Abstract
Chronic hyperoxia during the first 1–4 postnatal weeks attenuates the hypoxic ventilatory response (HVR) subsequently measured in adult rats. Rather than focusing on this long-lasting plasticity, the present study considered the influence of hyperoxia on respiratory control during the neonatal period. Sprague-Dawley rats were born and raised in 60% O2 until studied at postnatal ages (P) of 4, 6–7, or 13–14 days. Ventilation and metabolism were measured in normoxia (21% O2) and acute hypoxia (12% O2) using head-body plethysmography and respirometry, respectively. Compared with age-matched rats raised in room air, the major findings were 1) diminished pulmonary ventilation and metabolic O2 consumption in normoxia at P4 and P6–7; 2) decreased breathing stability during normoxia; 3) attenuation of the early phase of the HVR at P6–7 and P13–14; and 4) a sustained increase in ventilation during hypoxia (vs. the normal biphasic HVR) at all ages studied. Attenuation of the early HVR likely reflects progressive impairment of peripheral arterial chemoreceptors while expression of a sustained HVR in neonates before P7 suggests that hyperoxia also induces plasticity within the central nervous system. Together, these results suggest a complex interaction between inhibitory and excitatory effects of hyperoxia on the developing respiratory control system.
Keywords: developmental plasticity, breathing, biphasic hypoxic ventilatory response, heterokairy, heterochrony
oxygen levels experienced during the perinatal period can profoundly influence respiratory control development. In one such model of developmental plasticity, rats exposed to 60% O2 for the first 1–4 wk of life exhibit attenuated hypoxic ventilatory responses (HVR) as adults (4, 31). Similar effects on the HVR have been noted after milder exposures to hyperoxia (30% O2; 4, 19, 27) and after chronic intermittent hyperoxia (3) during development. There is a critical period for this plasticity during the first two postnatal weeks in rats (2, 6); exposure to 60% O2 appears to have no lasting effect on the HVR if begun after the second postnatal week (2, 31).
The impaired HVR of adult rats after perinatal hyperoxia reflects abnormal development of the carotid body. Specifically, hyperoxia-treated rats exhibit reduced numbers of the O2-sensitive glomus cells in the carotid body as well as fewer chemoafferent neurons in the carotid sinus nerve (CSN) (22, 42). Furthermore, the remaining glomus cells and chemoafferent neurons may have abnormal responses to acute hypoxia (37). As a result, whole nerve CSN responses to hypoxia, cyanide, and asphyxia are severely reduced (6, 32), even more than a year after return to normoxia (23).
Although numerous studies have examined the effects of perinatal hyperoxia on adult respiratory control (i.e., the persistent effects of chronic hyperoxia; reviewed in Ref. 1), few studies have considered the effects of chronic hyperoxia on the respiratory control of neonates. Hanson et al. (27) exposed kittens to 30% O2 from birth through 12–23 days of age (P12–23). These kittens exhibited reduced carotid body O2 sensitivity in single- and few-fiber recordings from the CSN and attenuated HVR compared to age-matched controls. Likewise, Donnelly et al. (16) observed reduced carotid body O2 sensitivity in rats immediately following a 2-wk exposure to 60% O2 beginning at birth. The impact of hyperoxia on the magnitude of the HVR has not been reported for neonatal rats. In a conference abstract, however, Eden and Hanson (19) noted that P5 rats exposed to 30% O2 since birth mounted a sustained increase in ventilation during an acute hypoxic exposure. In contrast, immature mammals normally exhibit a biphasic HVR in which an initial increase in ventilation is quickly followed by a return toward (or below) baseline levels (7, 20). The magnitude of the ventilatory decline in the late phase of the HVR diminishes with age, yielding a sustained increase in ventilation characteristic of the mature HVR (7, 20). It is now widely accepted that the early phase of the biphasic HVR is primarily mediated by excitation of the carotid body chemoreceptors while inhibitory central nervous system (CNS) mechanisms underlie ventilatory depression in the late phase (7). Taken together, these observations suggest that chronic hyperoxia could influence development of both the peripheral and central components of the HVR.
In the present study, we investigated time-dependent changes in the biphasic HVR that occur during a 2-wk exposure to hyperoxia in neonatal rats. We selected three age groups to study: P4, P6–7, and P13–14. These ages were chosen to span the period for normal postnatal maturation of the rat HVR (20); this age range also encompasses the critical period established for the long-lasting effects of hyperoxia on the HVR (2). Moreover, a recent study on rats exposed to 60% O2 beginning at P7 revealed that a reduction of carotid chemoreceptor O2 sensitivity occurs after 4 or 5 days of hyperoxic exposure (17). Thus we hypothesized that chronic hyperoxia would cause a progressive impairment of the early, carotid body-mediated phase of the HVR. Based on the preliminary observations by Eden and Hanson (19), we further hypothesized that hyperoxia would alter the late phase of the HVR.
METHODS
Experimental animals.
Timed-pregnant Sprague-Dawley rats (SAS SD, Charles River Laboratories, Wilmington, MA) were placed into environmental chambers maintained at 60% O2 approximately 1 day before giving birth; chambers were flushed with gases at sufficient flow rates to maintain <0.3% CO2. The resulting litters were raised in the chamber with their mothers for the first 14 postnatal days. Control rats were housed in the same room but were born and raised at ambient O2 levels (21% O2). Individual rat pups were removed from the cage at P4, P6–7, or P13–14 for determination of ventilation or metabolism (see below) and then returned to their home cages; although rats from the same litter were studied at multiple ages, rats were marked to ensure that each individual was studied only once. Rats were maintained on a 12:12-h light-dark cycle throughout the study and provided food and water ad libitum.
All experimental procedures were approved by the Animal Care and Use Committee at Bates College.
Ventilation measurements.
Ventilation was measured for neonatal rats aged P4, P6–7, and P13–14 from 10 control litters and 9 hyperoxia litters; individuals from 6–7 separate litters were used at each age per treatment group.
