Abstract
Biofilm formation is an important virulence factor that allows bacteria to resist host responses and antibacterial agents. The aim of the study was to assess the in vitro activities of several antimicrobials alone or in combination against two Staphylococcus aureus isolates in a novel pharmacokinetic/pharmacodynamic (PK/PD) model of biofilm for 3 days. One methicillin-susceptible S. aureus strain (SH1000) and one methicillin-resistant S. aureus strain (N315) were evaluated in a modified biofilm reactor with polystyrene coupons. Simulated regimens included vancomycin (VAN) plus rifampin (RIF), moxifloxacin (MOX), and high doses (10 mg/kg of body weight/day) of daptomycin (DAP) alone or combined with RIF or clarithromycin (CLA). Against viable planktonic bacteria (PB) and biofilm-embedded bacteria (BB) of SH1000, neither DAP nor MOX alone was bactericidal. In contrast, the combination of DAP or MOX with CLA significantly increased the activity of the two agents against both PB and BB (P < 0.01), and DAP plus CLA reached the limit of detection at 72 h. Against PB of N315, DAP alone briefly achieved bactericidal activity at 24 h, whereas sustained bactericidal activity was observed at 32 h with VAN plus RIF. Overall, only a minimal reduction was observed with both regimens against BB (<2.8 log10 CFU/ml). Finally, the combination of DAP and RIF was bactericidal against both PB and BB, achieving the limit of detection at 72 h. In conclusion, we developed a novel in vitro PK/PD model to assess the activities of antimicrobials against mature bacterial biofilm. Combinations of DAP or MOX with CLA were the most effective regimens and may represent promising options to treat persistent infections caused by S. aureus biofilms.
Biofilms are complex bacterial communities embedded in a self-produced glycocalyx slime that protects the cells from environmental and antimicrobial threats. It has been reported that antimicrobial MICs of bacteria embedded in biofilms can be 10 to 1,000 times higher than those in a planktonic state (4). The reasons for this decreased susceptibility are not completely understood. However, to explain the diminished activity of antimicrobials against biofilm-embedded cells, poor penetration of the drug through the layers of the matrix as well as heterogeneity of the growth within the biofilm have been suggested (22, 28, 36).
Because bacteria can produce biofilms on medical devices, biofilm-associated infections have a tremendous impact on the management of patient health. Staphylococcus aureus is a pathogen commonly associated with biofilm-related infections, such as osteomyelitis, prosthetic joint infections, endocarditis, and catheter-related infections (22). Various in vitro scenarios of biofilm formation have evaluated the activity of several antimicrobials and have reported different outcomes (13, 15, 31, 32).
Daptomycin (DAP) is a newly available lipopeptide antibiotic with potent in vitro and in vivo bactericidal activities against S. aureus, including methicillin-resistant S. aureus (MRSA) (5, 33). Daptomycin has been already evaluated in different in vitro biofilm models, and conflicting results have been reported (15, 18, 32). For instance, with an in vitro model of catheter-related infection, LaPlante and Mermel found that daptomycin at 5 mg/ml was able to eradicate S. aureus biofilms, whereas lower concentrations of daptomycin (clinically irrelevant) did not eradicate biofilm-embedded cells (18). With a static microtiter plate model, Hajdu et al. did not find any reduction in the viable count of Staphylococcus epidermidis biofilm cells with doses of daptomycin up to 128 times the MIC (15). Potential explanations for the discrepancies in these results might be differences in the regimens tested and in the models used for the evaluations.
Because there have been several reports about the safety and efficacy of doses of daptomycin up to 12 mg/kg of body weight/day (3, 9, 17, 21, 24), we designed the present study to evaluate a high-dose regimen of daptomycin (10 mg/kg/day) alone or in combination with other agents and compared its activity to that of vancomycin (VAN) for a MRSA isolate and to that of moxifloxacin (MOX) for a MSSA strain. Although there is no optimal therapy, these two drugs are considered useful by clinicians in the treatment of biofilm-associated infections caused by S. aureus. We investigated the effects of combination therapy, since association of vancomycin with other drugs, such as rifampin (RIF) and clarithromycin (CLA), has already been proved beneficial (11, 19, 25, 31). Finally, because most of the models developed so far present major limitations with regard to antibiotic pharmacokinetics simulations, we adapted a new in vitro pharmacokinetic/pharmacodynamic (PK/PD) model of biofilm formation to assess the antimicrobial activities of these drugs.
