Abstract
Measurement of body composition changes following bariatric surgery is complicated because of the difficulty of measuring body fat in highly obese individuals that have increased photon absorption and are too large for the standard dual-energy X-ray absorptiometry (DXA) table. We reproducibly measured body composition from half-body DXA scans and compared the values of total body fat estimated from total body water (TBW) and DXA measurements before and after Roux-en-Y gastric bypass surgery (RYGB). DXA, TBW (deuterium dilution), extracellular water (ECW; bromide dilution), and intracellular water (ICW) measurement (by subtraction) were made before surgery and at 2 wk, 6 wk, 6 mo, and 12 mo after surgery. Twenty individuals completed baseline and at least four follow-up visits. DXA appeared to underestimate the fat and bone mass in extreme obesity (before surgery), whereas at 6 and 12 mo after surgery, the DXA and TBW fat measurements were similar. The ECW-to-ICW ratio was increased in obese individuals and increased slightly more after surgery. We describe a new model that explains this abnormal water composition in terms of the normal physiological changes that occur in body composition in obesity and weight loss. This model is also used to predict the muscle mass loss following RYGB.
Keywords: bariatric, muscle mass, extracellular water, intracellular water, deuterium dilution, bromide dilution, fat mass, body composition
bariatric surgery not only results in dramatic weight loss but also relieves most of the comorbidities associated with obesity. Numerous studies have evaluated the changes in body composition following bariatric surgery (summarized in Tables 1 and 2) to determine the degree to which the body fat, muscle, and water distribution approach that of normal-weight individuals. The major limiting factor in these measurements is the difficulty of measuring body fat in the extremely obese. The three-compartment (3C) approach [from body density and total body water (TBW)] is usually considered the gold standard; however, because of technical difficulties, only 1 of the 21 total studies listed in Table 1 used it. Other methods for measuring body fat listed in Table 1 include TBW, body density, bioelectrical impedance analysis (BIA), and dual-energy X-ray absorptiometry (DXA).
Table 1.
Summary of measurements of weight and FM loss following bariatric surgery
| Weight, kg |
BMI, kg/m2 |
FM, kg |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | n | Surgery | Duration, mo | Initial | Final | %Loss | Initial | Final | Method | Initial | Final | ΔFM/ΔWt |
| Present study | 20 | RYGB | 12 | 132.4 | 87.8 | 34 | 47.7 | 31.69 | TBW | 71.27 | 33.34 | 0.85 |
| Present study | 20 | RYGB | 12 | 132.4 | 87.8 | 34 | 47.7 | 31.69 | DXA | 66.14 | 33.52 | 0.72 |
| 5 | 38 | RYGB | 6 | 114.9 | 82 | 29 | 44.0 | 31.6 | TBW | 60.0 | 34.0 | 0.79 |
| 8 | 20 | GB | 24 | 124.3 | 79.6 | 36 | 47.8 | 30.5 | 3C | 64.4 | 28.7 | 0.8 |
| 4 | 19 | RYGB | 12 | 140.8 | 89.9 | 36 | 48.7 | 30.8 | Density | 67.0 | 28.7 | 0.75 |
| 6 | 42 | RYGB | 12 | 113.9 | 74.5 | 35 | 45 | 29.5 | DXA | 54.9 | 26.3 | 0.73 |
| 7 | 149 | GB | 12 | 120.2 | 96.5 | 20 | 45.5 | 36.4 | DXA | 62.6 | 41.4 | 0.90 |
| 10 | 53 | GB | 24 | 95.2 | 74.9 | 21 | 33 | NR | DXA | 43.3 | 27.4 | 0.81 |
| 37 | 6 | GB | 6 | 107.1 | 90.0 | 16 | 42.8 | 36.6 | DXA | 50.3 | 36.2 | 0.83 |
| 40 | 17 | GB | 32 | 107.9 | 84.4 | 22 | 40.4 | 31.8 | DXA | 55.4 | 38.7 | 0.71 |
| 18 | 36 | GB | 24 | 117.0 | 77.4 | 34 | 43.8 | 32.6 | DXA | 63.5 | 30.8 | 0.83 |
| 19 | 31 | GB | 12 | 118.6 | NR | NR | 43.6 | NR | DXA | 64.7 | 46.7 | |
| 24 | 5 | RYGB | 12 | 134.3 | 85.8 | 36 | 49.8 | 32.1 | DXA | 68.5 | 32.4 | 0.74 |
| 31 | 36 | RYGB | 12 | 123.2 | NR | NR | 42.3 | NR | DXA | 54.1 | 26.9 | |
| 31 | 39 | VGB | 12 | 123.3 | NR | NR | 42.6 | NR | DXA | 56.0 | 36.0 | |
| 46 | 9 | GB | 24 | 117 | NR | 16 | 41 | 34 | DXA | 63.7 | NA | |
| 46 | 4 | RYGB | 24 | 113.3 | NR | 27 | 42.7 | 30.5 | DXA | 63.6 | NA | |
| 47 | 15 | GB | 12 | 130 | 93 | 28 | 45 | 32 | K+ | 65.0 | 36.2 | 79 |
| 47 | 15 | VBG | until 18% loss | 125 | 102 | 18 | NA | NA | K+ | 66.1 | 50.5 | 0.67 |
| 32 | 29 F | RYGB | 12 | 111.1 | 72.5 | 34.6 | 42.3 | 27.6 | BIA | 60.0 | 24.0 | |
| 32 | 21 M | RYGB | 12 | 148.9 | 93.8 | 37 | 47.4 | 29.6 | BIA | |||
| 15 | 26 | VGB | 18 | 143.5 | 95.5 | 33 | 52.5 | 35.7 | BIA | 67.5 | 34.7 | 0.68 |
| 17 | 73 | GB | 13.3 | 117.3 | 91.1 | 22 | 44.3 | 34.4 | BIA | |||
| 9 | 20 | RYGB | until 10% loss | 138 | 124 | 10 | 50.5 | 45.3 | BIA | |||
| 20 | 20 | GB | 24 | 120.2 | 86.1 | 28 | 46.1 | 32.6 | BIA | 61.3 | 30.7 | 0.90 |
BIA, bioelectrical impedance analysis; BMI, body mass index; 3C, 3 compartment; F, female; M, male; FM, fat mass; DXA: dual-energy X-ray absorptiometry; K+, total body potassium; VBG, vertical banded gastroplasty; RYGB, Roux-en-Y gastric bypass; GB, gastric band; NA, not available.
Table 2.
Summary of TBW and ECW measurements following bariatric surgery
| Weight, kg |
TBW, liters |
ECW, liters |
ECW/ICW |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | n | Surgery | Initial | Final | Initial BMI, kg/m2 | Method | Initial | Final | Method | Initial | Final | Initial | Final |
| Present study | 20 | RYGB | 132.4 | 87.7 | 46.7 | 2H2O | 46.38 | 40.64 | Br− | 20.81 | 20.89 | 0.82 | 1.085 |
| 5 | 38 | RYGB | 114.9 | 82.0 | 44.0 | 2H2O | 41.9 | NA | NA | ||||
| 8 | 20 | RYGB | 124.3 | 79.6 | 47.8 | H218O | 45.5 | 38.2 | Br− | 26.0 | 21.8 | 1.48 | 1.44 |
| 29 | 12 | VBG | 127.0 | 79.9 | 48 | 3H2O | 44.3 | 37.5 | 35SO4 | 19.7 | 16.9 | 0.81 | 0.83 |
| 29 | 13 | RYGB | 127 | 74.6 | 48.5 | 3H2O | 45.1 | 37.7 | 35SO4 | 20.2 | 19.5 | 0.82 | 1.09 |
| 37 | 6 | GB | 107.1 | 90.1 | 42.8 | 2H2O | 44.0 | 41.7 | Br− | 19.2 | 19.7 | 0.80 | 0.90 |
| 20 | 20 | GB | 120.2 | 86.7 | 46.1 | BIA | 43.2 | 40.5 | NA | ||||
| 32 | 29 F | RYGB | 111.1 | 72.5 | 42.3 | BIA | 41.43 | 36.61 | NA | ||||
| 32 | 21 M | RYGB | 148.9 | 93.8 | 47.4 | BIA | 58.2 | 51.89 | NA | ||||
TBW, total body water; ECW, extracellular water; ICW, intracellular water.