Ventilation measurements were made using a customized head-body plethysmograph similar to the design described by Mortola (34) but modified to include a separate head compartment for administering test gas mixtures. The plethysmograph consisted of two acrylic cylindrical chambers: a head chamber (4.75 mm ID; ∼70 ml) through which test gases flowed and a body chamber (3.1 cm ID; internal volume was adjusted using modeling clay to limit the backward movement of the animal). A flexible collar made from a layer of latex film (∼0.15 mm thick) and a layer of Parafilm (Pechiney Plastic Packaging, Menaha, WI) was fitted around the neck of the rat, thereby isolating the head and body compartments; a layer of petroleum jelly was applied around the rat's neck to prevent leaks. The body chamber contained a T-type thermocouple probe (IT-18, Physitemp Instruments, Clifton, NJ) to monitor air temperature as well as ports connected to a pneumotach (MLT1L, ADInstruments, Colorado Springs, CO) and a calibration syringe. The pneumotach was connected to a differential pressure transducer (ML141, ADInstruments) to monitor respiratory airflows; the pneumotach was calibrated at the start of each experiment using a 0.5-ml injection. Respiratory airflows and chamber temperature were recorded continuously to a computer at a sampling rate of 400–1,000 Hz (PowerLab 8SP and Chart 5.2 software with the spirometry extension, ADInstruments). Respiratory airflows were integrated and digitally filtered (high-pass, 0.1 Hz) to obtain respiratory volumes.
The plethysmograph was situated in a temperature-controlled incubator so that the air temperature in the body chamber could be maintained at 32–34°C. Although body temperature was not measured in this study, this ambient temperature range corresponds to the nest temperature and thermoneutral zone of neonatal rats (e.g., 29, 38). Gas flow through the head chamber was maintained at ∼1,000 ml/min using gas-mixing rotameters (Matheson Tri-Gas, Montgomeryville, PA) and a mass flow monitor (G265, Qubit Systems, Kingston, ON, Canada); the high gas flow rate coupled with the low-volume head chamber enabled rapid changes in inspired O2. After being weighed and sealed into the plethysmograph, the rat was exposed to 21% O2 (balance N2) for 8–15 min to settle. Once the rat appeared calm (based on relative stability of the breathing pattern), respiratory airflows were recorded for 2 min of normoxia (21% O2) followed by 8 min of hypoxia (12% O2, balance N2); inspired O2 concentrations were confirmed before and after each measurement (S-3A O2 analyzer; AEI Technologies, Pittsburgh, PA).
Ventilatory variables (tidal volume, respiratory frequency, and minute ventilation) were calculated for 30–45 s (typically 100–150 breaths) of the normoxia exposure (baseline) and for the last 10 s (typically 30–60 breaths) of each minute of hypoxia, excluding obvious movement artifacts, sighs, or vocalizations. To determine whether altered chemosensitivity might increase breathing variability, the coefficient of variation was calculated for 75 breaths that were either consecutive or interrupted only once by movement artifacts or vocalizations; only records that met these criteria were included in the variability analysis.
Metabolism measurements.
O2 consumption could not be measured at the same time as ventilation measurements due to the high gas flow rates required for the latter. Therefore, metabolic measurements were made on separate groups of neonatal rats aged P4, P7, and P14 from seven control litters and seven hyperoxia litters; individuals from three to four separate litters were used at each age per treatment group.
To measure O2 consumption, rats were placed into an acrylic, cylindrical respirometer chamber (5 cm ID, ∼225 ml); rats were able to move around within the chamber. The chamber was placed horizontally into a temperature-controlled incubator; air temperature within the chamber was monitored continuously and maintained at 32–34°C as described above. Airflow through the chamber was set at 200 ml/min STPD using a gas mixing mass flow controller (MFC-4; Sable Systems, Las Vegas, NV) and valves (series 840; Sierra Instruments, Monterey, CA). Fractional concentrations of O2 and CO2 in air entering and exiting the respirometer chamber were measured (S-3A and CD-3A analyzers; AEI Technologies) and recorded to computer at a sampling rate of 10 Hz (PowerLab 8SP and Chart 5.2 software, ADInstruments) to calculate metabolic O2 consumption; air was dried (Direrite, W.A. Hammond Drierite, Xenia, OH) before passing through the gas analyzers.
Even at the low gas flow rates used, it was necessary to place two P4 or two P7 rats (from the same litter) in the respirometer chamber simultaneously to achieve a sufficient change in the O2 of air entering and exiting the chamber; the O2 consumption of the pair was divided by their combined mass and used as a single replicate. P14 rats were studied individually. At all ages, rats were exposed to normoxia (21% O2) for 20 min followed by hypoxia (12% O2) for 20 min. O2 consumption was calculated using the average gas concentrations over the final 5 min of each exposure.
Statistical analysis.
Preliminary analysis revealed no interaction between the sex of the rat and the effects of hyperoxia on baseline or hypoxic ventilation at any age (2-way ANOVA; all sex × treatment, P > 0.05, data not shown). Therefore, data for male and female rats were pooled for all statistical comparisons. Similarly, preliminary analysis revealed no effect of ambient temperature (in the narrow range of 32–34°C) on any of the variables studied (linear regression; all P > 0.05, data not shown), so ambient temperature was not included as a covariate in our statistical analyses.
Body mass as well as baseline ventilation, breathing variability, and O2 consumption were compared among age and treatment groups using a two-way ANOVA and Student-Newman-Keuls (SNK) post hoc tests. Ventilation and metabolism during hypoxia were also expressed as a percentage change from baseline. The time course for changes in ventilation over the 8-min hypoxic exposure was first compared between treatment groups using two-way repeated-measures ANOVA and SNK post hoc tests separately at each age. Subsequently, the HVR was divided into early and late phases by restricting the analysis to minutes 1 and 8 of the hypoxic exposure, respectively. The early and late phases of the HVR, along with the metabolic response to hypoxia, were then compared among age and treatment groups using a two-way ANOVA and SNK post hoc tests. All statistical tests were run using SigmaStat 3.11 (SPSS, Chicago, IL), and P < 0.05 was considered significant. Values are reported as means ± SE.
