Abstract
The first study, described in the companion article, reports that acute exposure of rat hippocampal slices to either hyperbaric oxygen (HBO: 2.84 and 4.54 atmospheres absolute, ATA) or normobaric reoxygenation (NBOreox; i.e., normobaric hyperoxia: 0.6 or 0.0 → 0.95 ATA) stimulates synchronous orthodromic activity in CA1 neurons, which includes activation of O2-induced potentiation (OxIP) and, in some cases, hyperexcitability (secondary population spikes, sPS). In this second study we tested the hypothesis that HBO and NBOreox increase orthodromic activity of CA1 neurons (oPS, orthodromic population spike) and OxIP via a combination of both increased excitatory synaptic transmission (field excitatory postsynaptic potential, fEPSP) and intrinsic excitability (antidromic population spike, aPS). HBO and NBOreox increased the oPS but rarely increased or potentiated the fEPSP. HBO exposure produced epileptiform antidromic activity, which was abolished during inhibition of fast GABAergic and glutamatergic synaptic transmission. Decreasing O2 from 0.95 ATA (control) to 0.6 ATA (intermediate O2) or 0.0 ATA (hypoxia) reversibly abolished the fEPSP, and reoxygenation rarely induced potentiation of the fEPSP or aPS. Intracellular recordings and antidromic field potential recordings, however, revealed that synaptic transmission and neuronal excitability were preserved, albeit at lower levels, in 0.60 ATA O2. Together, these data indicate that 1) the changes in excitatory postsynaptic activity are not required for stimulation of the oPS during and HBO/NBOreox or for activation of OxIP, suggesting the latter is a form of intrinsic plasticity; 2) HBO disinhibits spontaneous synaptic transmission to induce epileptiform activity; and 3) although synchronous synaptic activation of the CA1 neuronal population requires hyperoxia (i.e., 0.95 ATA O2), synaptic activation of individual CA1 neurons does not.
Keywords: hyperbaric oxygen, oxygen toxicity, synaptic transmission, oxygen-induced potentiation, oxidative stress
our companion study (9) describes the excitatory effects of an acute hyperoxic stimulus, applied over a broad range of barometric pressure, on the orthodromic population spike (oPS) of CA1 neurons in rat hippocampal slices. Sensitivity to hyperbaric hyperoxia (hyperbaric oxygen, HBO) and normobaric hyperoxia (NBOreox; reoxygenation following acute exposure to hypoxia or an intermediate level of O2), was characterized by an increased amplitude of the oPS. In addition, both forms of hyperoxia led to the sustained (≤46 min) elevation in orthodromic excitability following exposure to HBO or NBOreox, referred to as “O2-induced potentiation” (OxIP), and could induce hyperexcitability (i.e., secondary population spikes, sPS). Thus CA1 neuronal excitability increases with increasing brain tissue Po2 (PtO2) at normobaric and hyperbaric pressures and persists beyond the period of hyperoxic exposure.
In the present study we tested the hypothesis that both NBOreox and HBO elevate orthodromic excitability in CA1 neurons, including activation of OxIP and induction of sPS activity, via mechanisms involving an increase in both excitatory synaptic transmission and postsynaptic excitability. Accordingly, we measured changes in the field excitatory postsynaptic potential (fEPSP) activated orthodromically by Schaffer collateral stimulation during O2 manipulation. To assess changes in postsynaptic excitability during O2 manipulation, we measured changes in the antidromic population spike (aPS) resulting from electrical stimulation of the axons of CA1 neurons. In addition, intracellular recordings were made in selected experiments to determine whether synaptic transmission was maintained in individual CA1 neurons exposed to an intermediate level of O2.
Our findings indicate that an increase in the fEPSP is not required for stimulation of the oPS during HBO or induction of OxIP during and following HBO or NBOreox. These findings suggest that OxIP is a form of intrinsic neural plasticity rather than synaptic plasticity. HBO, however, can induce mild epileptiform activity (i.e., sPS) in CA1 neurons by reduction in spontaneous inhibitory synaptic input, since sPS can be induced by blockade of GABA receptors alone. Therefore, the increased orthodromic excitability in CA1 neurons caused by HBO and NBOreox appears to involve changes in both intrinsic neuronal mechanisms (O2-induced stimulation of the oPS and induction of OxIP) and alterations in spontaneous synaptic activity (sPS during antidromic recordings). In addition, our findings indicate that synchronized synaptic activation of the population of CA1 neurons is contingent on the condition of normobaric hyperoxia (0.95 ATA).1 Conversely, synaptic activation of an individual CA1 neuron is not, which is maintained in an intermediate level of O2 (0.6 ATA).
METHODS
Preparation of hippocampal brain slices.
All procedures were performed with approval of the Wright State University Laboratory Animal Care and Use Committee guidelines. Wright State University is accredited by AAALAC and is covered by NIH assurance no. A362-0. Sagittal corticohippocampal tissue slices were prepared at 400-μm intervals from male Sprague-Dawley rats (>25 days, body weight >100 g) anesthetized with 70–100% CO2 followed by rapid decapitation. The preparation of brain slices, experimental oxygen conditions, and protocols for working at normobaric and hyperbaric pressures were previously described (9).
Solutions.
The artificial cerebrospinal fluid (aCSF) used in this study was composed of (in mM) 125 NaCl, 5.0 KCl, 1.3 MgSO4, 26 NaHCO3, 1.24 KH2PO4, 2.4 CaCl2, and 10 glucose. Picrotoxin, MK-801, and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) were obtained from Sigma Chemical (St. Louis, MO).
Electrophysiology.
Electrophysiological recordings were conducted using an Axoclamp 2A or 2B microelectrode clamp amplifier (headstage gain = 0.1×; Molecular Devices, Sunnyvale, CA). Recording electrodes were made of borosilicate glass (1B100F-4; WPI, Sarasota, FL) fabricated using a P87 Flaming/Brown micropipette puller (Sutter Instrument, Novato, CA). Extracellular recording electrodes were filled with 3 M NaCl and had a tip resistance of <4 MΩ, whereas intracellular recording electrodes were filled with 4 M potassium acetate and had a tip resistance >70 MΩ. The bipolar stimulating electrode was fabricated from two twisted platinum wires (50-μm diameter, Teflon coated; AM-Systems catalog no. 771000).
