Abstract
There is no consensus about whether making muscles abnormally large by reducing myostatin activity affects force-generating capacity or the ability to perform activities requiring muscular endurance. We therefore examined grip force, contractile properties of extensor digitorum longus (EDL) muscles, and voluntary wheel running in mice in which myostatin was depleted after normal muscle development. Cre recombinase activity was induced to knock out exon 3 of the myostatin gene in 4-mo-old mice in which this exon was flanked by loxP sequences (Mstn[f/f]). Control mice with normal myostatin genes (Mstn[w/w]) received the same Cre-activating treatment. Myostatin depletion increased the mass of all muscles that were examined (gastrocnemius, quadriceps, tibialis anterior, EDL, soleus, triceps) by ∼20–40%. Grip force, measured multiple times 2–22 wk after myostatin knockout, was not consistently greater in the myostatin-deficient mice. EDL contractile properties were determined 7–13 mo after myostatin knockout. Twitch force tended to be greater in myostatin-deficient muscles (+24%; P = 0.09), whereas tetanic force was not consistently elevated (mean +11%; P = 0.36), even though EDL mass was greater than normal in all myostatin-deficient mice (mean +36%; P < 0.001). The force deficit induced by eccentric contractions was approximately twofold greater in myostatin-deficient than in normal EDL muscles (31% vs. 16% after five eccentric contractions; P = 0.02). Myostatin-deficient mice ran 19% less distance (P < 0.01) than control mice during the 12 wk following myostatin depletion, primarily because of fewer running bouts per night rather than diminished running speed or bout duration. Reduced specific tension (ratio of force to mass) and reduced running have been observed after muscle hypertrophy was induced by other means, suggesting that they are characteristics generally associated with abnormally large muscles rather than unique effects of myostatin deficiency.
Keywords: muscle hypertrophy, exercise, eccentric contractions, extensor digitorum longus
it is well established that lack of myostatin signaling leads to increased muscle mass. This effect is most pronounced in mice with constitutive absence of myostatin (Mstn[−/−]), in which there is both muscle fiber hypertrophy and hyperplasia (21). A more modest muscle growth due to fiber hypertrophy occurs after myostatin is knocked out or blocked after muscle development (31, 32, 34). Most of the research on functional effects of myostatin deficiency has involved mouse models of neuromuscular diseases (28). Because athletes might attempt to employ anti-myostatin agents to enhance performance, there also is interest in functional effects of myostatin deficiency in otherwise normal muscles (9). There is not a consensus regarding whether the capacity for force generation is elevated in muscles that are abnormally large because of reduced myostatin signaling (1, 2, 5, 10, 17–19, 23, 27, 34). Myostatin deficiency can affect the ability to engage in activities requiring muscular endurance, but both negative and positive effects have been reported. Double-muscled cattle, which have mutations of the myostatin gene (15, 22), have difficulty with prolonged exercise (12). At a recent conference on myostatin, it was reported that Mstn[−/−] mice have reduced endurance for swimming (7). In contrast, whippet dogs that are hypermuscular due to a myostatin mutation tend to be better competitive racers than normal whippets (24), and treatment of old mice with an anti-myostatin antibody improved treadmill running performance (18). In the present study, we examined functional effects of myostatin deficiency by measuring grip force, in vitro contractile properties of extensor digitorum longus (EDL) muscles, and voluntary wheel running in mice in which the myostatin gene was knocked out after normal muscle development. The advantages of studying the effects of postdevelopmental rather than constitutive knockout are 1) the extent of hypertrophy after postdevelopmental myostatin knockout is closer to what might occur in adults receiving anti-myostatin therapies and 2) constitutive absence of myostatin significantly reduces the expression of slower isoforms of contractile proteins and of enzymes involved in oxidative energy metabolism (1, 2, 11, 29), whereas postdevelopmental knockout does not (33).
METHODS
Mice.
Procedures were approved by the University of Rochester and University at Buffalo animal research committees. Mstn[f/f] mice with a C57BL/6J background (minimum of six crosses with this strain), in which exon 3 of the Mstn gene is flanked by loxP sequences (floxed) (30), and control mice with normal myostatin genes (Mstn[w/w], also with C57BL/6J background) were hemizygous for the either the CAGG-CreERTM transgene (grip force and in vitro muscle contractile studies) or the Rosa26-CreERTM2 transgene (wheel running study) (31, 32). Genotyping was done as described previously (31, 32). Only male mice were studied. Mice had free access to food and water at all times. The cages were in rooms with a 12-h light/dark cycle (lights on 0600–1800). Room temperature was maintained at 23°C.
Myostatin depletion.
Stimulation of Cre recombinase activity, which leads to excision of the floxed exon, was initiated in both Mstn[f/f] and Mstn[w/w] mice by feeding them tamoxifen (0.025% of chow) when they were 4 mo old for a period of either 2 wk (CAGG-CreERTM+ mice) or 6 wk (Rosa26-CreERTM2+ mice) (32). The Rosa26-CreERTM2+ mice had 1-wk breaks on normal chow after weeks 2 and 4 of tamoxifen feeding. Promyostatin depletion in gastrocnemius or quadriceps muscles (snap frozen in liquid nitrogen at the time of euthanasia) was confirmed postmortem by immunoblotting, as described previously, except that glycoprotein selection with concanavalin A was not done (32).
Grip force.
Seventeen myostatin-deficient and 11 control mice were tested at 4-wk intervals between 2 and 22 wk after the end of tamoxifen feeding with a grip strength meter (Columbus Instruments). The test involved placing a mouse on a metal grid so that all paws were gripping a wire mesh grid, which was attached to a force transducer. The mouse was then pulled by the tail with increasing force until it lost its grip. The force transducer displayed the maximum force attained. This was done five times per session, and the highest of the five force values was recorded. The same person carried out all of the tests. Mice were euthanized for analysis of muscle mass within 2 wk after the final grip test.