RESULTS
Body mass.
No obvious differences were observed in the size or behavior of rats raised in normoxia (control) or 60% O2 (hyperoxia) at any of the ages studied. For the ventilation study, body mass was 9.0 ± 0.4 vs. 9.7 ± 0.3 g at P4, 12.9 ± 0.3 vs. 13.7 ± 0.4 g at P6–7, and 27.8 ± 0.8 vs. 28.6 ± 0.7 g at P13–14 for control and hyperoxia rats, respectively (see Fig. 1 for sample sizes). While there was a significant increase in mass with age as expected (P < 0.001), exposure to hyperoxia had no effect (treatment, P = 0.09; treatment × age, P = 0.99).
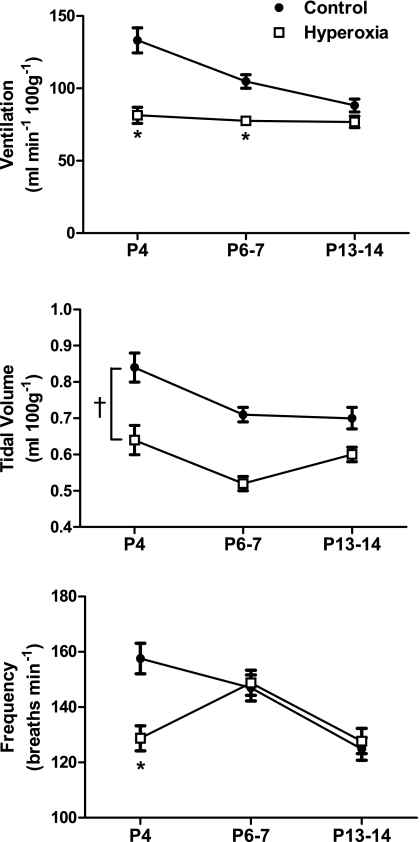
Fig. 1.
Minute ventilation, tidal volume, and respiratory frequency in normoxia for neonatal rats raised in 21% O2 (control) or 60% O2 (hyperoxia). Values are means ± SE. Sample sizes (P4, P6–7, and P13–14) are 17, 16, and 15 for control and 15, 15, and 17 for hyperoxia. Where treatment × age was significant, *P < 0.05 vs. control within the same age group. †P < 0.05 for treatment main effect. For clarity, only statistical comparisons between treatment groups are presented; please see the text for comparisons among age groups. P4, P6–7, and P13–14 refers to postnatal ages of 4 days, 6–7 days, and 13–14 days, respectively.
Normoxic ventilation.
The effect of hyperoxia on baseline ventilation varied depending on the age group being considered (treatment × age, P = 0.001) (Fig. 1). Hyperoxia rats had significantly lower ventilation at P4 (−39%) and P6–7 (−26%) compared with control rats (both P < 0.001). However, since baseline ventilation decreased with age in control rats (P6–7 and P13–14 vs. P4, both P < 0.001) but not in hyperoxia rats (all P > 0.05), there was no longer a difference in baseline ventilation between groups by P13–14 (P = 0.14) (Fig. 1). The reduced baseline ventilation in hyperoxia rats was mediated by lower tidal volumes at all ages (treatment, P < 0.001), while respiratory frequency was only lower at P4 (treatment × age, P = 0.001; post hoc comparison at P4, P < 0.001) (Fig. 1).
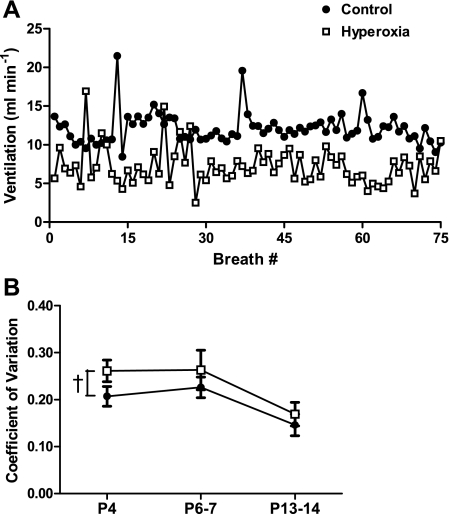
Variability in minute ventilation was quite high in neonatal rats (Fig. 2), with mean coefficients of variation (CV) ranging from 15 to 26% across ages and treatments. There was a significant effect of age (P < 0.001), with the CV for baseline ventilation being about one-third less at P13–14 than at P4 or P6–7 (both P < 0.001). Pooled across ages, however, the CV was ∼20% greater in hyperoxia rats than in control rats (23 vs. 19%; treatment, P = 0.03); no significant interaction between treatment and age was detected for breathing variability. This increased variability did not appear to be related to the occurrence of apneas (Fig. 2A). Neither the CV for tidal volume nor the CV for breath duration (i.e., instantaneous frequency) was statistically different between treatment groups when considered separately (treatment and treatment × age, all P > 0.05; data not shown).
Fig. 2.
Breathing variability in normoxia for neonatal rats raised in 21% O2 (control) or 60% O2 (hyperoxia). In A, instantaneous ventilation is plotted for 75 consecutive breaths for representative P4 control and hyperoxia rats. Although both individuals display periodic peaks in ventilation, note the greater breath-to-breath variability in the hyperoxia rat. In B, the coefficient of variation (CV) for instantaneous ventilation is presented for control and hyperoxia rats at P4, P6–7, and P13–14. Values are means ± SE. Sample sizes (P4, P6–7, and P13–14, respectively) are 16, 15, and 15 for control and 13, 12, and 16 for hyperoxia. †P < 0.05 for treatment main effect. For clarity, only statistical comparisons between treatment groups are presented; please see the text for comparisons among age groups.