The stimulating electrode was placed in the Schaffer collateral-commissural pathway to evoke the fEPSP, which was measured by placing a recording electrode in the stratum radiatum. To record the aPS, the stimulating electrode was placed at the border of stratum oriens (SO) and alveus while an extracellular recording electrode was placed in the stratum pyramidale. The electrical stimulus was set between 100 and 400 μA and ranged from 0.1 to 0.5 ms in duration (controlled by an A300 Pulsemaster and A-365 stimulus isolation unit; WPI). Stimulation intensity was selected on the basis of the minimum amount of current required to generate ∼50% of the maximal initial slope (mi) of either the fEPSP or the aPS amplitude.
For HBO experiments, extracellular recordings were established for a minimum of 6–10 min before the chamber was pressurized with helium. As reported in the first study (9), helium compression caused a transient inhibition of neural activity. Thus the control recordings for all HBO experiments were established at the new steady-state hyperbaric pressure. Pressurization to <4.13 ATA caused a transient inhibition of the fEPSP with less of an effect on the aPS (data not shown). At the new level of steady-state ambient pressure, the amount of electrical stimulation was adjusted to evoke a submaximal (∼50%) response of the fEPSP or aPS. In both normobaric and hyperbaric experiments, the initial control period in 0.95 ATA O2 lasted for 16 min. Recordings where the extracellular potential did not fluctuate more than ± 15% during the control period were considered to be stable and studied during O2 manipulation. After the control period, the superfusate was switched to one of the experimental O2 treatments for 16 min, followed by a 16-min recovery phase in 0.95 ATA O2. The ambient pressure (hyperbaric helium or normobaric air) surrounding the slice was maintained constant during all phases of the experiment. Likewise, bath temperature (36.5°C), Po2, and pH were constant, even during helium compression and decompression (9). Experiments using either 0.00 or 0.60 ATA O2 were conducted at ambient room pressure (∼1 ATA) in air, whereas HBO experiments were conducted at 2.43 and 4.13 ATA O2 in helium, as previously described (9).
Sharp microelectrode intracellular recordings of individual CA1 neurons were established at ambient pressure in 0.95 ATA O2 to assess the effects of intermediate oxygenation on synaptic transmission. Intracellular recordings were considered acceptable for study if, under the control condition at 0.95 ATA O2, the measured membrane potential (Vm) was more negative than −40 mV, the action potential overshot 0 mV, and an intracellular excitatory postsynaptic potential (EPSP) could be evoked by extracellular stimulation. To evoke an intracellular EPSP in a single neuron, the stimulating electrode was placed into the Schaffer collateral-commissural pathway and a submaximal amount of current (80 to 270 μA) was applied for 0.05–0.1 ms. Input resistance (Rin) was calculated from either a constant hyperpolarizing current injection (150–350 ms in duration, 0.1 Hz) or a current-voltage (I–V) curve generated from graded hyperpolarizing current injections.
Electrophysiological recordings were collected and stored using protocols previously described (9). Recordings of analog signals including ambient pressure inside the hyperbaric chamber, tissue bath temperature, and the electrical recordings were monitored continuously and stored for analyses.
Data analyses.
The aPS amplitude and the initial slope of the fEPSP (mi) were used to assess changes in postsynaptic excitability and excitatory synaptic transmission, respectively. The mi of the fEPSP was defined by the initial one-third of the fEPSP slope to avoid its contamination with the population spike whenever the population spike appeared during the latter phase of the fEPSP. Four sequential measurements of amplitude and mi values were averaged over 2 min. Data from individual experiments are expressed as a percentage of the baseline from that experiment (defined as the initial 16-min recording period in 0.95 ATA O2, which was assigned a value of 100%). The response of the CA1 population to a given O2 condition was summarized in an elapsed time plot where each data point is expressed as the mean ± SE. Differences between two means were determined using Student's t-test, whereas differences between three or more means were determined using one-way ANOVA followed by multiple comparisons testing (Dunnett's comparisons with control, 0.95 ATA O2). To determine whether a delta value was non-zero with respect to control conditions, a one-sample t-test was performed. In all cases, significance was determined if P < 0.05. Statistical analyses were conducted using either the KyPlot Data and Visualization software package (Kyence, Tokyo, Japan) or GraphPad InStat (GraphPad Software, San Diego, CA).
RESULTS
Effects of HBO on fEPSP.
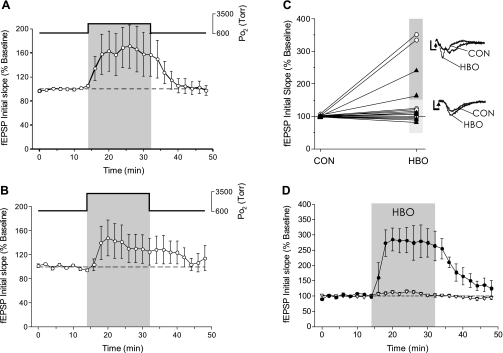
Increasing Po2 from 0.95 to 2.84 (n = 9, Fig. 1A) or 4.54 ATA O2 (n = 7, Fig. 1B) had no significant effect on the mean response of the fEPSP. Although both 2.84 and 4.54 ATA O2 appeared to increase the fEPSP response, the large variability of the response to HBO precluded statistical significance of the averaged responses. When the individual experiments from exposure to either 2.84 or 4.54 ATA O2 were grouped together (n = 16) and expressed as individual data pairs (ΔfEPSP in 0.95 → HBO), it is clear that in some cases HBO stimulated the fEPSP in excess of 150% (n = 4), whereas in most other slices it had no effect on the fEPSP (n = 12, Fig. 1C). Pooling the HBO data and dividing the 16 HBO experiments into fEPSP-stimulated and nonstimulated slices shows the reversible stimulation of the fEPSP by HBO in 25% of the hippocampal slices tested (Fig. 1D). In each case, the fEPSP rapidly decayed without any evidence of potentiation following HBO exposure.
Fig. 1.
Effect of hyperbaric oxygen (HBO) on the field excitatory postsynaptic potential (fEPSP) in CA1 neurons during electrical stimulation of Schaffer collaterals. A: the fEPSP response in CA1 neurons during HBO was variable. Although a 16-min exposure to 2.84 ATA O2 appeared to stimulate the fEPSP, no significance was found in the averaged response to 2.84 ATA O2 exposure (n = 9). B: similar to the response to 2.84 ATA O2, the fEPSP was variable and not significantly affected during a 16-min exposure to 4.54 ATA O2 (n = 7). C: plotting the individual fEPSP experiments before (control, CON; t = 0 min) and during the exposure (HBO; t = 32 min) to either 2.84 ATA O2 (○) or 4.54 ATA O2 (▴) revealed that HBO stimulated the fEPSP in a subset of experiments (n = 4/16). Raw data traces are shown from experiments where HBO exposure stimulated the fEPSP ≥150% or where HBO did not significantly affect the fEPSP. The arrowheads denote the stimulus artifact removed from trace. D: the response to HBO could be divided into 2 groups: stimulation (●) or no effect (▿); however, the predominant fEPSP response to HBO was no effect (n = 12/16).