EDL contractile properties.
Contractile properties were examined 7–13 mo (mean 9 mo for both genotypes) after tamoxifen administration (n = 13 Mstn[f/f], 14 Mstn[w/w]). After a mouse was anesthetized with Nembutal (100 mg/kg ip), its gasctrocnemius muscles were removed and frozen in liquid nitrogen, and its EDL and soleus muscles were removed bilaterally in cooled oxygenated (95% O2 and 5% CO2) Krebs solution (pH 7.2–7.4). Muscles from one limb were flash frozen at optimal length (Lo) in isopentane cooled by liquid nitrogen and stored at −70°C for subsequent histological analysis. Muscles from the opposite limb were used for contractile experiments. The muscles were then transferred to a water jacket bath filled with Krebs solution, aerated constantly with 95% O2 and 5% CO2, and maintained at 27°C. Muscles were tied at the musculotendon junction via 0.7-mm silk suture and attached proximally to a servo motor (300B Lever System, Aurora Scientific) and distally to a micromaniputator arm (26). Two stainless-steel electrodes were placed on either side of the muscle. Muscles were stimulated with supra-maximal voltage (80 V) with 2-ms square pulses (Grass S88 stimulator with custom current amplifier, Astro-Med,) while muscle length was adjusted to determine the muscle length that produced maximal force (Lo). Lo was measured using calipers (mean of three Lo values was used), and muscle cross-sectional area (CSA) was calculated based on Lo, muscle mass, and muscle density (25): CSA (mm2) = mass (mg) ÷ Lo (mm) ÷ density (1.063 mg/mm3). Muscles were held at Lo, and isometric force was recorded during 300-ms (EDL) or 900-ms (soleus) trains of electrical stimulation at increasing frequencies (10, 20, 35, 50, 65, 80, 100, 125, 150, and 200 Hz). Po was defined as the maximum force achieved. Peak twitch tension (Pt), time to peak twitch tension (TPTT), and time for tension to decay from maximum to half-maximum twitch tension (1/2RT) were determined from data collected at 10-Hz stimulation. Data were acquired and analyzed using Spike 2 (Windows, version 6, CED Products). The EDL contractile properties always were tested first; several of the soleus muscles had unusually low Pt and Po values, presumably related to the delayed testing. We therefore are presenting only the EDL contractile data.
Stretch-induced injury.
After the measurements of isometric force were completed, some of the EDL muscles (12 Mstn[w/w], 6 Mstn[f/f]) were subjected to five eccentric contractions with 1 min of rest between contractions. Muscles were stimulated at the frequency that had elicited maximal tetanic force and held at Lo for 100 ms, then immediately stretched through a 40% strain relative to fiber length (Lf). Lf was calculated based on the Lf-to-Lo ratio of 0.44 for EDL (23). The stretch velocity was 1 Lf/s, and the total stimulation time was 500 ms. After each lengthening contraction, maximal isometric force was determined.
Voluntary wheel running.
Cages with activity wheels, wheel rotation counters, and monitoring software were purchased from Lafayette Instrument. Mice were placed, one per cage, into these cages 1 wk after the end of tamoxifen feeding, when they were ∼6 mo old (n = 12 Mstn[w/w], 16 Mstn[f/f]). Their wheel-running activity was then monitored for a period of 12 wk. Activity summed over 1-h periods was recorded throughout the experiment. Dark-cycle activity was recorded in 10-s intervals every 2 wk to assess running speed and percentage of time spent running. One of the Mstn[w/w] mice never ran more than 2 km/day, ran <1 km/d over the final month of the study, ran only 97 km over the course of the study, and never spent >3% of the dark cycle running faster than 20 m/min. These values are more than three times lower than the lowest values in any of the other 27 mice given access to a running wheel, so data from this mouse were excluded from the analyses.
At the end of the wheel-running experiment, the mice were euthanized for analysis of muscle mass. Triceps muscles (which showed more pronounced metabolic adaptations to wheel running than gastrocnemius, quadriceps, and tibialis anterior muscles in mice with normal myostatin levels; unpublished data) from several mice of both genotypes were frozen in melting isopentane and stored at −70°C for subsequent histological analysis. Hearts were immersed in 10% neutral buffered formalin. Muscle mass also was examined in age-matched sedentary mice with the same genotypes as the runners (n = 6 Mstn[w/w]/Rosa26-CreERTM2, 8 Mstn[f/f]/Rosa26-CreERTM2). These sedentary mice also were fed tamoxifen for 6 wk as described above and were maintained in ordinary cages, 2–3 mice per cage.
Histology.
Cryosections of EDL, soleus, and triceps muscles were incubated either with anti-caveolin-3 (BD Transduction Laboratories) or with anti-dystrophin plus anti-merosin (Novocastra) primary antibodies, then with secondary antibodies labeled with Alexa Fluor 568 (Invitrogen) to define the perimeters of muscle fibers. Soleus muscles were co-stained for type 1 myosin heavy chain (MHC-s primary antibody, Novocastra; secondary antibody labeled with Alexa Fluor 488). Triceps muscles from wheel runners were co-stained for capillaries with Fluorescein-labeled Griffionia Simplifolia Lectin I (Vector Laboratories). Sections of formalin-fixed hearts of wheel runners were stained for collagen with picrosirius red.
Data analysis.
Student's t-tests were used to test the statistical significance of differences between Mstn[f/f] and Mstn[w/w] groups. ANOVA was used to examine the statistical significance of differences between these groups in force-frequency curves and the effect of exercise on the gains in muscle mass induced by myostatin depletion.
RESULTS
Myostatin depletion.