Hypoxic ventilation.
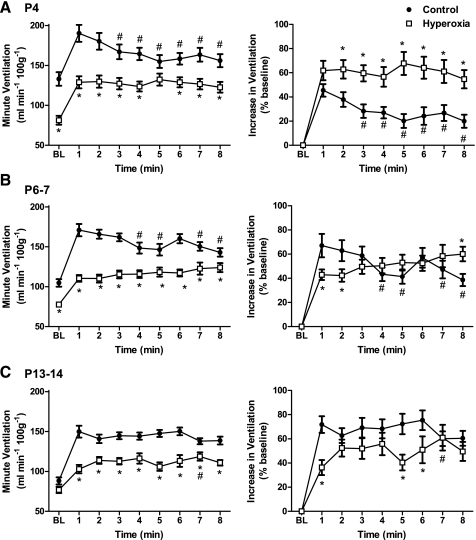
The pattern of the HVR differed between treatment groups at each age studied (treatment × time, all P < 0.01), and this was apparent in both the raw data and when the data were normalized as a percentage increase from baseline (Fig. 3). At P4 and P6–7, the ventilatory response to 12% O2 was biphasic in the control rats, with an initial peak in the first minute of hypoxia followed in subsequent minutes by a partial return toward baseline. The secondary decline in ventilation appeared reduced at P6–7 compared with P4, and control rats exhibited a sustained increase in ventilation at P13–14 (i.e., the HVR was no longer biphasic).
Fig. 3.
Changes in ventilation during an 8-min exposure to 12% O2 for P4 (A), P6–7 (B), and P13–14 rats (C) raised in 21% O2 (control) or 60% O2 (hyperoxia). Responses are presented both in units of raw ventilation (left panels) and as a percentage increase from baseline (BL) (right panels). Values are means ± SE. Sample sizes (P4, P6–7, and P13–14, respectively) are 17, 16, and 15 for control and 15, 15, and 17 for hyperoxia. *P < 0.05 vs. control within the same minute of hypoxia. #P < 0.05 vs. minute 1 of hypoxia within the same treatment group.
In contrast, hyperoxia rats exhibited a sustained increase in ventilation at all three ages (Fig. 3). At P4, this resulted in a greater increase in ventilation at minutes 2–8 (relative to baseline) in hyperoxia rats compared with control rats (all P ≤ 0.01). At P6–7 and P13–14, however, due to a smaller initial increase in ventilation (hyperoxia vs. control at minute 1, both P ≤ 0.01), differences in the HVR tended to be smaller between hyperoxia and control rats at minutes 2–8 (see Fig. 3, right, for statistical comparisons at each minute of hypoxia). When ventilation is expressed in raw units (Fig. 3, left), however, minute ventilation was reduced in hyperoxia rats throughout the hypoxic exposure at all ages (except minute 6 at P4). This is primarily explained by the lower baseline ventilation at P4 and P6–7 and the smaller initial increase in ventilation at P6–7 and P13–14.
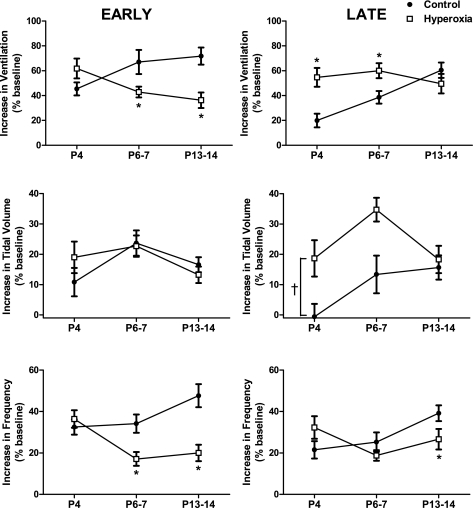
To compare the effects of hyperoxia on the HVR across ages, we divided the HVR (expressed as a percentage increase from baseline) into an early phase (minute 1) and a late phase (minute 8). For the early HVR (Fig. 4, left), the effect of hyperoxia varied with age (treatment × age, P < 0.001). Specifically, the early HVR was similar between hyperoxia and control rats at P4 (P = 0.10). However, the magnitude of this initial rise in ventilation increased with age in control rats (P = 0.03 for P6–7 and P = 0.02 for P13–14 vs. P4, respectively) while it decreased with age in hyperoxia rats (P = 0.03 for P13–14 vs. P4). Consequently, the early HVR was significantly reduced at P6–7 (P = 0.02) and P13–14 (P < 0.001) compared with control rats. This effect is nearly completely explained by a smaller increase in respiratory frequency during the first minute of hypoxia in hyperoxia rats (P < 0.01 at P6–7 and P < 0.001 at P13–14).
Fig. 4.
Early (left panels) and late (right panels) phases of the hypoxic ventilatory response to 12% O2 in neonatal rats raised in 21% O2 (control) or 60% O2 (hyperoxia). Values are means ± SE. Sample sizes (P4, P6–7, and P13–14, respectively) are 17, 16, and 15 for control and 15, 15, and 17 for hyperoxia. Where treatment × age was significant, *P < 0.05 vs. control within the same age group. †P < 0.05 for treatment main effect. For clarity, only statistical comparisons between treatment groups are presented; please see the text for comparisons among age groups.
The effect of perinatal hyperoxia also varied with age for the late phase of the HVR (treatment × age, P < 0.01) (Fig. 4, right). While the control rats exhibited a progressive increase in the magnitude of the late HVR with age (P = 0.04 for P6–7 and P < 0.001 for P13–14 vs. P4, respectively), the late HVR was similar across ages in hyperoxia rats. Consistent with their sustained increase in ventilation during acute hypoxia (see above), hyperoxia rats displayed a greater late HVR at P4 (P < 0.001) and P6–7 (P = 0.02) compared with control rats; no differences were observed at P13–14. Differences in the HVR between treatment groups at P4 and P6–7 reflect sustained, and consequently larger, increases in tidal volume in hyperoxia rats (treatment, P < 0.001) (Fig. 4).