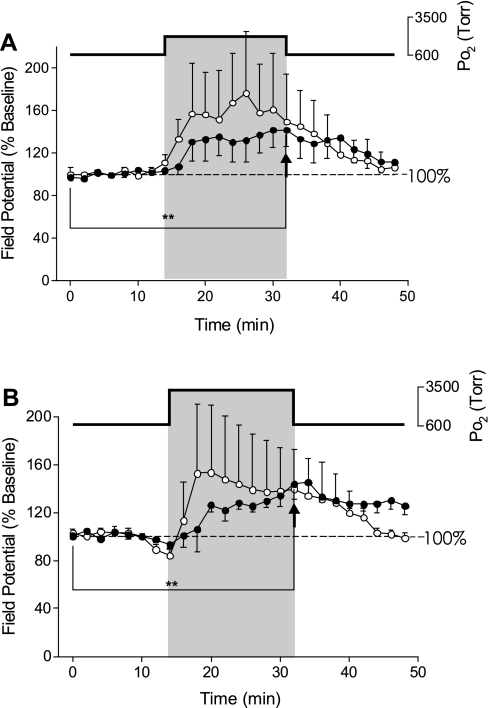
In a subset of the above experiments, we conducted simultaneous recordings of the fEPSP and oPS (Fig. 2). As previously reported (9), the oPS was significantly increased during exposure to 2.84 (Fig. 2A) and 4.54 ATA O2 (Fig. 2B) and exhibited OxIP on return to 0.95 ATA O2 from 4.54 ATA O2 (Fig. 2B). The magnitude of stimulation of the oPS was not significantly different between 2.84 and 4.54 ATA O2 (9). Again, as previously shown in Fig. 1, A and B, the fEPSP appeared to increase in parallel with the oPS during HBO, but the response was too variable, on average, to be significant. Thus, although the fEPSP is stimulated by HBO in some slices, an increase in the fEPSP is not a requirement for stimulation of the oPS in CA1 neurons during HBO or activation of OxIP.
Fig. 2.
Effects of HBO on simultaneous recordings of the fEPSP and orthodromic population spike (oPS). A: exposure to 2.84 ATA O2 caused variable effects on the fEPSP (○). While the fEPSP was stimulated in only 1 slice, the oPS (●) was significantly (**P < 0.01) stimulated by exposure to 2.84 ATA O2 compared with baseline (n = 5) as denoted by the arrowhead. B: simultaneous recordings (n = 4) during exposure to 4.54 ATA O2 caused a significant stimulation (**P < 0.01) of the oPS (●), as denoted by the arrowhead, with a significant O2-induced potentiation (OxIP) on return to 0.95 ATA O2, but again, the fEPSP (○) was stimulated by HBO in only 1 of the 4 preparations. The oPS data at both levels of HBO were pooled with experiments recording just the oPS as reported in Ref. 9.
Effects of normobaric O2 (0.00 and 0.60 ATA) and NBOreox on fEPSP.
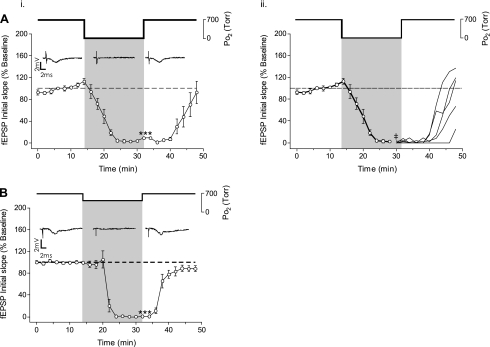
Lowering Po2 from 0.95 to 0.00 ATA O2 (hypoxia) significantly inhibited the fEPSP (n = 5, Fig. 3Ai). Recall that we have termed the use of 0% O2 as “hypoxia,” and not “anoxia,” because our measurements of PtO2 profiles in 400-μm-thick slices indicate a significant amount of O2 is present in the slice, which diffuses into the slice from the overlying atmosphere of room air (9, 15). At the end of the 16-min exposure to hypoxia, the initial slope of the fEPSP was 9.05 ± 5.23% of baseline. The fEPSP recovered toward initial baseline activity within 8 min following reoxygenation and reached 92.6 ± 22.8% of control activity by the end of the 16-min recovery period. No OxIP of the mean response of the fEPSP was observed following reoxygenation to 0.95 ATA (Fig. 3Ai), but the fEPSP responses were variable during the ensuing recovery period. Review of the individual records for each slice (Fig. 3Aii) reveals that some slices (n = 3/5) did overshoot the control baseline activity following reoxygenation (114–137%), whereas other slices (n = 2/5) exhibited only partial recovery (25–66%) toward the initial baseline level of activity 16 min after reoxygenation. Thus, in some slices, NBOreox following hypoxia did produce a potentiated fEPSP response that could be classified as OxIP.
Fig. 3.
Effect of 0.60 and 0.00 ATA O2 on the fEPSP. Ai: exposure to 16 min of 0.00 ATA O2 significantly inhibited the fEPSP (n = 5, ***P > 0.001). The fEPSP recovered to baseline within 16 min on return to the control 0.95 ATA O2. ii: the mean recovery response of the fEPSP from 0.00ATA O2 (i) was separated into the individual recoveries (beginning at ‡) showing that recovery of the fEPSP from hypoxia was variable, with some (n = 2/5) preparations not recovering to baseline and others overshooting baseline (OxIP) by the end of the 16-min recovery period (t = 48 min). B: exposure to 16 min of 0.60 ATA O2 also significantly inhibited the fEPSP (n = 5, ***P > 0.001), which recovered to baseline within 16 min on return to the control 0.95 ATA O2 with no preparation exhibiting an overshoot of baseline activity.
Lowering O2 from 0.95 to 0.60 ATA for 16 min also completely inhibited the fEPSP (n = 5, Fig. 3B). This inhibitory response was reversible; the fEPSP was no longer significantly different from preexposure baseline level after 6 min of reoxygenation. By the end of the 16-min recovery period, the fEPSP was 88.2 ± 5.1% of the initial baseline and not significantly different from the initial baseline response. Overshoot of the initial baseline activity, that is, OxIP, was not observed in any slice following reoxygenation from 0.6 ATA O2 and during the ensuing recovery period. In 4 of the 5 experiments, the fEPSP was followed for an additional 16 min of recovery and no OxIP of the fEPSP was observed following reoxygenation (data not shown).