(A portion of this work was presented at the 20th European Conference of Clinical Microbiology and Infectious Diseases in Vienna, Austria, 10 to 13 April 2010, as poster P3153.)
MATERIALS AND METHODS
Bacterial strains and culture media.
Two well-described S. aureus strains, one MSSA (SH1000) (20) and one MRSA (N315) (26), were evaluated in this study. Kazuya Morikawa from Tsukuba University, Japan, generously provided MRSA N315. Isolates were stored in CryoCare vials (KEY Scientific Products, Inc., TX) at −80°C. Tryptic soy agar (TSA; Difco, Detroit, MI) plates were used to grow isolates and perform bacterial enumeration. Tryptic soy broth supplemented with 1% or 10% glucose (gSTSB) was used for the 24-h batch or 16-h continuous flow modes of the conditioning phase, respectively. Mueller-Hinton broth (MH; Difco, Detroit, MI) supplemented with 25 μg/ml calcium and 12.5 μg/ml magnesium (SMHB) was used for all in vitro PK/PD experiments and susceptibility testing involving vancomycin, moxifloxacin, clarithromycin, and rifampin. For simulated regimens and susceptibility testing with daptomycin, MHB was supplemented with 50 μg/ml calcium and 12.5 μg/ml magnesium because of the calcium dependency of daptomycin.
Antimicrobial agents.
Vancomycin, clarithromycin, and rifampin were obtained from Sigma-Aldrich (St. Louis, MO). Daptomycin and moxifloxacin were commercially purchased. Drug powders were reconstituted following the CLSI guidelines (6), using sterile distilled water for vancomycin and daptomycin and methanol for rifampin and clarithromycin. Moxifloxacin was purchased as a 1.6-mg/ml solution in 0.9% NaCl. Except for moxifloxacin, stock solutions of each antibiotic were freshly prepared prior to each experiment and stored at −4°C. Moxifloxacin was kept at room temperature.
Susceptibility testing.
Susceptibility testing of all antimicrobials was performed in duplicate by broth microdilution following CLSI M7-A8 guidelines (6). Biofilm MIC testing of all antimicrobials was performed using the modified Calgary biofilm device method, as previously described (4).
In vitro PK/PD model.
We based our protocol for biofilm growth upon the methods previously published by Donlan et al. and McLeod and Sandvik (14, 23). Briefly, a CDC biofilm reactor (CBR) model (BioSurfaces Technologies, Bozeman, MT) was set up with 24 polycarbonate coupons inserted into eight rods (3 coupons per rod). A 40-h biofilm conditioning phase was performed prior to evaluation of the antimicrobials and consisted of a 24-h incubation at 37°C of inoculated 1% gSTSB, followed by 16 h of continuous flow with 10% gSTSB performed with peristaltic pumps (Masterflex; Cole-Parmer Instrument Co., Chicago, IL) set up at a rate of 13.3 ml/min to achieve a 30-min residence time. Once the continuous flow phase was completed, inflow medium with SMHB was used for antibiotic simulations. Boluses of antibiotics were injected into the reactor after the biofilm conditioning phase was completed. Peristaltic pumps were then set up to simulate the half-lives of the antibiotics. Regimens evaluated were daptomycin at 10 mg/kg every 24 h (q24h; free drug peak concentration [fCmax], 11.3 mg/liter; t1/2, 8 h; protein binding, 92%) (Cubicin package insert, Cubist Pharmaceuticals) alone or in combination with clarithromycin at 250 mg q12h (fCmax, 1 mg/liter; t1/2, 3.5 h; protein binding, 50%) (Cleocin package insert [http://dailymed.nlm.nih.gov/dailymed/archives/fdaDrugInfo.cfm?archiveid=843]) and moxifloxacin at 400 mg q24h (fCmax, 2.5 mg/liter; t1/2, 12.5 h; protein binding, 50%) (2) alone or in combination with clarithromycin at 250 mg q12h for MSSA SH1000. For MRSA N315, we evaluated daptomycin at 10 mg/kg q24h alone or in combination with rifampin at 600 mg q24h (fCmax, 3.5 mg/liter; t1/2, 3 h; protein binding, 90%) (Rifadin package insert, http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=7821) and vancomycin at q12h (fCmax, 20 mg/liter; t1/2, 6 h; protein binding, 50%) in combination with rifampin at 600 mg q24h. A growth control was performed with no drug, and each model was evaluated in duplicate to ensure reproducibility.
