Abstract
Breathing hyperbaric oxygen (HBO) is common practice in hyperbaric and diving medicine. The benefits of breathing HBO, however, are limited by the risk of central nervous system O2 toxicity, which presents as seizures. We tested the hypothesis that excitability increases in CA1 neurons of the rat hippocampal slice (400 μm) over a continuum of hyperoxia that spans normobaric and hyperbaric pressures. Amplitude changes of the orthodromic population spike were used to assess neuronal O2 sensitivity before, during, and following exposure to 0, 0.6, 0.95 (control), 2.84, and 4.54 atmospheres absolute (ATA) O2. Polarographic O2 electrodes were used to measure tissue slice Po2 (PtO2). In 0.95 ATA O2, core PtO2 at 200 μm deep was 115 ± 16 Torr (mean ± SE). Increasing O2 to 2.84 and 4.54 ATA increased core PtO2 to 1,222 ± 77 and 2,037 ± 157 Torr, respectively. HBO increased the orthodromic population spike amplitude and usually induced hyperexcitability (i.e., secondary population spikes) and, in addition, a long-lasting potentiation of the orthodromic population spike that we have termed “oxygen-induced potentiation” (OxIP). Exposure to 0.60 ATA O2 and hypoxia (0.00 ATA) decreased core PtO2 to 84 ± 6 and 20 ± 4 Torr, respectively, and abolished the orthodromic response. Reoxygenation from 0.0 or 0.6 ATA O2, however, usually produced a response similar to that of HBO: hyperexcitability and activation of OxIP. We conclude that CA1 neurons exhibit increased excitability and neural plasticity over a broad range of PtO2, which can be activated by a single, hyperoxic stimulus. We postulate that transient acute hyperoxia stimulus, whether caused by breathing HBO or reoxygenation following hypoxia (e.g., disordered breathing), is a powerful stimulant for orthodromic activity and neural plasticity in the CA1 hippocampus.
Keywords: oxygen toxicity, hyperbaric oxygen, neural plasticity, oxidative stress
hyperbaric oxygen (HBO) is breathed during HBO therapy (82) and during underwater diving operations and disabled submarine rescue (41, 61). What limits the use of HBO in each case is the potential risk of central nervous system (CNS) O2 toxicity. The hallmark sign of CNS O2 toxicity is unpredictable tonic clonic seizures, which can begin when neural tissue Po2 (PtO2) increases from the normoxic range (<10–45 Torr) to ∼240 Torr or more (7, 83). One key factor in the pathogenesis of CNS O2 toxicity appears to be the accumulation of reactive oxygen species (ROS) and reactive nitrogen species (RNS). Increased production and accumulation of ROS/RNS leads to oxidation of essential cellular constituents such as enzymes, membrane lipid bilayers, and proteins (19), which in turn, alters intrinsic membrane properties and synaptic transmission (4, 5, 12, 51, 56). Electrophysiological studies in rat brain slices indicate that HBO decreases membrane conductance and stimulates firing rate in neurons of the hippocampus and medulla oblongata (25, 50, 65). The excitatory effect of HBO on neurons is blocked by antioxidants and mimicked by prooxidants, which supports the hypothesis that during hyperoxia, increases in ROS/RNS cause elevated neuronal excitability (65).
The exact cellular mechanisms underlying O2-induced seizure (O2 toxicity) are poorly understood because of the paucity of electrophysiological studies that have examined the effects of hyperoxia on neurons (50, 65). By contrast, a vast amount of literature exists on the electrophysiological effects of hypoxia on neurons, which has been reviewed elsewhere (57, 76). The main reason for the lack of information on how hyperoxia affects neurons under in vitro conditions is that electrophysiological studies conducted in brain slices employ a hyperoxic control condition using ∼0.95 atmospheres absolute (ATA) O2; i.e., nutrient medium equilibrated with 95% O2 at barometric pressure (Pb) ≅ 1 ATA (1, 64). Consequently, the only way to study the effects of further hyperoxia (>0.95 ATA O2)1 in the brain slice preparation is to use hyperbaric pressure to produce HBO (25, 50, 65, 66). In addition, the effects of relative hyperoxia on PtO2 and excitability can be studied at normobaric pressure (Pb ≅ 1 ATA) during reoxygenation following acute exposure to an intermediate level of O2 (0.6 ATA) or anoxia (0.0 ATA), as in previous studies (17, 18, 42, 44), which we have termed “normobaric reoxygenation” (NBOreox).
In the present study, we used HBO and NBOreox to study the effects of a broad range of hyperoxia on PtO2 and neuronal excitability in the CA1 region of the rat hippocampal tissue slice. We tested the hypothesis that neuronal excitability increases over a broad continuum of PtO2 at normobaric and hyperbaric pressures. Although such an excitatory effect of increasing PtO2 on CA1 electrical activity in the hippocampus might be predicted, to our knowledge, no single study has described the O2 sensitivity of CA1 neurons over a range of PtO2 that spans both normobaric and hyperbaric pressures. Few in vitro studies, in fact, have examined O2 sensitivity of neurons in tissue slices at levels of PtO2 >0.95 ATA O2 (25, 50, 65) or <0.95 ATA but ≥0.20 ATA O2 (33, 87). To accomplish this, we recorded the orthodromic population spike (oPS) from the CA1 neuronal population over a broad range of PtO2 using superfusate equilibrated with 0.00 (hypoxia), 0.60 (intermediate oxygenation), 0.95 (control), and 2.84 and 4.54 ATA O2 (HBO). In an additional set of experiments, PtO2 profiles in 400-μm-thick hippocampal slices were measured under identical conditions of Pb, PtO2, pH, temperature, and artificial cerebral spinal fluid (aCSF) flow rate.
Our findings demonstrate that beginning in 0.95 ATA O2, neuronal excitability, in general, increased as PtO2 increased and decreased as PtO2 decreased; however, the greatest sensitivity to O2 manipulation occurred between 0.6 and 0.95 ATA O2. Our results also indicate that a single hyperoxic stimulus delivered at normobaric pressure (NBOreox) or hyperbaric pressure (HBO), initiated from 0.0, 0.6, or 0.95 ATA O2, induces hyperexcitability (i.e., secondary population spikes, sPS) and long-lasting neural plasticity. We have termed this form of neural plasticity “O2-induced potentiation” (OxIP) of the oPS. Our findings further support an earlier report (64) that experimental O2 conditions (95% O2) commonly used with the brain slice preparation (∼400 μm thick) are hyperoxic compared with the normoxic CNS of an air-breathing animal. A second study, described in the companion article (40), reports the effects of O2 manipulation on the field excitatory postsynaptic potential and the antidromic population spike and provides evidence for our hypothesis that the excitatory effects of hyperoxia on the oPS occur primarily by postsynaptic, intrinsic mechanisms of plasticity, whereas the stimulatory effects of hyperoxia to produce sPS (hyperexcitability) occur through disinhibition of spontaneous neurotransmission (40).
METHODS
Preparation of Hippocampal Brain Slices
All procedures were performed with approval of the Wright State University Laboratory Animal Care and Use Committee. Wright State University is accredited by AAALAC and is covered by National Institutes of Health assurance no. A362-01. Sagittal corticohippocampal tissue slices were prepared at 400-μm intervals from male Sprague-Dawley rats (27–40 days old) anesthetized with 70–100% CO2 and euthanized by rapid decapitation. The cortex was extracted, and the frontal and temporal lobes were dissected free from the rest of the cerebrum. The remaining cerebrum was bisected at the central fissure to yield two tissue blocks, each containing one lobe of the hippocampus. Both tissue blocks were glued (cyanoacrylate cement) to the specimen tray of the vibratome and supported against and glued to two agar blocks previously glued to the chilled specimen tray. The tissue blocks were oriented such that the midline surface of the brain was uppermost and parallel to the cutting blade. The tissue blocks were then submerged in chilled (4–6°C) aCSF, which was composed of (in mM) 125 NaCl, 5.0 KCl, 1.3 MgSO4, 26 NaHCO3, 1.24 KH2PO4, 2.4 CaCl2, and 10 glucose. When the aCSF was equilibrated with a gas mixture containing ∼38 Torr CO2 (i.e., 0.05 ATA CO2), the pH of the aCSF ranged between 7.40 and 7.44. Corticohippocampal slices were cut, collected, and incubated in aCSF (∼22°C) equilibrated with 95% O2 + 5% CO2. Slices were allowed at least 1 h of recovery before experiments were conducted at ∼37°C. Slices were used for study up to 8 h following dissection.
Experimental O2 Conditions
Experimental O2 conditions were established by equilibrating aCSF with various gas mixtures (Weiler Gas, Dayton, OH) whose percentages of O2 and CO2 were verified with gas analyzers (O2: model N-22M; CO2: model P-61B; AEI, Pittsburgh, PA). The O2 tensions used for equilibrating aCSF are reported in ATA, assuming Pb = 1 ATA and without correction for Pb and water vapor pressure; however, measurements for PtO2 given below in Torr are corrected for Pb and water vapor pressure. Control superfusate was prepared by continuous aeration of aCSF with 95% O2 + 5% CO2 (0.95 ATA O2). An intermediate level of oxygenated superfusate was prepared by continuous aeration of aCSF with 60% O2+ 35% N2 + 5% CO2 (0.60 ATA O2), whereas hypoxic superfusate was prepared by continuous aeration of aCSF with 95% N2 + 5% CO2 (0.00 ATA O2). Aeration of aCSF (37°C) with these gas mixtures at normobaric pressure was done for a minimum of 20 min before the start of the experiment. Isocapnic-HBO media (0.05 ATA CO2 with 2.84 and 4.54 ATA O2) were prepared by decreasing the concentration of CO2 from 5 to 1.78 and 1.11% (balance O2), respectively, in the gas mixture used to pressurize aCSF inside a 1-liter volume, high-pressure sample cylinder (24, 26). Decreasing the fractional concentration of CO2 was necessary to maintain Pco2 (0.05 ATA) and pH constant in the aCSF at HBO test pressures. The aCSF was allowed to equilibrate a minimum of 20 min at hyperbaric pressure before use in experiments. Prewarming of the sample cylinders was not necessary; however, aCSF was warmed as it flowed into the brain slice chamber via an in-line heater, which did not cause significant bubble formation (Warner Instruments, Hamden, CT).
Hyperbaric Chamber
Extracellular recordings and O2 measurements were conducted in a refurbished Bethlehem hyperbaric animal research chamber (model NB-878) certified with a maximum working pressure of 11 ATA (Hyperbaric Clearinghouse, Lorton, VA). The hyperbaric chamber was modified for in vitro electrophysiology similar to the chamber previously described (24, 26) with the addition of a custom-built IR-video microscope. The present study used low power (×2 and ×4 objectives) with darkfield illumination (via low-angle side lighting) and light microscopy (transmitted light) to visualize the CA1 cell layer for positioning the stimulating electrode and recording microelectrode.
The brain slice was stabilized in the heated tissue bath (model RC-27L; Warner Instruments) between a bottom stationary nylon mesh support and a removable top nylon mesh grid. The aCSF was equilibrated with normobaric O2 (0.00, 0.60, and 0.95 ATA O2) and delivered at a constant rate of 2.0 ml/min (35.5–36.5°C) to the brain slice at normobaric and hyperbaric pressures using a high-pressure liquid chromatography (HPLC) pump (model 582; ESA, Chelmsford, MA). Hyperbaric pressures (2.84 and 4.54 ATA O2) were produced by compressing the hyperbaric chamber with 100% helium as in previous studies (30, 31, 62, 63, 66, 77, 78). Helium is inert and without any partial pressure effects (i.e., narcotic potency) on the intact CNS unless used at >100 ATA (reviewed in Ref. 25). To test the effects of HBO on the oPS response, aCSF from the HPLC pump was diverted away from the slice and replaced by flow-matched (Omega rotameter, model FL211) hyperbaric oxygenated aCSF that was delivered from the high-pressure sample cylinder with the use of a +0.43 atmosphere gauge pressure differential (pressure in the high-pressure sample cylinder > ambient pressure inside the hyperbaric chamber). No significant off-gassing at the brain slice was observed during superfusion with HBO medium as evidenced by the lack of significant bubble formation within the superfusate within the chamber (24). A new steady-state medium Po2 (PmO2) was established in the tissue slice bath within 6 min of initiating media exchange.
Electrode Positioning
Recording and stimulating electrodes were positioned over the slice preparation, but not within the tissue slice, with the hyperbaric chamber door open. For hyperbaric experiments, the chamber door was closed and sealed. Recording and stimulating electrodes were then driven into the tissue slice by using remote control microdrives (model DC3001; WPI, Sarasota, FL) under visual guidance with the hyperbaric video microscope. Six to ten minutes after either an electrophysiological or a polarographic recording was established, the experimental protocol began.