Effects of HBO on aPS.
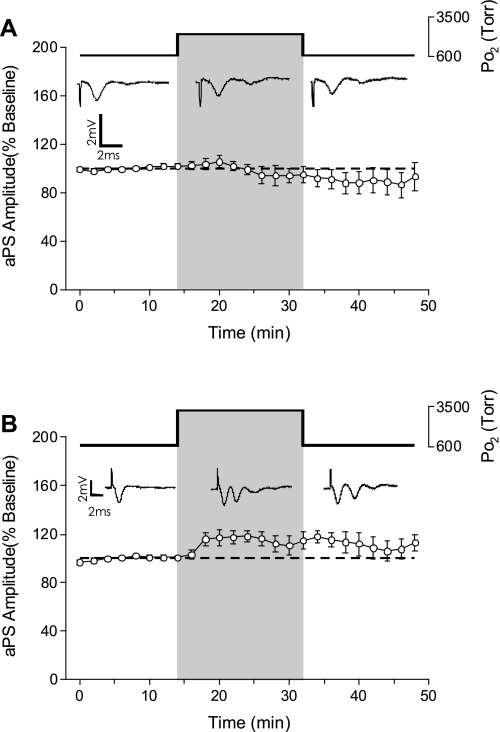
To determine the effects of hyperbaric hyperoxia on postsynaptic activity in CA1 neurons, the antidromic population spike (aPS) was recorded while the slice was exposed to either 2.84 or 4.54 ATA O2. Acute exposure to 2.84 ATA O2 did not affect the amplitude of the aPS (n = 6, Fig. 4A). During exposure to 2.84 ATA O2, however, sPS were evoked in 16% of the slices tested, which increased to 33% of the slices tested during the ensuing recovery period. Exposure to 4.54 ATA O2 also had no significant effect on aPS amplitude (n = 6, Fig. 4B); however, sPS were activated in 50% of the slices tested, which decreased to 33% of the slices during the recovery period. Thus, whereas the aPS did not exhibit any stimulation during HBO or potentiation following HBO, the sPS activated during HBO continued during the 16-min recovery period in approximately one-third of the slices tested.
Fig. 4.
Effects of HBO on the antidromic population spike (aPS). A: a 16-min exposure to 2.84 ATA O2 did not affect the amplitude of the aPS (n = 6). B: a 16-min exposure to 4.54 ATA O2 likewise did not significantly stimulate the amplitude of the aPS (n = 6). However, note the occurrence of secondary population spikes (sPS) during and following exposure to 4.54 ATA O2 (n = 3/6).
To determine whether the sPS activity during HBO (antidromic recordings) resulted from a change in spontaneous synaptic activity, a cocktail to block GABA- and glutamate-activated channels was added to the superfusate while recording the aPS before, during, and following exposure to 4.54 ATA O2. The cocktail consisted of 50 μM picrotoxin (GABAA receptor antagonist), 100 μM MK-801 [N-methyl-d-aspartate (NMDA)receptor antagonist], and 20 μM CNQX (3-hydroxy-5-methyl-4-isoxazole proprionic acid receptor antagonist). In the presence of this chemical synaptic blockade cocktail, exposure to 4.54 ATA O2 neither induced sPS nor stimulated the primary aPS (n = 7, Fig. 5A). The application of picrotoxin (50–65 μM) alone (n = 5, Fig. 5B) at 0.95 ATA O2, without further O2 manipulation, however, did induce secondary and tertiary population spikes in antidromic recordings without stimulation of the primary aPS.
Fig. 5.
Synaptic blockade medium prevents the occurrence of sPS induced during and following exposure to 4.54 ATA O2. A: in the presence of synaptic blockade (50 μM picrotoxin, 100 μM MK-801, 50 and 20 μM CNQX), exposure to 4.54 ATA O2 had no effect on the aPS amplitude, but the occurrence of sPS were eliminated (n = 7). Arrowhead denotes stimulus artifact removed from the raw data trace. B: exposure to the GABAA receptor antagonist picrotoxin (50–65 μM) alone while maintaining medium O2 tension (PmO2) constant at 0.95 ATA O2 caused no significant change in the aPS but consistently produced sPS (asterisk) in all slices tested during exposure and recovery (n = 5). Arrowhead denotes stimulus artifact removed from the raw data trace.
Effects of normobaric O2 (0.60 and 0.00 ATA) and NBOreox on aPS.
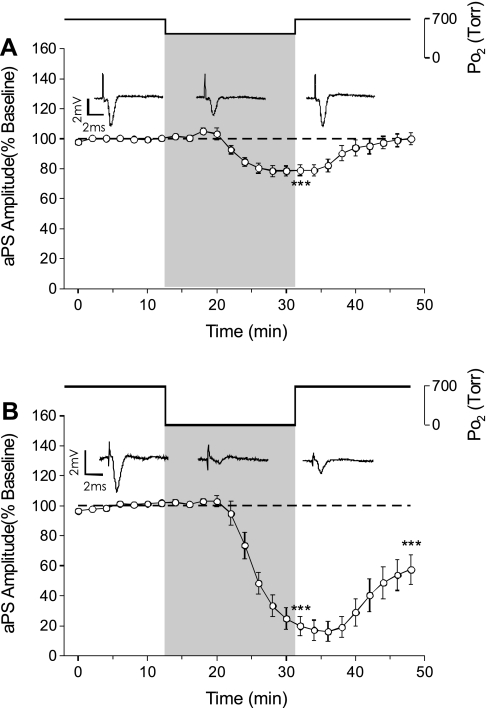
The aPS was measured during exposure of the slice to an intermediate level of oxygenation (0.60 ATA O2) and hypoxia (0.00 ATA O2) to identify the O2 sensitivity of postsynaptic activity at normobaric pressure and the effect of NBOreox. Reducing O2 from 0.95 to 0.60 ATA O2 progressively reduced the amplitude of the aPS. By the end of exposure to 0.60 ATA O2 (16 min), the aPS was reduced to 78.9 ± 3.5% of its baseline amplitude. The aPS returned to baseline level by the end of the recovery period without any OxIP of the antidromic activity or induction of sPS (n = 7, Fig. 6A). Thus decreasing O2 from 0.95 to 0.60 ATA reduces excitability of CA1 neurons but does not block action potential generation or axonal conduction.