PD analysis.
Three coupons were aseptically removed at 0, 4, 8, 24, 32, 48, 56, and 72 h. Each coupon was washed twice in sterile normal saline to remove excess planktonic cells. Then, each coupon was placed in a sterile tube containing 10 ml of normal saline. Biofilm bacteria were recovered by three alternating 60-s cycles of vortexing and sonication at 20 Hz (Bransonic 12; Branson Ultrasonic Corporation) and a final 60 s of vortexing. Samples were then serially diluted with normal saline and drop plated onto TSA to allow enumeration of viable colonies. Planktonic bacteria recovered at the different time points (in ≈1 ml of medium) were serially diluted and spiral plated onto TSA by using an automated spiral plater (Don Whitley Scientific Ltd., West Yorkshire, England). Plated samples were incubated at 37°C for 24 h, and colony counts (log10 CFU per milliliter) were determined using a laser colony counter (ProtoCOL, version 2.05.02; Symbiosis, Cambridge, England). For all samples, antimicrobial carryover was accounted for by serial dilution of the plated samples or by vacuum filtration if the drug level of the anticipated dilution was near the MIC value of the organism. Briefly, samples were washed through a 0.45-μm filter with normal saline, plated onto TSA, and incubated at 37°C for 24 h. The limit of detection of these methods of colony count determination was 2 log10 CFU/ml extended to 1 log10 CFU/ml by vacuum filtration for both planktonic and biofilm cells. Concentrations of both biofilm-embedded and planktonic cells (means and standard deviations in CFU/ml) were computed and plotted to graph time-kill curves. Absolute reductions in colony counts were determined over the 72-h period and compared between regimens. Bactericidal (99.9% kill) and bacteriostatic effects were defined as a ≥3-log10 CFU/ml reduction and a <3-log10 CFU/ml reduction in the colony count compared to the starting inoculum, respectively. The time required to achieve 99.9% killing was determined by linear regression (if r2 was ≥0.95) or by visual inspection. Inactivity was defined as no observed reduction in colony counts. Enhancement and improvement of activity by the addition of a drug were defined as a ≥2-log10 CFU/ml increase and a 1- to 2-log10 CFU/ml increase in kill compared to the most active single agent of the combination, respectively. Combinations that resulted in a ≥-1 log10 CFU/ml reduction in bacterial growth in comparison to the least-active single agent were considered to represent antagonism.
PK analysis.
Vancomycin concentrations were measured by fluorescence polarization immunoassay (TDx; Abbott Diagnostics). This assay has a lower limit of detection of 2 μg/ml. Between-day coefficients of variation (CV%) were 0.9%, 2.5%, and 3.0% for high, medium, and low standards (75, 35, and 7 μg/ml), respectively. Concentrations of rifampin, daptomycin, and moxifloxacin were determined by bioassay using Micrococcus luteus ATCC 9341 and antibiotic medium 11. Clarithromycin concentrations were measured by bioassay using Bacillus subtilis ATCC 6631 and antibiotic medium 11. The elimination half-lives, areas under the concentration-time curves (AUCs), and peak and trough concentrations were determined by the trapezoidal method with PKAnalyst software (version 1.10; MicroMath Scientific Software, Salt Lake City, UT).
SEM.
One coupon of each rod recovered at 0, 24, and 72 h was evaluated for the presence and structure of biofilm by scanning electron microscopy (SEM). After removal, the coupon was washed in normal saline to remove nonadherent cells and immersion fixed in a solution of 2.5% glutaraldehyde, 2% paraformaldehyde in 0.1 M sodium phosphate buffer. Coupons were then dehydrated in a graded ethanol series and carbon coated at 30 A for 3 min by using a SeeVac Conductavac IV sputter coater. The coupons were imaged using a Hitachi S570 SEM at 2,000× magnification and evaluated for the presence and characteristics of biofilm.
Emergence of resistance.
Susceptibility testing of colonies recovered at 48 and 72 h was performed according to CLSI guidelines in order to evaluate any change in the MIC or minimal bactericidal concentration. Similarly, the biofilm MIC was performed to evaluate any changes in the minimum biofilm inhibitory concentration (MBIC) (6).
Statistical analysis.
Changes in the log10 CFU/ml for planktonic and biofilm-embedded bacteria at 72 h were evaluated for each regimen by analysis of variance with Tukey's post hoc test. A P value of ≤0.05 was considered significant. All statistical analyses were performed using SPSS statistical software (release 17.0; SPSS, Inc., Chicago, IL).