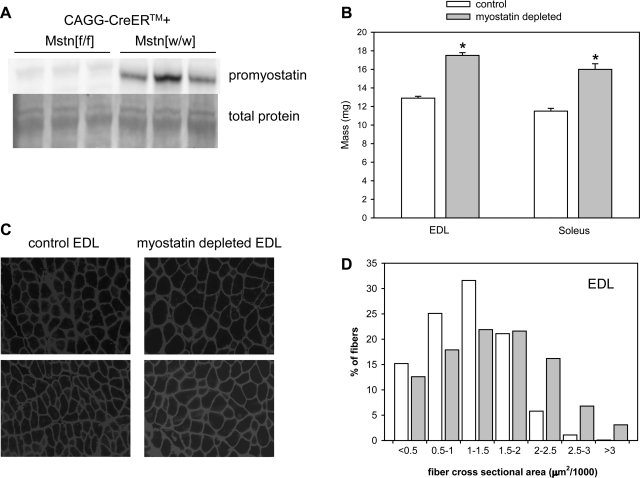
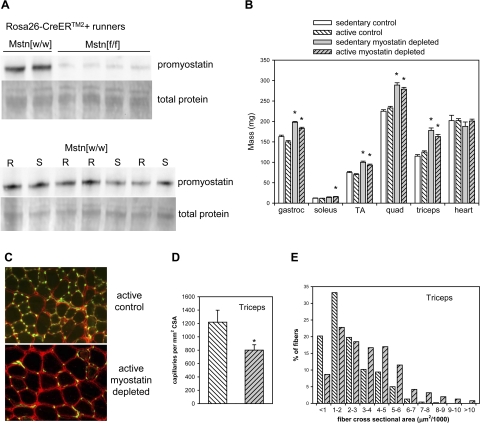
In all tamoxifen-treated Mstn[f/f] mice with the CAGG-CreERTM transgene (studies of grip force and EDL force), promyostatin either was undetectable by immunoblotting or levels were reduced by >95% relative to control levels (Fig. 1A). In tamoxifen-treated Mstn[f/f] mice with the Rosa26-CreERTM2 transgene (wheel running study), promyostatin expression was reduced by ∼80–90% (see Fig. 3A). In Mstn[w/w] mice, wheel running did not affect promyostatin levels in quadriceps muscles (mean promyostatin per milligram total extracted protein in runners was 98% of mean promyostatin level in sedentary mice; representative blot shown in Fig. 3A).
Fig. 1.
Muscle hypertrophy after postdevelopmental myostatin depletion. A: representative immunoblot confirming myostatin depletion in muscles of tamoxifen-treated mice with floxed myostatin genes (Mstn[f/f]). Total protein transferred to the blot in the region of the promyostatin dimer bands (∼100 kDa, determined by the Pierce Memcode procedure) also is shown. B: mass of EDL and soleus muscles was significantly greater in myostatin-deficient mice (Mstn[f/f]) 7–13 mo after tamoxifen administration than in age-matched tamoxifen-treated Mstn[w/w] control mice. Bars represent means. Whiskers represent standard errors. *P < 0.001 for comparison between control and myostatin-deficient muscles. C: representative micrographs illustrating increased fiber size in myostatin-deficient extensor digitorum longus (EDL) muscle. Immunostaining of dystrophin and merosin was done to define the perimeters of the muscle fibers. All micrographs were obtained from the same microscope, objective, and camera and represent an identical total area. D: fiber size [cross-sectional area (CSA)] distributions in normal (open bars) and myostatin-deficient (shaded bars) EDL muscles. The difference in distributions was highly significant according to a χ2 test (P < 0.001).
Fig. 3.
Increased muscle mass and reduced capillary density in normal and myostatin-deficient mice with access to running wheels. A: representative immunoblot showing that promyostatin protein expression in skeletal muscle was markedly reduced after tamoxifen treatment in Mstn[f/f] mice relative to Mstn[w/w] controls. Promyostatin expression was similar in wheel-running (R) and sedentary (S) Mstn[w/w] mice. B: skeletal, but not cardiac, muscle mass of both sedentary and wheel-running mice increased significantly after myostatin depletion. Bars represent means. Whiskers represent standard errors. *P < 0.05 for comparison between control and myostatin-deficient muscles within the same activity condition. C: representative micrograph illustrating that myostatin-deficient runners had larger muscle fibers (fiber perimeters stained for caveolin, red) and fewer capillaries per CSA (stained with Fluorescein-labeled Griffionia Simplifolia Lectin I, green) in triceps muscles. Both micrographs were obtained from the same microscope, objective, and camera and represent an identical total area. D: mean capillary density was reduced in triceps muscles of myostatin-deficient wheel runners. Bars represent means. Whiskers represent standard errors. *P < 0.05. E: fiber size (CSA) distributions in normal (open bars) and myostatin-deficient (shaded bars) triceps muscles of wheel runners. The difference in distributions was highly significant according to a χ2 test (P < 0.001).
Effects of myostatin depletion on body and muscle mass in sedentary mice.