Metabolic rate.
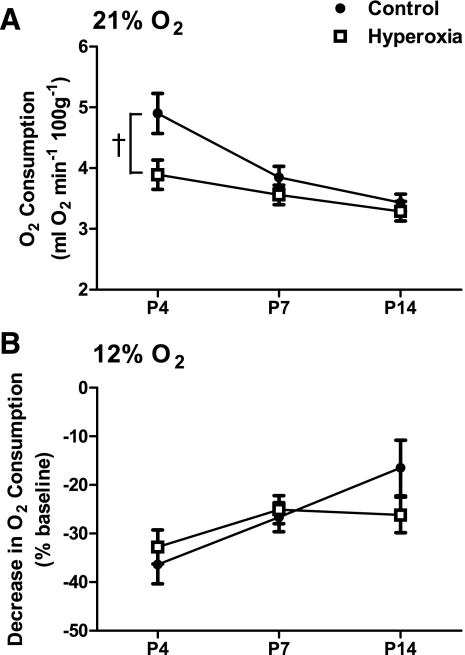
Baseline mass-specific O2 consumption decreased with age independent of the perinatal treatment group (age, P < 0.001). Overall, hyperoxia rats exhibited significantly lower O2 consumption during normoxia than control rats (treatment, P = 0.01) (Fig. 5A). Although we were unable to detect a statistically significant interaction between treatment and age (P = 0.12), visual inspection of Fig. 5A suggests that this effect is driven by differences at P4 (−21%) and, to a much lesser extent, P6–7 (−8%). These trends for O2 consumption across age groups appear to parallel those observed for minute ventilation (see Fig. 1).
Fig. 5.
Metabolic O2 consumption for neonatal rats raised in 21% O2 (control) or 60% O2 (hyperoxia). O2 consumption is presented in absolute units for rats in normoxia (21% O2; A) and as a percentage decrease from baseline for the same rats in 12% O2 (B). Values are means ± SE. Sample sizes (P4, P7, and P14) are 13, 9, and 9 for control and 11, 12, and 13 for hyperoxia. †P < 0.05 for treatment main effect. For clarity, only statistical comparisons between treatment groups are presented; please see the text for comparisons among age groups.
Neonatal rats decreased their mass-specific O2 consumption during a 20-min exposure to 12% O2 in both treatment groups (Fig. 5B). This response was reduced in the older rats (age, P < 0.01), but hyperoxia and control rats exhibited similar degrees of hypoxic hypometabolism (treatment, P = 0.64; treatment × age, P = 0.19).
DISCUSSION
This study revealed several novel effects of chronic hyperoxia on normoxic ventilation and the acute HVR of neonatal rats. The major findings were 1) diminished pulmonary ventilation and metabolic O2 consumption in normoxia at the younger ages studied (i.e., P4 and P6–7); 2) increased breathing variability during normoxia; 3) attenuation of the early HVR by P6–7, but after P4; and 4) a sustained increase in ventilation during hypoxia at all ages studied (vs. the normal biphasic HVR expected at the younger ages). Together, these results suggest a complex interaction between inhibitory and excitatory effects of chronic hyperoxia on the developing respiratory control system: hyperoxia enhances the late HVR, on one hand, while progressively impairing the early, carotid body-mediated phase of the HVR on the other.
Normoxic ventilation in hyperoxia-treated rats.
Rats exposed to 60% O2 from birth displayed substantially reduced ventilation in normoxia during the first postnatal week. This effect was no longer evident at P14, which is consistent with previous studies reporting no lasting effect of perinatal hyperoxia (2 wk, 60% O2) on normoxic ventilation in adult rats (3, 5). Across all ages, hyperoxia-treated rats also exhibited a more variable breathing pattern. It is not clear, however, if this modest (∼20%) increase in variability is biologically significant since there was no obvious increase in the occurrence of apneas. Although impaired sensitivity of peripheral chemoreceptors may lead to breathing instability (24), variability was also increased at P4 despite an apparently normal early HVR. This could indicate that chronic hyperoxia alters other components of the respiratory control system that regulate eupneic breathing as well.
The unexpectedly low ventilation at P4 and P6–7 may not reflect a true hypoventilation since it is at least partially explained by the lower metabolic rates observed in hyperoxia-treated rats. It is not clear, however, why chronic hyperoxia would lower the rate of O2 consumption. There was no evidence that hyperoxia impaired postnatal growth in neonates in the present study. Moreover, previous studies of rats acutely exposed to hyperoxia have found no change (25) or an increase in O2 consumption (18) in rats during the exposure, so there is no reason to predict a lingering decrease in metabolism on return to normoxia.
It is interesting to note that mass-specific metabolic rate and ventilation generally decrease in parallel between P4 and P14 in Sprague-Dawley rats (13, 33), which is also the pattern observed for control rats in the present study. Mass-specific O2 consumption changes little between P14 and weaning (P21) in rats (33). Thus, with the exception of a transient increase at P13, neonatal rats maintain a fairly stable ratio of ventilation to metabolism (i.e., convection requirement) over this period of postnatal development (i.e., P4-P21) (33). In contrast, mass-specific metabolic rate and ventilation changed little with age in hyperoxia-treated rats, causing the values for hyperoxia-treated and control rats to converge around P14. One potential interpretation, therefore, is that hyperoxia-treated rats obtained a mature phenotype for normoxic ventilation and metabolism at an earlier age.