Although DXA is now a well-accepted method for measuring body fat, there are problems with the use of standard DXA instruments in extremely obese individuals. Instrument size, weight limitations, and the high degree of photon absorption with increasing trunk thickness may produce errors in the DXA measurements (8, 14, 37, 40). One purpose of this study was to investigate the accuracy of the DXA measurement by comparing it with the TBW measurements of body fat. We minimized the problem of the size and weight limitations of the standard DXA table by making measurements on only one-half of the body.
A second purpose of this study was to investigate the changes in intracellular (ICW) and extracellular water (ECW) that occur following bariatric surgery. It is well recognized that the ratio ECW/ICW in obese individuals is significantly greater than the normal-weight reference value of 0.76 (8). Most interestingly, this high ratio does not decrease following bariatric surgery, and in most of the studies reviewed in Table 2, it actually increases. This is usually explained as some sort of obesity-related chronic alteration in fluid regulation (27, 29, 37, 51). We have reexamined this question with a combination of measurements of DXA, TBW, and ECW and show that the water distribution can be explained in terms of the normal physiological changes in body composition that occur in obese subjects before and after surgery.
An important question about the weight loss following bariatric surgery is the degree of muscle mass loss. Using whole body MRI as well as 40K and [13C]leucine kinetics, Gallagher et al. (16) observed a muscle loss of ∼17% of the total weight loss following a hypocaloric diet (∼1,200 kcal/day) in mildly obese individuals. We are not aware of any quantitative measurements of muscle loss in highly obese individuals following bariatric surgery. In this study we describe a new body composition model that allows us to predict the muscle loss based on the TBW and ECW measurements along with some reasonable assumptions about fat, muscle, and skin body composition.
MATERIALS AND METHODS
Subjects.
Extremely obese women who planned to undergo the laparoscopic Roux-en-Y gastric bypass (RYGB) procedure were recruited from the Weight Loss Surgery Center at the University of Minnesota Medical Center, Fairview. Individuals for this longitudinal analysis were participating in a follow-up study investigating body composition and metabolic changes after RYGB surgery. Partial body composition data from a subset of this study population has been reported elsewhere (26). This study included five visits that were ∼24 h in duration: visit 1, 30–70 days pre-RYGB; visit 2, 2 wk post-RYGB; visit 3, 6 wk post-RYGB; visit 4, 6 mo post-RYGB; and visit 5, 12 mo post-RYGB. Of the 29 subjects who were enrolled in the study, 17 individuals completed all 5 time points; 20 completed visits 1, 3, 4, and 5; 1 dropped out of the study after visit 2; and 8 completed only visit 1. Exclusion criteria included the following: use of corticosteroids, testosterone, or anabolic agents; internally placed biomedical device (e.g., pacemaker); liver, renal, or heart failure; pulmonary hypertension; thyroid disease (included if treated and within normal limits); neoplastic disease; type 1 or uncontrolled type 2 diabetes mellitus (defined by HbA1c >7%); pregnancy; and/or history of previous weight loss surgery. The study protocol was reviewed and approved by the Institutional Review Board and the General Clinical Research Center (GCRC) at the University of Minnesota, and individuals provided written, informed consent before participating in the study.
Participants were instructed the day before admission to the GCRC to avoid caffeine, alcohol, and vigorous exercise for 24 h before testing. On admission to the GCRC, a peripheral intravenous (IV) line was inserted and remained in place for the following 18 h. To promote euhydration by day 2, participants received 1,600 ml of IV hydration at a rate of 200 ml/h during the afternoon of day 1 of their admission. They were provided a dinner meal at the GCRC and were fasted after 8:00 PM with the exception of water.
Height and weight were measured using standardized procedures. Height was measured to the nearest 0.1 cm with the use of a wall-mounted stadiometer (model S100; Ayrton, Prior Lake, MN) at visit 1 only. After the first morning urine collection at all testing visits, weight was measured to the nearest 0.1 kg on a digital scale (model 5002, Scale-Tronix, White Plains, NY).
ECW measurements.
ECW was determined by bromide (Br) dilution. A fasted baseline blood sample was collected at 7:00 AM of day 2. Immediately thereafter, a 3% (wt/vol) sodium bromide solution (NaBr; Sigma Chemical, St. Louis, MO) was infused by IV at a rate of 300 ml/h to provide a dose of 1 ml 3% NaBr solution/kg body wt. The bag and tubing set containing the NaBr were weighed before and after administration to determine the amount that was administered to the subject. Completion of the NaBr infusion marked the start of a 4-h equilibration period during which individuals remained fasted. A final blood sample at 4 h postinfusion was collected. All serum samples were centrifuged, tightly sealed, packed on dry ice, and stored at −80°C until analysis. Serum samples were shipped to Pennington Biomedical Research Center (Baton Rouge, LA) for analysis; Br enrichment was measured by high-performance liquid chromatography (30). The coefficient of variation (CV) for Br measurements with this technique is ∼5% (30).
Two different equations were used to relate the Br dilution space to the ECW (kg). The first is the standard approach (22):
| (1) |
where 0.95 is the Donnan equilibrium correction and 0.9 corrects for the intracellular distribution (including red blood cells). Silva et al. (38) have shown that this Br calculation underestimated the ECW in obese individuals by ∼1 liter relative to estimates based on total body potassium. They suggested that the 0.9 intracellular correction is too large because obese individuals have a fractional ICW space and a corresponding fractional TBW (fTBW) that is reduced relative to the normal-weight individuals for which this correction was derived. As an approximate correction for the dependence of the intracellular distribution of the subject's body composition, we assumed that the experimental value of fTBW is a surrogate measure of body fat and used this to scale the intracellular correction:
| (2) |
where the factor 1 − β is used in place of 0.9 in Eq. 1. For normal-weight subjects with 20% body fat, fTBW = 0.584 [assuming TBW/fat free mass = 0.73 (39)] and 1− β reduces to the normal value of 0.9 (Eq. 1). For the obese subjects in this study (Table 2), fTBW = 0.35 and 1 − β = 0.94, which produces a 4.5% increase (∼1 liter) in the estimate of ECW relative to the prediction of Eq. 1. Both the “standard” (Eq. 1) and the “corrected” (Eq. 2) values for ECW are reported in the results.
TBW measurements.
TBW was determined by deuterium dilution. A fasted baseline urine sample was collected at 7:00 AM of day 2, just before the initiation of the NaBr infusion. Approximately 10 min before completion of the NaBr infusion, a weighed dose of deuterium oxide (99.9 atom%; Isotec, Miamisburg, OH) providing 0.1 g/kg body wt, was IV pushed over a 5-min period. The syringe containing the deuterium oxide was weighed before and after administration to determine the amount that was administered to the subject. Hourly urine samples were collected, as well as a final urine sample at 4 h postinfusion. All urine samples were tightly sealed, packed on dry ice, and stored at −80°C until analysis. Baseline and 3- and 4-h-postdose urine samples were shipped to Dr. Michael Jensen's laboratory at the Mayo Clinic (Rochester, MN) for analysis. Urine (0.5 ml) was added to ∼40 mg of activated charcoal, vortexed, and spun in a refrigerated centrifuge for 10 min. The supernatant was then passed through a 0.22-μm filter by spinning in a microcentrifuge for 10 min at 8,000 rpm. The filtered sample (1.5 μl) was then injected into a chromium furnace held at 825°C (Thermo H/Device). The resulting hydrogen was then passed into the dual inlet of a Thermo DeltaS isotope ratio mass spectrometer (Bremen, Germany) and measured against a deuterium reference gas (Oztech Trading, Safford, AZ). Values (δ‰) were corrected for response by using a five-point calibration curve prepared from secondary reference standards (Iso Analytical, Crewe, UK). Interassay variation of three quality controls measured over a period of 6 mo (n = 28) using a Thermo DeltaS isotope ratio mass spectrometer via Thermo H/Device and dual inlet system was <1% for the two higher enrichments (892 and 1,738 δ‰) and 3.5% for the lower value (62 δ‰). TBW was calculated from the average deuterium enrichment of the 3- and 4-h urine samples (21, 35) and then reduced by 4% to correct for exchange with the nonaqueous compartment (35, 36).
DXA measurements.