Myostatin-deficient mice in the grip force study had a greater mean body mass than control mice (34.6 vs. 31.1 g at end of study; P < 0.001). Myostatin deficiency was not associated with significantly greater body mass in the sedentary mice in the wheel-running study (33.8 g in myostatin depleted mice, 33.1 g in control mice; P = 0.66) or in the mice from which EDL muscles were obtained (37.0 vs. 35.3 g; P = 0.26). Myostatin depletion increased the mass of quadriceps, gastrocnemius, triceps, tibialis anterior, EDL, and soleus muscles(Figs. 1B, 2B, and 3B) by an average of 28–44% (P < 0.001) according to data pooled from sedentary mice in all of the studies. Fiber size distributions were examined in EDL muscles from several myostatin-deficient and control mice. As expected from our previous demonstration of fiber hypertrophy in quadriceps muscles of mice with postdevelopmental myostatin knockout (31–33), there was a marked shift toward larger fibers in the myostatin-deficient mice (Fig. 1, C and D). Mean fiber CSA was 34% larger in EDL muscles of myostatin-deficient mice than in those of control mice (P < 0.01). Although Mstn[−/−] mice (constitutive absence of myostatin) have a reduced number and percentage of type 1 fibers in their soleus muscles (11), there was no loss of type 1 fibers after 7 mo or more of postdevelopmental myostatin deficiency (means ± SE: 199 ± 17 type 1 fibers/cross section, 36 ± 2% of total in control mice; 191 ± 11 type 1 fibers/cross section, 36 ± 1% of total in myostatin-deficient mice).
Fig. 2.
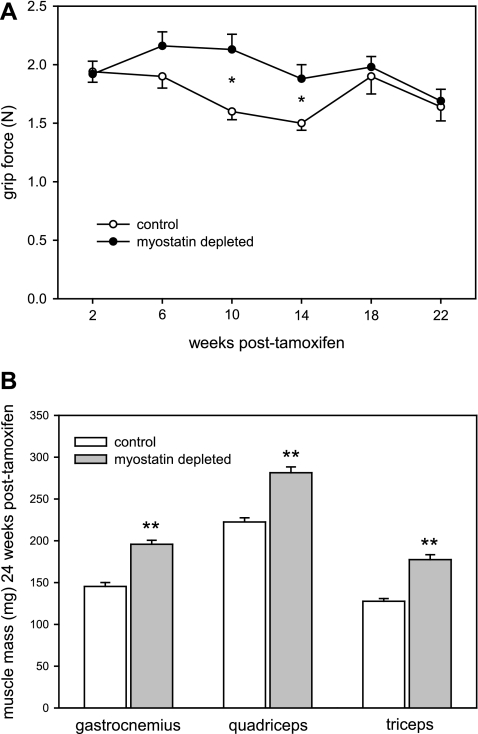
Myostatin deficiency was not associated with a consistent increase in grip force during the 5 mo after myostatin knockout (A), even though muscle mass increased by >25% (B). Bars and circles represent means. Whiskers represent standard errors. Significant difference for comparison between control and myostatin-deficient mice: *P < 0.05; **P < 0.001.
Effects of myostatin depletion on body and muscle mass in wheel-running mice.
Mice with access to running wheels had reduced body mass relative to their age- and genotype-matched sedentary controls. At the end of 12 wk of running, this reduction in body mass was 11% (P < 0.01) in mice with normal myostatin levels but only 5% (P < 0.05) in myostatin-deficient mice. Thus final body weights of active myostatin-deficient mice (mean 32.1 g) were greater than those of active control mice (29.5 g; P < 0.001). Gastrocnemius muscles were slightly smaller in runners than in sedentary controls (P < 0.05) regardless of myostatin status (Fig. 2B), but the hypertrophic effect of myostatin depletion on the gastrocnemius was similar in runners (21%) and in sedentary age- and Cre-genotype-matched controls (22%). Myostatin depletion caused a smaller increase in triceps mass in runners than in sedentary mice (P = 0.03 for genotype × activity level interaction), but the 30% increase in triceps mass in myostatin-deficient runners was similar in magnitude to the effect of myostatin depletion on other muscles (20–34%). Fiber size distributions and capillary densities in the triceps muscles were determined in several wheel-running mice. The expected increase in the proportion of larger fibers was observed in myostatin-deficient runners (Fig. 3E). The ratio of the number of capillaries to the number of muscle fibers was 2.6 in the triceps muscles of both myostatin-deficient and control runners. Because fibers were larger in the myostatin-deficient mice, it follows that capillary density was reduced (by 34%; P < 0.05) in these mice (Fig. 3, C and D).
Wheel running did not affect heart mass in either myostatin-deficient or control mice (Fig. 3B). There were no histological abnormalities in the hearts of myostatin-deficient runners. Collagen staining was similar in hearts of myostatin-deficient (1.4 ± 0.1% of tissue CSA; mean ± SE) and control runners (1.5 ± 0.2%).
Grip force.
The mean grip force tended to be greater in the myostatin-deficient mice from 6 to 14 wk after the end of tamoxifen administration (Fig. 2A). However, the mean grip force in the final test 22 wk after tamoxifen adnministration was only 3% greater in the myostatin-deficient mice (P = 0.77), even though their limb muscles were ∼30% larger than normal (P < 0.001) at the end of the study (Fig. 2B).
EDL contractile properties.
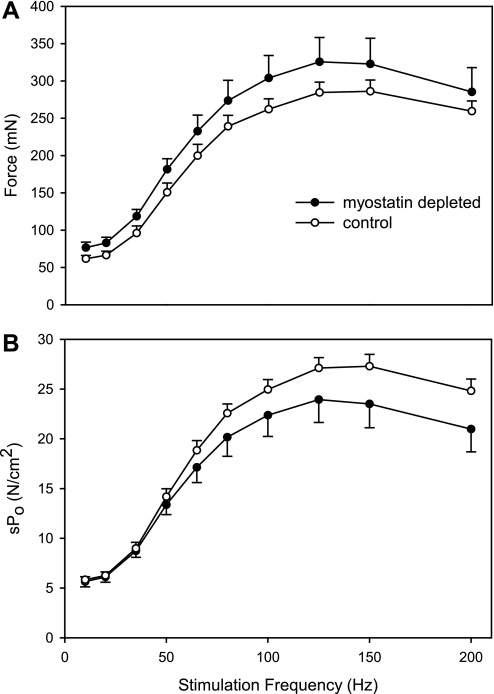
The force-frequency curve (Fig. 4A) demonstrated that myostatin depletion did not affect the stimulation frequency required to elicit peak force in the EDL. Myostatin depletion tended to increase force generation, but the effect was not statistically significant overall (P > 0.2 for genotype main effect by ANOVA) or at any single stimulation frequency (P = 0.08–0.46). The mean Po was only 11% higher in myostatin-deficient EDL muscles even though mean mass was 36% higher. Thus mean specific tension (Po per CSA or per milligram of muscle) tended to be less in myostatin-deficient EDL (Fig. 4B; Table 1). There was a small reduction in mean 1/2RT in the myostatin-deficient muscles, but there was no effect of myostatin depletion on TTPT (Table 1).