Ventilation and metabolism were measured on separate groups of rats in the present study, precluding the calculation of the convection requirement for individuals. Using the group means for ventilation and O2 consumption at each age, however, the O2 convection requirement tended to be lower in hyperoxia-treated rats than in age-matched controls (P4: 21 vs. 27, P6–7: 22 vs. 27, P13–14: 24 vs. 26). Although these ratios must be interpreted cautiously since they are based on different animals studied under different experimental conditions, this analysis suggests that metabolism may not entirely explain the reduced ventilation observed in hyperoxia-treated rats. It is possible, for example, that chronic hyperoxia diminishes the carotid body's contribution to normoxic ventilatory drive (8, 38) by reducing the number of carotid chemoafferent neurons (22) and basal chemoreceptor activity (16). Carotid body activity remains low at P14 (16) despite normalization of normoxic ventilation. Therefore, if carotid body dysfunction does contribute to reduced normoxic ventilation in the first postnatal week (i.e., P4 and P6–7), compensatory plasticity would need to emerge by P13–14 elsewhere in the respiratory control system. Consistent with this hypothesis, normoxic ventilation spontaneously recovers in neonatal rats within a week following carotid body denervation (38).
HVR in hyperoxia-treated rats.
The HVR is biphasic in neonatal rats as in other mammals (7, 20). The initial increase in ventilation reflects the rapid activation of peripheral arterial chemoreceptors, primarily the carotid body. In immature mammals, however, hypoxia also activates inhibitory mechanisms in the CNS that gradually depress ventilation (7, 39). In our study, we observed changes in both the early, carotid body-mediated phase of the HVR and the late phase of the HVR in response to chronic hyperoxia. Hyperoxia-treated and control rats had similar metabolic responses to hypoxia, so observed differences in the HVR are unlikely to involve changes in O2 demand.
The magnitude of the early phase of the HVR increased with age in control rats but decreased with age in hyperoxia-treated rats. The early HVR often increases during the neonatal period in mammals (7) and mirrors the well-established postnatal maturation of carotid body O2 sensitivity (11, 15). The decrease in the early HVR in hyperoxia-treated rats, however, likely reflects progressive impairment of carotid body function. The time course for changes in carotid body function during chronic hyperoxia is only beginning to be elucidated but correlates well with the observed changes in the early HVR. The number of carotid body glomus cells and chemoafferent neurons decreases within the first week of exposure to 60% O2 (22), and these effects may be apparent in as few as 4 days (Dmitrieff EF, Broge TA Jr, Piro SE, Bavis RW, unpublished observations). In addition, Donnelly et al. (17) studied carotid chemoreceptor single-unit activity and glomus cell calcium responses to hypoxia in rats exposed to 60% O2 beginning at P7. These experiments revealed diminished O2 sensitivity of carotid chemoreceptors after only 5 days in hyperoxia. Although additional plasticity downstream of the carotid body cannot be excluded, these data indicate that attenuation of the early HVR at P6–7 and P13–14 is caused by abnormal carotid body development. Indeed, carotid body dysfunction is primarily responsible for the long-lasting, if not permanent, attenuation of hypoxic responses observed in adult rats after perinatal hyperoxia (23, 32).
Control rats exhibited a biphasic ventilatory response to 12% O2 at P4 and P6–7, but not at P13–14. The observed decrease in the magnitude of ventilatory depression with advancing age is consistent with normal postnatal maturation of the HVR (7, 20). For example, Eden and Hanson (20) reported a significant ventilatory decline between minutes 1 and 4 of hypoxia in their P5 rats, but not in P7 or P14 rats, in response to 12% O2; at younger ages (P1 and P3), ventilation often fell below the baseline, normoxic value by the fourth minute of hypoxia. While the age at which the biphasic HVR transitioned into a mature, sustained increase in ventilation varied with the severity of hypoxia (i.e., the biphasic HVR was evident in older rats when lower inspired O2 levels were used), the HVR appeared fully mature by P14.
In contrast to our control rats, we did not observe a biphasic HVR in hyperoxia-treated rats at any of the ages studied; this result confirms and extends a similar observation for P5 hyperoxia-treated rats (30% O2 from birth) reported by Eden and Hanson (19) in a conference abstract. Whereas previous studies have emphasized changes in carotid body function after perinatal hyperoxia (reviewed in Ref. 1), the sustained increase in ventilation in young hyperoxia-treated rats suggests that hyperoxia is also altering central components of the HVR. Indeed, it appears as though hyperoxia is hastening maturation of the late HVR, perhaps shifting the balance from inhibitory toward excitatory neuromodulation in the CNS at an earlier age. Along these lines, it is important to note that the enhanced late HVR in hyperoxia-treated rats at P4 and P6–7 is due to a sustained increase in tidal volume (consistent with the normal maturation of the late HVR in rats; see Ref. 20) rather than an abnormally high frequency response.
Whether or not hyperoxia alters the rate or timing of normal maturational processes, it is also possible that some other form of excitatory plasticity contributes to the enhanced late HVR of hyperoxia-treated neonatal rats. Hyperoxia has been shown to have a variety of effects on respiratory control in adult animals, often mediated by reactive oxygen species (ROS) (reviewed in Ref. 14). For example, brief exposures to hyperoxia (10–15 min of 100% O2) have been shown to enhance the HVR in adult humans and rats (26, 28, 36). This plasticity is abolished by inhibitors of the O2-dependent enzyme neuronal nitric oxide synthase, implicating nitric oxide in the mechanism (26). Moreover, Mulkey et al. (35), using the brain stem slice preparation, demonstrated that hyperoxia increases the firing rate in a subpopulation of neurons in the solitary complex linked to respiratory control. Although neither vitamin E nor a powerful superoxide dismutase mimetic were effective at blocking long-lasting effects of perinatal hyperoxia on the adult carotid body and/or HVR (5), these experiments cannot exclude a role for ROS in transient plasticity in neonatal respiratory control.
O2 as a stimulus for postnatal maturation of respiratory control.