DXA scans were performed on the same instrument (GE Lunar Prodigy; GEMedical Systems, Madison, WI) by an experienced technician at the GCRC Body Composition Laboratory. All scans were performed in “thick” mode, using software version 8.8. A modified protocol for conducting half-body scans was developed based on the method described by Tataranni and Ravussin (41). At each testing visit, individuals were positioned on the left side of the DXA table to undergo a complete scan of the right side of the body, including the entirety of the head, spine, right arm, and right leg. At visits 1–3, a gurney was placed directly next to, and at the same height as, the DXA table to support the left side of the subject's body during scans. The gurney was typically not required at visits 4 and 5 because individuals had lost a substantial amount of body weight by these time points. For individuals experiencing back pain while prone, sand bags were placed under the knees and neck as needed. For the scans, individuals wore a hospital gown or lightweight cotton clothing, with all metal, jewelry, accessories, and brassiere removed. DXA scans provided information regarding whole body and regional fat mass (FM), percent fat (%fat), bone-free lean body mass (LBM), bone mineral content (BMC), and bone mineral density. For the analysis of each DXA scan, a sagittal line was positioned along the spine to demarcate the right side of the body, and regional lines were placed to delineate the right-sided data into trunk, arm, and leg regions of interest (ROI) (Fig. 1). Only the right-sided total and regional data were utilized. To avoid intraobserver variation (11, 43), each scan was analyzed by the same researcher.
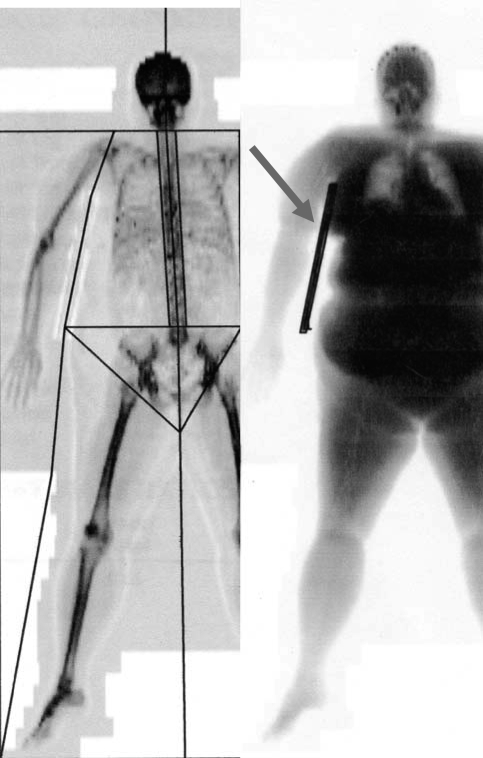
Fig. 1.
Example of dual-energy X-ray absorptiometry (DXA) scan analysis. The image at left illustrates how the half-body scan line was determined and how the trunk, arm, and leg were defined using the region of interest (ROI) feature. The image at right illustrates the density contribution of the acrylic board.
One problem with DXA regional scans in the obese population is that breast tissue can overlap onto the arm during scanning. To address the problem of breast overlap, we applied three techniques for the comparison of regional estimates of body composition. Technique 1 (no support) provided no breast support to the subject during the scan. Technique 2 (taping) involved wrapping and securing the breast tissue with flexible gauze bandage in a crisscross pattern around the upper torso/shoulder blades; this procedure was performed by trained GCRC staff. Technique 3 (acrylic board) required the placement of a transparent, 0.5-in.-thick 14 × 12-in. acrylic board under the armpit of the subject to provide a physical barrier between the arm and the trunk. The subject was repositioned between the scans for each of the three techniques. The three different scanning methodologies were performed at baseline testing (visit 1) for 17 individuals to establish intramethod variation for the separation of ROI. Analysis of the difference between these three methods determined that the acrylic board was superior to that of taping for reducing breast overlay and estimating both regional and total right-sided estimates of FM. In addition, the acrylic board was a noninvasive technique that could be easily applied to this population (44). Consequently, for the duration of the study, the acrylic board method was used.
The DXA density contributed by the acrylic board was estimated by using the following procedure. The board was aligned parallel to the X-ray beam so that the regions of the DXA scan that represented just the board could be identified using the ROI function (Fig. 1). From measurements of the densities in these regions, it was determined that the board density was interpreted by the software as 100% fat. From measurements on seven individuals for whom the board could be clearly delineated (Fig. 1), the total board density was 0.919 ± 0.096 kg. This density was subtracted from the measured total DXA fat density. In addition, it was determined that the fraction of the board that contributed to the arm, trunk, and leg was 0.50, 0.42, and 0.08 respectively, and these fractions of the total board density were subtracted from the fat values for these regions.
To minimize rounding errors, all calculations were performed in grams, and final values for FM and fat-free mass (FFM) were converted to kilograms. The right-side mass values for FM, LBM, and BMC were multiplied by two to derive total body FM and FFM (FFM = BMC + LBM). Although Tataranni and Ravussin (41) observed a small difference between right-and left-side measurements, in a recent study using an iDXA scanner, Rothney et al. (34) did not observe any significant difference for the two sides or for twice the half-body measurement vs. the directly measured total.
FM estimated from TBW measurement.
Two different relations were used for determining FM from TBW measurements. The standard procedure is to assume that the water fraction of FFM (TBW/FFM) is a constant (0.738), independent of body composition (2):
| (3) |
However, using 3C measurements of body fat, Das et al. (8) found that TBW/FFM increased to an average value of 0.756 in highly obese subjects. As an approximate correction for the dependence of TBW/FFM of the subject's body composition, we assumed that TBW/FFM has a linear dependence on fTBW and used the normal and obese values to scale this dependence:
| (4) |
The parameter (TBW/FFM)cor increases from the standard value of 0.738 (Eq. 3) for normal-weight subjects [20% fat, fTBW = 0.584 (39)] to the Das et al. (8) value of 0.756 for the highly obese individuals(fTBW = 0.37). The Das et al. individuals, after weight reduction, had an average fTBW value of 0.48 and an experimental TBW/FFM of 0.747, which is identical to the value of (TBW/FFM)cor predicted by Eq. 4. The FM estimates based on both the standard (Eq. 3) and the corrected relation (Eq. 4) are reported in the results.
Physiological model of body composition changes after bariatric surgery: estimate of the change in skeletal muscle mass from measurements of ECW and TBW.
A body composition model was developed that uses the change in TBW and ECW after RYGB to estimate the corresponding change in skeletal muscle mass. The basis of the model is described below, and the details are included in the appendix.
The model assumes that the total body composition is divided into skeletal muscle (M), adipose (A), bone (B), and all the other organs (E; skin, gastrointestinal tract, nervous system, etc.). It is assumed that 1) the only weight changes that occur after surgery are changes in the adipose and muscle mass, with no significant changes in E or B, and 2) as the body fat decreases, there is a corresponding increase in the ECW fraction of adipose tissue as the adipose cell volume decreases. This dependence of human adipose tissue extracellular volume on the degree of obesity is well documented (1, 13, 28, 33, 42). The adipose ECW is equal to the total adipose mass minus the FM:
| (5) |
where a is defined as the extracellular fraction of adipose tissue. Solving Eq. 5 for adipose ECW gives
| (6) |
This relation assumes that the adipose ICW is negligible, which is consistent with the results of Wang and Pierson (48), who found no significant difference between the total adipose water and the adipose ECW determined by the Br space.
The muscle is described by the two parameters M (total muscle mass) and m (extracellular fraction of muscle). It is assumed that m is constant, whereas the extracellular fraction of adipose (a) changes after bariatric surgery. These assumptions lead to the following two equations describing the changes in TBW (ΔTBW) and ECW (ΔECW) between the first visit before surgery (visit 1) and visit i after surgery:
| (7) |
Given this model (Eq. 7) and some reasonable assumptions about the parameters M1, a1, m, and c, one can estimate the change in M and a between visit 1 (M1, a1) and visit i (Mi, ai) using the experimental measurements of ΔECW and ΔTBW. The details of this derivation, the basis for the choice of parameters, and a sensitivity analysis of parameter dependence are described in the appendix.