Chamber Compression
In experiments involving hyperbaric compression, all recordings were established at normobaric pressure with the chamber door closed. After a control period (16 min) at Pb = 1 ATA, room air in the sealed hyperbaric chamber atmosphere was flushed away with the use of pure helium for 2 min to remove nitrogen and oxygen (i.e., room air) from the overlying atmosphere. Next, the decompression valve was closed so that the chamber was compressed with helium at a rate of ∼0.5–1.0 ATA min−1 to a final chamber pressure of either 2.41 or 4.11 ATA. We previously reported (64) that O2 diffuses from the hyperoxygenated aCSF (≥0.95 ATA O2) into the essentially infinite volume of the helium-filled anoxic atmosphere of the hyperbaric chamber (∼0.0 ATA O2). Regardless, the PmO2 remained stable in the tissue bath before, during, and following helium compression because of the constant supply to the brain slice of oxygenated aCSF using the HPLC pump (43). Similarly, Pco2/pH of the aCSF remained unchanged during helium compression (24). Despite any temperature fluctuations that may have occurred in the overlying atmosphere of helium, the temperature of the brain slice preparation remained stable (Fig. 1) (24). After 6–10 min at the new ambient level of pressure, an additional 16 min of control data were collected, followed by exposure to HBO (16 min) and a recovery period (16–32 min). Hyperbaric electrophysiological experiments could be conducted typically for up to 2 h or more under tightly controlled conditions of Pb, temperature, oxygen, and pH.
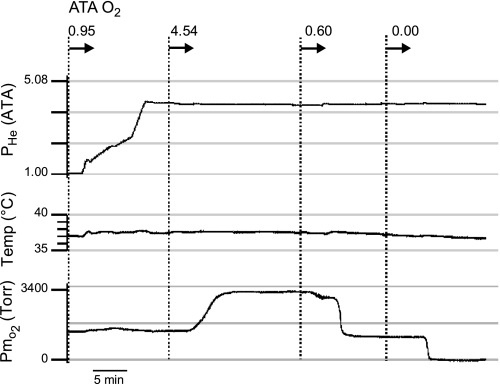
Fig. 1.
Temperature regulation and oxygenation of the artificial cerebrospinal fluid (aCSF) in the slice bath before and during helium compression and while at steady-state hyperbaric pressure (4.54 ATA He). During helium pressurization (top trace: 1 → 4.08 ATA He), both temperature (middle trace) and Po2 of the aCSF (bottom trace) in the brain slice bath remain stable. Hyperbaric helium, which is inert, is used to mimic the effects of hydrostatic compression (see text for further details). In this example, 3 of the 4 levels of test O2 are shown: 4.54, 0.6, and 0.0 ATA. Hence, the Po2 of the aCSF can be varied over a wide range while barometric pressure (i.e., helium pressure, Phe) remains unchanged. Media were equilibrated with 0.95, 0.60, and 0.00 ATA O2 at normobaric pressure and pumped to the brain slice at constant flow rate (∼2 ml/min) using an HPLC pump regardless of the level of ambient pressure (1 to ∼4.08 ATA He) produced by increasing Phe. In the actual study, however, 0.00 and 0.60 were administered to the brain slice at room pressure (normobaric pressure) with the chamber door open. Thus, in this example, the level of O2 measured in the aCSF (bottom trace) during exposure to “anoxia” (0.00 ATA) is lower than that reported in Table 1 (media O2 tension, PmO2) and Fig. 7A due to the lack of O2 from air (i.e., 100% He + 0% O2) diffusing into the brain slice bath. In contrast, with the chamber door open and the chamber's atmosphere replaced with air (i.e., 20% O2), then the level of PmO2 (and tissue O2 tension, PtO2) achieved is higher due to diffusion of O2 from the atmosphere into the aCSF and brain slice.
O2 Measurements
Media and tissue slice O2 measurements were made using polarographic electrodes, fabricated within the laboratory from glass capillary tubing (1.5-mm outer diameter) and Teflon-coated platinum wire (100-μm outer diameter). The bare platinum sensing surface (50-μm outer diameter) was located at the tip of the electrode. Oxygen electrode calibrations were conducted in the brain slice bath, using a range that spanned from nominally 0.00 ATA O2 (95% N2 + 5% CO2, 2 mM Na2SO3) to the highest PmO2 value used in that particular experiment, including at least one midpoint level of PmO2. Hence, calibration curves used three to four different PmO2 calibration points. A Ag-AgCl reference electrode was positioned downstream of the brain slice in an adjoining fluid chamber.
After the calibration points were recorded, the values were plotted (PmO2 vs. current) and the relationship of current to PmO2 was described using a second-degree polynomial function (Fig. 2A). Although a linear function is commonly used to describe the relationship between Po2 and electrode current (55, 64), we presently report that a curvilinear function better describes the Po2-to-current relationship given that electrode sensitivity plateaus at high O2 tensions. The O2 electrode was polarized at −525, −640, or −675 mV depending on the range of PtO2 to be measured (Fig. 2B). More positive polarization values were used during HBO experiments to avoid 1) saturation of current signal and 2) oxygen poisoning of the electrode. The electrode was calibrated before and after each PtO2 profile measurement.
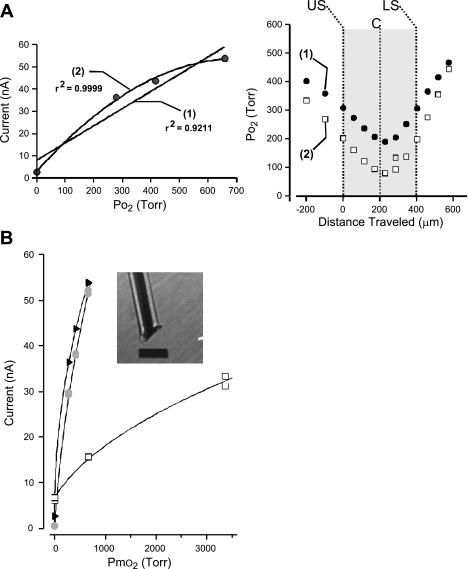
Fig. 2.
Calibration of O2 electrode and measurement of Po2 profiles in aCSF and tissue. A: a representative Po2 experiment using linear and curvilinear calibration functions to calculate Po2 of the tissue slice and aCSF. Left, the linear fitting function (1) of the calibration points overestimates Po2 over most of the range compared with the values derived using the curvilinear fitting function described by a second-degree polynomial (2). Right, plotting calculated Po2 values using either linear (1, solid circles) or curvilinear fitting functions (2, open squares) shows that although the general shape of the profiles are similar to one another, the calculated values can differ. Specifically, the calculated PtO2 values throughout the tissue slice (large shaded area: US, upper surface of slice; C, core of slice; LS, lower surface of slice) are greater when the linear fitting function is used compared with the values generated by the curvilinear function. B: curvilinear calibrations were used to describe the relationship between PmO2 and current generated at various polarization values. Examples of O2 electrode calibration plot (PmO2 vs. current generated) at 3 different polarization values: −675 mV (shaded circles), −640 mV (solid triangles), and −525 mV (open squares). Reduction of polarization potential reduced the amount of current generated per Torr O2. A second-degree polynomial equation was used to describe all calibration curves. Shown in the inset is the tip of the O2 electrode that was constructed from a platinum wire (50-μm outer diameter) insulated with Teflon (25 μm thick). The electrode's sensing surface was located at the end of the exposed wire and consisted of a bare platinum surface. Calibration bar = 125 μm.
Each O2 profile measurement began 200 μm above the top surface of the slice after 16 min of exposure to the level of test PmO2. Next, the electrode was advanced toward the upper slice surface in 100-μm increments. Once at the tissue slice, which was determined by visual assessment using the video microscope, the electrode was driven into the tissue using 40- to 50-μm steps that were continued until the electrode tip passed through the lower surface of the slice and back into the superfusate. The current was allowed to stabilize at each step for 1–3 min before the electrode was moved. The calculated PmO2 and slice PtO2 values were reported in Torr and corrected for both ambient pressure in Dayton, OH, which is slightly above sea level, and for water vapor pressure at 37°C.
Electrophysiology
Extracellular recordings were conducted using an Axoclamp 2A or 2B microelectrode amplifier (headstage gain = 0.1×; Molecular Devices, Sunnyvale, CA). Borosilicate glass (1B100F-4; WPI) recording electrodes were fabricated using a P87 Flaming/Brown micropipette puller (Sutter Instrument, Novato, CA). Recording electrodes were filled with 3 M NaCl and had a tip resistance of <4 MΩ. The bipolar stimulating electrode was fabricated from two twisted platinum wires (50 μm, Teflon coated; catalog no. 771000, AM-Systems). The stimulating electrode was placed in the Schaffer collateral-commissural pathway to evoke the oPS in the CA1 hippocampal cell layer, which was measured by placing a recording electrode in the stratum pyramidale. The stimulus was delivered at 0.033 Hz. The intensity of the electrical stimulus ranged from 100 to 400 μA and had a duration ranging from 0.1 to 0.5 ms (controlled by an A300 Pulsemaster and A-365 stimulus isolation unit; WPI). Intensity was selected on the basis of the minimum amount of current required to activate the oPS to ∼50% maximal amplitude. After 16 min of stable activity in 0.95 ATA O2, at either normobaric or hyperbaric pressure, the superfusate was switched to 0.60, 2.84, or 4.54 ATA O2 for an additional 16 min. The test level of O2 was followed by a recovery phase in 0.95 ATA O2 at either normobaric or hyperbaric pressure for at least another 16 min. In some cases the recovery period was extended to 46 min when following the time course of OxIP. Only stable extracellular recordings were included in this study. Stable recordings were defined as any recording that met the following criteria in control 95%O2: 1) no spontaneous interictal discharge was observed; 2) the oPS amplitude remained unchanged for at least 16 min; and 3) sPS activity, which is indicative of repetitive firing in response to a single orthodromic stimulation (hyperexcitability) in the brain slice preparation (6), was not observed (Fig. 3B). (For raw data examples of sPS evoked by orthodromic stimulation during hyperoxia, see Fig. 5, A and B, traces ii and iii). Orthodromic recordings exhibiting interictal-like bursting or seizure-like activity during initial control conditions, if observed, were discontinued, and the slice was discarded. We defined interictal discharge as a spontaneous burst consisting of three or more action potentials (having duration of at least 50 ms). We rarely ever observed this type of activity under control conditions, however.
Fig. 3.
Stability of the extracellular recording of the orthodromic population spike (oPS) in 0.95 ATA O2 during helium compression (A) and at steady-state pressure (B). A: before helium compression, air was flushed from the hyperbaric chamber and replaced with pure helium to avert any narcotic actions of hyperbaric N2. Helium compression of the chamber and slice bath to 2.41 or 4.51 ATA, while superfusing the slice with aCSF (PmO2 = 0.95 ATA O2), caused a transient depression of the oPS amplitude, which recovered back toward normobaric values after 10–12 min at steady-state hyperbaria. The amplitude of the oPS thereafter remained stable at hyperbaric pressure for an additional 16 min; e.g., see control periods in Fig. 5, A and C. On the basis of these results, the effect of compression on CA1 neuronal excitability was considered negligible under the steady-state hyperbaric conditions used in this study. Consequently, the control condition used for all hyperbaric oxygen (HBO) tests was PmO2 = 0.95 ATA O2 (in aCSF), and the chamber was pressurized to 2.41 or 4.11 ATA He. Raw data traces of the oPS correspond to the elapsed time at 14 (inset i) and 30 min (inset ii). Insets: calibration bars = 2 mV × 2 ms. B: data were collected from slices at normobaric pressure and both levels of hyperbaric pressure and were pooled together. Raw data traces of the oPS (top) correspond to the elapsed time at 4 (inset i), 22 (inset ii), and 40 min (inset iii). Note that no secondary spikes were observed (n = 5). The tightly grouped averaged data (bottom) indicate that the amplitude of the oPS response remained stable throughout the 50-min period under control conditions, which was the duration of the typical experiment. Insets: calibration bars = 2 mV × 2 ms.
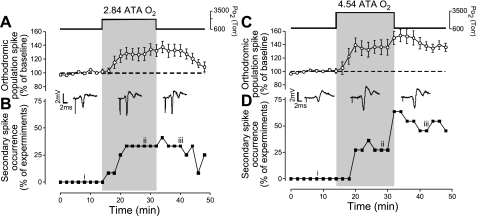
Fig. 5.