In placental mammals, birth is associated with a rapid rise in arterial O2. This “relative hyperoxia” is recognized as an important stimulus for physiological changes that mark the transition from fetal to neonatal life, including alterations in circulation (12), brain neurochemistry (30), and breathing (10). Perhaps the best evidence for this in respiratory control has been heterokairy in the postnatal maturation of carotid body O2 sensitivity. Heterokairy is defined as plasticity in the timing and/or rate of physiological development (40), akin to heterochrony but occurring at the level of individuals instead of species. For example, hypoxia [mimicking partial pressures of O2 (Po2) in utero] delays resetting of carotid body O2 sensitivity at the level of the glomus cell in rats (41). It appears that O2 sensitivity will reset normally once the rat is returned to normoxia (41) or (eventually) even if maintained in hypoxia (21). Conversely, sheep ventilated with hyperoxic gas mixtures in utero exhibited increased carotid body sensitivity to hypoxia compared with sheep ventilated with gases to maintain normal fetal Po2 (9), suggesting premature resetting of O2 sensitivity. These effects are presumably obscured during longer periods of hyperoxia in which carotid body function becomes progressively impaired through an independent mechanism (1).
The findings of the present study indicate that postnatal O2 may also regulate other aspects of respiratory control development. While other forms of plasticity cannot be excluded yet, changes in normoxic ventilation and metabolism and in the late HVR are consistent with earlier maturation of the respiratory phenotype during moderate hyperoxia (i.e., heterokairy). At least one other study supports the possibility that O2 regulates development of the biphasic HVR. Eden and Hanson (21) exposed rats to chronic hypoxia (13–15% O2) for the first 5–10 wk of life. This treatment nearly abolished the early HVR, as previously demonstrated in a variety of species (reviewed in Ref. 1). Interestingly, these rats showed a weak HVR to severe hypoxia (8% O2) that was distinctly biphasic (21); rats typically exhibit a sustained increase in ventilation to 8% O2 by 2 wk of age (20). In other words, it is possible that hypoxia delays the transition from the biphasic to sustained HVR, directly opposite of the effects proposed for hyperoxia in the present study. Taken together, these data suggest that perinatal O2 levels modulate the developmental programs for both the peripheral and central components of the respiratory control system.
GRANTS
This work was supported by National Institutes of Health Grant R15-HL-083972 and by the Biology Department at Bates College.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Bavis RW, Mitchell GS. Long-term effects of the perinatal environment on respiratory control. J Appl Physiol 104: 1220–1229, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Bavis RW, Olson EB, Jr, Mitchell GS. Critical developmental period for hyperoxia-induced blunting of hypoxic phrenic responses in rats. J Appl Physiol 92: 1013–1018, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Bavis RW, Russell KER, Simons JC, Otis JP. Hypoxic ventilatory responses in rats after hypercapnic hyperoxia and intermittent hyperoxia. Respir Physiol Neurobiol 155: 193–202, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Bavis RW, Olson EB, Jr, Vidruk EH, Bisgard GE, Mitchell GS. Level and duration of developmental hyperoxia influence impairment of hypoxic phrenic responses in rats. J Appl Physiol 95: 1550–1559, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Bavis RW, Wenninger JM, Miller BM, Dmitrieff EK, Olson EB, Jr, Mitchell GS, Bisgard GE. Respiratory plasticity after perinatal hyperoxia is not prevented by antioxidant supplementation. Respir Physiol Neurobiol 160: 301–312, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bisgard GE, Olson EB, Jr, Wang ZY, Bavis RW, Fuller DD, Mitchell GS. Adult carotid chemoafferent responses to hypoxia after 1, 2, and 4 wk of postnatal hyperoxia. J Appl Physiol 95: 946–952, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Bissonnette JM. Mechanisms regulating hypoxic respiratory depression during fetal and postnatal life. Am J Physiol Regul Integr Comp Physiol 278: R1391–R1400, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Blain GM, Smith CA, Henderson KS, Dempsey JA. Contribution of the carotid body chemoreceptors to eupneic ventilation in the intact, unanesthetized dog. J Appl Physiol 106: 1564–1573, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Blanco CE, Hanson MA, McCooke HB. Effects on carotid chemoreceptor resetting of pulmonary ventilation in the fetal lamb in utero. J Dev Physiol 10: 167–174, 1988 [PubMed] [Google Scholar]
- 10. Carroll JL. Developmental plasticity in respiratory control. J Appl Physiol 94: 375–389, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Carroll JL, Kim I. Postnatal development of carotid body glomus cell O2 sensitivity. Respir Physiol Neurobiol 149: 201–215, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Cornfield DN. Developmental regulation of oxygen sensing and ion channels in the pulmonary vasculature. Adv Exp Med Biol 661: 201–220, 2010 [DOI] [PubMed] [Google Scholar]
- 13. Davis SE, Solhied G, Castillo M, Dwinell M, Brozoski D, Forster HV. Postnatal developmental changes in CO2 sensitivity in rats. J Appl Physiol 101: 1097–103, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Dean JB, Mulkey DK, Henderson RA, 3rd, Potter SJ, Putnam RW. Hyperoxia, reactive oxygen species, and hyperventilation: oxygen sensitivity of brainstem neurons. J Appl Physiol 96: 784–791, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Donnelly DF. Development of carotid body/petrosal ganglion responses to hypoxia. Respir Physiol Neurobiol 149: 191–199, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Donnelly DF, Kim I, Carle C, Carroll JL. Perinatal hyperoxia for 14 days increases nerve conduction time and the acute unitary response to hypoxia of rat carotid body chemoreceptors. J Appl Physiol 99: 114–119, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Donnelly DF, Bavis RW, Kim I, Dbouk HA, Carroll JL. Time course of alterations in pre- and post-synaptic chemoreceptor function during developmental hyperoxia. Respir Physiol Neurobiol 168: 189–197, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dotta A, Mortola JP. Effects of hyperoxia on the metabolic response to cold of the newborn rat. J Dev Physiol 17: 247–250, 1992 [PubMed] [Google Scholar]