By using the values ai obtained with this modeling, the DXA measurements can be used to estimate the arm and leg skeletal muscle mass loss. Assuming that the change in DXA LBM results entirely from the change in skeletal muscle mass plus the change in the adipose ECW water:
| (8) |
where LBM1, LBMi; FM1, FMi; and M1, Mi are the lean, fat, and skeletal muscle mass at visits 1 and i, respectively. By using the DXA measurements for the LBM and FM, Eq. 8 can then be solved for the change in muscle mass.
Statistics.
The only statistical test used was the standard unequal variance, two-sample, two-tailed t-test as implemented in Maple (Maplesoft).
RESULTS
DXA measurements.
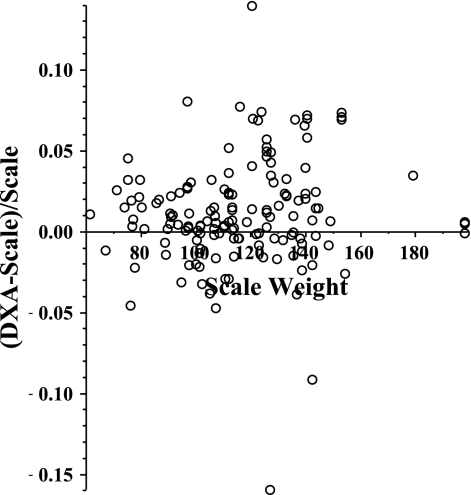
One measure of the accuracy and reliability of DXA measurements is obtained by comparing the scale weight with the total DXA mass (defined as twice the value of the sum of the total DXA half-body FM, bone, and LBM). The scatter plot of (DXA weight − scale weight)/(scale weight) is shown in Fig. 2. The average value of this parameter is 0.012, indicating that the average DXA weight is 1.2% greater than the scale weight. This error cannot be explained by a DXA bias for the extremely obese individuals, because there was there was no significant difference (P > 0.3) between the error for the first visit (0.0127 ± 0.035) vs. the last visit (0.0083 ± 0.016). This small error in the DXA measurements could result from either an error in the DXA calibration or a bias in the procedure used to determine the body midline for the half-body density measurements. In any case, all the DXA weights were adjusted by a factor of 0.988 to make the average DXA weight equal the scale weight. As shown in Fig. 2, there were three outliers (out of a total 145) in which the difference between the scale and DXA weight was >8%, and these measurements were discarded. When these three measurements were removed, the standard deviation of (DXA − scale weight)/scale weight was reduced from 0.035 to 0.027. These scaled weights with the three outliers removed are used in all the rest of the analyses.
Fig. 2.
Fractional difference between the DXA and scale weight as a function of the scale weight for all of the DXA measurements before and after Roux-en-Y gastric bypass (RYGB) surgery.
The mean scale weight, BMI, and total DXA fat, lean, and bone mass (in kg) for each visit are presented in Table 3. The average values of the DXA fat, lean, and bone mass for the arm, leg, and trunk regions are shown in Table 4. There was no significant change in the arm and leg bone mass between the first and last visit. However, there was an apparent 63% increase in the trunk bone mass between the visit before surgery (0.55 kg) and the 6-mo postoperative visit (0.9 kg). Table 5 lists the values of the DXA total fat, lean, and bone mass fraction (defined as DXA mass/scale weight).
Table 3.
Average value of scale weight and total DXA FM, LBM, and bone mass at each visit
| Visit | n | Weight, kg | BMI, kg/m2 | FM, kg | LBM, kg | Bone Mass, kg |
|---|---|---|---|---|---|---|
| 1 | 29 | 127.20 | 46.71 | 62.68 | 61.97 | 2.58 |
| 1 | 20 | 132.43 | 47.88 | 66.28 | 64.52 | 2.62 |
| 2 | 17 | 119.21 | 42.93 | 60.32 | 56.39 | 2.85 |
| 3 | 20 | 118.4 | 42.82 | 59.18 | 56.31 | 2.85 |
| 4 | 20 | 97.89 | 35.38 | 42.31 | 51.61 | 3.00 |
| 5 | 20 | 87.71 | 31.69 | 33.53 | 50.73 | 2.88 |
Values of the half-body DXA mass have been multiplied by 2. LBM, lean body mass.
Table 4.
Average DXA-measured LBM, FM, and bone mass for arm, leg, and trunk regions
| LBM, kg |
FM, kg |
Bone Mass, kg |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Visit | n | Arm | Leg | Trunk | Arm | Leg | Trunk | Arm | Leg | Trunk |
| 1 | 29 | 6.20 | 19.81 | 32.74 | 6.20 | 24.05 | 31.26 | 0.36 | 1.14 | 0.55 |
| 1 | 20 | 6.47 | 20.90 | 34.14 | 6.66 | 26.25 | 32.02 | 0.36 | 1.19 | 0.51 |
| 2 | 17 | 5.74 | 18.49 | 28.92 | 5.85 | 22.95 | 30.35 | 0.37 | 1.17 | 0.75 |
| 3 | 20 | 5.39 | 19.12 | 29.29 | 5.59 | 22.78 | 29.70 | 0.37 | 1.19 | 0.75 |
| 4 | 20 | 4.95 | 17.15 | 26.40 | 3.79 | 16.70 | 20.80 | 0.36 | 1.19 | 0.90 |
| 5 | 20 | 4.81 | 16.80 | 25.95 | 2.94 | 13.68 | 16.02 | 0.35 | 1.15 | 0.85 |
Values of the half-body DXA mass have been multiplied by 2. For the initial visit (visit 1), values are listed for either all the subjects (n = 29) or for those subjects that had subsequent water and DXA measurements at visits 3–5 (n = 20).
Table 5.
Average value of total DXA FM, LBM, and bone mass fraction for each visit
| Visit | n | FM Fraction | LBM Fraction | Bone Fraction |
|---|---|---|---|---|
| 1 | 29 | 0.491 ± 0.042 | 0.487 ± 0.043 | 0.0207 ± 0.0047 |
| 1 | 20 | 0.498 ± 0.035 | 0.491 ± 0.038 | 0.0200 ± 0.0042 |
| 2 | 17 | 0.503 ± 0.045 NS | 0.474 ± 0.047 NS | 0.0242 ± 0.0038 P = 0.006 |
| 3 | 20 | 0.496 ± 0.050 NS | 0.480 ± 0.046 NS | 0.0244 ± 0.0048 P = 0.005 |
| 4 | 20 | 0.424 ± 0.072 P < 0.001 | 0.535 ± 0.074 P = 0.024 | 0.0313 ± 0.0051 P < 0.0001 |
| 5 | 20 | 0.370 ± 0.090 P < 0.0001 | 0.591 ± 0.091 P = 0.001 | 0.0336 ± 0.0052 P < 0.0001 |
Values are means ± SD of FM, LBM, and bone mass divided by the scale weight. For the initial visit, values are listed for either all the subjects (n = 29) or for those subjects that had subsequent water and DXA measurements at visits 3–5 (n = 20). P value indicates significant difference in comparison with value at the first visit that had subsequent measurements. NS, no significant difference.
At the first visit for 22 of the individuals, three separate scans were obtained using no support, taping, or the acrylic board to investigate the effectiveness of the breast constraint in separating the trunk and arm mass. Although it was determined that the acrylic board provided the most direct separation and this was used in all the other individuals and at all subsequent visits, there were no significant differences for any of the constraints for the DXA arm, leg, and trunk measurements. These independent duplicate measurements can be used to estimate the reproducibility of the DXA results, including the assignment of the anatomical half-body arm, trunk, and leg regions. The coefficient of variation for all the measurements (fat, lean, and bone for leg, arm, and trunk) was 5% or less, with the exception of the trunk bone measurement, which had a large variation (18%). When there were multiple measurements for the whole body DXA for the first visit, averages of these measurements were used in all the following results. For the arm, trunk, and leg measurements, only the results with the acrylic board were used.
TBW and ECW measurements.
The average values of the TBW and the standard and corrected ECW estimates are listed in Table 6. TBW decreased while ECW remained unchanged over the course of the five visits. Table 7 lists the values of weight fraction of the TBW, ECW, ICW, and the ratio ECW/ICW.
Table 6.