Fig. 6.
Effects of normobaric oxygen manipulation on the aPS. A: exposure to 0.60 ATA O2 caused a significant suppression in the aPS that was reversible upon return to control O2 (n = 7, ***P < 0.001). B: exposure to 0.00 ATA O2 caused a significant suppression in the aPS that was greater than the inhibition during exposure to 0.60 ATA O2 (n = 7, ***P < 0.001). On return to 0.95 ATA O2, the aPS did not return to its baseline level but continued to be suppressed (***P < 0.001).
Hypoxia (0.00 ATA O2) caused a reduction in the amplitude of the aPS. By the end of hypoxia, the aPS was significantly reduced to 19.8 ± 6.5% of its baseline value, but it was not completely blocked. Inhibition of the aPS was greater in hypoxia to that during exposure to 0.60 ATA O2 (n = 7, Fig. 6B). Moreover, by the end of the recovery phase, the mean aPS amplitude was only 57.5 ± 10.1% of baseline amplitude. Thus, although hypoxia significantly decreases the aPS like an intermediate level of O2, hypoxia can cause a sustained depression of excitability following reoxygenation.
Effects of intermediate O2 manipulation on individual CA1 neurons: intracellular recordings.
The complete inhibition of fEPSP during exposure to an intermediate level of oxygenation (0.60 ATA O2) suggests that glutamatergic synaptic transmission has become silent. Conversely, the moderate, graded reduction in the aPS when O2 is decreased from 0.95 to 0.60 ATA O2 suggests that postsynaptic excitability is only partially reduced. These two observations raise the issue of whether orthodromic activity in 0.6 ATA O2 is fully abolished by ≤0.95 O2 or, alternatively, reduced to the point where extracellularly recorded field potentials are no longer detected. We were interested in this issue because the range of PtO2 established in the 400-μm-thick hippocampal slice, which is superfused on both sides with aCSF aerated with 0.6 ATA O2, is still relatively high and overlaps in part with that which occurs with 0.95 ATA O2 (9). By contrast, the PtO2 profile during hypoxia is significantly lower and flattened and does not overlap with those measured in 0.6 and 0.95 ATA O2 (9). Thus, to determine whether synchronized synaptic activation of CA1 neurons was fully inhibited at an intermediate level of O2, we conducted a series of intracellular recording experiments in CA1 neurons during CA3 electrical stimulation before, during, and following exposure to 0.60 ATA O2. We reasoned that intracellular recording of Vm in single CA1 neurons would enable detection of synaptic transmission with higher resolution than extracellular field potential recordings.
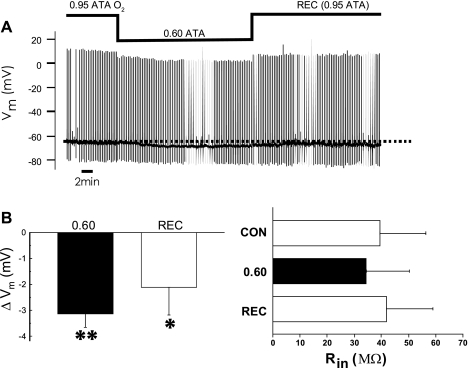
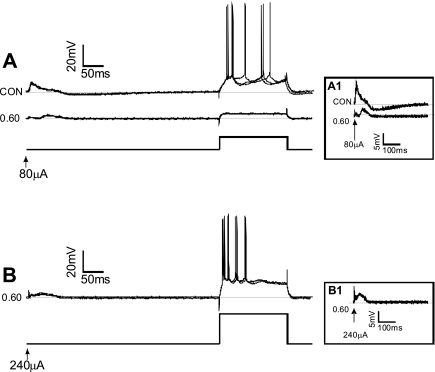
Stable intracellular recordings were obtained from five individual CA1 neurons, from five different slices, during O2 manipulation at normobaric pressure (e.g., Fig. 7A). At the end of the control period (0.95 ATA O2), Vm values were −65 ± 2 mV and the Rin values were 39 ± 17 MΩ. After 16 min of 0.60 ATA O2, Vm significantly hyperpolarized to −70 ± 1 mV, but Rin was unchanged on average (34 ± 16 MΩ) (Fig. 7B). Sixteen minutes after reoxygenation to 0.95 ATA O2, Rin was 42 ± 17 MΩ and Vm depolarized to −68 ± 2 mV, which was not significantly different from that measured in control O2. In addition, during exposure to 0.60 ATA O2, the amount of depolarizing current injected to elicit action potentials increased along with the amount of extracellular current required to evoke an EPSP (Fig. 8). When the extracellular current stimulus was increased threefold at 0.60 ATA O2, the EPSP could still be evoked, but it was attenuated in amplitude compared with that which could be evoked at 0.95 ATA (Fig. 8, A1 and B1). These measures of reduced excitability in 0.6 ATA O2 were reversible on reoxygenation to 0.95 ATA O2, but no potentiation of excitability was observed during the recovery period (data not shown). Thus CA1 neurons in a slice equilibrated with an intermediate level of O2 maintain the capacity for synaptic activation, albeit at a lower level of neurotransmission compared with that which is possible in 0.95 ATA O2. Likewise, excitability during depolarizing current injection is decreased as O2 is decreased below 0.95 ATA.
Fig. 7.
Effects of intermediate levels of O2 on the membrane potential (Vm) and input resistance (Rin) of individual CA1 neurons. The rationale for recording Vm during acute exposure to 0.60 ATA O2 was to determine whether CA1 neurons remained electrophysiologically viable during intermediate oxygenation, since orthodromic field potential recordings (oPS, fEPSP) suggested that they did not, but antidromic field potential recordings (aPS) suggested that they did. A: raw data of a nonspontaneously firing CA1 neuron. Action potentials could be evoked with current injection, whereas excitatory postsynaptic potentials (EPSPs) could be evoked with extracellular stimulation. To evoke action potentials and EPSPs during intermediate oxygenation, the amount of current injected intracellularly or extracellularly had to be increased (see Fig. 8). The trace also illustrates the hyperpolarization below the mean Vm value under initial control conditions (dashed line) without a measurable change in Rin. B, left: summary bar graph showing that exposure to 0.60 ATA O2 caused a reversible (P > 0.05) hyperpolarization that was significantly different from control as tested by a one-sample t-test (**P < 0.05) during exposure to 0.60 ATA O2 (n = 5). Right, summary bar graph showing Rin was not significantly altered on average during or following (P > 0.05) exposure to 0.60 ATA O2 (n = 5). Rec, recovery.