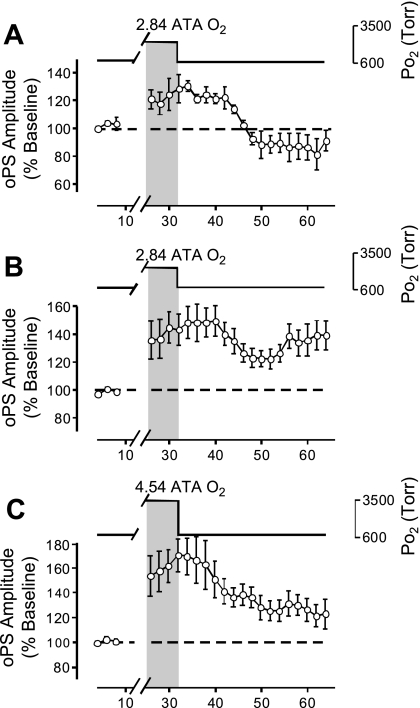
Effects of HBO on the oPS amplitude and induction of secondary population spikes (sPS), which are indicative of hyperexcitability. A: acute exposure for 16 min to 2.84 ATA O2 stimulated the amplitude of the oPS (n = 12). This stimulation decayed to baseline after 16 min of recovery in control O2 (0.95 ATA). B: acute exposure to 2.84 ATA O2 induced sPS activity in some cases (n = 3/12) coincident with the increased amplitude of the oPS (A), which persisted into the recovery period. Representative raw data traces were sampled before (i), during (ii), and after exposure (iii) to 2.84 ATA O2, showing both the stimulation of the oPS amplitude and the onset of the sPS, which follows the primary spike. C: acute exposure to 4.54 ATA O2 increased the amplitude of the oPS (n = 11). This stimulatory effect of HBO, however, did not return to baseline level after 16 min of recovery in 0.95 ATA O2. The potentiation in the oPS following HBO exposure was referred to as “oxygen-induced potentiation” (OxIP) and suggests that a form of O2-dependent neural plasticity exists in the CA3 → CA1 hippocampal circuitry. D: acute exposure to 4.54 ATA O2 induced sPS hyperexcitability in a fraction of experiments during HBO, which persisted and increased throughout the recovery period in several of the slices (n = 5/11). Representative raw data traces of the oPS and sPS activity were sampled before (i), during (ii), and after exposure (iii) to 4.54 ATA O2.
Extracellular responses were collected and stored using two pClamp 9.0 data acquisition systems (Molecular Devices). Axoscope 9.0 software was used to record all experimental parameters in continuous gap free mode (sample rate = 13.3 kHz), whereas Clampex 9.0 (sample rate = 50.0 kHz) and A300 Pulsemaster were used to trigger the stimulating electrode repetitively (0.033 Hz). Recordings of analog signals including chamber ambient pressure (helium), tissue slice bath temperature, and evoked orthodromic activity were monitored continuously and stored for offline analyses to determine the oPS amplitude, the presence of O2-induced sPS activity, and induction of OxIP of the orthodromic response.
Data Analysis
The oPS amplitude was used to assess relative changes in excitability of CA1 neurons stimulated during synaptic activation. The amplitudes of four consecutively evoked orthodromic responses were averaged over 2 min. Data from individual experiments were expressed as a percentage of the baseline (defined as the initial 16-min recording period in 0.95 ATA O2, which was assigned a value of 100%) from that experiment. The response of the CA1 population to a given O2 condition was summarized in an elapsed time plot, where each data point is expressed as the mean ± SE. All differences between two means were determined using Student's t-test and are considered significant at P < 0.05. All differences (P < 0.05) between three or more means were determined using one-way repeated-measures ANOVA followed by multiple comparisons testing (electrophysiology: Dunnett's comparisons with control, 0.95 ATA O2; O2 profiles: Tukey's comparison) using KyPlot Data and Visualization software package (Kyence, Tokyo, Japan) or GraphPad InStat (La Jolla, CA).
RESULTS
Effects of Helium Pressurization at 0.95 ATA Control O2 on oPS
Compression with hyperbaric helium to either 2.43 or 4.11 ATA at constant O2 (0.95 ATA) typically caused a transient decrease in the amplitude of the oPS, which recovered back toward the normobaric level of orthodromic activity within 10–12 min of reaching steady-state hyperbaric pressure. Figure 3A demonstrates the typical response in a subset of experiments (n = 4 slices) conducted to identify the transient depression of the oPS response during and following helium compression at constant O2 tension. No distinction was made in Fig. 3A between helium pressurization from 1.0 to 2.43 or 4.11 ATA, since both levels of hyperbaric helium led to similar outcomes in orthodromic activity: a transient inhibition of the oPS of similar magnitude in each case. For experiments using HBO protocols (e.g., see Fig. 5), the maximal oPS amplitude was reassessed at steady-state hyperbaria to determine if there was a persistent effect of hyperbaric helium on orthodromic activity; however, there was not. The stimulus intensity needed to evoke an oPS of ∼50% of maximal amplitude under hyperbaric pressure was unchanged from that required at normobaric pressure. Stability of the orthodromic recording was determined by the unchanging amplitude of the oPS (Fig. 3B) and the lack of sPS during exposure to 0.95 ATA O2 at hyperbaric pressure.
Effect of HBO (2.84 and 4.54 ATA O2) on PtO2
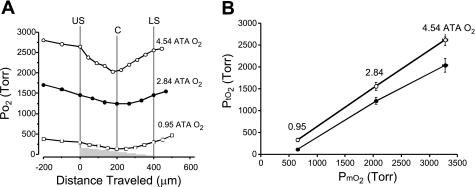
To evaluate the relationship between PmO2 and PtO2, we measured Po2 in submerged, 400-μm-thick hippocampal slices maintained with two-sided superfusion. The PmO2 measured above the upper surface of the slice increased significantly with 2.84 and 4.54 ATA O2 relative to that measured in 0.95 ATA O2 (Fig. 4). The PtO2 profile within the CA1 hippocampus, in 0.95 ATA O2, produced a V-shaped profile with a minimum value of 115 ± 16 Torr at the core of the slices (∼200 μm deep; Fig. 4A). A similar V-shaped profile has been reported in brain slices from other regions of the CNS maintained under similar experimental conditions of two-sided superfusion (9, 64). V-shaped PtO2 profiles occurred at both 2.84 and 4.54 ATA O2, as well (Fig. 4A). The mean core values of 1,222 ± 77 and 2,037 ± 157 Torr at 2.84 and 4.54 ATA O2, respectively, were significantly different from the mean control core value measured at 0.95 ATA (Fig. 4B). At each level of O2, a Tukey's test revealed that the mean upper and lower surface PtO2 values did not significantly differ from one another, as expected, but PtO2 values at both surfaces did differ significantly from the core PtO2 value (Table 1).
Fig. 4.
Oxygen tension profiles measured in 400-μm-thick hippocampal slices during 2-sided superfusion in aCSF equilibrated with 0.95 ATA O2 (control) in an air atmosphere and HBO in a pure helium atmosphere. A: representative PmO2 and PtO2 profiles made at 0.95, 2.84, and 4.54 ATA O2 in 3 different brain slices. Each PtO2 profile measured at each level of PmO2 yielded a similar V-shaped profile that shifted upward as PmO2 was increased. At each level of PmO2 tested, the upper and lower surfaces of the slice had equivalent values, whereas the minimum Po2 measured occurred at the core of the brain slice. The values at the upper and lower surfaces of the slice were statistically different from the core values at 2.84 and 0.95 ATA O2; see Table 1 for further details. B: a plot of average upper surface (○) and core (●) PtO2 values in all slices tested as a function of the average PmO2 in the aCSF. Neurons in each slice are exposed to a range of PtO2 values, which is indicated by the vertical distance separating the 2 averaged values for the upper surface and core PtO2 values at each level of PmO2 tested.
Table 1.
Po2 values measured in the tissue preparation chamber at different depths in the bath and tissue slice during exposure to various levels of oxygenation
| O2, ATA | PmO2, Torr | n | PmO2 200 μm Above Slice, Torr | Upper Surface PtO2, Torr | Core PtO2, Torr | Lower Surface PtO2, Torr |
|---|---|---|---|---|---|---|
| 0.00 | 94 ± 20 | 5 | 69 ± 24 | 43 ± 12 | 20 ± 4 | 24 ± 4 |
| 0.60 | 379 ± 14 | 6 | 293 ± 11‡ | 211 ± 21‡ | 84 ± 6 | 187 ± 22† |
| 0.95 | 655 ± 8 | 10 | 436 ± 36‡ | 330 ± 35‡ | 115 ± 16 | 315 ± 32‡ |
| 2.84 | 2,049 ± 22 | 5 | 1,761 ± 64‡ | 1,555 ± 91* | 1,222 ± 77 | 1,513 ± 85* |
| 4.54 | 3,280 ± 48 | 6 | 2,824 ± 156† | 2,618 ± 139* | 2,037 ± 157 | 2,665 ± 70* |
Values are means ± SE representing medium (PmO2) or tissue slice Po2 (PtO2). PmO2 was measured 500 μm above the slice unless otherwise indicated. Oxygenation levels of 0.00, 0.60, and 0.95 ATA O2 represent air atmosphere; those at 2.84 and 4.54 ATA O2 represent helium atmosphere. Statistically significant differences (*P ≤ 0.05;†P ≤ 0.01;‡P ≤ 0.001) were determined using Tukey's test to compare Po2 values with the core PtO2 value of the slice at agiven PmO2. No differences were found between upper and lower surfaces at any PmO2. All values of Po2 reported were derived from a curvilinear calibration function as described in methods (see Fig. 2).
Effects of HBO (2.84 and 4.54 ATA O2) on CA1 Neuronal Excitability
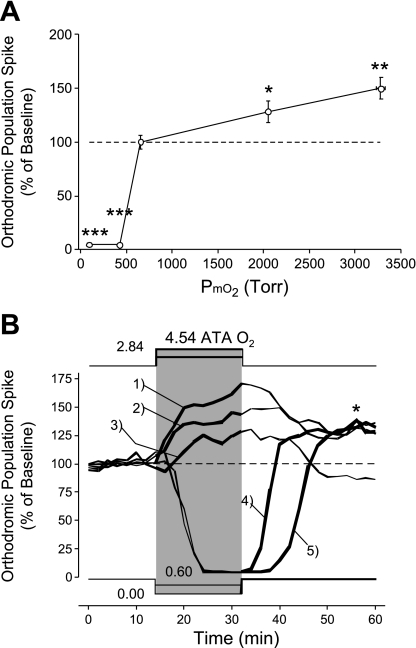
Raising PmO2 from 0.95 to 2.84 ATA for 16 min increased the amplitude of the oPS (n = 12, Fig. 5A). After 16 min at 2.84 ATA O2, the mean oPS amplitude was 32.4 ± 8.3% (P ≤ 0.01) greater than baseline activity, and 33% of the slices tested displayed sPS (Fig. 5B). The sPS was defined as a clear downward deflection following the primary oPS, which had an amplitude >25% of the oPS amplitude during control conditions. On returning to the control O2 condition, the elevated amplitude of the oPS, on average, decayed toward the original baseline value (Fig. 5A), and ≥25% of slices continued to exhibit sPS (Fig. 5B).
Raising Po2 from 0.95 to 4.54 ATA O2 likewise increased the amplitude of the oPS (n = 11, Fig. 5C). After 16 min of 4.54 ATA O2, the mean oPS amplitude was increased significantly by 49.8 ± 9.8% (P ≤ 0.01) above baseline, and 64% of the slices tested exhibited sPS (Fig. 5D). All slices exposed to 4.54 ATA O2 exhibited OxIP, that is, the amplitude of the oPS remained significantly elevated above the original baseline (36.2 ± 6.0%, P ≤ 0.01) on return to 0.95 following a single bout of 4.54 ATA O2. In addition to OxIP of the oPS, 45% of the slices continued to exhibit sPS activity during the recovery phase in 0.95 ATA O2.
A comparison of oPS amplitude during exposure to each dose of HBO (2.84 and 4.54 ATA O2) showed no statistically significant difference from one another. Furthermore, neither 2.84 nor 4.54 ATA O2 caused regular interictal discharge (>2 periods) during 20 of 23 HBO exposures. However, in 3 of 23 HBO experiments (2.84 ATA O2, n = 2/12; 4.54 ATA O2, n = 1/11), hyperoxia induced one to two periods of interictal discharge throughout the HBO exposure (not shown). In these three cases, interictal discharge did not persist following exposure to HBO. Likewise, no statistical differences were found between sPS incidence following exposure to either level of HBO. However, a significant difference was found when comparing the effect of 2.84 to that of 4.54 ATA O2 on the oPS amplitude during the recovery period (i.e., return to 0.95 ATA O2 condition for 16 min). The mean postexposure amplitude of the oPS following 4.54 ATA O2 remained significantly elevated, that is, exhibited OxIP compared with the respective mean oPS response from experiments using 2.84 ATA O2 (P ≤ 0.01).