Average values of scale weight, TBW, and standard and corrected ECW
| Visit | n | Weight, kg | TBW, kg | ECW, kg | ECWcor, kg |
|---|---|---|---|---|---|
| 1 | 28 | 127.20 | 45.36 | 19.31 | 20.14 |
| 1 | 20 | 132.43 | 46.38 | 19.94 | 20.81 |
| 2 | 18 | 119.63 | 41.99 | 18.81 | 19.64 |
| 3 | 20 | 118.44 | 42.11 | 20.42 | 21.30 |
| 4 | 20 | 97.89 | 41.21 | 19.44 | 20.02 |
| 5 | 20 | 87.71 | 40.64 | 20.39 | 20.89 |
Table 7.
Weight fractions of TBW, ECW, and ECWcor and the ratios ECW/ICW and (ECW/ICW)cor
| Visit | n | fTBW, l/kg | fECW, l/kg | fECWcor, l/kg | fICW, l/kg | fICWcor, l/kg | ECW/ICW | (ECW/ICW)cor, l/kg |
|---|---|---|---|---|---|---|---|---|
| 1 | 29 | 0.36 ± 0.034 | 0.15 ± 0.018 | 0.16 ± 0.018 | 0.21 ± 0.035 | 0.20 ± 0.036 | 0.76 ± 0.168 | 0.82 ± 0.192 |
| 2 | 18 | 0.35 ± 0.044 NS | 0.16 ± 0.016 NS | 0.17 ± 0.017 NS | 0.19 ± 0.039 NS | 0.19 ± 0.040 NS | 0.84 ± 0.166 NS | 0.91 ± 0.195 NS |
| 3 | 20 | 0.36 ± 0.038 NS | 0.17 ± 0.018 P < 0.001 | 0.18 ± 0.018 P < 0.001 | 0.18 ± 0.032 P = 0.034 | 0.18 ± 0.033 P = 0.032 | 0.96 ± 0.177 P < 0.001 | 1.05 ± 0.208 P < 0.001 |
| 4 | 20 | 0.43 ± 0.059 P < 0.001 | 0.20 ± 0.031 P < 0.001 | 0.21 ± 0.030 P < 0.001 | 0.22 ± 0.043 NS | 0.22 ± 0.045 NS | 0.92 ± 0.185 P = 0.004 | 0.97 ± 0.204 P = 0.012 |
| 5 | 20 | 0.48 ± 0.069 P < 0.001 | 0.24 ± 0.043 P < 0.001 | 0.24 ± 0.042 P < .0.001 | 0.24 ± 0.052 P = 0.03 | 0.23 ± 0.054 P = 0.026 | 1.04 ± 0.293 P < 0.001 | 1.08 ± 0.317 P = 0.0023 |
Body fat estimates.
Three different estimates of FM mass were made: 1) DXA total FM, 2) from TBW using the standard TBW/FFM relation (Eq. 3), and 3) from TBW using the corrected TBW/FFM relation (Eq. 4). For this comparison, only individuals that had both DXA and TBW measurements at visits 1, 3, 4, and 5 were used. Table 8 lists these three estimates of FM along with the scale weight, the ratio DXA FM/weight, and the ratio TBW/(DXA FFM).
Table 8.
Scale weight, DXA FM, FM/weight fraction, and TBW/DXA FFM as well as three different estimates of FM
| Visit | Weight, kg | DXA FM, kg | DXA FM/Weight | TBW/(DXA FFM) | FMtbw, kg | FMtbwcor, kg |
|---|---|---|---|---|---|---|
| 1 | 132.4 ± 21.1 | 66.1 ± 12.403 | 0.50 ± 0.035 | 0.70 ± 0.058 | 69.58 ± 15.4 | 71.27 ± 15.73 |
| 3 | 118.4 ± 19.8 | 59.2 ± 12.84 P = 0.09 | 0.50 ± 0.050 NS | 0.72 ± 0.069 NS | 61.37 ± 15.3 P = 0.09 | 62.87 ± 15.21 P = 0.09 |
| 4 | 97.9 ± 18.3 | 42.3 ± 12.89 P < 0.0001 | 0.42 ± 0.072 P = 0.0003 | 0.74 ± 0.059 P = 0.039 | 42.04 ± 14.5 P < 0.0001 | 43.04 ± 14.86 P < 0.0001 |
| 5 | 87.7 ± 18.5 | 33.5 ± 13.77 P < 0.0001 | 0.37 ± 0.090 P < 0.0001 | 0.75 ± 0.034 P = 0.003 | 32.63 ± 14.84 P < 0.0001 | 33.34 ± 14.80 P < 0.0001 |
Values are means ± SD. Only data for subjects (n = 20) that had water and DXA measurements at visits 1, 3, 4, and 5 are included. Fat-free mass (FFM) = scale weight − DXA FM; FM estimates included the DXA FM as well as the standard (FMtbw) and corrected (FMtbwcor) body fat estimated from TBW. P value indicates significant difference in comparison with value at the first visit.
Physiological model prediction of skeletal muscle weight loss.
Table 9 lists the model predictions (Eq. 7) of the change in muscle mass and ECW adipose fraction for visits 3–5 based on the experimental TBW and ECWcor measurements (Table 6). Table 10 lists the values of arm and leg muscle loss (Eq. 8) estimated from the DXA LBM and FM measurements (Table 4) and the model ECW adipose fraction (Table 9).
Table 9.
Model estimate of total skeletal muscle mass, percentage of muscle loss, and the ECW fraction of adipose tissue
| Visit | Muscle Mass, kg | Muscle Loss, % | Adipose ECW Fraction |
|---|---|---|---|
| 1 | 24.60 | 0 | 0.12 |
| 3 | 17.41 | 29.2 | 0.149 |
| 4 | 17.99 | 26.9 | 0.184 |
| 5 | 15.81 | 35.7 | 0.245 |
Model estimates of total skeletal muscle mass and the percentage of muscle mass lost at visits 1, 3, 4, and 5 were determined from the change in TBW and ECW. The ECW fraction of adipose tissue was also determined by this model calculation.
Table 10.
Estimates of arm and leg skeletal muscle weight loss from DXA measurements and model values for ECW adipose weight fraction at each visit
| Arm |
Leg |
|||
|---|---|---|---|---|
| Visit | Muscle loss, kg | Fraction weight loss | Muscle loss, kg | Fraction weight loss |
| 3 | 1.258 | 0.0937 | 2.59 | 0.054 |
| 4 | 1.466 | 0.1087 | 3.89 | 0.081 |
| 5 | 1.702 | 0.126 | 4.92 | 0.102 |
Estimates of arm and leg skeletal muscle weight loss at visits 3, 4, and 5 were determined from DXA measurements and model values for ECW adipose weight fraction at each visit. Absolute values of the muscle weight loss and the fractional muscle loss (defined as muscle weight loss/total DXA mass before surgery) are listed. The half-body model results have been multiplied by 2.
DISCUSSION
The weight and fat loss results of this study are compared with previously published results in Table 1. The 34% weight loss 12 mo after RYGB surgery and the DXA and TBW estimates of FM loss are similar to those reported previously. The TBW estimate of the change in FM after 12 mo (visit 5) determined using either the standard (ΔFM = 36.95 kg) or corrected (ΔFM = 37.93 kg) TBW relation (Eqs. 3 and 4) is significantly larger than that for the DXA measurement (ΔFM = 32.62 kg) (Table 8). As discussed below, there are several reasons for suspecting that for the highly obese individuals, the TBW estimates of FM are more accurate than the DXA measurements.
The TBW and ECW results of this study were compared with previously published bariatric surgery results in Table 2. The initial and final TBW were similar to those of other studies using deuterium dilution. With the exception of the Das et al. (8) study, our measurements of initial and final ECW measurements are in general agreement with other Br or SO42− dilution results, with very little change in ECW with the ratio ECW/ICW in the range of 0.8 to 0.82 before surgery and increasing to a value in the range of 0.83 to 1.09 after surgery. The Das et al. (8) result differs substantially from the rest of the measurements in Table 2, with a larger initial ECW and a greater change after surgery. All of these values for ECW/ICW are significantly larger than the standard reference value for normal-weight individuals of 0.76 (8). As discussed below, these changes in TBW and ECW in obesity and following RYGB can be explained by the normal physiological body composition changes that occur in obesity and during weight loss.
Limitations of DXA measurements in extremely obese individuals.