Fig. 8.
Effects of intermediate level of O2 on the action potential generation and the evoked EPSP of individual CA1 neurons measured during intracellular recording. The rationale for these experiments was to determine whether CA1 neurons retained the capacity for synaptic activation during acute exposure to 0.6 ATA O2, since orthodromic field potential recordings suggested that they did not. A: action potentials and EPSPs could be evoked by depolarizing current and extracellular stimulation of Schaffer collaterals under control conditions (0.95 ATA O2). on exposure to 0.60 ATA O2, the same depolarizing current protocol could not evoke action potentials, whereas the extracellular stimulating current could only intermittently evoke attenuated EPSPs. In this example, Rin decreased during exposure to 0.6 ATA O2; however, the change in Rin during exposure to intermediate oxygenation, on average, was not significant (Fig. 7B). Inset A1: high-gain images of the evoked EPSPs under control and 0.60 ATA O2 conditions. B: by increasing the amount of depolarizing current injected, it was possible to evoke repetitive firing of action potentials in 0.60 ATA O2. Moreover, increasing the extracellular stimulation current also increased the amplitude of the EPSP, although it was still attenuated compared with the EPSPs generated under control O2 conditions. Inset B1: high-gain images of an EPSP generated in 0.60 ATA O2 using 3× the stimulation current in the same cell as shown in A.
DISCUSSION
On the basis of our findings in the first study (9), we have tested the hypothesis that hyperoxic stimulation of CA1 neurons is dependent on a combination of increased excitatory synaptic activity and increased excitability of the postsynaptic membrane. For each extracellular measure of excitability (oPS, OxIP, and sPS), our hypothesis was only partially correct, however. Our findings show that 1) an increase in the fEPSP is not required for hyperoxic stimulation of the oPS or 2) induction of OxIP. 3) Furthermore, induction of spontaneous sPS activity during and following HBO is due to disinhibition of spontaneous synaptic activity, which we postulate to be GABAergic. On the basis of these findings, we hypothesize (see Fig. 10) that the excitatory effects of hyperoxia on the oPS occur primarily by postsynaptic, intrinsic mechanisms of excitability and plasticity, whereas the stimulatory effect of hyperoxia that produce sPS (hyperexcitability) occur through disinhibition of spontaneous neurotransmission.
Fig. 10.
Working model derived from our two studies (Ref. 9 and present study) that postulates how acute exposure to HBO may stimulate CA1 neurons causing increased excitability and OxIP. This model may also be applied to the phenomenon of OxIP of the oPS caused by NBOreox from 0.60 to 0.95 ATA O2. A: in 0.95 ATA O2 (control conditions), excitability of CA1 pyramidal neurons (open and shaded bars) is regulated by active synaptic input from GABAergic interneurons [open box, inhibitory (−) neurons] and from Schaffer-commissural collaterals and intrinsic membrane properties of the pyramidal neurons themselves. Moreover, with submaximal stimulation of the Schaffer-commissural collaterals, as used in our studies, only a fraction of neurons are activated (open), whereas others are silent (shaded). B: during HBO, our study shows that the oPS is stimulated (1), and thus more CA1 pyramidal neurons are activated with Schaffer-commissural collateral stimulation. The elevated excitability of CA1 neurons during HBO is not dependent on a significant increase in the excitatory synapses of the Schaffer-commissural collaterals as measured by the lack of significant change on average of the fEPSP (2) or lack of change in axonal conduction as measured by the primary aPS (3). Finding that onset of antidromic sPS (3) during and following HBO exposure could be blocked by chemical synaptic blockade and mimicked by the application of picrotoxin (Ptx), which inhibits GABAergic ionotropic receptors, suggests that epileptiform activity occurs through GABAergic interneurons (shaded box) (4). The lack of effect of HBO on the primary aPS and fEPSP suggests that elevated excitability of CA1 pyramidal neurons results from a change in conduction from synapse to soma (5). Although it has yet to be determined, potential targets of O2 (and presumably ROS/RNS) that would increase orthodromic activation and induction of OxIP of the oPS include voltage-gated channels that regulate dendritic conduction and the threshold for action potential generation and gap junctions (shaded area).
Hyperoxia and the fEPSP contribution to increased CA1 excitability including OxIP.
We found that HBO and NBOreox stimulate postsynaptic activity of CA1 neurons during Schaffer collateral stimulation (9) without increasing CA3 synaptic input (fEPSP) in 75% of the slices tested. The only other CA1 hippocampal slice study to use HBO (13) also reported that HBO increased orthodromic activity; however, HBO increased the fEPSP while decreasing synaptic efficacy. The method of gas compression they used, however, would have also increased CO2 concentration concurrently with O2 (6). Unfortunately, these investigators did not consider the stimulatory effects of CO2/H+ on reactive oxygen species (ROS) and reactive nitrogen species (RNS) production and thus cellular O2 sensitivity; that is, hypercapnic acidosis would increase redox and nitrosative stress during hyperoxia (5). Thus these results (13) are difficult to compare with the current study. Overall, our findings indicate that increased glutamatergic synaptic transmission is not a requirement for expression of hyperoxia-induced excitation of CA1 neurons.
That said, HBO did cause a strong stimulation of the fEPSP in 25% of HBO experiments (Fig. 1C), and a small proportion of slices also exhibited an increased fEPSP subsequent to NBOreox following relative hypoxia. Variability in the fEPSP response to hyperoxia contrasts with the consistent stimulatory effects that hyperoxia had on the oPS response (9). Moreover, variability in the fEPSP occurred only during hyperoxia, which became stable once more during the ensuing recovery period. Thus we conclude that the variability in the fEPSP between slices reflected an effect of hyperoxia. Presumably, this was due to differences in the relative balance of ROS and RNS and thus redox and nitrosative stress between slices at any given time. The effect of ROS on the fEPSP in the CA1 region, whether it be excitatory or inhibitory, depends on the particular species of ROS present (14, 16, 17). Superoxide anion stimulates the fEPSP (14), whereas hydrogen peroxide is either stimulatory or inhibitory on the fEPSP depending on the concentration (12). It is known that increasing the fractional concentration of O2, increasing barometric pressure, or, alternatively, increasing exposure time to a given level of O2 increases redox and nitrosative stress in the hippocampus based on production of superoxide (3), nitric oxide (10), and isoprostanes (7). The cumulative effects of hyperoxia and ROS/RNS in a brain tissue slice will be exacerbated by the concurrent washout of endogenous antioxidants such as ascorbate (2, 3, 7). Consequently, variability in fEPSP measurements could reflect the sensitivity of synaptic transmission to redox and nitrosative stress that increases over time in the slice preparation.