Fig. 4.
A: EDL muscles of myostatin-deficient mice tended to have higher force production at all stimulation frequencies, but the increases were not statistically significant at any frequency (P > 0.05). B: force per CSA (sPo) tended to be reduced in EDL muscles of myostatin-deficient mice, but the differences were not statistically significant (P > 0.05 for all stimulation frequencies). Circles represent means; whiskers represent standard errors.
Table 1.
Contractile properties of EDL muscles
| Mstn[w/w] | Mstn[f/f] | P Value (t-test) | |
|---|---|---|---|
| EDL mass, mg | 12.9 ± 0.2 | 17.5 ± 0.3 | <0.001 |
| CSA, mm2 | 1.07 ± 0.03 | 1.36 ± 0.03 | <0.001 |
| Lo, mm | 11.7 ± 0.2 | 12.2 ± 0.2 | 0.120 |
| TTPT, ms | 17.9 ± 0.7 | 17.5 ± 0.7 | 0.749 |
| 1/2RT, ms | 19.1 ± 0.9 | 15.3 ± 0.9 | 0.008 |
| Twitch force, mN | 61.6 ± 4.5 | 76.6 ± 7.4 | 0.089 |
| Maximal Po, mN | 300 ± 16 | 334 ± 34 | 0.364 |
| Maximal Po per CSA, sPo, N/cm2 | 28.4 ± 1.2 | 24.5 ± 2.3 | 0.149 |
| Maximal Po per mass, mN/mg | 22.8 ± 1.1 | 18.9 ± 1.8 | 0.073 |
Values are means ± SE of 14 Mstn[w/w] and 13 Mstn[f/f] extensor digitorum longus (EDL) muscles. Lo, optimal length; CSA, cross-sectional area; TTPT, time to peak twitch tension; 1/2RT, one-half relaxation time; Po maximum tetanic force.
Stretch injury.
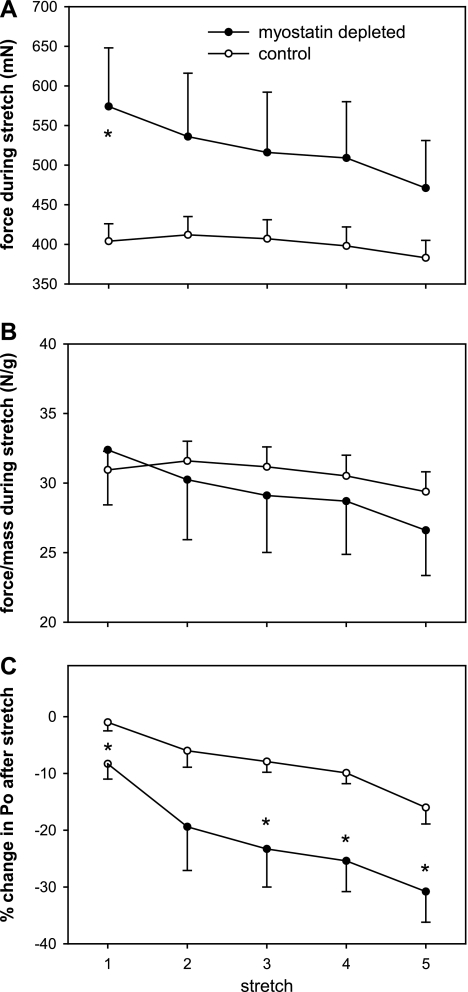
In the set of EDL muscles subjected to stretch injury, the mean mass was 36% greater (P < 0.001) and the prestretch Po was 24% greater (P = 0.06) in the myostatin-deficient than in the normal muscles. The force generated during the first stretch was 42% greater (P < 0.01) in the myostatin-deficient muscles (Fig. 5A). The differences between myostatin-deficient and control muscles in force during stretch were not as large for stretches 2 through 5 (23–30%; P = 0.07–0.11). There was no significant effect of myostatin depletion on the ratio of force to EDL mass for any of the stretches (Fig. 5B). The decline in force caused by the eccentric contractions, as a percent of initial Po, was approximately twofold greater in the myostatin-deficient muscles than in the muscles with normal myostatin levels (Fig. 5C).
Fig. 5.
Force during five consecutive eccentric contractions of EDL muscles, and postcontraction Po after each stretch. Force per whole muscle (A), but not force per gram of muscle (B), during the eccentric contractions tended to be greater in the myostatin-deficient muscles. The force deficits after the eccentric contractions were significantly greater in the myostatin-deficient muscles (C). Circles represent means; whiskers represent standard errors. *Significant difference between control and myostatin-deficient muscles (P < 0.05).
Voluntary wheel running.