OxIP Following Acute Exposure to HBO
Although the mean response of the oPS following all exposures to 2.84 ATA O2 showed a progressive return toward baseline activity (Fig. 5A), analysis of the individual experiments revealed that 67% of the exposed slices (n = 8/12) did not fully recover to baseline during the 16-min recovery period (not shown). This suggested that OxIP was activated by 2.84 ATA O2 in some of the hippocampal slices. Consequently, to resolve this issue and to further characterize OxIP following cessation of HBO, the recovery period following acute exposure to HBO (both 2.84 and 4.54 ATA O2) was extended from 16 to 32 min. Extending the oPS recovery period in a subset of experiments (n = 10) revealed that the oPS response to 2.84 ATA O2 expressed two distinct patterns of recovery. The oPS amplitude either returned to baseline (n = 4/10, Fig. 6A) or exhibited OxIP for at least 32 min of the recovery period (n = 6/10, Fig. 6B). Of the slices that exhibited OxIP following 2.84 ATA O2, 67% (n = 4/6) also exhibited sustained sPS activity during the 32 min of recovery (not shown). At the highest level of HBO tested (4.54 ATA O2), all slices exhibited OxIP throughout the entire 32 min of recovery (n = 6, Fig. 6C). Of these, 50% also exhibited sustained sPS activity during the recovery period (not shown). In some experiments (n = 3), the recovery period was extended and OxIP following 4.54 ATA O2 persisted for at least 46 min (not shown). Longer recovery periods were not tested. A comparison between the magnitude of OxIP induced in a subset of the slices exposed to 2.84 ATA O2 (Fig. 6B) and in all the slices exposed to 4.54 ATA O2 (Fig. 6C) indicated no significant difference in the magnitude of OxIP caused by either level of HBO.
Fig. 6.
The recovery period following HBO was extended from 16 to 32 min to determine the incidence of OxIP. Slices were divided into those that did not exhibit (A) and those that did exhibit OxIP (B and C). A: exposure to 2.84 ATA O2 induced 1 of 2 types of recovery patterns. As shown, a fraction of the slices tested (n = 4/10) exhibited full recovery of the oPS response that eventually undershoots the original baseline activity. B: exposure to 2.84 ATA O2 activated OxIP in over one-half of the slices tested (n = 6/10), which was sustained for at least 32 min. Four of 6 slices also exhibited sPS activity during HBO that was sustained throughout OxIP. This population of slices with OxIP of the oPS was not evident in Fig. 5A when all the data were pooled and a shorter recovery period was analyzed. C: exposure to the highest level of HBO tested (4.54 ATA O2) activated OxIP for at least 32 min in all of the slices tested (n = 6). Three of 6 slices also exhibited sPS activity during HBO that was sustained throughout OxIP.
Effect of Normobaric Oxygen (0.00 and 0.60 ATA O2) on PtO2
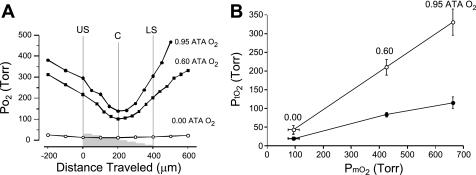
Exposure to 0.6 ATA O2 during two-sided superfusion produced a V-shaped O2 profile that was significantly lower than the PtO2 profile measured in 0.95 ATA O2, whereas exposure to 0.00 ATA O2 produced a relatively flat PtO2 profile (Fig. 7A). In addition, the range of PtO2 values encompassing the profile in 0.60 ATA O2 partially overlapped the range of PtO2 values encompassing the profile range at 0.95 ATA O2, whereas the range of PtO2 values encompassing the profile range in 0.00 were distinctly different from those in 0.95 ATA O2 and the profile range measured in 0.60 ATA O2 (Fig. 7B). The PmO2 values measured 200 μm above the upper surface of the slice, in aCSF equilibrated in 0.00 ATA O2, revealed that O2 was still present in the aCSF. Thus, during 16 min of exposure to 0.00 ATA O2, the slice was not anoxic but ranged from 20 ± 4 to 24 ± 4 Torr at the core and lower surface of the slice, respectively. This relatively flat O2 profile contrasted with the V-shaped profile at 0.60 ATA O2, where the mean core value of 211 ± 21 Torr was significantly different from both upper and lower surfaces of the slice (Table 1).
Fig. 7.
Oxygen tension profiles measured in 400-μm-thick hippocampal slices during 2-sided superfusion in aCSF equilibrated with 0.95 (control), 0.60, and 0.00 ATA O2 in an overlying air atmosphere. A: representative PmO2 and PtO2 profiles made at 0.95, 0.60, and 0.00 ATA O2 in 3 different brain slices. Each of the PtO2 profiles measured at a level of PmO2 >0.00 ATA O2 yielded a similar V-shaped profile that shifted downward as PmO2 was decreased. At each level of O2 >0.00 ATA O2 tested, the upper and lower surfaces of the slice had equivalent values, whereas the minimum Po2 measured occurred at the core of the brain slice. At 0.00 ATA O2, however, the O2 profile appears to be flat compared with the V-shaped profiles observed at 0.60 ATA O2 and above. The values at the upper and lower surfaces of the slice were statistically different from the core values at 0.95 and 0.60 ATA O2; see Table 1 for further details. B: a plot of average upper surface (○) and core (●) PtO2 values in all slices tested as a function of the average PmO2 in the aCSF at normobaric pressure. Each slice is exposed to a range of PtO2, which is indicated by the vertical distance separating the 2 averaged values for upper surface and core PtO2 values at each O2 level tested. Notice that the values of PtO2 in control O2 (0.95 ATA) are hyperoxic (ranging from 115 to 330 Torr) compared with values for PtO2 measured in the intact central nervous system of an animal breathing normobaric air (see text). The data for 0.95 ATA O2 were also plotted in Fig. 4 on a compressed y-axis.
Effects of Normobaric O2 (0.00 and 0.60 ATA) and NBOreox (0.0 or 0.6 → 0.95 ATA) on CA1 Neuronal Excitability
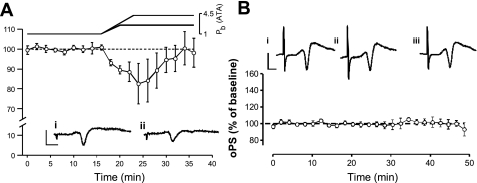
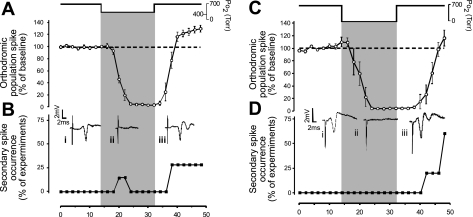
Decreasing PmO2 from 0.95 ATA to an intermediate level of O2 (0.60 ATA) led to near complete inhibition of the oPS within 10 min, which persisted throughout the 16 min of exposure (2.966 ± 1.1% of baseline control, n = 7, P ≤ 0.01; Fig. 8A). Restoration of the oPS response was first observed after 2-min exposure to 0.95 ATA O2. The amplitude of the oPS was still significantly greater than control baseline (30.2 ± 6.2%, P ≤ 0.01) 16 min into the recovery period in 0.95 ATA O2. Hence, another form of OxIP was observed after O2 tension was increased from an intermediate level of O2 (0.60 to 0.95 ATA); i.e., NBOreox. Moreover, 28.6% of the slices tested exhibited sPS following NBOreox (Fig. 8B).
Fig. 8.
Effects of 0.6 and 0.00 ATA O2 and “normobaric reoxygenation” (NBOreox) on the oPS amplitude and induction of sPS hyperexcitability. A: acute exposure for 16 min to 0.60 ATA O2 completely inhibited the oPS response in all slices tested. The oPS response returned toward control activity on reoxygenation and overshot the original baseline by 30.2% after 16 min of recovery (n = 7), which we interpreted as OxIP. B: in parallel with the stimulation of the oPS amplitude during reoxygenation from 0.6 to 0.95 ATA O2, one-quarter of the slices tested also displayed sPS activity or hyperexcitability. Representative raw data traces were sampled before (i), during (ii), and after exposure (iii) to 0.60 ATA O2, showing the effects of O2 manipulation on the oPS and sPS activity. C: similar to the synaptic response to 0.60 ATA O2, acute exposure to 0.00 ATA O2 completely inhibited the oPS as previously reported by many investigators (see text). In contrast to the response in 0.6 ATA O2, recovery of the oPS response back toward control activity required a longer time. Eventually, after 14 min of recovery, the oPS fully returned and overshot baseline activity by 16.3%, which is more evident in Fig. 9B, trace 5, where a longer period of recovery is shown. D: in parallel with the stimulation of the oPS amplitude during reoxygenation from 0.00 to 0.95 ATA O2, one-half of the slices tested also displayed sPS hyperexcitability. Representative raw data traces were sampled before (i), during (ii), and after exposure (iii) to hypoxia, showing the effects of O2 manipulation on the oPS and sPS activity.
As previously reported (9, 33, 73, 74, 76, 87), switching PmO2 from 0.95 to 0.00 ATA also led to complete inhibition of the oPS response. The inhibition of the oPS occurred within 8 min of hypoxic exposure and persisted throughout the 16-min exposure (4.6 ± 0.6% of baseline activity, n = 5, P ≤ 0.01; Fig. 8C). On reoxygenation, the oPS was first noted after 8 min of exposure to 0.95 ATA O2 and eventually recovered to baseline (116.3 ± 12.7%) after 16 min in 0.95 ATA O2. Moreover, 60% of the slices tested exhibited sPS during the reoxygenation period (Fig. 8D). Although no statistical differences were found when comparing the magnitude or rate of inhibition of the oPS during exposure to 0.60 vs. 0.00 ATA O2 (both levels of normobaric oxygen abolished the oPS response), a comparison of the mean oPS amplitudes 8 min following exposure to 0.60 and 0.00 ATA O2 indicated that it recovered more quickly following 0.60 than 0.00 ATA O2 (P < 0.001). With only one exception, normobaric reoxygenation from 0.60 to 0.95 ATA O2 did not lead to spontaneous regular interictal discharge (>2 periods). Although not illustrated clearly in Fig. 8C, OxIP also was observed following reoxygenation, but only after 16 min of recovery (see Fig. 9B, trace 5).
Fig. 9.
Summary of how orthodromic activity changes in CA1 neurons during and following O2 manipulation at normobaric and hyperbaric pressures. A: graph shows the effects of 16 min of O2 manipulation on the amplitude of the oPS with PmO2 = 0.95 ATA as the initial control O2 tension. The greatest O2 sensitivity occurs between 0.6 and 0.95 ATA (*P < 0.05; **P < 0.01; ***P < 0.001). Notice that CA1 excitability increases in a nonlinear fashion as the level of PmO2 used during O2 manipulation increases from 0.6 (∼380 Torr) through 4.54 ATA (∼3,280 Torr) O2. Both 0.0 and 0.6 ATA O2 completely blocked the oPS response. Orthodromically stimulated activity in the O2 range of 0.6 to 0.95 ATA is especially O2 sensitive (see text). B: data replotted from Figs. 5, 6, and 8 without the standard error bars. Notice that any hyperoxic stimulus (NBOreox and HBO), regardless of the initial PmO2 value, increases excitability for at least 16 min (and as long as 46 min). This point is emphasized with the use of thick lines in the PmO2 traces and oPS amplitude traces, which demarcate the time period when a hyperoxic stimulus, covering a different range of PmO2 each time, is being applied to the hippocampal slice. This includes initiating the hyperoxic stimulus from either 0.95 ATA O2 via HBO (oPS trace 1, 4.5 ATA O2; oPS trace 2, 2.8 ATA O2) or 0.60 ATA O2 (oPS trace 4) and 0.00 ATA O2 (oPS trace 5) via NBOreox. Trace 3 is the subsample of slices that did not exhibit OxIP following acute exposure to 2.8 ATA O2 (Fig. 6A). The asterisk highlights the similarity in magnitude of OxIP activated by HBO or NBOreox in oPS traces 1, 2, 4, and 5.
Summary of Changes in CA1 Neuronal Excitability During and Following Acute O2 Manipulation
Neuronal O2 sensitivity during O2 manipulation from the control level of 0.95 ATA is a complex, nonlinear function that exists over a broad range of O2 tension. Based on changes in the oPS amplitude, the excitability of CA1 neurons is most sensitive between 0.60 to 0.95 ATA O2 and decreases to zero as PmO2 is lowered toward 0.60 ATA. As PmO2 is increased above 0.95 ATA using HBO, O2 sensitivity increases in a linear fashion, but the overall O2 sensitivity is less between 0.95 and 4.54 ATA O2 compared with that measured between 0.60 and 0.95 ATA (Fig. 9A). Regardless of these differences in O2 sensitivity over different ranges of O2 manipulation, the magnitude of OxIP measured during the recovery period (0.95 ATA) is the same regardless of the hyperoxic stimulus used (Fig. 9B). For example, with the exception of oPS trace 3, the magnitude of the oPS response following HBO (oPS traces 1 and 2) and during NBOreox (oPS traces 4 and 5) all converge in the range of 125–130% stimulation of control activity in 0.95 ATA O2.