Although the recently developed iDXA scanner (GE Lunar Medical Systems, Madison, WI) allows measurements on subjects weighing up to 500 lb. (34), the great majority of DXA measurements in most clinical settings are still made on standard-sized instruments. We were able to reproducibly define the half-body anatomical markers and found that doubling the half-body total DXA mass produced values that were in good agreement with the individual's scale weight (Fig. 2). We anticipated that this approach would provide an accurate method for fat measurement in these individuals. However, there are two features of the DXA measurements that suggest that DXA fat and bone calibration in these highly obese individuals is not reliable.
The first feature comes from measurements of bone mass. The total bone mass of 2.58 kg for the first visit (presurgery) was 16% less than the value at the fourth visit (6 mo), when the individuals had lost 24% of their weight (Table 3). Because an increase of this magnitude in bone mass after bariatric surgery is highly unlikely, this result suggests that the initial DXA measurement underestimates the true bone mass. The error seems to be limited to the trunk region, where there was a 63% increase in bone mass between the first and fourth (6 mo) visit (Table 4). In contrast, there was no significant change in bone mass in the arm and leg regions (Table 4). The total bone mass values at visits 4 (6 mo) and 5 (12 mo) were between 3.00 and 2.88 kg and are close to the reported normal values (39), suggesting that the DXA error only occurs in highly obese individuals.
The second feature that indicates an error in the DXA measurements in these highly obese individuals comes from a comparison of the DXA and TBW FM estimates. The ratio TBW/(DXA FFM) before surgery is 0.70 (Table 8), which was significantly less than the standard value of 0.738 (2) for normal-weight individuals. One would predict that in obese individuals, the ratio TBW/FFM should, if anything, be greater than the standard 0.738 value because of the additional adipose ECW. This prediction was confirmed by Das et al. (8), who found a TBW/FFM value of 0.756 for highly obese individuals by using 3C measurements of fat. The ratio TBW/(DXA FFM) is close to the standard value of 0.738 for the individuals at visits 4 (6 mo, 0.743) and 5 (12 mo, 0.752), again suggesting that the DXA calibration is accurate for these less obese individuals. This too small value for the ratio TBW/(DXA FFM) should produce an underestimate of the true FM value (assuming the TBW measurement is correct). In support of this prediction, before surgery, the DXA FM was ∼7% less than the TBW estimate (Table 8). Again, there was good agreement between the DXA and TBW FM predictions at the visits 6 and 12 mo after the individuals had lost weight. This is consistent with the results of Williams et al. (49), who found good agreement between Lunar DXA and 4C fat measurements in moderately obese individuals. This error in DXA measurements is consistent with the recent results of Valentine et al. (45), who determined the ability of DXA to detect changes in total fat when 2-kg lard packets were placed on the trunk or legs of normal-weight individuals. They found a large error in the trunk measurements, with only 59% of the exogenous weight correctly interpreted as fat, whereas the thigh measurements were more accurate, with 94% correctly interpreted as fat.
Implications of physiological model analysis: changes in muscle mass and ECW/ICW following bariatric surgery.
The model has two basic assumptions. The first is that only the weight of adipose (including all organ fat deposits) and muscle tissue changes following surgery, whereas the weight of the skin, gastrointestinal tract, nervous system, and other tissues remains constant. This leads to an increase in the relative skin weight after weight loss and corresponding increases in ECW/ICW because skin has a high ECW fraction (∼0.42) relative to other tissues (25). Although it is generally recognized that there is an increase in the relative skin mass after bariatric surgery, we are not aware of any quantitative measurements that support this assumption. Although there are large changes in, for example, liver and abdominal organ weights following bariatric surgery, most of this weight loss can be attributed to the loss of organ fat deposits (including decreases in liver steatosis) and not lean organ weight (3, 12).
The second assumption is that the ECW fraction of adipose tissue increases as the adipose cell volume shrinks following weight loss. Biopsies from normal and obese subjects have shown that the adipose ECW fraction is greater in normal-weight individuals (1, 28, 33, 42), and Entenman et al. (13) have shown that the ECW fraction increases after weight loss with the reported values for adipose ECW fraction increasing from a range of 0.08 to 0.15 in obese individuals to 0.20 to 0.3 in normal-weight individuals. These direct biopsy measurements are similar to our model predictions of an increase of the adipose ECW fraction from 0.12 before surgery to 0.245 by 1 yr after surgery (Table 9).
This model allows us to use the experimental TBW and ECW measurements (Table 6) to predict the change in muscle mass following bariatric surgery (Eq. 7). The predicted muscle mass decreases from 24.6 kg before surgery to 15.81 kg after surgery, a decrease of 35% (Table 9). This result is consistent with the reported 33.5% decrease in maximal lower limb force 1 yr after bariatric surgery (23). This predicted muscle loss corresponds to 19% of the total weight loss. Using whole body MRI, 40K, and [13C]leucine kinetics, Gallagher et al. (16) reported a muscle loss of ∼17% of the total weight loss following dieting in mildly obese individuals. Our model results should be regarded as the maximum estimate of muscle loss, since the model is based on the assumption that only the muscle and adipose tissue weight decreases during weight loss. Any skin or other organ weight loss would decrease the calculated muscle loss.
By using this estimate of the ECW adipose fraction before and after surgery, the arm and leg muscle mass loss can be estimated from the DXA lean and FM measurements (Tables 4) using Eq. 8. (The trunk is not used in this calculation because of the unreliability of trunk DXA measurements as discussed above). One year after surgery there was a loss of ∼1.7 kg of arm muscle and 4.92 kg of leg muscle (Table 10). This corresponds to ∼13% of the initial arm weight and 10% of the initial leg weight.
As summarized in Table 2, the ratio ECW/ICW in highly obese individuals is significantly greater than that in normal-weight individuals (0.76), and this ratio slightly increases after bariatric surgery in most studies. Although this is usually explained as an obesity-related chronic alteration in fluid regulation (27, 29, 37, 51), our model analysis suggests that this result can be explained in terms of normal physiological changes in body composition. Our experimental measurements of TBW and ECW are consistent with the body composition model and the predicted changes in muscle mass.
These model calculations depend on a number of assumptions and should be regarded only as approximate. The important point is that our experimental value of ECW/ICW of 0.82 before surgery and its increase to 1.08 after weight loss (Table 2) are consistent with physiological body composition changes, and one does not need to invoke any abnormal change in water balance or regulation. The key reason why the ratio ECW/ICW does not decrease toward normal after bariatric surgery is that the skin mass, with its high ECW space, remains relatively constant.
Conclusions and summary.
Although the half-body DXA FM, bone, and LBM can be reproducibly measured, the bone and FM are probably underestimated in highly obese individuals because of the increased photon absorption. The DXA total FM before bariatric surgery is ∼7% less than that based on TBW measurements. At 6 and 12 mo after surgery, the DXA and TBW fat measurements were in good agreement. The ECW/ICW was significantly increased above normal before surgery, and it increased slightly after surgery. These changes are consistent with the expected physiological changes in body composition after surgery, particularly the relative increase in skin mass and the increase in the adipose ECW fraction as fat cells shrink. A quantitative body composition model of the changes in ECW and TBW can be used to predict the changes in muscle mass that occur following bariatric surgery. This model predicts a maximum 35% muscle mass loss corresponding to 19% of the total weight loss. Our analysis suggests that the water composition changes can be explained entirely on the basis of the physiological changes that occur in body composition following bariatric surgery and do not represent abnormal changes in fluid balance.
GRANTS
Funding for this study was provided by the Rhoads Research Foundation of the American Society for Parenteral and Enteral Nutrition; Center for Translational Science Activities Grant ULI-RR24150 and National Institutes of Health (NIH) Grant DK50456 (to M. D. Jensen); NIH Grant M01-RR00400, which supported the GCRC at the University of Minnesota; the Minnesota Agricultural Experiment Station; and the Midwest Dairy Association.
DISCLAIMERS
None of the study sponsors had involvement in any aspect of the study design, data collection or analyses, or writing of this manuscript.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank the study participants for their commitment to this study and the team at the Fairview Obesity Surgery Center and the General Clinical Research Center (GCRC) at the University of Minnesota for ongoing support of this study. We thank Dr. Michael Jensen, Charles Ford, and Jaime Gransee at the Mayo Clinic for assistance with the deuterium analyses. We also thank Dr. Dale Schoeller for input on the TBW method.
APPENDIX
Derivation and analysis of the physiological model of body composition.
