators reported that doripenem regimens attaining 23% fT>MIC result in a static effect against members of the Enterobacteriaceae family that produce KPC carbapenemase; however, there are many apparent differences between our study and that of Craig et al. The first is that the isolates used in the study by Craig et al. exhibited MICs that were lower than the MICs of our isolates. In this study, we utilized KPC isolates with doripenem BMD MICs in the range of 4 to 32 μg/ml, because this phenotypic profile is now more frequently observed in the clinical setting. We also performed MICs by the Etest methodology in the hopes that this would provide a more exacting pharmacodynamic profile; however, these values were similar to that obtained with BMD (Table 1). Studies have demonstrated that K. pneumoniae with elevated carbapenem MICs have other mechanisms, such as intrinsic impermeability or efflux mechanisms, contributing to the carbapenem resistance in addition to the presence of other β-lactamase enzymes (15). These added resistance mechanisms have the potential to elevate the amount of drug required to achieve pharmacodynamic efficacies similar to those of isolates with lower MICs. Another blatant difference between the studies is that we employed human simulated dosing regimens, while Craig et al. experimented with a wide range of dosing strategies. These different dosing strategies employed differing drug dosages in addition to various dosing intervals. Although these differences may not fully explain the differences in results between the two studies, the variations in methodologies cannot be ignored.
When evaluating the efficacy of these simulated human dosing regimens against KPC isolates, it is important to consider the infection model utilized. In our analysis, we chose to employ both immunocompromised and immunocompetent murine thigh infection models to fully evaluate the efficacy of the doripenem regimens. Past studies have demonstrated that the presence of neutrophils has enhanced the efficacy of β-lactams (4, 5). In our analysis, when examining the effect of neutrophils on the 1-g doripenem dose, there is a statistically significant difference seen between the immunocompetent and immunocompromised murine models for both isolates tested (P < 0.05). With both KP 354 and KP 356, the presence of the neutrophils, in addition to the 1-g doripenem dose produces an approximately 1-log-greater decrease in bacterial density compared to the immunocompromised murine model. However, when comparing the effect of the presence of neutrophils on the 2-g doripenem regimen, although the difference between the immunocompromised and immunocompetent murine models was statistically significant (P < 0.05) for each KPC isolate involved (KP 354, KP 356, and KP 359), overall, the magnitude of difference was not as great as that seen with the 1-g dose regimen. This decreased effect of the presence of neutrophils is not unique to this analysis and has been demonstrated with other β-lactams in other studies as well (13, 14). When considering which model is most accurate to apply to human outcomes, one may argue that the results of immunocompetent studies in animals would best define the outcomes in humans, because only a minority of patient populations are lacking the effects of neutrophils. If this is true, then the results of our studies with the presence of neutrophils enhancing the efficacy of doripenem in immunocompetent animals may prove to give hope for the use of this agent against KPC bacteria in patients.
An aspect of this study that some may consider a limitation is the fact that pharmacokinetic (PK) studies were not completed to confirm estimated doripenem exposures. The humanized dosing regimens that were employed were determined and rigorously validated by our group in the same murine species, and since this work was completed in our laboratory, we had no humane justification for the utilization of additional animals for this purpose. While the in vivo PK profile may have been altered by the organism and thus may have resulted in increased required exposures, the dose exposure delivered was that of a humanized regimen, which was the question asked in the study design.
Although the ideal endpoint for this model is profound bactericidal activity, the ability of doripenem to maintain static effects in severely compromised hosts and modest CFU reductions in the competent model for organisms harboring carbapenemases suggests the potential utility, albeit most likely in combination with another agent, of the doripenem agent against this formidable pathogen.
Acknowledgments
We thank Henry Christensen, Lindsay Tuttle, Debora Santini, Jennifer Hull, Dora Wiskirchen, and Rebecca Keel for their assistance with the conduct of the animal experimentation.
This study was undertaken with funds from the Center for Anti-Infective Research and Development, Hartford Hospital, and was not funded by Johnson & Johnson Pharmaceuticals, the manufacturer of doripenem.
Footnotes
Published ahead of print on 26 July 2010.