DISCUSSION
Our study produced four major findings focused on the O2 sensitivity of CA1 neurons throughout a range of physiologically relevant O2 tensions. 1) All forms of O2 manipulation tested caused significant changes in orthodromic excitability as determined by changes in the amplitude of the oPS. As we hypothesized, excitability of CA1 neurons, in general, increased as PtO2 increased; however, the greatest increase in excitability during O2 manipulation occurred over the range of 0.60 to 0.95 ATA O2. 2) Both forms of hyperoxic stimuli, HBO and NBOreox, produce OxIP, a form of neural plasticity defined by a sustained potentiation of the oPS amplitude (lasting ≥46 min) on return to control O2 conditions (0.95 ATA O2), which represents increased neuronal excitability. 3) A single exposure to HBO and NBOreox often produces sPS that usually persists during OxIP. 4) The CA1 region in the 400-μm hippocampal slice, bathed in aCSF equilibrated with O2 ≥0.60 ATA over both surfaces, is hyperoxic throughout the entire slice compared with the intact CNS. Surprisingly, acute exposure to aCSF equilibrated with anoxic gas mixture (95% N2 + 5% CO2) produces a range of PtO2 values in the 400-μm slice that resemble that during normoxia (in vivo). The core of the 400-μm-thick brain slice equilibrated with anoxic gas is not anoxic.
Neuronal Barosensitivity
In previous studies (30, 31, 62, 63, 77, 78, 86), 100% helium has been used to mimic the effects of hydrostatic compression. Helium is virtually insoluble in lipid membranes and exhibits no noticeable narcotic effects over the range of physiologically tolerable pressures (11, 25, 66). We have previously reported, however, that certain neurons in the dorsal medulla oblongata in slices are also stimulated by ≤4 ATA of helium, having either a sustained or transient increase in firing rate (66). In that case, barosensitivity of medullary neurons did not correlate with sensitivity to HBO. Therefore, it was necessary to consider what effect, if any, moderate hyperbaric pressures of helium had on the oPS originating from CA1 neurons (Fig. 3A).
As indicated in Fig. 3A, we found that during helium compression, the oPS amplitude was inhibited but then fully recovered when a new level of steady-state hyperbaria was established at ≤4.5 ATA. This transient inhibitory response during helium compression was related to the effect of pressure per se and was not the result of fluctuations in temperature and PmO2, since both conditions remained stable during the “diving descent” (Fig. 1) and “surfacing” of the hippocampal slice inside the chamber (24). The effects of >1 to 4 ATA helium compression on the electrophysiology of CA1 hippocampal neurons has rarely been studied. Southan and Wann (77) reported that the oPS generated in the CA1 hippocampus is partially depressed during compression through 4.0 ATA helium on the way to a maximum compression of 100 ATA. Therefore, it is likely that they did not observe the transient inhibition of the oPS response reported in the present study because steady-state compression at 4 ATA was never tested in their study. Moreover, Mor and Grossman (62, 63) used steady-state 2–4 ATA helium as the control condition and then studied the effects of much greater levels of helium compression (≥100 ATA) on synaptic activation of CA1 neurons. Such studies, however, likely activate additional mechanisms of barosensitivity that involve thermodynamic reaction intensity parameters that simply cannot occur at the moderate levels of compression used in the present study (22, 59, 60). Current ideas on how pressure per se affects the cellular membrane to alter ionic channels are presented elsewhere (19, 22, 25). The functional significance of barosensitivity in the CA1 hippocampus, in this case, transient inhibition of orthodromic activity during moderate helium compression, remains to be determined. One possibility is that barometric pressure, like temperature, is an environmental cue that enables the organism to adapt to changes in its external environment (22, 25).
Excitability Under Control Conditions
Given the general excitatory effects of NBOreox and HBO on CA1 neurons, we are obliged to comment on the control conditions used, which would be expected to produce elevated baseline excitability in the hippocampal slice preparation used in this study: 0.95 ATA control O2, which is hyperoxia, and elevated extracellular potassium concentration ([K+]e) of 6.24 mM. Based on this study and the companion study (40), we now know that normobaric hyperoxia, that is, equilibration of aCSF with 95% O2 at Pb = 1 ATA, plays a permissive role in synchronous orthodromic activation of CA1 neurons in hippocampal slices. Likewise, enhanced tissue slice oxygenation through improved O2 delivery to the tissue using high flow rate and two-sided superfusion enables spontaneous synchronous network activity in hippocampal tissue slices (43). (Two-sided superfusion at 2 ml/min was used in the present study.) In addition, if hippocampal slices are maintained in aCSF equilibrated with 20% control O2 for many hours, acute exposure to normobaric hyperoxia (0.95 ATA) stimulates spontaneous and evoked firing rate in individual CA1 pyramidal neurons (21). It is unknown, however, how oPS activity is affected by the use of 20% control O2. For example, it is unknown whether OxIP occurs in CA1 neurons in slices maintained in a lower level of control O2. We would predict that it does occur, however, since OxIP is activated by NBOreox following hypoxia and intermediate oxygenation (13, 14; present study). Loss of OxIP at a lower level of control O2, however, if it occurs, would support the hypothesis that this form of neural plasticity is dependent on a background of increased ROS/RNS production during the preceding control period.
Normal [K+]e is ∼3 mM in the CNS, but it can increase as high as 10–12 mM during periods of intense neural activity (52, 53). We selected the level of 6.24 mM [K+]e a priori to enhance activity in a deafferented brain slice preparation, an approach that is commonly used in in vitro electrophysiology studies. For example, the control level of [K+]e for brain slice experiments can range from 2 to 8 mM (2, 28, 29, 84). Rutecki et al. (72) demonstrated that a steeply graded relationship exists between the log of [K+]e and frequency of interictal discharge in the range between 6.5 and 10 mM. Moreover, they observed that the amplitude of interictal discharge was markedly smaller at 6.5 mM [K+]e compared with that observed at ≥7.5 mM [K+]e. In agreement with the observations made by Rutecki et al.(72), we found that from slice to slice, neither sPS nor interictal discharge was consistently observed during control conditions in 6.24 mM [K+]e. In cases where sPS or interictal discharge was observed during control conditions, those experiments were considered unstable and were terminated. Hence, [K+]e = 6.24 mM did not induce a measurable state of hyperexcitability (sPS or spontaneous bursting) during the initial control period in any of the slice preparations included in this study. As we reported, sPS and spontaneous bursting did occur as a result of acute exposure to normobaric reoxygenation or following HBO; however, interictal bursting was not a component of the oxygen-induced increased activity.
Excitability During O2 Manipulation
Because the inhibitory effect of pressure per se on the oPS was transient, any change in excitability during O2 manipulation at steady-state helium compression was attributed to the direct effects of molecular O2 and/or the secondary reaction products of O2 (i.e., ROS/RNS) on CA1 neurons (21), and not the effects of pressure per se. As a general statement, our hypothesis that CA1 neuronal excitability increases over a continuum of PtO2 was correct; however, the relationship between PtO2 and excitability is a complex one (e.g., Fig. 9, A and B). Oxygen manipulation had multiple effects on the field potential that were influenced, to varying degrees, by the range of O2 tested and the timing during the O2 manipulation (i.e., specific patterns of excitability occurred during and following O2 manipulation).
Hyperbaric hyperoxia (0.95 → 2.84 or 4.54 ATA O2).
Signs of CNS O2 toxicity occur in awake rats breathing 4.0–8.0 ATA O2, which manifest as tonic clonic seizures (16, 75, 83). The PtO2 measurements made in 400-μm-thick hippocampal slices superfused with aCSF equilibrated with 0.95, 2.84, and 4.54 ATA O2, using polarographic electrodes approximated the range of PtO2 measured in intact rats breathing >2 to 8 ATA O2 (47, 64). Hence, the levels of HBO used were physiologically relevant for studying cellular activity (in vitro) during early exposure to a level of hyperoxia that causes CNS O2 toxicity (in vivo). The fact that these levels of HBO increased CA1 excitability but did not induce seizure-like activity may be attributed to the acute, relatively short duration of HBO stimuli. The dose of hyperoxia (and thus ROS/RNS) that elicits CNS O2 toxicity is determined by the O2 concentration product, which is the product of 1) the level of PtO2 and 2) the duration of hyperoxic exposure. Perhaps longer HBO exposures than those used in the this study (15–16 min) will in future studies induce seizure-like activity in CA1 neurons in hippocampal tissue slices.
How accurate are the PtO2 measurements reported in this study given the limitations of polarographic electrodes, the primary one of which is O2 consumption by the sensing tip (85)? Despite these limitations, polarographic electrodes are nonetheless considered the gold standard for measurement of brain PtO2 (80). O2 consumption by the polarographic electrode becomes problematic at low levels of PtO2, particularly in tissues with low metabolism (85). In the present study, however, low levels of PtO2 were not encountered, even during acute exposures to anoxic gas mixture. Moreover, O2 consumption was an ongoing process at ∼37°C in the CA1 region of the 400-μm-thick slice in aCSF equilibrated with ≥0.60 ATA O2 as indicated by the steep surface-to-core PtO2 profile through the hippocampal slice. The steepness of the PtO2 profile is used as an estimate of cellular metabolism in brain slices (64). Interestingly, studies that compare polarographic electrodes to other PtO2 measurements find no significant differences, on average, in the level of PtO2 measured. Polarographic electrodes, however, do tend to show greater heterogeneity in PtO2 values. This is attributed to the greater temporal resolution of the electrode tip relative to other types of O2 sensors and, consequently, detection of spatial differences in O2 delivery caused by the differences in local cellular metabolism and capillary density (3, 69).
This broad range of tissue hyperoxia during acute O2 manipulation led to 1) an increase in the oPS amplitude, 2) the occurrence of sPS and, rarely, spontaneous fictive interictal bursting, and 3) the induction of OxIP. Of these responses, the increase in oPS and onset of sPS are consistent with generalized CNS hyperexcitability that occurs during O2 toxicity in vivo (27). The latter effect, OxIP, is a new observation following exposure to HBO (in vitro). Whether OxIP contributes to the off-latency of seizure (in vivo) that follows reduction in inspired Po2 is unknown at this time. The functional significance of OxIP is considered further in the last section of the discussion.
Because the oPS represents synchronized action potential generation from many CA1 neurons, the increased oPS amplitude in response to both levels of HBO indicated that more CA1 neurons are activated and/or in the number of action potentials generated (25) compared with the preceding control period in 0.95 ATA O2. A surprising finding was that the magnitude of stimulation between 0.95 → 2.84 ATA O2 and 0.95 → 4.54 ATA O2 was not statistically significant from each other. This was unexpected given the significant increase in PtO2 between 2.84 and 4.54 ATA and, presumably, the significant increase in production of ROS and RNS that would be expected to occur (21). Reduction or blunting of O2 sensitivity of the oPS response between 0.95 and 4.54 ATA O2 (Fig. 9A) may be the result of the inherent narcotic potency of O2 arising from either the interference of molecular O2 with lipid-lipid and/or lipid-protein interactions in the plasma membrane (8, 19) or the increase in O2-derived free radicals and membrane lipid peroxidation (19–21, 67).
Alternatively, the blunted stimulation of the oPS in HBO may have resulted from prolonged exposure to the hyperoxic control condition (0.95 ATA) during slicing, incubation, and recording, which may have induced “oxidative preconditioning.” Our measurements of PtO2 profiles in 0.95 ATA O2 confirm our previous reports (25, 64) that 300- to 400-μm-thick brain slices, superfused over both surfaces with aCSF equilibrated with 0.95 ATA O2, are hyperoxic at all levels in the tissue. The range of PtO2 in the slice equilibrated with 0.95 ATA O2 is equivalent to that in a rat breathing more than 2.2 ATA O2 (25, 47, 64; Table 2). In fact, exposure of hippocampal slices to 0.95 ATA O2 for 4 h causes a significant increase in markers of oxidative stress as determined by isoprostane production (32) and increased superoxide production in the CA1 region (21). Increased ROS production is a critical step in the induction of oxidative preconditioning (35).
Table 2.