Given values for the parameters M1, a1, m, and c (Eq. 7) can be solved (using the “solve” feature of Maple; Maplesoft) for Mi and ai:
The experimental measurements for TBW, ECWcor (Table 6), and FM (Table 8, TBWcor) for visits 1, 3, 4, and 5 were used as input to these equations.
The parameters M1, a1, m, and c were estimated as follows. The reference normal female value of the muscle mass/LBM is 0.423 (50). Assuming that the skeletal muscle relative to “lean” body weight of the average obese subjects at visit 1 is also 0.423, a value for M1 of 24.6 is obtained for the average obese subject (weight = 132 kg, fat = 76.43). The value of a1 was assumed to be 0.12, similar to what is found experimentally from adipose samples from obese individuals (1, 13, 28, 33, 42). A value of m (ECW muscle weight fraction) of 0.117 and a value of c (muscle cell water fraction) of 0.75 were assumed based on measurements on skeletal muscle (25).
An analysis of the sensitivity of the calculated changes in muscle mass to the assumed initial parameters is described in Table A1, where the dependence of muscle mass value as a function of the range of values of the various parameters is listed. This analysis shows that the calculation of the fraction of muscle loss after surgery is relatively robust and insensitive to the model assumptions.
Table A1.
Sensitivity analysis of the dependence of the calculation of adipose ECW fraction and fractional change in muscle massfor various combinations of the assumed model parameters
| Input Parameters |
Solution |
|||||
|---|---|---|---|---|---|---|
| M1, kg | a1 | m | c | a5 | M5 | (M1 − M5)/M1 |
| 24.60 | 0.12 | 0.117 | 0.75 | 0.245 | 15.81 | 0.357 |
| 28.00 | 0.12 | 0.117 | 0.75 | 0.245 | 19.21 | 0.314 |
| 24.60 | 0.18 | 0.117 | 0.75 | 0.334 | 15.81 | 0.357 |
| 24.60 | 0.12 | 0.16 | 0.75 | 0.252 | 15.36 | 0.375 |
| 24.60 | 0.12 | 0.117 | 0.68 | 0.247 | 14.91 | 0.394 |
Values represent sensitivity analysis of the dependence of the calculation of the adipose ECW fraction at visit 5 (a5) and the fractional change in muscle mass [(M1 − M5)/M1] for various combinations of the assumed model parameters: M1, muscle mass at first visit; a1, adipose ECW fraction at first visit; m, muscle ECW fraction; and c, muscle cell water fraction.
Glossary
- a1, ai
- Adipose ECW fraction at visits 1 and i
- c
- Muscle ICW fraction
- ECW, ECWcor
- Standard and corrected ECW
- ΔECW, ΔTBW
- Change in ECW and TBW between visits 1 and i
- FM, FM1, FMi
- Fat mass and fat mass at visits 1 and i
- FMtbw, FMtbwcor
- Standard and corrected estimates of body fat from TBW measurements
- fTBW
- Fractional TBW (TBW/scale weight)
- ICW, ICWcor
- Standard and corrected ICW (TBW − ECW)
- m
- Muscle ECW fraction
- M1, Mi
- Skeletal muscle mass at visits 1 and i
- LBM1, LBMi
- Lean body mass at visits 1 and i
- TBW
- Total body water (kg)
REFERENCES
- 1. Bjorntorp P, Hood B, Martinsson A. The sucrose space of human subcutaneous adipose tissue in obesity. Acta Med Scand 180: 123–127, 1966 [DOI] [PubMed] [Google Scholar]
- 2. Brozek J, Grande F, Anderson JT, Keys A. Densitometric analysis of body composition: revision of some quantitative assumptions. Ann NY Acad Sci 110: 113–140, 1963 [DOI] [PubMed] [Google Scholar]
- 3. Busetto L, Tregnaghi A, De Marchi F, Segato G, Foletto M, Sergi G, Favretti F, Lise M, Enzi G. Liver volume and visceral obesity in women with hepatic steatosis undergoing gastric banding. Obes Res 10: 408–411, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Carey DG, Pliego GJ, Raymond RL. Body composition and metabolic changes following bariatric surgery: effects on fat mass, lean mass and basal metabolic rate: six months to one-year follow-up. Obes Surg 16: 1602–1608, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Carrasco F, Papapietro K, Csendes A, Salazar G, Echenique C, Lisboa C, Diaz E, Rojas J. Changes in resting energy expenditure and body composition after weight loss following Roux-en-Y gastric bypass. Obes Surg 17: 608–616, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Carrasco F, Ruz M, Rojas P, Csendes A, Rebolledo A, Codoceo J, Inostroza J, Basfi-Fer K, Papapietro K, Rojas J, Pizarro F, Olivares M. Changes in bone mineral density, body composition and adiponectin levels in morbidly obese patients after bariatric surgery. Obes Surg 19: 41–46, 2009 [DOI] [PubMed] [Google Scholar]
- 7. Coupaye M, Bouillot JL, Poitou C, Schutz Y, Basdevant A, Oppert JM. Is lean body mass decreased after obesity treatment by adjustable gastric banding? Obes Surg 17: 427–433, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Das SK, Roberts SB, Kehayias JJ, Wang J, Hsu LK, Shikora SA, Saltzman E, McCrory MA. Body composition assessment in extreme obesity and after massive weight loss induced by gastric bypass surgery. Am J Physiol Endocrinol Metab 284: E1080–E1088, 2003 [DOI] [PubMed] [Google Scholar]
- 9. del Genio F, Alfonsi L, Marra M, Finelli C, del Genio G, Rossetti G, del Genio A, Contaldo F, Pasanisi F. Metabolic and nutritional status changes after 10% weight loss in severely obese patients treated with laparoscopic surgery vs integrated medical treatment. Obes Surg 17: 1592–1598, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Dixon JB, Strauss BJ, Laurie C, O'Brien PE. Changes in body composition with weight loss: obese subjects randomized to surgical and medical programs. Obesity (Silver Spring) 15: 1187–1198, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Economos CD, Nelson ME, Fiatarone MA, Dallal GE, Heymsfield SB, Wang J, Yasumara S, Ma R, Vaswani AN, Russell-Aulet M, Pierson RN. A multi-center comparison of dual energy X-ray absorptiometers: in vivo and in vitro soft tissue measurement. Eur J Clin Nutr 51: 312–317, 1997 [DOI] [PubMed] [Google Scholar]
- 12. Engl J, Sturm W, Sandhofer A, Kaser S, Tschoner A, Tatarczyk T, Weiss H, Tilg H, Patsch JR, Ebenbichler CF. Effect of pronounced weight loss on visceral fat, liver steatosis and adiponectin isoforms. Eur J Clin Invest 38: 238–244, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Entenman C, Goldwater WH, Ayres NS, Behnke AR., Jr Analysis of adipose tissue in relation to body weight loss in man. J Appl Physiol 13: 129–134, 1958 [DOI] [PubMed] [Google Scholar]
- 14. Evans EM, Saunders MJ, Spano MA, Arngrimsson SA, Lewis RD, Cureton KJ. Body-composition changes with diet and exercise in obese women: a comparison of estimates from clinical methods and a 4-component model. Am J Clin Nutr 70: 5–12, 1999 [DOI] [PubMed] [Google Scholar]
- 15. Gahtan V, Goode SE, Kurto HZ, Schocken DD, Powers P, Rosemurgy AS. Body composition and source of weight loss after bariatric surgery. Obes Surg 7: 184–188, 1997 [DOI] [PubMed] [Google Scholar]
- 16. Gallagher D, Kovera AJ, Clay-Williams G, Agin D, Leone P, Albu J, Matthews DE, Heymsfield SB. Weight loss in postmenopausal obesity: no adverse alterations in body composition and protein metabolism. Am J Physiol Endocrinol Metab 279: E124–E131, 2000 [DOI] [PubMed] [Google Scholar]