OxIP averages 125–130% of the control oPS response in 0.95 ATA O2 before hyperoxia, and once activated, it is the same magnitude regardless of the level of normobaric oxygen from which it is initiated (9). We determined that hyperoxia rarely potentiated the fEPSP response. Thus potentiation of the fEPSP is not a prerequisite for induction of OxIP. This is in contrast to other forms of synaptic plasticity exhibited in 0.95 ATA O2 by CA1 neurons that depend on increased presynaptic release of neurotransmitter and enhanced postsynaptic sensitivity to glutamate, such as long-term potentiation, which involves mechanisms of Ca2+ influx through NMDA receptors and the initiation of intracellular signaling leading to sustained elevation of excitatory synaptic communication. By contrast, OxIP most likely occurs by mechanism(s) of intrinsic plasticity (4, 21), as hypothesized in the model presented in Fig. 10.
Elevated excitability following the SO-alvear stimulation, antidromic recordings.
Neither HBO nor NBOreox caused an increase in the amplitude of the aPS. SO-alvear stimulation antidromically activated CA1 neurons, but it may also have activated cortical inputs that orthodromically innervate the hippocampus. We doubt that this was the case, however, since the primary population spike arising from SO-alvear stimulation was neither attenuated nor blocked in the presence of synaptic blockade medium. We concluded therefore that SO-alvear stimulation predominantly produced antidromic activation of CA1 neurons. The inability of hyperoxia to cause an increase in the amplitude of the aPS indicates that the intrinsic mechanism(s) responsible for hyperoxia-induced enhanced excitability is not triggered by antridromic activation of the CA1 population.
HBO, however, did induce sPS activity during SO-alvear stimulation in some experiments, indicating that HBO increased postsynaptic excitability of CA1 neurons. Although the occurrence of sPS was not observed with each exposure to HBO, the percentage of experiments exhibiting sPS increased with the degree of HBO exposure. We believe that the sPS activity induced following the primary aPS is caused by disinhibition of spontaneous synaptic input, because synaptic blockade (glutamatergic and GABAergic receptor antagonists) prevented onset of sPS activity during exposure to HBO. Likewise, inhibition of GABA receptors in control O2 induced sPS activity. It has been proposed that disruption of the balance between excitatory (glutamatergic) and inhibitory (GABAergic) synapses may facilitate onset of epileptiform activity observed during CNS O2 toxicity (1, 18–20).
Hypoxia and intermediate oxygenation vs. hyperoxia and CA1 excitability.
Two additional observations were made concerning the effects of relative hypoxia (0.0 ATA O2) and intermediate oxygenation (0.6 ATA O2) vs. hyperoxia (≥0.95 ATA O2) on CA1 excitability in the hippocampal slice preparation. First, above 0.95 ATA O2, the aPS and fEPSP responses are, on average, insensitive to further increases in O2 tension. This suggests that these two mechanisms of excitability become saturated in CA1 neurons maintained in hyperoxic control medium. Beginning at 0.95 ATA O2, reducing O2 tension to ≤0.60 ATA O2 causes significant reduction/complete inhibition in the fEPSP, aPS, and oPS (Fig. 9). Conversely, increasing O2 tension above 0.95 ATA (control) using HBO stimulates the oPS, but to a lesser extent, whereas the fEPSP and aPS are not stimulated further, on average, by HBO. As previously discussed (9), the reduction or blunting of neural sensitivity to oxidative stress (e.g., extreme hyperoxia) could result from the acute effects of oxidative preconditioning with mild hyperoxia (0.95 ATA O2) (8). In addition, our discovery that the aPS (postsynaptic activity) and intracellular synaptic activity are only reduced and not blocked in 0.60 ATA O2 (Figs. 6 and 8) suggest that the conventional hyperoxic control O2 condition (0.95 ATA) can be reduced without inducing hypoxia, which agrees with our previous measurements of PtO2 profiles in slices (9, 15).
Fig. 9.
Mean responses of field potentials as a function of measured PmO2 at normobaric pressure (NBO) and HBO. Mean responses of oPS, fEPSP, and aPS are plotted as a function of PmO2. The oPS data are reproduced from Fig. 9A in the first of the companion articles (9). Multiple measures of CA1 neuronal excitability indicate that hippocampal slices maintained in normobaric hyperoxia (0.95 ATA O2) exhibit their greatest O2 sensitivity in the range of normobaric oxygenation (NBO: oPS, fEPSP, and aPS). In contrast, O2 sensitivity of the mechanisms underlying the aPS and fEPSP plateaus are saturated beginning at 0.95 ATA O2. Likewise, the O2 sensitivity of the oPS is significantly reduced beginning at 0.95 ATA O2 and above (see Ref. 9 for statistics).
Second, we discovered that normobaric hyperoxia (0.95 ATA O2) is a precondition for synchronous activation of CA1 neurons during extracellular recording. That is, synaptic activation of individual CA1 neurons is possible at an intermediate level of oxygenation (0.6 ATA), as revealed by intracellular recording, but synchronous firing of the population of CA1 neurons during extracellular recording requires 0.95 ATA O2. Reducing O2 from 0.95 to 0.00 or 0.60 ATA completely abolished the population fEPSP, which correlated with the complete loss of the oPS (9). Hence, by first approximation, 0.60 ATA O2 reduces neuronal excitability similar to that observed using nominal hypoxia. Our intracellular recording experiments, however, indicate that these two O2 stimuli are not equivalent in their effects on CA1 neurons. Intracellular recordings conducted at 0.60 ATA O2 demonstrate that synaptic transmission is not inhibited completely but is reduced. Synchronized firing of neurons in brain slices has been reported to be a conditional property, dependent on stimuli that increase excitability; for example, synchronous bursting activity within local circuits of the locus coeruleus appears to require electrotonic coupling within dendrites outside the soma (11). Likewise, we postulate that hyperoxia (0.95 ATA O2) may act as the conditional stimulus for synchronized synaptic activation in the CA1 by promoting an increase in electrotonic coupling and/or a decrease in membrane conductance (10).
Summary of possible mechanisms of hyperoxic neuronal sensitivity.