Polarographic electrode measurements of PtO2 in the intact CNS
| CNS Region Measured | Pb, ATA | Inspired O2, %O2 | PtO2, Torr | Study Ref. |
|---|---|---|---|---|
| Cortex | 1.0 | Air | 34 ± 4 | 47 |
| 100 | 90 ± 13 | |||
| 2.0 | 100 | 244 ± 34 | ||
| 4.0 | 100 | 643 ± 89 | ||
| 6.0 | 100 | 1,293 ± 170 | ||
| Globus | 1.0 | Air | 11 ± 3.2 | 45 |
| pallidus | 5.0 | 100 | 1,250* | |
| Neostriatum | 1.0 | Air | 19 ± 9 | 45 |
| 5.0 | 100 | 1,010* | ||
| Hypothalamus | 1.0 | Air | 13 ± 3.3 | 14 |
| 1.0 | 100 | 41 ± 6 | ||
| Hippocampus | 1.0 | Air | 19 ± 1 | 34 |
| 1.0 | 14 | 13 ± 3 | ||
| 9 | 2 ± 1 | |||
| Cortex | 1.0 | 26 | 28 ± 6 | 69 |
| Cerebellar cortex | 1.0 | 30 | 38 ± 3 | 68 |
Values are means ± SE. Barometric pressure (Pb), assuming ambient room pressurewhere measurements were made, was equivalent to 1 atmosphere absolute (ATA; 760 Torr). “Air” refers to 20–21% O2-balance N2. Asterisks indicate that only maximal value was reported because there were transient changes in PtO2. Note that values reported in the hypothalams in Ref. 14 were taken from calculated values reported by Mulkey et al. (64). CNS, central nervous system.
The anticipated effect of hyperoxic oxidative preconditioning, if it occurs in CA1 neurons, would be that neurons are rendered less sensitive to subsequent bouts of oxidative stress, such as when HBO is breathed (35). Oxidative preconditioning seems plausible given that a single bout of acute hyperoxia, lasting only minutes, induces long-lasting changes in CA1 excitability (17, 18, 73, 76). It is not unreasonable, therefore, to postulate that 1–5 h of sustained exposure to normobaric hyperoxia (0.95 ATA O2), which is equivalent to breathing HBO (in vivo) can, likewise, alter the O2 sensitivity of hippocampal CA1 neurons to an ensuing bout of acute hyperoxia (Fig. 9A; a decrease in the stimulatory effect of HBO?). This may also explain why seizure-like activity was not observed during HBO in the present study. This hypothesis, however, needs to be tested by preconditioning hippocampal slices to lower levels of O2 [for example, it is clear that brain slices maintained in 20–40% control O2 exhibit normal electrophysiology for several hours (21, 23)] and, in addition, using longer bouts of HBO stimuli.
Intermediate oxygenation at normobaric pressure (0.95 → 0.60 ATA O2).
The present study was designed to determine the effects of hyperoxia on orthodromic excitability, of which NBOreox represented a second form of hyperoxic stimulation. For purposes of comparison, however, we also analyzed the effects of decreasing O2 below 0.95 ATA on orthodromic activity. Our findings indicate surprisingly that decreasing O2 from 0.95 ATA to an intermediate level of oxygenation (0.60 ATA) completely inhibited the oPS response. The greatest increase in O2 sensitivity of CA1 neurons, therefore, occurred between PmO2 >0.60 and 0.95 ATA O2; however, the exact minimum threshold of PmO2 at which the oPS can be evoked was not determined (Fig. 9A). As discussed below, this inhibitory effect of 0.60 ATA on the oPS response is identical to that reported for nominal hypoxia using 95% N2 + 5% CO2 (17, 18, 42, 44, 57, 76). The strong inhibitory effect of an intermediate level of oxygenation (0.60 ATA O2) might be interpreted to mean that any level of PmO2 <0.95 ATA induces tissue slice “hypoxia,” resulting in a loss of general mechanisms of neuronal excitability (33, 87). However, on the basis of our findings, we do not think this is the case for the following two reasons. First, reduction of PmO2 from 0.95 to 0.60 ATA produced a range of PtO2 that overlaps in part with the range of PtO2 measured at 0.95 ATA O2 and, likewise, exceeded the PtO2 measured in the intact CNS of an animal breathing normobaric air (Table 2). The surface-to-core PtO2 profile was similar in magnitude and shape to the PtO2 profile measured in 0.95 ATA O2, suggesting that cells throughout all depths in the slice are respiring and consuming O2 in both 0.60 and 0.95 ATA O2 (64). By comparison, the PtO2 profile in 0.00 ATA O2 is essentially flat, suggesting that energy metabolism was compromised during hypoxia despite the relatively elevated level of PtO2 (64).
Second, our laboratory also reported that cell death in the CA1 region is significantly less over a 4-h period in 0.60 ATA O2 than it is in 0.95 ATA O2 (21). In fact, cell death is lowest when 400-μm-thick hippocampal slices are incubated in 20–40% O2 and greatest when incubated in 95% O2 (21). Furthermore, new evidence presented in the companion article (40) supports the hypothesis that mechanisms of excitability in CA1 neurons are reduced during exposure to 0.60 ATA O2 but are not abolished; for example, antidromic activation of the CA1 population and orthodromic synaptic activation of individual CA1 neurons are both retained during exposure to 0.60 ATA O2. Similarly, D'Agostino et al. (21) reported that CA1 neurons in slices continue to generate action potentials for many hours in 0.20 ATA O2 (∼37°C) and are stimulated to fire faster during acute exposure to 0.95 ATA O2. Thus CA1 neurons remain excitable in 0.6 ATA O2 (and less). What is eliminated in 0.60 ATA O2, we propose, is the ability for synchronized synaptic activation of the population of CA1 neurons based on extracellular field potential recordings, which is further discussed in the companion article (40).
Few studies have examined the effects of O2 <95% but >15% at normobaric pressure. Three studies have reported that the oPS amplitude decreases proportionally with the reduction of O2 from 95% through 0% O2 and that the oPS is not abolished until O2 is lowered to 24% or below (1, 33, 44, 87). The lack of PtO2 measurements in those studies, however, prevents us knowing the absolute range of PtO2 values that were established and the relative magnitude of the PtO2 profile under their experimental O2 conditions. This is a critical consideration, since slice PtO2 is influenced not only by the equilibrating O2 mixture but also by other experimental parameters that will vary between laboratories, including thickness of the slice preparation, bath temperature, bath depth (i.e., submerged vs. interface slice), type of slice bath (1-sided vs. 2-sided superfusion), the relative surface area of the tissue slice exposed to aCSF (unpublished observations), and the rate of superfusion with aCSF (1, 25, 43, 87). Therefore, without companion measurements of PtO2 with orthodromic activity, an accurate comparison of their findings to ours regarding the O2 threshold for turning off/on of neural activity cannot be made.
Normobaric hypoxia (0.95 → 0.00 ATA O2).
For purposes of comparison of CA1 neuronal O2 sensitivity to HBO, we also analyzed the effects of anoxic gas mixture (95% N2 + 5% CO2) on CA1 activity, which has been reported on extensively (33, 36, 37, 54, 57, 74, 76, 81, 87). Not surprisingly, equilibration of aCSF with an anoxic gas mixture completely abolished the oPS in CA1 neurons. What was surprising, however, was that aCSF containing no O2 carrier (e.g., hemoglobin or perfluorocarbon) still yielded a level of PtO2 throughout all depths of the 400-μm tissue slice that was neither anoxic nor hypoxic; that is, the PtO2 profile decreased to ∼20–43 Torr (Table 1), which overlaps with the range of normoxic PtO2 measured in the intact CNS of an animal breathing normobaric air (Table 2).
The likely explanation is the diffusion of atmospheric O2 into the aCSF, since all experiments conducted at normobaric pressure exclusively were performed with the hyperbaric chamber open to ambient room air. In future studies, the PmO2 could be reduced further by sealing the pressure vessel and replacing the air with pure helium or nitrogen while aerating the medium with 95% N2 + 5% CO2 (25).
Regardless, we called aCSF equilibrated with anoxic gas mixture “hypoxia” for two reasons. First, changing PmO2 from 0.95 to 0.00 ATA O2 is a relative hypoxic stimulus; i.e., a decreasing O2 stimulus initiated from what we would call hyperoxia (0.95 ATA). Second, our measurements of the magnitude of the PtO2 profile in a 400-μm-thick slice suggest that despite the apparently normoxic level of PtO2 (∼20–43 Torr), the brain slice is less metabolically active and thus consuming less O2 than at ≥0.60 ATA O2. As already stated above, an actively respiring slice will have a steeper PtO2 surface-to-core profile (64), yet at 0.00 ATA O2, the PtO2 is comparatively flat relative to that measured at ≥0.60 ATA O2 (∼84–200 Torr). The flat PtO2 profile is likely due in part to the smaller O2 diffusion gradient from the aCSF to the slice with the use of anoxic gas mixture vs. intermediate O2 gas mixture. This effect, however, would be offset in part by the larger O2 diffusion gradient from the ambient air (0.21 ATA O2) into the aCSF (43).
Why PtO2 = 20–40 Torr O2 (at PmO2 = 0% O2) is insufficient to sustain metabolic and electrical activity in the brain slice is unclear. It suggests, however, that endogenous factors other than absolute level of molecular O2 are important in maintaining normal function in the isolated brain slice. For example, endogenous levels of antioxidants, such as ascorbate (10), are depleted by superfusion of brain slices with aCSF (71). Also, as already mentioned, it is unknown how continuous exposure to 95% O2 during the first several hours of the experiment modifies responsiveness of the slice preparation to acute O2 manipulation over the duration of a typical experiment (35).
Excitability After O2 Manipulation: Neural Plasticity
Hyperoxia (HBO and NBOreox) also activated persistent stimulation of the oPS in 0.95 ATA O2 for at least 46 min. We first identified OxIP following exposure to HBO. OxIP was evoked in the majority of the slices tested following exposure to 2.84 ATA O2, and it was evoked in all of the slices following exposure to 4.54 ATA O2. This finding suggests that a threshold dose of hyperbaric hyperoxia (i.e., O2 concentration × duration of exposure to hyperoxia) may be required to consistently induce this form of excitatory neural plasticity. However, OxIP was not unique to HBO exposure, since it also was activated by a single bout of NBOreox from hypoxia or an intermediate level of oxygenation. The surprising finding was that despite the variability in O2 sensitivity during O2 manipulation (Fig. 9A), the magnitude of OxIP measured following O2 manipulation was the same regardless of the initial O2 level from which the hyperoxic stimulus was initiated (Fig. 9B). These findings suggest that the mechanism(s) activated by HBO and NBOreox to induce OxIP are possibly the same, although this hypothesis requires further testing.
Previous studies in acutely harvested hippocampal slices have reported, based on measurements of the field excitatory postsynaptic potential, that neural plasticity is activated by reoxygenation following hypoxia (73), which was termed “anoxic long-term potentiation,” or “anoxic LTP” (17, 18). We have confirmed this form of O2-induced plasticity and have expanded this concept, however, by showing that it is really a form of oxygen-hyperoxia-induced potentiation rather than a form of anoxic LTP; that is, OxIP is activated by a single upswing in PtO2 that is initiated over a broad range of normobaric hyperoxia and hyperbaric hyperoxia. The critical stimulus is not hypoxia or anoxia, but rather reoxygenation or, if initiated from 0.95 ATA, acute HBO exposure. However, we recognize that OxIP caused by reoxygenation from hypoxia (0.00 to 0.95 ATA O2) may involve additional cellular changes uniquely related to a (relative) hypoxic insult (17, 18). Thus OxIP activated by NBOreox following hypoxia potentially differs in the underlying mechanisms from the other forms of OxIP that are activated by NBOreox from an intermediate level of O2 and HBO. For example, such differences unique to relative hypoxia may account for the significantly longer delay before activation of OxIP following NBOreox (Figs. 8C and 9B, trace 5). It is interesting to note that in organotypic hippocampal slices prepared from immature rats, NBOreox did not induce OxIP (42, 44) and, in fact, the oPS was inhibited more in 0.95 than in 0.19 ATA O2. This suggests a fundamental difference in O2 sensitivity of the organotypic hippocampal slice preparation from the acute hippocampal slice preparation studies (42, 44).
As discussed earlier, elevated [K+]e enhances excitability in CA1 neurons [vs. 3 mM K+ (48, 49)], but we do not believe that elevated excitability mediated by high K+ in the aCSF underlies OxIP. This is supported by the fact that anoxic LTP (long-term potentiation), that is, OxIP activated by NBOreox, occurs in hippocampal slices maintained in [K+]e 3.5 mM (17, 18). Thus O2-induced plasticity occurs in low-K+ (17, 18) and relatively higher K+ media (present study). Moreover, the present study used hippocampal slices that 1) did not exhibit hyperexcitability during the initial control period and 2) exhibited stable activity during the initial control run for a minimum of 48 min (Fig. 2B). This approach indicated that OxIP was only observed following NBOreox and acute exposure to HBO.