RESULTS
Susceptibilities of the isolates are displayed in Table 1. Because MRSA N315 was resistant to moxifloxacin (MIC, 8 mg/liter), we did not run moxifloxacin against this isolate. No changes in susceptibilities were observed throughout the experiments. Observed PK parameters are summarized in Table 2. Overall, PK values were within 15% of the targeted values.
TABLE 1.
MIC and MBIC values of each antimicrobial agent evaluated against the two isolates of S. aureus
| Antimicrobial agent | MSSA SH1000 |
MRSA N315 |
||
|---|---|---|---|---|
| MIC (μg/ml) | MBIC (μg/ml) | MIC (μg/ml) | MBIC (μg/ml) | |
| Daptomycin | 0.125 | 2 | 0.125 | 2 |
| Moxifloxacin | 0.0625 | 0.5 | NDa | ND |
| Vancomycin | ND | ND | 0.25 | 2 |
| Rifampin | ND | ND | <0.0625 | <0.0625 |
| Clarithromycin | >128 | >128 | ND | ND |
ND, not done.
TABLE 2.
PK results for daptomycin, vancomycin, moxifloxacin, clarithromycin, and rifampin obtained with the PK/PD model
| Antimicrobial agent | fCmax (μg/ml)a (targeted value) | t1/2 (h) (targeted value) | AUC0-24h (μg/ml·h) |
|---|---|---|---|
| Daptomycin | 11.79 ± 0.22 (11.3) | 8.21 (8) | 199.77 |
| Moxifloxacin | 2.52 ± 0.14 (2.5) | 12.13 (12.5) | 44.15 |
| Vancomycin | 22.6 ± 0.17 (20) | 5.91 (6) | 192.88 |
| Rifampin | 3.82 ± 1.21 (3.5) | 3.56 (3) | 19.65 |
| Clarithromycin | 1.15 ± 0.6 (1) | 3.91 (3.5) | 6.53 |
Data are means ± standard deviations.
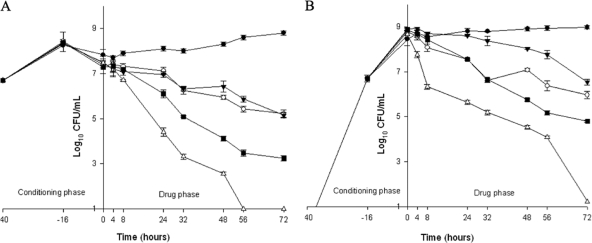
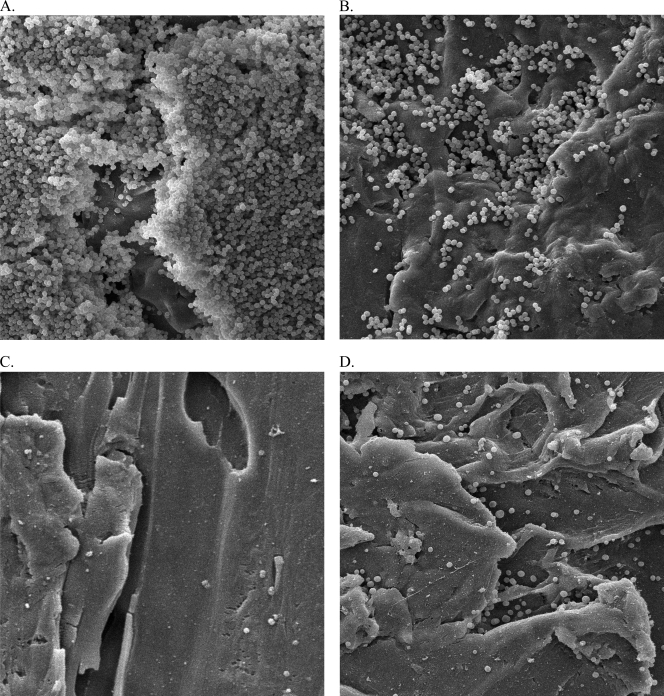
Against MSSA SH1000, daptomycin and moxifloxacin alone demonstrated similar activities and never achieved bactericidal activity against either PB or BB. Absolute reductions in PB and BB, respectively, were 2.2 ± 0.16 and 2.83 ± 0.15 log10 CFU/ml for daptomycin and 2.33 ± 0.24 and 2.38 ± 0.21 log10 CFU/ml for moxifloxacin. Combinations of daptomycin or moxifloxacin with clarithromycin significantly increased the activity compared to the drug alone, achieving bactericidal activity against both PB and BB at 24 h for daptomycin and at 48 h for moxifloxacin. Only daptomycin plus clarithromycin significantly reduced the bacterial density of PB and BB to the limit of detection at 72 h (P = 0.01 and 0.008, respectively), whereas the absolute reduction in PB and BB with moxifloxacin was 4.04 ± 0.14 and 4.06 ± 0.08 log10 CFU/ml, respectively (Fig. 1). SEM imaging confirmed these pharmacodynamic results. Images were collected at 2,000× magnification and demonstrated a net reduction in the organisms and biofilm present at the surfaces of the different coupons (Fig. 2 B to D) compared to the initial coupon (Fig. 2A).