The increased oPS amplitude (9) that occurred during and following NBOreox and HBO indicates that there is an increase in the number of action potentials elicited during synaptic activation. This may reflect an increase in the number of neurons activated during Schaffer collateral stimulation, an increase in the number of action potentials generated by individual neurons (6), or some combination of both scenarios. Our present findings indicate that HBO and NBOreox do not consistently increase excitatory synaptic transmission. Thus OxIP, as defined by the long-lasting stimulation of the oPS, is a form of intrinsic plasticity (4, 21). The exact target of O2 and ROS/RNS has yet to be determined. Figure 10 summarizes our various findings and leads to the working hypothesis that stimulation of orthodromic activity by HBO (and NBOreox) is mediated through a change in the conduction of dendritic information to the soma, which lowers the threshold for firing action potentials. Thus this change increases general postsynaptic excitability and results in recruiting of more CA1 neurons during Schaffer collateral stimulation during and following HBO and NBOreox. Synaptic mechanisms nonetheless occur in some cases, which we attribute to the relative redox state of the slice (3), as discussed above, and the high incidence of spontaneous, epileptiform activity (Ref. 9; present study). Additional details of our hypothesis are provided in the text of the legend for Fig. 10.
GRANTS
This work was supported by Office of Naval Research Grants DoD/ONR DURIP N000140210643, ONR N000140410172, and ONR N000140710890 and National Institutes of Health Grant R01 HL 56683-09.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We acknowledge the advice and assistance of Dr. L. Hartzler, and the technical assistance of P. Douglas was greatly appreciated.
All experiments were conducted at Wright State University before J. B. Dean's relocation to the University of South Florida and A. J. Garcia's departure for the University of Chicago. The manuscript was prepared thereafter.
Footnotes
As described in the companion article, Garcia et al. (9), oxygen tension of aCSF is expressed without correction for true barometric pressure and water vapor content. Hence, the use of 95 and 60% O2 at normobaric pressure (1 ATA) is expressed as 0.95 ATA and 0.6 ATA O2, respectively.
REFERENCES
- 1. Bitterman N, Halpern P. The effect of flumazenil on CNS oxygen toxicity in the rat. Methods Find Exp Clin Pharmacol 17: 169–174, 1995 [PubMed] [Google Scholar]
- 2. Brahman B, Forman RE, Stewart EE, Nicholson C, Rice ME. Ascorbate inhibits edema in brain slices. J Neurochem 74: 1263–1270, 2000 [DOI] [PubMed] [Google Scholar]
- 3. D'Agostino DP, Putnam RW, Dean JB. Superoxide (•O2−) production in CA1 neurons of rat hippocampal slices exposed to graded levels of oxygen. J Neurophysiol 98: 1030–1041, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Daoudal G, Dubane D. Long-term plasticity of intrinsic excitability: learning rules and memory. Learn Mem 10: 456–465, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Dean J. Hypercapnia causes cellular oxidation and nitrosation in addition to acidosis: implications for CO2 chemoreceptor function and dysfunction. J Appl Physiol 108: 1786–1795, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dean JB, Mulkey DK, Garcia AJ, III, Putnam RW, Henderson RA., III Neuronal sensitivity to hyperoxia, hypercapnia and inert gases at hyperbaric pressures. J Appl Physiol 95: 883–909, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Fessel J, Arbeson WC, Winder D, Roberts LJ. Standard brain slice protocols result in oxidative injury in hippocampal brain slices (Abstract). Free Radic Biol Med 33: S433, 2002 [Google Scholar]
- 8. Freiberger JJ, Suliman HB, Sheng H, McAdoo J, Piantadosi CA, Warner DS. A comparison of hyperbaric oxygen versus hypoxic cerebral preconditioning in neonatal rats. Brain Res 1075: 213–222, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Garcia AJ, III, Putnam RW, Dean JB. Hyperbaric hyperoxia and normobaric reoxygenation increases excitability and activates oxygen-induced potentiation in CA1 hippocampal neurons. J Appl Physiol (June 17, 2010). doi:10.1152/japplphysiol.91429.2008 [DOI] [PMC free article] [PubMed]
- 10. Hwang SH. Effects of normobaric and hyperbaric oxygen on nitric oxide production in rat brain slices (M.S. thesis). Dayton, OH: Wright State University, 2004 [Google Scholar]
- 11. Ishimatsu M, Williams JT. Synchronous activity in locus coeruleus results from dendritic interactions in pericoerulear regions. J Neurosci 16: 5196–5204, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kamsler A, Segal M. Hydrogen peroxide modulation of synaptic plasticity. J Neurosci 23: 269–276, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. King GL, Parmentier JL. Oxygen toxicity of hippocampal tissue in vitro. Brain Res 260: 139–142, 1983 [DOI] [PubMed] [Google Scholar]
- 14. Klann E, Roberson E, Knapp L, Sweatt J. A role for superoxide in protein kinase C activation and induction of long-term potentiation. J Biol Chem 273: 4516–4522, 1998 [DOI] [PubMed] [Google Scholar]
- 15. Mulkey DK, Henderson RA, Olson JE, Putnam RW, Dean JB. Oxygen measurements in brain stem slices exposed to normobaric hyperoxia and hyperbaric oxygen. J Appl Physiol 90: 1887–1899, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Sah R, Galeffi F, Ahrens R, Jordan G, Schwartz-Bloom RD. Modulation of the GABAA-gated chloride channel by reactive oxygen species. J Neurochem 80: 383–391, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Sah R, Schwartz-Bloom RD. Optical imaging reveals elevated intracellular chloride in hippocampal pyramidal neurons after oxidative stress. J Neurosci 19: 9209–9217, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tzuk-Shina T, Bitterman N, Harel D. The effect of vigabatrin on central nervous system oxygen toxicity in rats. Eur J Pharmacol 202: 171–175, 1991 [DOI] [PubMed] [Google Scholar]
- 19. Wood JD. The role of gamma-aminobutyric acid in the mechanism of seizures. Prog Neurobiol 5: 77–95, 1975 [DOI] [PubMed] [Google Scholar]
- 20. Zhang S, Takeda Y, Hagioka S, Keiji Goto, Kiyoshi M. The close relationship between decreases in extracellular GABA concentrations and increases in the incidence of hyperbaric oxygen-induced electrical discharge. Acta Med Okayama 58: 91–95, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Zhang W, Linden D. The other side of the engram: experience-driven changes in intrinsic neuronal excitability. Nat Rev Neurosci 4: 885–900, 2003 [DOI] [PubMed] [Google Scholar]