Implications for General Neurophysiology, Disease, and Future Studies
Because CA1 neurons exhibit 1) several forms of neural plasticity (58, 70) and 2) a high degree of O2 sensitivity, it is not surprising to discover that O2 manipulation involving an increase in O2, such as HBO or NBOreox, activates neural plasticity in this region of the CNS. Thus OxIP may be a potentially important mechanism for modulating CA1 hippocampal output, especially during conditions that perturb tissue oxygenation, such as stroke, ischemia, and reperfusion injury or disordered breathing patterns (e.g., sleep apnea), which produce a recurring pattern of hypoxia and reoxygenation (i.e., relative hyperoxia). Is there an in vivo correlate to OxIP in the CA1 hippocampus? Perhaps, but the evidence conflicts between studies. Electrophysiological recordings in the CA1 region of anesthetized animals indicate that transient cerebral ischemia lasting 5–15 min activates hyperactivity for up to 48 h within minutes to hours of reperfusion by increasing synaptic potentiation and spontaneous firing rate (15, 39, 79, 88). In contrast, other studies report that neuronal activity and synaptic transmission are depressed following transient ischemia (13, 38, 46).
It remains to be determined what role, if any, O2-induced plasticity may have in the pathogenesis of CNS O2 toxicity. Typically, O2 toxicity seizures in rats occur after they breathe 3 ATA O2 for 3–5 h or 6 ATA O2 for 9–20 min (74).2 Unfortunately, few studies have documented the details of the off-response of CNS O2 toxicity (seizure) in unanesthetized animals. Typically, a study of CNS O2 toxicity in rats is designed to induce seizure as the end point without carefully describing the temporal characteristics of the off-response once inspired Po2 is lowered. In other words, the primary interest to date has been in conditions that delay onset of seizure or neurological processes activated by seizure. Thus the temporal characteristics of recovery from O2-induced seizure are largely unstudied. If hyperoxic breathing is terminated when the first electrical discharge is noted and decompression is commenced immediately, O2-induced seizures stop within 1–14 min (3). The postictal period, however, can last for many minutes, especially if the rat has been seizing for a while (Piantadosi CA, personal communication). Likewise, in a subset of animals, seizures can continue during the decompression period and also once reaching normobaric pressure (Demchenko IP, personal communication). Thus, at this time, there is no published evidence of a consistently occurring, persistent neural equivalent to OxIP following HBO in the unanesthetized rat. Regardless, the discovery of OxIP emphasizes the importance of further identifying the electrophysiological and biochemical changes that occur in real time in hyperoxic environments, such as during brain slice incubation in 0.95 ATA O2, to have a thorough understanding of neuronal O2 sensing in this popular in vitro tissue model of mammalian CNS function.
GRANTS
This work was supported by Office of Naval Research Undersea Medicine Program Grants DoD/ONR DURIP N000140210643, ONR N000140410172, and ONR N000140710890 and National Heart, Lung, and Blood Institute Grant R01 HL 56683-09.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We acknowledge the advice and assistance of Dr. L. Hartzler, and the technical assistance of P. Douglas was greatly appreciated. We acknowledge personal communications from C. A. Piantadosi and I. P. Demchenko, Duke Center for Hyperbaric Medicine and Environmental Physiology, Durham, NC.
All experiments were conducted at Wright State University before J. B. Dean's departure for the University of South Florida and A. J. Garcia's departure for postdoctoral training. The manuscript was prepared thereafter.
Footnotes
Uncorrected for water vapor pressure, expressed as Pb = 1 ATA multiplied by the fractional concentration of oxygen.
As mentioned above, the range of hyperoxia used in 400-μm-thick hippocampal slices in this study (in vitro: 0.95 to 4.54 ATA O2) is equivalent to the range of PtO2 in intact rat breathing >2 to 8 ATA HBO (in vivo).
REFERENCES
- 1. Aitken PG, Breese GR, Dudek FF, Edwards F, Espanol MT, Larkman PM, Lipton P, Newman GC, Nowak TSJ, Panizzon KL. Preparative methods for brain slices: a discussion. J Neurosci Methods 59: 139–149, 1995 [DOI] [PubMed] [Google Scholar]
- 2. Alger BE, Dhanjal SS, Dingledine R, Garthwaite J, Henderson G, King GL, Lipton P, North A, Schwartzkronin PA, Sears TA, Segal M, Whittingham TS, Williams J. Appendix, brain slice methods. In: Brain Slices, edited by Dingledine R. New York: Plenum, 1984, p. 381–437 [Google Scholar]
- 3. Arieli R, Gutterman A. Recovery time constant in central nervous system O2 toxicity in the rat. Eur J Appl Physiol 75: 182–187, 1997 [DOI] [PubMed] [Google Scholar]
- 4. Atkins CM, Sweatt JD. Reactive oxygen species mediate activity-dependent neuron-glia signaling in output fibers of the hippocampus. J Neurosci 19: 7241–7248, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Avshalumov MV, Rice ME. Activation of ATP-sensitive K+ (KATP) channels by H2O2 underlies glutamate-dependent inhibition of striatal dopamine release. Proc Natl Acad Sci USA 100: 11729–11734, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Avshalumov MV, Rice ME. NMDA receptor activation mediates hydrogen peroxide-induced pathophysiology in rat hippocampal slices. J Neurophysiol 87: 2896–2903, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Balentine JD. Pathology of Oxygen Toxicity. New York: Academic, 1982 [Google Scholar]
- 8. Bennett P, Papahadjopoulos D, Bangham A. The effect of raised pressure of inert gases on phospholipid membranes. Life Sci 6: 2527–2533, 1967 [DOI] [PubMed] [Google Scholar]
- 9. Bingmann D, Kolde G. Po2 profiles in hippocampal slices of the guinea pig. Exp Brain Res 48: 89–96, 1982 [DOI] [PubMed] [Google Scholar]
- 10. Brahma B, Forman RE, Stewart E, Rice ME. Ascorbate inhibits edema in brain slices. J Neurochem 74: 1263–1270, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Brauer RW, Hogan PM, Hugon M, Macdonald AG, Miller KW. Patterns of interaction of effects of light metabolically inert gases with those of hydrostatic pressure as such-a review. Undersea Biomed Res 9: 353–396, 1982 [PubMed] [Google Scholar]
- 12. Butterfield DA, Koppal T, Howard B, Subramaniam R, Hall N, Hensley K, Yatin S, Allen K, Aksenov M, Aksenova M, Carney J. Structural and functional changes in proteins induced by free radical-mediated oxidative stress and protective action of the antioxidants N-tert-butyl-alpha-phenylnitrone and vitamin E. Ann NY Acad Sci 854: 448–462, 1998 [DOI] [PubMed] [Google Scholar]
- 13. Buzsaki G, Freund TF, Bayardo F, Somogyi P. Ischemia-induced changes in the electrical activity of the hippocampus. Exp Brain Res 78: 268–278, 1989 [DOI] [PubMed] [Google Scholar]
- 14. Cater D, Garattini S, Marina F, Silver I. Changes of oxygen tension in brain and somatic tissues induced by vasodilator and vasoconstrictor drugs. Proc R Soc Lond B Biol Sci 155: 136–157, 1961 [Google Scholar]
- 15. Chang HS, Sasaki T, Kassell NF. Hippocampal unit activity after transient cerebral ischemia in rats. Stroke 20: 1051–1058, 1989 [DOI] [PubMed] [Google Scholar]
- 16. Cragg PA, Drysdale DB, Hamilton JH. Ventilation in intact and glossopharyngeal nerve sectioned anaesthetized rats exposed to oxygen at high pressure. J Physiol 370: 489–499, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Crepel V, Hammond C, Chinestra P, Diabira D, Ben-Ari Y. A selective LTP of NMDA receptor-mediated currents induced by anoxia in CA1 hippocampal neurons. J Neurophysiol 70: 2045–2055, 1993 [DOI] [PubMed] [Google Scholar]
- 18. Crepel V, Hammond C, Krnjevic K, Chinestra P, Ben-Ari Y. Anoxia-induced LTP of isolated NMDA receptor-mediated synaptic responses. J Neurophysiol 69: 1774–1778, 1993 [DOI] [PubMed] [Google Scholar]
- 19. D'Agostino DP, Colomb DG, Dean JB. Effects of hyperbaric gases on membrane nanostructure and function in neurons. J Appl Physiol 106: 996–1003, 2009 [DOI] [PubMed] [Google Scholar]
- 20. D'Agostino DP, Olson JE, Dean JB. Acute hyperoxia increases lipid peroxidation and induces plasma membrane blebbing in human U87 glioblastoma cells. Neuroscience 159: 1011–1022, 2009 [DOI] [PubMed] [Google Scholar]
- 21. D'Agostino DP, Putnam RW, Dean JB. Superoxide (•O2−) production in CA1 neurons of rat hippocampal slices exposed to graded levels of oxygen. J Neurophysiol 98: 1030–1041, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Dean JB, D'Agostino DP. Pressure effects on human physiology. In: Physiology and Medicine of Hyperbaric Oxygen Therapy, edited by Newman T, Thom S. Philadelphia, PA: Elsevier, 2008, p. 189–202 [Google Scholar]
- 23. Dean JB, D'Agostino DP, Landon C, Matott M, Hartzler LK, Putnam RW. Superoxide production increases in nucleus tractus solitarius (NTS) neurons in rat brain slices during acute normobaric hyperoxia and hypoxia. Soc Neurosci Abstr. Program No. 481.20, 2008 [Google Scholar]
- 24. Dean JB, Mulkey DK. Continuous intracellular recording from mammalian neurons exposed to hyperbaric helium, oxygen, or air. J Appl Physiol 89: 807–822, 2000 [DOI] [PubMed] [Google Scholar]
- 25. Dean JB, Mulkey DK, Garcia AJ, III, Putnam RW, Henderson RA., III Neuronal sensitivity to hyperoxia, hypercapnia and inert gases at hyperbaric pressures. J Appl Physiol 95: 883–909, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Dean JB, Mulkey DK, Arehart JD. Details on building a hyperbaric chamber for intracellular recording in brain tissue slices. J Appl Physiol 89: 807–822, 2000. [Supplemental Material for Ref. 24] [DOI] [PubMed] [Google Scholar]
- 27. Demchenko IT, Boso AE, Whorton AR, Piantadosi CA. Nitric oxide production is enhanced in rat brain before oxygen-induced convulsions. Brain Res 917: 253–261, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Dingledine R, Dodd J, Kelly JS. The in vitro brain slice as a useful neurophysiological preparation for intracellular recording. J Neurosci Methods 2: 323–362, 1980 [DOI] [PubMed] [Google Scholar]
- 29. DiScenna P. Method and myth in maintaining brain slices. Brain Slices: Fundamentals, Applications, and Implications, edited by Schurr A. Louisville, KY: Karger, 1987, p. 10–21 [Google Scholar]
- 30. Fagni L, Soumireu-Mourat B, Carlier E, Hugon M. A study of spontaneous and evoked activity in the rat hippocampus under helium-oxygen high pressure. Electroencephalogr Clin Neurophysiol 60: 267–275, 1985 [DOI] [PubMed] [Google Scholar]
- 31. Fagni L, Zinebi F, Hugon M. Helium pressure potentiates the N-methyl-d-aspartate- and d,l-homocysteate-induced decreases of field potentials in the rat hippocampal slice preparation. Neurosci Lett 81: 285–290, 1987 [DOI] [PubMed] [Google Scholar]
- 32. Fessel J, Arbeson WC, Winder D, Roberts LJ. Standard brain slice protocols result in oxidative injury in hippocampal brain slices (Abstract). Free Radic Biol Med 33: S433, 2002 [Google Scholar]
- 33. Fowler JC. Changes in extracellular adenosine levels and population spike amplitude during graded hypoxia in the rat hippocampal slice. Naunyn Schmiedebergs Arch Pharmacol 347: 73–78, 1993 [DOI] [PubMed] [Google Scholar]