FIG. 1.
Activity of DAP at 10 mg/kg/day q24h and 400 mg MOX daily, alone or in combination with CLA at 250 mg q12h against SH1000. (A) Planktonic bacteria; (B) biofilm-embedded bacteria. White circles, DAP; black inversed triangles, MOX; white triangles, DAP plus CLA; black squares, MOX plus CLA; black circles, GC, growth control (organism growth with no drug added).
FIG. 2.
SEM images of coupon surfaces to assess the presence and structure of the matrix of a SH1000 biofilm. Images were collected at 2,000× magnification. (A) Before any drug exposure; (B) after 72 h of daptomycin exposure; (C) after 72 h of daptomycin plus clarithromycin exposure; (D) after 72 h of moxifloxacin plus clarithromycin exposure.
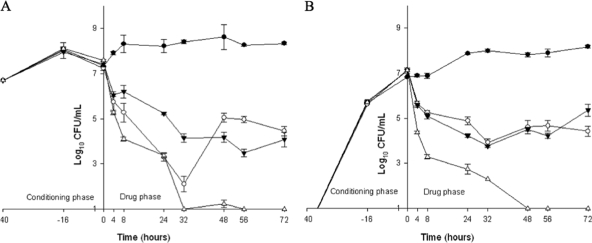
Against MRSA N315, daptomycin was bactericidal at 24 h against PB, but bacterial regrowth uncorrelated to the emergence of resistance was observed after 48 h. The absolute final reduction was therefore 2.78 ± 0.14 and 2.73 ± 0.13 log10 CFU/ml for PB and BB, respectively. In contrast, sustained bactericidal activity was observed with the combination of vancomycin plus rifampin at 32 h against PB, whereas the reduction in BB was only minimal. Overall, the absolute reductions in PB and BB achieved with this combination were 3.34 ± 0.24 and 1.76 ± 0.15 log10 CFU/ml, respectively. A significant increase in the killing effect (P = 0.04 and P = 0.04 for PB and BB, respectively) was obtained when daptomycin was combined with rifampin. Bactericidal activity was achieved at 8 h for both PB and BB, and the reductions in the viable bacterial densities reached the limit of detection at 72 h for both PB and BB (Fig. 3).
FIG. 3.
Activity of DAP at 10 mg/kg/day q24h, alone or in combination with RIF, and of VAN in combination with RIF against N315. (A) Planktonic bacteria; (B) biofilm-embedded bacteria. White circles, DAP; black inversed triangles, VAN plus RIF; white triangles, DAP plus RIF; black circles, GC, growth control (organism growth with no drug added).
DISCUSSION
Staphylococcus spp. account for the majority of biofilm-associated infections (1, 7, 12, 27). In a recent review of case series and case reports, both Staphylococcus spp. and Pseudomonas spp. were the most frequent causative pathogens, and resolution of the infections was not achieved without removal of the infected implants (8), highlighting the need for new agents or strategies to treat biofilm-associated infections.