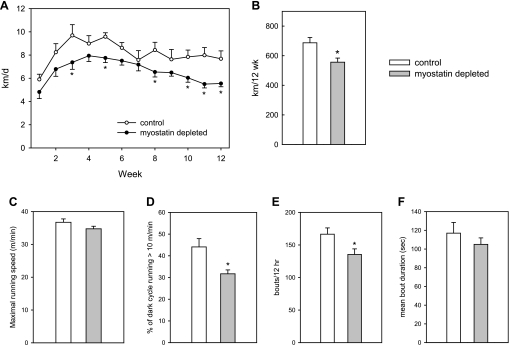
Both Mstn[f/f] and Mstn[w/w] mice exhibited the diurnal running pattern typical of laboratory mice, with almost all of the activity occurring during the dark cycle. The total distance run over the course of the 12-wk period was 19% less in the myostatin-deficient mice (Fig. 6, A and B). Maximal running speed was not significantly affected by myostatin deficiency (Fig. 6C). Myostatin-deficient mice spent a smaller proportion of the dark cycle running than did mice with normal myostatin levels (Fig. 6D). To evaluate effects on exercise bout frequency and duration, we defined the start of a running bout as rotation of the wheel at an average speed of >10 m/min over a 10-s period. The end of a bout was defined as a 10-s period with an average rotation speed of <10 m/min. Myostatin-deficient mice had 19% fewer running bouts than mice with normal myostatin levels (Fig. 6E). The small (10%) reduction in mean bout duration in myostatin-deficient mice (Fig. 6F) was not statistically significant (P = 0.35). Thus the reduced running distances in myostatin-deficient mice generally were the result of longer rest breaks between running bouts rather than early termination of running bouts. However, one myostatin-deficient mouse had a large number of running bouts (228 per night) with a very short mean duration (51 s).
Fig. 6.
Myostatin depletion reduced wheel-running distances (A and B). The effect was not accounted for by a slower running speed (C) but by a reduction in the time spent running (D) primarily because of fewer running bouts (E) rather than shorter bouts (F). Bars and circles represent means; whiskers represent standard errors. *Significant difference between control and myostatin-deficient mice (P < 0.05).
DISCUSSION
A potential benefit of muscle hypertrophy is increased capacity to generate force. We observed trends for increased grip force and electrically stimulated contractile force after muscle hypertrophy was induced by postdevelopmental myostatin depletion, but the effects were not consistent and were proportionally less than the increases in muscle mass. The mean effect of myostatin depletion on grip force in the present study was similar to the 10% increase in grip force after an anti-myostatin antibody was administered to young adult mice for 8 wk (34). Studies of normal mice have had mixed results in terms of whether electrically stimulated force production can be increased by inhibiting myostatin activity, but in general there was no major effect of treatments that increased muscle mass by 15–18% (10, 18, 19). The present study extends these findings by showing that a twofold larger increase in muscle mass after loss of myostatin signaling (i.e., 36% increase in EDL mass) did not consistently increase Po. In contrast, it was reported recently that blocking myostatin activity by localized follistatin expression in quadriceps muscles of macaque monkeys increased force-generating capacity (both twitch and tetanic tension) by 12–78%, even though muscle mass increased only ∼10–15% (17). The study involved only three monkeys, and the untreated muscles did not receive injections of a control vector, so additional research is needed to confirm that myostatin blockade enhances force-generating capacity in muscles of primates.
The failure of muscle hypertrophy to increase force production in proportion to the increase in muscle mass could be a structural issue. When muscles grow without an increase in length, the pennation angle increases, and this reduces the efficiency of contractions. Mendias et al. suggested that this factor explained the 18% reduction in force per CSA that they observed in EDL muscles of Mstn[−/−] mice (23). This phenomenon appears to be a general feature of muscles that have enlarged beyond the normal level rather than a specific effect of myostatin deficiency. For example, the mean reduction in EDL-specific tension in the present study was similar to the mean reduction in gasctrocnemius-specific tension associated with muscle hypertrophy induced by activated Akt (4). Reduced specific tension was reported as an effect of growth hormone-induced muscle hypertrophy nearly 60 years ago (3). The hypertrophy associated with muscle overload also leads to a reduction in specific tension (6, 16).
Amthor et al. reported a very large deficit in the ratio of force to muscle mass or CSA in EDL muscles of Mstn[−/−] mice (1), far greater than the deficit in EDL-specific tension reported by Mendias et al. (23) and the effect observed in the present study. They concluded that an altered pennation angle was not the only factor that limited force production and proposed that depletion of mitochondria contributed to the reduced specific tension in the Mstn[−/−] muscles. We have observed that postdevelopmental myostatin knockout does not reduce markers of mitochondrial abundance, including intensity of SDH and COX staining and expression of hundreds of mRNAs encoding mitochondrial proteins (33). Thus it is unlikely that mitochondrial depletion limits the capacity for force generation after postdevelopmental myostatin depletion.
Mendias et al. reported that the extent of hypertrophy in soleus muscles of Mstn[−/−] mice was far less than the hypertrophy of EDL muscles (36 vs. 66%), and this difference was attributed to a lower level of expression of the activin type IIB receptor in soleus muscles than in EDL muscles (23). In the present study, the extent of hypertrophy after postdevelopmental myostatin depletion was similar in EDL and soleus muscles. This discrepancy probably is explained by the fact that part of the excessive muscle growth in Mstn[−/−] mice is from hyperplasia (21, 23), but this is limited to type 2 fibers (11). Because mice have a greater proportion of type 2 fibers in EDL than in soleus muscles, selective hyperplasia of type 2 fibers can explain why hypertrophy of EDL muscles was greater than hypertrophy of soleus muscles in Mstn[−/−] mice. We did not observe a decline in the number or proportion of type 1 fibers in soleus muscles after ≥7 mo of postdevelopmental myostatin deficiency, indicating that the deficiency of type 1 fibers in Mstn[−/−] mice results from influences that are limited to the period of muscle development.