REFERENCES
- 1.Andes, D., and W. A. Craig. 1998. In vivo activities of amoxicillin and amoxicillin-clavulanate against Streptococcus pneumoniae: application to breakpoint determinations. Antimicrob. Agents Chemother. 42:2375-2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bulik, C. C., H. Christensen, P. Li, C. A. Sutherland, D. P. Nicolau, and J. L. Kuti. 2010. Comparison of the activity of a human simulated, high-dose, prolonged infusion of meropenem against Klebsiella pneumoniae producing the KPC carbapenemase versus that against Pseudomonas aeruginosa in an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 54:804-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clinical and Laboratory Standards Institute. 2008. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 8th ed. CLSI publication M07-A8. Clinical and Laboratory Standard Institute, Wayne, PA.
- 4.Craig, W. A. 2003. Basic pharmacodynamics of antibacterials with clinical applications to the use of beta-lactams, glycopeptides, and linezolid. Infect. Dis. Clin. North Am. 17:479-501. [DOI] [PubMed] [Google Scholar]
- 5.Craig, W. A., and D. R. Andes. 2008. In vivo pharmacodynamics of ceftobiprole against multiple bacterial pathogens in murine thigh and lung infection models. Antimicrob. Agents Chemother. 52:3492-3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Craig, W. A., S. Kethireddy, D. R. Andes, T. Stamstad, K. Marchilo, J. Asbeck, and R. N. Jones. 2008. Impact of KPCs on the in vivo activity of three carbapenems in the neutropenic mouse-thigh infection model, abstr. A-029. Abstr. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (IDSA) 46th Annu. Meet. American Society for Microbiology and Infectious Diseases Society of America, Washington, DC.
- 7.Crandon, J. L., C. C. Bulik, and D. P. Nicolau. 2009. In vivo efficacy of 1- and 2-gram human simulated prolonged infusions of doripenem against Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 53:4352-4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deshpande, L. M., P. R. Rhomberg, H. S. Sader, and R. N. Jones. 2006. Emergence of serine carbapenemases (KPC and SME) among clinical strains of Enterobacteriaceae isolated in the United States medical centers: report from the MYSTIC program (1999-2005). Diagn. Microbiol. Infect. Dis. 56:367-372. [DOI] [PubMed] [Google Scholar]
- 9.Drusano, G. L., F. Sorgel, J. Quinn, B. Mason, and D. Melnick. 2005. Impact of pharmacodynamic dosing of meropenem on emergence of resistance during treatment of ventilator-associated pneumonia (VAP): a prospective clinical trial, abstr. K-127. Abstr. 45th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 10.Holmberg, S. D., S. L. Solomon, and P. A. Blake. 1987. Health and economic impacts of antimicrobial resistance. Rev. Infect. Dis. 9:1065-1078. [DOI] [PubMed] [Google Scholar]
- 11.Kim, A., M. A. Banevicius, and D. P. Nicolau. 2008. In vivo pharmacodynamic profiling of doripenem against Pseudomonas aeruginosa by simulating human exposures. Antimicrob. Agents Chemother. 52:2497-2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mattoes, H. M., J. L. Kuti, G. L. Drusano, and D. P. Nicolau. 2004. Optimizing antimicrobial pharmacodynamics: dosage strategies for meropenem. Clin. Ther. 26:1187-1198. [DOI] [PubMed] [Google Scholar]
- 13.Meinen, J. B., J. T. McClure, and E. Rosin. 1995. Pharmacokinetics of enrofloxacin in clinically normal dogs and mice and drug pharmacodynamics in neutropenic mice with Escherichia coli and staphylococcal infections. Am. J. Vet. Res. 56:1219-1224. [PubMed] [Google Scholar]
- 14.Pruul, H., and P. J. McDonald. 1996. Uptake of cefepime by phagocytosing polymorphonuclear neutrophils and subsequent intracellular killing. Antimicrob. Agents Chemother. 40:1870-1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Queenan, A. M., and K. Bush. 2007. Carbapenemases: the versatile β-lactamases. Clin. Microbiol. Rev. 20:440-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rhomberg, P. R., and R. N. Jones. 2009. Summary trends for the Meropenem Yearly Susceptibility Test Information Collection Program: a 10-year experience in the United States (1999-2008). Diagn. Microbiol. Infect. Dis. 65:414-426. [DOI] [PubMed] [Google Scholar]