In the present study, we report the in vitro activities of antimicrobials currently available, used alone or in combination, against two strains of S. aureus grown in mature biofilms. Consistent with previous data (13, 15, 31, 32), we found that none of the agents alone (including high-dose daptomycin) was able to reduce the biofilm bacterial burden below the limit of detection. Combination therapy resulted, however, in a complete eradication of planktonic and biofilm-embedded staphylococci. Combinations of daptomycin or moxifloxacin with rifampin or clarithromycin at physiologically achievable concentrations resulted in a significant and pronounced improvement in the in vitro activity of daptomycin against both planktonic and biofilm-embedded cells, reducing the bacterial density below the limit of detection. Also consistent with other findings reported in the literature (16, 30, 34), we found that daptomycin plus rifampin resulted in better killing than vancomycin plus rifampin against MRSA grown in biofilm, and combination regimens performed better than daptomycin alone. Daptomycin has been already evaluated alone and in combination in different in vitro models of biofilms. Conflicting results have been reported, presumably due to the differences in the models and doses investigated (15, 18, 32), but of interest, a high dose of daptomycin always resulted in an enhancement in the activity (18, 32). This may suggest and highlight the need for higher doses to cure these difficult-to-treat infections.
Similarly, moxifloxacin demonstrated promising activity against MSSA grown in mature biofilm. In this study, we found that moxifloxacin and daptomycin were equally active against planktonic and biofilm-embedded bacteria, but a significant enhancement in the killing effect was observed when combined with clarithromycin. However, only daptomycin was able to reduce the bacterial count below the limit of detection at 72 h. Of note, clarithromycin was not used as monotherapy in this model, since the isolate of MSSA SH1000 was resistant to this drug, with a MIC greater than 128 μg/ml, and no killing effect for clarithromycin. These results are consistent with those reported by Gander et al. (13), who assessed the activities of different antimicrobials, including telavancin, moxifloxacin, vancomycin, teicoplanin, and linezolid in an in vitro model of biofilms. They reported that moxifloxacin was the most active agent against moxifloxacin-susceptible strains of S. aureus, with the greatest reduction in biofilm-embedded bacteria (range, 0 to 6 log10). Frank et al. (10) also found that moxifloxacin at clinically achievable concentrations was the only antimicrobial agent able to kill more than 70% of Staphylococcus lugdunensis isolates grown in biofilm. However, Pérez-Giraldo et al. (29) found different results in an in vitro model of biofilm and reported that moxifloxacin concentrations up to 100× MIC were needed to achieve more than a 1-log10 CFU/ml reduction in biofilm-embedded bacteria.
In conclusion, we found that neither moxifloxacin nor high-dose daptomycin alone exhibited bactericidal activity against staphylococcal biofilms. Combination therapy such as daptomycin or moxifloxacin plus clarithromycin significantly improved the bacterial killing effect of daptomycin against biofilms of staphylococci. However, this study presented some limitations that need to be carefully considered, including the low variety of tested organisms (two strains of S. aureus) and the short length of therapy, which might preclude the emergence of resistance. Finally, the mechanism by which clarithromycin enhanced daptomycin and moxifloxacin activity is not completely understood yet. Previous studies reported that clarithromycin suppressed glycocalyx production and therefore biofilm formation (35, 37). Further investigations are now warranted to clarify the mechanism of action of clarithromycin against biofilms and to determine the clinical impact of such preliminary results.
Acknowledgments
J.P.-R. was supported by Sociedad Andaluza de Enfermedades Infecciosas and Consejería de Salud de la Junta de Andalucía.
Footnotes
Published ahead of print on 9 August 2010.
REFERENCES
- 1.Aslam, S. 2008. Effect of antibacterials on biofilms. Am. J. Infect. Control 36:S175.e9-S175.e11. [DOI] [PubMed] [Google Scholar]
- 2.Balfour, J. A., and L. R. Wiseman. 1999. Moxifloxacin. Drugs 57:363-373. [DOI] [PubMed] [Google Scholar]
- 3.Benvenuto, M., D. P. Benziger, S. Yankelev, and G. Vigliani. 2006. Pharmacokinetics and tolerability of daptomycin at doses up to 12 milligrams per kilogram of body weight once daily in healthy volunteers. Antimicrob. Agents Chemother. 50:3245-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ceri, H., M. E. Olson, C. Stremick, R. R. Read, D. Morck, and A. Buret. 1999. The Calgary biofilm device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J. Clin. Microbiol. 37:1771-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cha, R., W. J. Brown, and M. J. Rybak. 2003. Bactericidal activities of daptomycin, quinupristin-dalfopristin, and linezolid against vancomycin-resistant Staphylococcus aureus in an in vitro pharmacodynamic model with simulated endocardial vegetations. Antimicrob. Agents Chemother. 47:3960-3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clinical and Laboratory Standards Institute. 2009. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 9th ed. Clinical and Laboratory Standards Institute, Wayne, PA.