EDL muscles of Mstn[−/−] mice have a greater force deficit than normal mice after eccentric contractions, which reflects increased stretch-induced damage to sarcomeres (23). In the present study, we observed that postdevelopmental myostatin knockout also increased the stretch-induced force deficit. Mendias et al. attributed this phenomenon to a decrease in the collagen content of myostatin-deficient muscles (23). We have observed that, after postdevelopmental myostatin knockout, there is reduced expression in muscle of several procollagen genes, especially those encoding type I and type III collagens, and a 25–30% reduction in muscle hydroxyproline levels (a marker of collagen content) (33). If the extracellular matrix of muscle is more fragile in myostatin-deficient mice because of reduced collagen production, it could be damaged more during stretches and therefore afford less protection to the sarcomeres. Because the extracellular matrix is an integral part of transmitting the force of muscle contractions to tendons and bones, it is possible that reduced collagen content also contributes to the reduced specific tension observed in myostatin-deficient mice.
The reduced voluntary running in myostatin-deficient mice is consistent with reports that cattle and mice with constitutive lack of myostatin have reduced exercise capacity (7, 12). Mice with postdevelopmental myostatin knockout do not have the deficits in expression of enzymes involved in oxidative energy metabolism that are observed in Mstn[−/−] mice (33), so this is not a likely explanation for the reduced voluntary running. A potential factor in the reduced wheel running of myostatin-deficient mice is the fact that their capillary-to-fiber ratio was the same as that of control runners (assuming that triceps is representative of all limb muscles). Because their fibers were significantly larger, it follows that oxygen and nutrients from the blood had to diffuse farther to reach the center of a fiber and metabolic products (e.g., CO2, lactate) had to diffuse farther to enter the circulation. The greater body mass (+9%) of myostatin-deficient runners also might have had an inhibitory influence on running because of the increased workload.
Reduced treadmill running has been observed in rodents in which muscle hypertrophy was induced by expression of constitutively active Akt1 (14) or the anabolic drug clenbuterol (8, 13). Clenbuterol also inhibited voluntary wheel running in rats (8). Cardiac pathology is a potential contributor to the reduced endurance capacity associated with clenbuterol administration (8), but cardiac mass was normal and there was no fibrosis in the hearts of myostatin-deficient runners in the present study. Reduced voluntary wheel running also has been observed in rats treated with the anabolic steroid nandrolone, although it is unclear whether this treatment induced muscle hypertrophy (20). Based on these other studies, we suspect that reduced running is a typical consequence of abnormally large muscles rather than a specific effect of myostatin deficiency.
The studies described in this report indicate that blocking myostatin activity in otherwise-normal muscles, by itself, is unlikely to produce significant functional benefits. Of course, the present results do not diminish the considerable evidence that blocking myostatin activity in dystrophic or atrophied muscles can have functional benefits (28) and do not rule out the possibility that myostatin inhibition would enhance some types of exercise training programs.
GRANTS
This study was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant AR-054366.
DISCLOSURES
S. Welle has received research support from GlaxoSmithKline. He accepts full responsibility for the research, had full access to all the data, and controlled the decision to publish.
ACKNOWLEDGMENTS
We thank Don Henderson for assistance with the muscle histology.
REFERENCES
- 1. Amthor H, Macharia R, Navarrete R, Schuelke M, Brown SC, Otto A, Voit T, Muntoni F, Vrbova G, Partridge T, Zammit P, Bunger L, Patel K. Lack of myostatin results in excessive muscle growth but impaired force generation. Proc Natl Acad Sci USA 104: 1835–1840, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baligand C, Gilson H, Menard JC, Schakman O, Wary C, Thissen JP, Carlier PG. Functional assessment of skeletal muscle in intact mice lacking myostatin by concurrent NMR imaging and spectroscopy. Gene Ther 17: 328–337, 2010 [DOI] [PubMed] [Google Scholar]
- 3. Bigland B, Jehring B. Muscle performance in rats, normal and treated with growth hormone. J Physiol 116: 129–136, 1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blaauw B, Canato M, Agatea L, Toniolo L, Mammucari C, Masiero E, Abraham R, Sandri M, Schiaffino S, Reggiani C. Inducible activation of Akt increases skeletal muscle mass and force without satellite cell activation. FASEB J 23: 3896–3905, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Byron CD, Borke J, Yu J, Pashley D, Wingard CJ, Hamrick M. Effects of increased muscle mass on mouse sagittal suture morphology and mechanics. Anat Rec A Discov Mol Cell Evol Biol 279: 676–684, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Donovan CM, Faulkner JA. Muscle grafts overloaded by ablation of synergistic muscles. J Appl Physiol 61: 288–292, 1986 [DOI] [PubMed] [Google Scholar]
- 7. Dumonceaux J, Amthor H. Current advances in the development of therapies for neuromuscular disorders based on myostatin signaling, 3rd International Institute of Myology Workshop, Paris, September 12th, 2008. Neuromuscul Disord 19: 797–799, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Duncan ND, Williams DA, Lynch GS. Deleterious effects of chronic clenbuterol treatment on endurance and sprint exercise performance in rats. Clin Sci (Lond) 98: 339–347, 2000 [PubMed] [Google Scholar]