- 17. Galtier F, Farret A, Verdier R, Barbotte E, Nocca D, Fabre JM, Bringer J, Renard E. Resting energy expenditure and fuel metabolism following laparoscopic adjustable gastric banding in severely obese women: relationships with excess weight lost. Int J Obes (Lond) 30: 1104–1110, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Gasteyger C, Suter M, Calmes JM, Gaillard RC, Giusti V. Changes in body composition, metabolic profile and nutritional status 24 months after gastric banding. Obes Surg 16: 243–250, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Giusti V, Suter M, Heraief E, Gaillard RC, Burckhardt P. Effects of laparoscopic gastric banding on body composition, metabolic profile and nutritional status of obese women: 12-months follow-up. Obes Surg 14: 239–245, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Guida B, Belfiore A, Angrisani L, Micanti F, Mauriello C, Trio R, Pecoraro P, Falconi C. Laparoscopic gastric banding and body composition in morbid obesity. Nutr Metab Cardiovasc Dis 15: 198–203, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Halliday D, Miller AG. Precise measurement of total body water using trace quantities of deuterium oxide. Biomed Mass Spectrom 4: 82–87, 1977 [DOI] [PubMed] [Google Scholar]
- 22. Heymsfield SB, Lohman TG, Wang Z, Going SB. editors. Human Body Composition. Champaign, IL: Human Kinetics, 2005, p. 164 [Google Scholar]
- 23. Hue O, Berrigan F, Simoneau M, Marcotte J, Marceau P, Marceau S, Tremblay A, Teasdale N. Muscle force and force control after weight loss in obese and morbidly obese men. Obes Surg 18: 1112–1118, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Inge T, Wilson KA, Gamm K, Kirk S, Garcia VF, Daniels SR. Preferential loss of central (trunk) adiposity in adolescents and young adults after laparoscopic gastric bypass. Surg Obes Relat Dis 3: 153–158, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Levitt DG. The pharmacokinetics of the interstitial space in humans. BMC Clin Pharmacol 3: 3, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mager JR, Sibley SD, Beckman TR, Kellogg TA, Earthman CP. Multifrequency bioelectrical impedance analysis and bioimpedance spectroscopy for monitoring fluid and body cell mass changes after gastric bypass surgery. Clin Nutr 27: 832–841, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marken Lichtenbelt WD, Fogelholm M. Increased extracellular water compartment, relative to intracellular water compartment, after weight reduction. J Appl Physiol 87: 294–298, 1999 [DOI] [PubMed] [Google Scholar]
- 28. Martin AD, Daniel MZ, Drinkwater DT, Clarys JP. Adipose tissue density, estimated adipose lipid fraction and whole body adiposity in male cadavers. Int J Obes Relat Metab Disord 18: 79–83, 1994 [PubMed] [Google Scholar]
- 29. Mazariegos M, Kral JG, Wang J, Waki M, Heymsfield SB, Pierson RN, Jr, Thornton JC, Yasumura S. Body composition and surgical treatment of obesity. Effects of weight loss on fluid distribution. Ann Surg 216: 69–73, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Miller ME, Cappon CJ. Anion-exchange chromatographic determination of bromide in serum. Clin Chem 30: 781–783, 1984 [PubMed] [Google Scholar]
- 31. Olbers T, Bjorkman S, Lindroos A, Maleckas A, Lonn L, Sjostrom L, Lonroth H. Body composition, dietary intake, and energy expenditure after laparoscopic Roux-en-Y gastric bypass and laparoscopic vertical banded gastroplasty: a randomized clinical trial. Ann Surg 244: 715, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Palazuelos-Genis T, Mosti M, Sanchez-Leenheer S, Hernandez R, Garduno O, Herrera MF. Weight loss and body composition during the first postoperative year of a laparoscopic Roux-en-Y gastric bypass. Obes Surg 18: 1–4, 2008 [DOI] [PubMed] [Google Scholar]
- 33. Pawan GLS, Clode M. The gross chemical composition of subcutaneous adipose tissue in the lean and obese human subject. Biochem J 74: 9p, 1960 [Google Scholar]
- 34. Rothney MP, Brychta RJ, Schaefer EV, Chen KY, Skarulis MC. Body composition measured by dual-energy X-ray absorptiometry half-body scans in obese adults. Obesity (Silver Spring) 17: 1281–1286, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schoeller DA. Hydrometry. In: Human Body Composition, edited by Roche AF, Heymsfield SB, Lohman TG. Champaign, IL: Human Kinetics, 1996, p. 25–43 [Google Scholar]
- 36. Schoeller DA, van Santen E, Peterson DW, Dietz W, Jaspan J, Klein PD. Total body water measurement in humans with 18O and 2H labeled water. Am J Clin Nutr 33: 2686–2693, 1980 [DOI] [PubMed] [Google Scholar]
- 37. Sergi G, Lupoli L, Busetto L, Volpato S, Coin A, Bertani R, Calliari I, Berton A, Enzi G. Changes in fluid compartments and body composition in obese women after weight loss induced by gastric banding. Ann Nutr Metab 47: 152–157, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Silva AM, Heymsfield SB, Gallagher D, Albu J, Pi-Sunyer XF, Pierson RN, Jr, Wang J, Heshka S, Sardinha LB, Wang Z. Evaluation of between-methods agreement of extracellular water measurements in adults and children. Am J Clin Nutr 88: 315–323, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Silva AM, Shen W, Wang Z, Aloia JF, Nelson ME, Heymsfield SB, Sardinha LB, Heshka S. Three-compartment model: critical evaluation based on neutron activation analysis. Am J Physiol Endocrinol Metab 287: E962–E969, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Strauss BJ, Marks SJ, Growcott JP, Stroud DB, Lo CS, Dixon JB, O'Brien PE. Body composition changes following laparoscopic gastric banding for morbid obesity. Acta Diabetol 40, Suppl 1: S266–S269, 2003 [DOI] [PubMed] [Google Scholar]
- 41. Tataranni PA, Ravussin E. Use of dual-energy X-ray absorptiometry in obese individuals. Am J Clin Nutr 62: 730–734, 1995 [DOI] [PubMed] [Google Scholar]
- 42. Thomas LW. The chemical composition of adipose tissue of man and mice. Q J Exp Physiol Cogn Med Sci 47: 179–188, 1962 [DOI] [PubMed] [Google Scholar]
- 43. Thomsen TK, Jensen VJ, Henriksen MG. In vivo measurement of human body composition by dual-energy X-ray absorptiometry (DXA). Eur J Surg 164: 133–137, 1998 [DOI] [PubMed] [Google Scholar]
- 44. Valentine BJ. The use of dual-energy X-ray absorptiometry for the assessment of body composition in extremely obese women undergoing roux-en-Y gastric bypass surgery (Master's thesis). St. Paul, MN: University of Minnesota, 2007, p. vii, 115 leaves [Google Scholar]
- 45. Valentine RJ, Misic MM, Kessinger RB, Mojtahedi MC, Evans EM. Location of body fat and body size impacts DXA soft tissue measures: a simulation study. Eur J Clin Nutr 62: 553–559, 2008 [DOI] [PubMed] [Google Scholar]
- 46. von Mach MA, Stoeckli R, Bilz S, Kraenzlin M, Langer I, Keller U. Changes in bone mineral content after surgical treatment of morbid obesity. Metabolism 53: 918–921, 2004 [DOI] [PubMed] [Google Scholar]
- 47. Wadström C, Backman L, Forsberg AM, Nilsson E, Hultman E, Reizenstein P, Ekman M. Body composition and muscle constituents during weight loss: studies in obese patients following gastroplasty. Obes Surg 10: 203–213, 2000 [DOI] [PubMed] [Google Scholar]
- 48. Wang J, Pierson RN., Jr Disparate hydration of adipose and lean tissue require a new model for body water distribution in man. J Nutr 106: 1687–1693, 1976 [DOI] [PubMed] [Google Scholar]
- 49. Williams JE, Wells JC, Wilson CM, Haroun D, Lucas A, Fewtrell MS. Evaluation of Lunar Prodigy dual-energy X-ray absorptiometry for assessing body composition in healthy persons and patients by comparison with the criterion 4-component model. Am J Clin Nutr 83: 1047–1054, 2006 [DOI] [PubMed] [Google Scholar]
- 50. Williams LR, Leggett RW. Reference values for resting blood flow to organs of man. Clin Phys Physiol Meas 10: 187–217, 1989 [DOI] [PubMed] [Google Scholar]
- 51. Zimmerman ME, Andersson H, Lundell L, Olbe L. Alterations in body composition after gastroplasty for morbid obesity. Scand J Gastroenterol 25: 263–268, 1990 [PubMed] [Google Scholar]