- 19. Eden GJ, Hanson MA. Effect of hyperoxia from birth on the carotid chemoreceptor and ventilatory responses of rats to acute hypoxia (Abstract). J Physiol 374: 24P, 1986 [Google Scholar]
- 20. Eden GJ, Hanson MA. Maturation of the respiratory response to acute hypoxia in the newborn rat. J Physiol 392: 1–9, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eden GJ, Hanson MA. Effects of chronic hypoxia from birth on the ventilatory response to acute hypoxia in the newborn rat. J Physiol 392: 11–19, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Erickson JT, Mayer C, Jawa A, Ling L, Olson EB, Jr, Vidruk EH, Mitchell GS, Katz DM. Chemoafferent degeneration and carotid body hypoplasia following chronic hyperoxia in newborn rats. J Physiol 509: 519–526, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fuller DD, Bavis RW, Vidruk EH, Wang ZY, Olson EB, Jr, Bisgard GE, Mitchell GS. Life-long impairment of hypoxic phrenic responses in rats following 1 month of developmental hyperoxia. J Physiol 538: 947–955, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gauda EB, Cristofalo E, Nunez J. Peripheral arterial chemoreceptors and sudden infant death syndrome. Respir Physiol Neurobiol 157: 162–170, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Gautier H, Murariu C. Metabolic and ventilatory responses to CO hypoxia at different levels of oxygenation in the rat. Respir Physiol 129: 307–315, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Gozal D. Potentiation of hypoxic ventilatory response by hyperoxia in the conscious rat: putative role of nitric oxide. J Appl Physiol 85: 129–132, 1998 [DOI] [PubMed] [Google Scholar]
- 27. Hanson MA, Eden GJ, Nijhuis JG, Moore PJ. Peripheral chemoreceptors and other oxygen sensors in the fetus and newborn. In: Chemoreceptors and Reflexes in Breathing: Cellular and Molecular Aspects, edited by Lahiri S, Forster RE, Davies RO, Pack AI. New York: Oxford Univ. Press, 1989, p. 113–120 [Google Scholar]
- 28. Honda Y, Tani H, Masuda A, Kobayashi T, Nishino T, Kimura H, Masuyama S, Kuriyama T. Effect of prior O2 breathing on ventilatory response to sustained isocapnic hypoxia in adult humans. J Appl Physiol 81: 1627–1632, 1996 [DOI] [PubMed] [Google Scholar]
- 29. Huang YH, Brown AR, Costy-Bennett S, Luo Z, Fregosi RF. Influence of prenatal nicotine exposure on postnatal development of breathing pattern. Respir Physiol Neurobiol 143: 1–8, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Lagercrantz H, Pequignot J, Pequignot JM, Peyrin L. The first breaths of air stimulate noradrenaline turnover in the brain of the newborn rat. Acta Physiol Scand 144: 433–438, 1992 [DOI] [PubMed] [Google Scholar]
- 31. Ling L, Olson EB, Jr, Vidruk EH, Mitchell GS. Attenuation of the hypoxic ventilatory response in adult rats following one month of perinatal hyperoxia. J Physiol 495: 561–571, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ling L, Olson EB, Jr, Vidruk EH, Mitchell GS. Developmental plasticity of the hypoxic ventilatory response. Respir Physiol 110: 261–268, 1997 [DOI] [PubMed] [Google Scholar]
- 33. Liu Q, Fehring C, Lowry TF, Wong-Riley MT. Postnatal development of metabolic rate during normoxia and acute hypoxia in rats: implication for a sensitive period. J Appl Physiol 106: 1212–1222, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mortola JP. Breathing pattern in newborns. J Appl Physiol 56: 1533–1540, 1984 [DOI] [PubMed] [Google Scholar]
- 35. Mulkey DK, Henderson RA, 3rd, Ritucci NA, Putnam RW, Dean JB. Oxidative stress decreases pHi and Na+/H+ exchange and increases excitability of solitary complex neurons from rat brain slices. Am J Physiol Cell Physiol 286: C940–C951, 2004 [DOI] [PubMed] [Google Scholar]
- 36. Pokorski M, Kolesnikova E, Marczak M, Budzinska K. Neurotransmitter mechanisms in the enhancement of the hypoxic ventilatory response by antecedent hyperoxia in the anesthetized rat. J Physiol Pharmacol 56: 433–446, 2005 [PubMed] [Google Scholar]
- 37. Prieto-Lloret J, Caceres AI, Obeso A, Rocher A, Rigual R, Agapito MT, Bustamante R, Castañeda J, Perez-Garcia MT, López-López JR, González C. Ventilatory responses and carotid body function in adult rats perinatally exposed to hyperoxia. J Physiol 554: 126–144, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Serra A, Brozoski D, Hedin N, Franciosi R, Forster HV. Mortality after carotid body denervation in rats. J Appl Physiol 91: 1298–1306, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Simakajornboon N, Kuptanon T. Maturational changes in neuromodulation of central pathways underlying hypoxic ventilatory response. Respir Physiol Neurobiol 149: 273–286, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Spicer JI, Rundle SD. Plasticity in the timing of physiological development: physiological heterokairy—what is it, how frequent is it, and does it matter? Comp Biochem Physiol A 148: 712–719, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Sterni LM, Bamford OS, Wasicko MJ, Carroll JL. Chronic hypoxia abolished the postnatal increase in carotid body type I cell sensitivity to hypoxia. Am J Physiol Lung Cell Mol Physiol 277: L645–L652, 1999 [DOI] [PubMed] [Google Scholar]
- 42. Wang ZY, Bisgard GE. Postnatal growth of the carotid body. Respir Physiol Neurobiol 149: 181–190, 2005 [DOI] [PubMed] [Google Scholar]