- 9. Fedoruk MN, Rupert JL. Myostatin inhibition: a potential performance enhancement strategy? Scand J Med Sci Sports 18: 123–131, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Foster K, Graham IR, Otto A, Foster H, Trollet C, Yaworsky PJ, Walsh FS, Bickham D, Curtin NA, Kawar SL, Patel K, Dickson G. Adeno-associated virus-8-mediated intravenous transfer of myostatin propeptide leads to systemic functional improvements of slow but not fast muscle. Rejuvenation Res 12: 85–93, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Girgenrath S, Song K, Whittemore LA. Loss of myostatin expression alters fiber-type distribution and expression of myosin heavy chain isoforms in slow- and fast-type skeletal muscle. Muscle Nerve 31: 34–40, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Holmes JH, Ashmore CR, Robinson DW. Effects of stress on cattle with hereditary muscular hypertrophy. J Anim Sci 36: 684–694, 1973 [DOI] [PubMed] [Google Scholar]
- 13. Ingalls CP, Barnes WS, Smith SB. Interaction between clenbuterol and run training: effects on exercise performance and MLC isoform content. J Appl Physiol 80: 795–801, 1996 [DOI] [PubMed] [Google Scholar]
- 14. Izumiya Y, Hopkins T, Morris C, Sato K, Zeng L, Viereck J, Hamilton JA, Ouchi N, Lebrasseur NK, Walsh K. Fast/glycolytic muscle fiber growth reduces fat mass and improves metabolic parameters in obese mice. Cell Metab 7: 159–172, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kambadur R, Sharma M, Smith TPL, Bass JJ. Mutations in myostatin (GDF-8) in double-muscled Belgian Blue and Piedmontese cattle. Genome Res 7: 910–915, 1997 [DOI] [PubMed] [Google Scholar]
- 16. Kandarian SC, White TP. Mechanical deficit persists during long-term muscle hypertrophy. J Appl Physiol 69: 861–867, 1990 [DOI] [PubMed] [Google Scholar]
- 17. Kota J, Handy CR, Haidet AM, Montgomery CL, Eagle A, Rodino-Klapac LR, Tucker D, Shilling CJ, Therlfall WR, Walker CM, Weisbrode SE, Janssen PML, Clark KR, Sahenk Z, Mendell JR, Kaspar BK. Follistatin gene delivery enhances muscle growth and strength in nonhuman primates. Sci Transl Med 1: 6ra15, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lebrasseur NK, Schelhorn TM, Bernardo BL, Cosgrove PG, Loria PM, Brown TA. Myostatin inhibition enhances the effects of exercise on performance and metabolic outcomes in aged mice. J Gerontol A Biol Sci Med Sci 64: 940–948, 2009 [DOI] [PubMed] [Google Scholar]
- 19. Matsakas A, Foster K, Otto A, Macharia R, Elashry MI, Feist S, Graham I, Foster H, Yaworsky P, Walsh F, Dickson G, Patel K. Molecular, cellular and physiological investigation of myostatin propeptide-mediated muscle growth in adult mice. Neuromuscul Disord 19: 489–499, 2009 [DOI] [PubMed] [Google Scholar]
- 20. McGinnis MY, Lumia AR, Tetel MJ, Molenda-Figueira HA, Possidente B. Effects of anabolic androgenic steroids on the development and expression of running wheel activity and circadian rhythms in male rats. Physiol Behav 92: 1010–1018, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-β superfamily member. Nature 387: 83–90, 1997 [DOI] [PubMed] [Google Scholar]
- 22. McPherron AC, Lee SJ. Double muscling in cattle due to mutations in the myostatin gene. Proc Natl Acad Sci USA 94: 12457–12461, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mendias CL, Marcin JE, Calerdon DR, Faulkner JA. Contractile properties of EDL and soleus muscles of myostatin-deficient mice. J Appl Physiol 101: 898–905, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mosher DS, Quignon P, Bustamante CD, Sutter NB, Mellersh CS, Parker HG, Ostrander EA. A mutation in the myostatin gene increases muscle mass and enhances racing performance in heterozygote dogs. PLoS Genet 3: e79, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Personius KE, Nautiyal J, Reddy S. Myotonia and muscle contractile properties in mice with SIX5 deficiency. Muscle Nerve 31: 503–505, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Personius KE, Sawyer RP. Variability and failure of neurotransmission in the diaphragm of mdx mice. Neuromuscul Disord 16: 168–177, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Qiao C, Li J, Jiang J, Zhu X, Wang B, Li J, Xiao X. Myostatin propeptide gene delivery by adeno-associated virus serotype 8 vectors enhances muscle growth and ameliorates dystrophic phenotypes in mdx mice. Hum Gene Ther 19: 241–254, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Rodino-Klapac LR, Haidet AM, Kota J, Handy C, Kaspar BK, Mendell JR. Inhibition of myostatin with emphasis on follistatin as a therapy for muscle disease. Muscle Nerve 39: 283–296, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Steelman CA, Recknor JC, Nettleton D, Reecy JM. Transcriptional profiling of myostatin-knockout mice implicates Wnt signaling in postnatal skeletal muscle growth and hypertrophy. FASEB J 20: 580–582, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Welle S, Bhatt K, Pinkert CA. Myofibrillar protein synthesis in myostatin-deficient mice. Am J Physiol Endocrinol Metab 290: E409–E415, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Welle S, Bhatt K, Pinkert CA, Tawil R, Thornton CA. Muscle growth after postdevelopmental myostatin gene knockout. Am J Physiol Endocrinol Metab 292: E985–E991, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Welle S, Burgess K, Thornton CA, Tawil R. Relation between extent of myostatin depletion and muscle growth in mature mice. Am J Physiol Endocrinol Metab 297: E935–E940, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Welle S, Cardillo A, Zanche M, Tawil R. Skeletal muscle gene expression after myostatin knockout in mature mice. Physiol Genomics 38: 342–350, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Whittemore LA, Song K, Li X, Aghajanian J, Davies M, Girgenrath S, Hill JJ, Jalenak M, Kelley P, Knight A, Maylor R, O'Hara D, Pearson A, Quazi A, Ryerson S, Tan XY, Tomkinson KN, Veldman GM, Widom A, Wright JF, Wudyka S, Zhao L, Wolfman NM. Inhibition of myostatin in adult mice increases skeletal muscle mass and strength. Biochem Biophys Res Commun 300: 965–971, 2003 [DOI] [PubMed] [Google Scholar]