- 7.Costerton, J. W., L. Montanaro, and C. R. Arciola. 2005. Biofilm in implant infections: its production and regulation. Int. J. Artif. Organs 28:1062-1068. [DOI] [PubMed] [Google Scholar]
- 8.Falagas, M. E., A. M. Kapaskelis, V. D. Kouranos, O. K. Kakisi, Z. Athanassa, and D. E. Karageorgopoulos. 2009. Outcome of antimicrobial therapy in documented biofilm-associated infections: a review of the available clinical evidence. Drugs 69:1351-1361. [DOI] [PubMed] [Google Scholar]
- 9.Figueroa, D. A., E. Mangini, M. Amodio-Groton, B. Vardianos, A. Melchert, C. Fana, W. Wehbeh, C. M. Urban, and S. Segal-Maurer. 2009. Safety of high-dose intravenous daptomycin treatment: three-year cumulative experience in a clinical program. Clin. Infect. Dis. 49:177-180. [DOI] [PubMed] [Google Scholar]
- 10.Frank, K. L., E. J. Reichert, K. E. Piper, and R. Patel. 2007. In vitro effects of antimicrobial agents on planktonic and biofilm forms of Staphylococcus lugdunensis clinical isolates. Antimicrob. Agents Chemother. 51:888-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujimura, S., T. Sato, T. Mikami, T. Kikuchi, K. Gomi, and A. Watanabe. 2008. Combined efficacy of clarithromycin plus cefazolin or vancomycin against Staphylococcus aureus biofilms formed on titanium medical devices. Int. J. Antimicrob. Agents 32:481-484. [DOI] [PubMed] [Google Scholar]
- 12.Fux, C. A., P. Stoodley, L. Hall-Stoodley, and J. W. Costerton. 2003. Bacterial biofilms: a diagnostic and therapeutic challenge. Expert Rev. Anti Infect. Ther. 1:667-683. [DOI] [PubMed] [Google Scholar]
- 13.Gander, S., A. Kinnaird, and R. Finch. 2005. Telavancin: in vitro activity against staphylococci in a biofilm model. J. Antimicrob. Chemother. 56:337-343. [DOI] [PubMed] [Google Scholar]
- 14.Goeres, D. M., L. R. Loetterle, M. A. Hamilton, R. Murga, D. W. Kirby, and R. M. Donlan. 2005. Statistical assessment of a laboratory method for growing biofilms. Microbiology 151:757-762. [DOI] [PubMed] [Google Scholar]
- 15.Hajdu, S., A. Lassnigg, W. Graninger, A. M. Hirschl, and E. Presterl. 2009. Effects of vancomycin, daptomycin, fosfomycin, tigecycline, and ceftriaxone on Staphylococcus epidermidis biofilms. J. Orthop. Res. 27:1361-1365. [DOI] [PubMed] [Google Scholar]
- 16.John, A. K., D. Baldoni, M. Haschke, K. Rentsch, P. Schaerli, W. Zimmerli, and A. Trampuz. 2009. Efficacy of daptomycin in implant-associated infection due to methicillin-resistant Staphylococcus aureus: importance of combination with rifampin. Antimicrob. Agents Chemother. 53:2719-2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kullar, R., S. Davis, D. P. Levine, et al. 2009. Safety of high-dose daptomycin for gram-positive infections, abstr. 1983. Abstr. 49th. Intersci. Conf. Antimicrob. Agents Chemother., San Francisco, CA, 12 to 15 September 2009. American Society for Microbiology, Washington, DC.