- 34. Fowler JC, Gervitz LM, Hamilton ME, Walker JA. Systemic hypoxia and the depression of synaptic transmission in rat hippocampus after carotid artery occlusion. J Physiol 550: 961–972, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Freiberger JJ, Suliman HB, Sheng H, McAdoo J, Piantadosi CA, Warner DS. A comparison of hyperbaric oxygen versus hypoxic cerebral preconditioning in neonatal rats. Brain Res 1075: 213–222, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Friedman JE, Haddad GG. Anoxia induces an increase in intracellular sodium in rat central neurons in vitro. Brain Res 663: 329–334, 1994 [DOI] [PubMed] [Google Scholar]
- 37. Fung ML, Haddad GG. Anoxia-induced depolarization in CA1 hippocampal neurons: role of Na+-dependent mechanisms. Brain Res 762: 97–102, 1997 [DOI] [PubMed] [Google Scholar]
- 38. Furukawa K, Yamana K, Kogure K. Postischemic alterations of spontaneous activities in rat hippocampal CA1 neurons. Brain Res 530: 257–260, 1990 [DOI] [PubMed] [Google Scholar]
- 39. Gao TM, Xu ZC. In vivo intracellular demonstration of an ischemia-induced postsynaptic potential from CA1 pyramidal neurons in rat hippocampus. Neuroscience 75: 665–669, 1996 [DOI] [PubMed] [Google Scholar]
- 40. Garcia AJ, III, Putnam RW, Dean JB. Hyperoxic stimulation of synchronous orthodromic activity and induction of neural plasticity does not require changes in excitatory synaptic transmission. J Appl Physiol (June 17, 2010). doi:10.1152/japplphysiol.91430.2008 [DOI] [PMC free article] [PubMed]
- 41. Gennser M, Blogg SL. Oxygen or carbogen breathing before simulated submarine escape. J Appl Physiol 104: 50–56, 2008 [DOI] [PubMed] [Google Scholar]
- 42. Graulick J, Hoffmann U, Maier RF, Ruscher K, Pomper JK, Ko HK, Gabriel S, Obladen M, Heinemann U. Acute neuronal injury after hypoxia is influenced by the reoxygenation mode in juvenile hippocampal slice cultures. Dev Brain Res 137: 35–42, 2002 [DOI] [PubMed] [Google Scholar]
- 43. Hajos N, Ellender TJ, Zemankovics R, Mann EO, Exley R, Cragg SJ, Freund TF, Paulsen O. Maintaining network activity in submerged hippocampal slices: importance of oxygen supply. Eur J Neurosci 29: 319–327, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hoffmann U, Pomper J, Graulich J, Zeller M, Schuchmann S, Gabriel S, Maier RF, Heinemann U. Changes of neuronal activity in areas CA1 and CA3 during anoxia and normoxic or hyperoxic reoxygenation in juvenile rat organotypic hippocampal slice cultures. Brain Res 1069: 207–215, 2006 [DOI] [PubMed] [Google Scholar]
- 45. Hunt R, Blackburn J, Ogilvie R, Balentine D. Oxygen tension in the globus pallidus and neostriatum of unanesthetized rats during exposure to hyperbaric oxygen. Exp Neurol 62: 698–707, 1978 [DOI] [PubMed] [Google Scholar]
- 46. Imon H, Mitani A, Andou Y, Arai T, Kataoka K. Delayed neuronal death is induced without postischemic hyperexcitability: continuous multiple-unit recording from ischemic CA1 neurons. J Cereb Blood Flow Metab 11: 819–823, 1991 [DOI] [PubMed] [Google Scholar]
- 47. Jamieson D, Van den Brenk A. Measurement of oxygen tensions in cerebral tissues of rats exposed to high pressures of oxygen. J Appl Physiol 18: 869–876, 1963 [DOI] [PubMed] [Google Scholar]
- 48. Jensen MS, Azouz R, Yaari Y. Variant firing patterns in rat hippocampal pyramidal cells modulated by extracellular potassium. J Neurophysiol 71: 831–839, 1994 [DOI] [PubMed] [Google Scholar]
- 49. Jensen MS, Yaari Y. Role of intrinsic burst firing, potassium accumulation, and electrical coupling in the elevated minpotassium model of hippocampal epilepsy. J Neurophysiol 77: 1224–1233, 1997 [DOI] [PubMed] [Google Scholar]
- 50. King GL, Parmentier JL. Oxygen toxicity of hippocampal tissue in vitro. Brain Res 260: 139–142, 1983 [DOI] [PubMed] [Google Scholar]
- 51. Klann E, Roberson E, Knapp L, Sweatt J. A role for superoxide in protein kinase C activation and induction of long-term potentiation. J Biol Chem 273: 4516–4522, 1998 [DOI] [PubMed] [Google Scholar]
- 52. Krnjevic K. Electrophysiology of cerebral ischemia. Neuropharmacology 55: 319–333, 2008 [DOI] [PubMed] [Google Scholar]
- 53. Krnjevic K, Morris ME, Reiffenstein RJ. Changes in extracellular Ca2+ and K+ activity accompanying hippocampal discharges. Can J Physiol Pharmacol 58: 579–582, 1980 [DOI] [PubMed] [Google Scholar]
- 54. Krnjevic K, Xu Y. Mechanisms underlying anoxic hyperpolarization of hippocampal neurons. Can J Physiol Pharmacol 68: 1609–1613, 1990 [DOI] [PubMed] [Google Scholar]
- 55. Le Fevre ME. Calibration of Clark oxygen electrode for use in aqueous solutions. J Appl Physiol 26: 844–846, 1969 [DOI] [PubMed] [Google Scholar]
- 56. Lei SZ, Pan ZH, Aggarwal SK, Chen HS, Hartman J, Sucher NJ, Lipton SA. Effect of nitric oxide production on the redox modulatory site of the NMDA receptor-channel complex. Neuron 8: 1087–1099, 1992 [DOI] [PubMed] [Google Scholar]
- 57. Lipton P. Ischemic cell death in brain neurons. Physiol Rev 79: 1431–1568, 1999 [DOI] [PubMed] [Google Scholar]
- 58. Lynch MA. Long-term potentiation and memory. Physiol Rev 84: 87–136, 2004 [DOI] [PubMed] [Google Scholar]
- 59. MacDonald AG. Hydrostatic pressure as an environmental factor in life processes. Comp Biochem Physiol 116A: 291–297, 1997 [Google Scholar]
- 60. MacDonald AG, Fraser PJ. The transduction of very small hydrostatic pressures. Comp Biochem Physiol 122A 13–36, 1999 [DOI] [PubMed] [Google Scholar]
- 61. Mahon RT, Dainer HM, Gibellato MG, SES Short oxygen pre-breathe periods reduce or prevent severe decompression sickness in a 70kg swine saturation model. J Appl Physiol 106: 1459–1463, 2009 [DOI] [PubMed] [Google Scholar]
- 62. Mor A, Grossman Y. High pressure modulation of NMDA receptor dependent excitability. Eur J Neurosci 25: 2045–2052, 2007 [DOI] [PubMed] [Google Scholar]
- 63. Mor A, Grossman Y. Modulation of isolated N-methyl-d-aspartate receptor response under hyperbaric conditions. Eur J Neurosci 24: 3453–3462, 2006 [DOI] [PubMed] [Google Scholar]
- 64. Mulkey DK, Henderson RA, Olson JE, Putnam RW, Dean JB. Oxygen measurements in brain stem slices exposed to normobaric hyperoxia and hyperbaric oxygen. J Appl Physiol 90: 1887–1899, 2001 [DOI] [PubMed] [Google Scholar]
- 65. Mulkey DK, Henderson RA, Putnam RW, Dean JB. Hyperbaric oxygen and chemical oxidants stimulate CO2/H+-sensitive neurons in rat brain stem slices. J Appl Physiol 95: 910–921, 2003 [DOI] [PubMed] [Google Scholar]
- 66. Mulkey DK, Henderson RA, Putnam RW, JBD Pressure (<4 ATA) increases membrane conductance and firing rate in the rat solitary complex. J Appl Physiol 95: 922–930, 2003 [DOI] [PubMed] [Google Scholar]
- 67. Noda Y, McGeer PL, McGeer EG. Lipid peroxide distribution in brain and the effect of hyperbaric oxygen. J Neurochem 4: 1329–1332, 1983 [DOI] [PubMed] [Google Scholar]
- 68. Offenhauser N, Thomsen K, Caesar K, Lauritzen M. Activity-induced tissue oxygenation changes in rat cerebellar cortex: interplay of postsynaptic activation and blood flow. J Physiol 565: 279–294, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. O'Hara JA, Khan N, Hou H, Wilmo CM, Demidenko E, Dunn JF, Swartz HM. Comparison of EPR oximetry and Eppendorf polarographic electrode assessments of rat brain PtO2. Physiol Meas 25: 1413–1423, 2004 [DOI] [PubMed] [Google Scholar]
- 70. Pittenger C, Kandel ER. In search of general mechanisms for long-lasting plasticity: aplysia and the hippocampus. Philos Trans R Soc Lond B Biol Sci 358: 757–763, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Rice ME. Use of ascorbate in the preparation and maintenance of brain slices. Methods 18: 144–149, 1999 [DOI] [PubMed] [Google Scholar]
- 72. Rutecki PA, Lebeda FJ, DJ Epileptiform activity induced by changes in extracellular potassium in hippocampus. J Neurophysiol 54: 1363–1374, 1985 [DOI] [PubMed] [Google Scholar]
- 73. Schiff SJ, Somjen GG. Hyperexcitability following moderate hypoxia in hippocampal tissue slices. Brain Res 337: 337–340, 1985 [DOI] [PubMed] [Google Scholar]
- 74. Sick TJ, Solow EL, Roberts ELJ. Extracellular potassium ion activity and electrophysiology in the hippocampal slice: paradoxical recovery of synaptic transmission during anoxia. Brain Res 418: 227–234, 1987 [DOI] [PubMed] [Google Scholar]
- 75. Simon AJ, Torbati D. Effects of hyperbaric oxygen on heart, brain and lung functions in rat. Undersea Biomed Res 9: 263–275, 1982 [PubMed] [Google Scholar]
- 76. Somjen GG, Aitken PG, Czeh G, Jing J, Young JN. Cellular physiology of hypoxia of the mammalian central nervous system. Res Publ Assoc Res Nerv Ment Dis 71: 51–65, 1993 [PubMed] [Google Scholar]
- 77. Southan AP, Wann KT. Effects of high helium pressure on intracellular and field potential responses in the CA1 region of the in vitro rat hippocampus. Eur J Neurosci 8: 2571–2581, 1996 [DOI] [PubMed] [Google Scholar]
- 78. Southan AP, Wann KT. Methods for intracellular recording from hippocampal brain slices under high helium pressure. J Appl Physiol 71: 365–371, 1991 [DOI] [PubMed] [Google Scholar]
- 79. Suzuki R, Yamaguchi T, Li CL, Klatzo I. The effects of 5-minute ischemia in Mongolian gerbils. II. Changes of spontaneous neuronal activity in cerebral cortex and CA1 sector of hippocampus. Acta Neuropathol 60: 217–222, 1983 [DOI] [PubMed] [Google Scholar]
- 80. Swartz HM, Dunn JF. Measurements of oxygen in tissues: overview and perspectives on methods. Adv Exp Med Biol 530: 1–12, 2003 [DOI] [PubMed] [Google Scholar]
- 81. Taylor MD, Millert TK, Parmentier JL, Lynne EJ. Pharmacological protection of reoxygenation damage to in vitro brain slice tissue. Brain Res 347: 286–273, 1985 [DOI] [PubMed] [Google Scholar]
- 82. Tibbles PM, Edelsberg JS. Hyperbaric-oxygen therapy. N Engl J Med 334: 1642–1648, 1996 [DOI] [PubMed] [Google Scholar]
- 83. Torbati D, Mokashi A, Lahiri S. Effects of acute hyperbaric oxygenation on respiratory control in cats. J Appl Physiol 67: 2351–2356, 1989 [DOI] [PubMed] [Google Scholar]
- 84. Tryba AK, Peña F, Lieske SP, Viemari JC, Thoby-Brisson M, Ramirez JM. Differential modulation of neural network and pacemaker activity underlying eupnea and sigh-breathing activities. J Neurophysiol 99: 2114–2125, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Vanderkooi JM, Ereccinska M, Silver IA. Oxygen in mammalian tissue: methods of measurement and affinities of various reactions. Am J Physiol Cell Physiol 260: C1130–C1150, 1991 [DOI] [PubMed] [Google Scholar]
- 86. Wann KT, Southan AP. The action of anaesthetics and high pressure on neuronal discharge patterns. Gen Pharmacol 23: 993–1004, 1992 [DOI] [PubMed] [Google Scholar]
- 87. Watson PL, Weiner JL, Carlen PL. Effects of variations in hippocampal slice preparation protocol on the electrophysiological stability, epileptogenicity and graded hypoxia responses of CA1 neurons. Brain Res 775: 134–143, 1997 [DOI] [PubMed] [Google Scholar]
- 88. Xu ZC, Gao TM, Ren Y. Neurophysiological changes associated with selective neuronal damage in hippocampus following transient forebrain ischemia. Biol Signals Recept 8: 294–308, 1999 [DOI] [PubMed] [Google Scholar]