- 18.LaPlante, K. L., and L. A. Mermel. 2007. In vitro activity of daptomycin and vancomycin lock solutions on staphylococcal biofilms in a central venous catheter model. Nephrol. Dial. Transplant. 22:2239-2246. [DOI] [PubMed] [Google Scholar]
- 19.LaPlante, K. L., and S. Woodmansee. 2009. Activities of daptomycin and vancomycin alone and in combination with rifampin and gentamicin against biofilm-forming methicillin-resistant Staphylococcus aureus isolates in an experimental model of endocarditis. Antimicrob. Agents Chemother. 53:3880-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lauderdale, K. J., B. R. Boles, A. L. Cheung, and A. R. Horswill. 2009. Interconnections between sigma B, agr, and proteolytic activity in Staphylococcus aureus biofilm maturation. Infect. Immun. 77:1623-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lichterfeld, M., M. J. Ferraro, and B. T. Davis. 2010. High-dose daptomycin for the treatment of endocarditis caused by Staphylococcus aureus with intermediate susceptibility to glycopeptides. Int. J. Antimicrob. Agents 35:96. [DOI] [PubMed] [Google Scholar]
- 22.Mah, T. F., and G. A. O'Toole. 2001. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 9:34-39. [DOI] [PubMed] [Google Scholar]
- 23.McLeod, B. R., and E. L. Sandvik. 2010. A biofilm growth protocol and the design of a magnetic field exposure setup to be used in the study of magnetic fields as a means of controlling bacterial biofilms. Bioelectromagnetics 31:56-63. [DOI] [PubMed] [Google Scholar]
- 24.Moise, P. A., E. Hershberger, M. I. Amodio-Groton, and K. C. Lamp. 2009. Safety and clinical outcomes when utilizing high-dose (≥ 8 mg/kg) daptomycin therapy. Ann. Pharmacother. 43:1211-1219. [DOI] [PubMed] [Google Scholar]
- 25.Monzon, M., C. Oteiza, J. Leiva, and B. Amorena. 2001. Synergy of different antibiotic combinations in biofilms of Staphylococcus epidermidis. J. Antimicrob. Chemother. 48:793-801. [DOI] [PubMed] [Google Scholar]
- 26.Morikawa, K., A. Maruyama, Y. Inose, M. Higashide, H. Hayashi, and T. Ohta. 2001. Overexpression of sigma factor, sigma(B), urges Staphylococcus aureus to thicken the cell wall and to resist beta-lactams. Biochem. Biophys. Res. Commun. 288:385-389. [DOI] [PubMed] [Google Scholar]
- 27.Otto, M. 2008. Staphylococcal biofilms. Curr. Top. Microbiol. Immunol. 322:207-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel, R. 2005. Biofilms and antimicrobial resistance. Clin. Orthop. Relat. Res. 437:41-47. [DOI] [PubMed] [Google Scholar]
- 29.Perez-Giraldo, C., C. Gonzalez-Velasco, R. M. Sanchez-Silos, C. Hurtado, M. T. Blanco, and A. C. Gomez-Garcia. 2004. Moxifloxacin and biofilm production by coagulase-negative staphylococci. Chemotherapy 50:101-104. [DOI] [PubMed] [Google Scholar]
- 30.Raad, I., H. Hanna, Y. Jiang, T. Dvorak, R. Reitzel, G. Chaiban, R. Sherertz, and R. Hachem. 2007. Comparative activities of daptomycin, linezolid, and tigecycline against catheter-related methicillin-resistant Staphylococcus bacteremic isolates embedded in biofilm. Antimicrob. Agents Chemother. 51:1656-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rose, W. E., and P. T. Poppens. 2009. Impact of biofilm on the in vitro activity of vancomycin alone and in combination with tigecycline and rifampicin against Staphylococcus aureus. J. Antimicrob. Chemother. 63:485-488. [DOI] [PubMed] [Google Scholar]
- 32.Roveta, S., A. Marchese, and G. C. Schito. 2008. Activity of daptomycin on biofilms produced on a plastic support by Staphylococcus spp. Int. J. Antimicrob. Agents 31:321-328. [DOI] [PubMed] [Google Scholar]
- 33.Safdar, N., D. Andes, and W. A. Craig. 2004. In vivo pharmacodynamic activity of daptomycin. Antimicrob. Agents Chemother. 48:63-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sakoulas, G., G. M. Eliopoulos, J. Alder, and C. Thauvin-Eliopoulos. 2003. Efficacy of daptomycin in experimental endocarditis due to methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 47:1714-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sano, M., T. Hirose, M. Nishimura, S. Takahashi, M. Matsukawa, and T. Tsukamoto. 1999. Inhibitory action of clarithromycin on glycocalyx produced by MRSA. J. Infect. Chemother. 5:10-15. [DOI] [PubMed] [Google Scholar]
- 36.Stewart, P. S., and J. W. Costerton. 2001. Antibiotic resistance of bacteria in biofilms. Lancet 358:135-138. [DOI] [PubMed] [Google Scholar]
- 37.Yasuda, H., Y. Ajiki, T. Koga, and T. Yokota. 1994. Interaction between clarithromycin and biofilms formed by Staphylococcus epidermidis. Antimicrob. Agents Chemother. 38:138-141. [DOI] [PMC free article] [PubMed] [Google Scholar]