Abstract
Skin blood flow responses in the human forearm, assessed by three commonly used technologies—single-point laser-Doppler flowmetry, integrated laser-Doppler flowmetry, and laser-Doppler imaging—were compared in eight subjects during normothermic baseline, acute skin-surface cooling, and whole body heat stress (Δ internal temperature = 1.0 ± 0.2°C; P < 0.001). In addition, while normothermic and heat stressed, subjects were exposed to 30-mmHg lower-body negative pressure (LBNP). Skin blood flow was normalized to the maximum value obtained at each site during local heating to 42°C for at least 30 min. Furthermore, comparisons of forearm blood flow (FBF) measures obtained using venous occlusion plethysmography and Doppler ultrasound were made during the aforementioned perturbations. Relative to normothermic baseline, skin blood flow decreased during normothermia + LBNP (P < 0.05) and skin-surface cooling (P < 0.01) and increased during whole body heating (P < 0.001). Subsequent LBNP during whole body heating significantly decreased skin blood flow relative to control heat stress (P < 0.05). Importantly, for each of the aforementioned conditions, skin blood flow was similar between the three measurement devices (main effect of device: P > 0.05 for all conditions). Similarly, no differences were identified across all perturbations between FBF measures using plethysmography and Doppler ultrasound (P > 0.05 for all perturbations). These data indicate that when normalized to maximum, assessment of skin blood flow in response to vasoconstrictor and dilator perturbations are similar regardless of methodology. Likewise, FBF responses to these perturbations are similar between two commonly used methodologies of limb blood flow assessment.
Keywords: heat stress, blood flow, laser-Doppler flowmetry
the control of human skin blood flow is critical for the regulation of internal temperature and, during heat stress, for blood pressure regulation (8, 16, 17). Mechanisms that regulate skin blood flow are impaired in the normal ageing process and in a variety of physiological diseases such as diabetes and congestive heart failure (3, 7, 18, 19, 22, 27). Therefore, important research is ongoing toward a greater understanding of mechanisms regulating skin blood flow.
A number of methodologies have been utilized to provide indexes of skin blood flow (2, 5, 9, 13, 14). While each method offers unique advantages and disadvantages, currently the most widely used technique is laser-Doppler flowmetry. A primary advantage of this method is that it provides a continuous index of skin blood flow, which is beneficial particularly when the dynamic response to acute perturbations is of interest (9). A disadvantage of laser-Doppler flowmetry is the relatively small sample area; for example, a single-point probe samples from an area as little as ∼1 mm3 of tissue. Anatomic studies indicate that the ascending arterioles on the ventral surface of the human forearm are separated by an average of 1.7 mm (1). Therefore, it is possible that variability in skin blood flow measurements obtained from multiple sites within a small area of the forearm using single-point laser-Doppler flow probes (9, 13, 21) are at least partially attributed to a heterogeneous pattern of the underlying vasculature. This disadvantage has been minimized in recent years by the use of integrating laser-Doppler flow probes. In contrast to the single-point probe, which has only one emitting and one receiving fiber, the integrating probes have multiple emitting/receiving fibers and thus sample from a larger area of tissue, (i.e., upward to ∼7 mm in diameter).
Besides laser-Doppler flowmetry, another technique for assessment of skin blood flow that has emerged in recent years is topographical perfusion mapping by laser-Doppler imaging systems (13). The advantage of these imaging systems is that they provide an index of skin blood flow over a much larger sample area (i.e., capacities well over 100 cm2). The downside, though, is that the measurements are not continuous and, depending on the size of the region evaluated, can take anywhere from seconds to minutes to complete.
Given differences in sampling area between the aforementioned devices, coupled with heterogeneity of cutaneous vascular responses, differing findings between studies, and even within a subject, may be observed for a given perturbation depending on whether skin blood flow is assessed from a single point probe, an integrating probe, or an imager. Therefore, the first objective of this study was to address the hypothesis that skin blood flow responses to vasoconstricting and dilating perturbations will be different among these three commonly used methodologies.
Limb vascular responses to vasoconstricting and dilating perturbations have for decades been evaluated via venous occlusion plethysmography, and more recently via Doppler ultrasound (6, 11, 12, 24). However, we are unaware of studies that have evaluated responses between these techniques during combined thermal and hypotensive challenges known to alter limb blood flow. Therefore, the secondary objective of this study was to examine the hypothesis that forearm blood flow responses during the aforementioned perturbations would be similar between venous occlusion plethysmography and Doppler ultrasound techniques.
METHODS
Eight healthy normotensive subjects (6 male and 2 female) participated in this study. Average subject characteristics were age, 33 ± 13 years; height, 177 ± 11 cm; and weight, 73 ± 9 kg (mean ± SD). Subjects were not taking medications and were free of any known cardiovascular, metabolic, or neurological diseases. Subjects were informed of the purpose and risks of the study before providing their informed written consent. The protocol and consent were approved by the Institutional Review Boards at the University of Texas Southwestern Medical Center at Dallas and Texas Health Presbyterian Hospital Dallas. Subjects refrained from alcohol, caffeine, and exercise for 24 h before the study.
Instrumentation and Measurements
Immediately on arrival to the lab, subjects swallowed an ingestible telemetry pill for the measurement of intestinal temperature (HQ, Palmetto, FL). Each subject was fitted with a water-perfused tube-lined suit (Med-Eng, Ottawa, Canada) and was placed into a lower-body negative pressure (LBNP) chamber, sealed at the iliac crest, while in the supine position. The suit covered the entire body except for the head, hands, both arms, and feet. The suit permitted the control of skin and internal temperatures by adjusting the temperature of the water perfusing the suit. Mean skin temperature was measured from the weighted average of six thermocouples attached to the skin under the water-perfused suit (20). The thermocouples were attached to the lateral calf, lateral thigh, lower back, lower abdomen, upper back, and chest.
Skin blood flow.
Skin blood flow was continuously indexed from the ventral portion of the forearm via a single-point laser-Doppler flow probe (Periflux401; Perimed, North Royalton, OH), which utilizes one emitting light source and one receiving source and samples an area of ∼1 mm3 of tissue, and at an adjacent site using an integrating laser-Doppler flow probe (Periflux413; Perimed), which provides an integrated value from seven different emitting/receiving light sources and thus samples from a larger area of tissue (i.e., ∼7 mm in diameter). Each probe was housed within a heating element (3-cm diameter, Peritemp 4005; Perimed) for control of local skin temperature during assessment of maximal skin blood flow (see Experimental Protocol). Both probes were connected to a dual-channel laser-Doppler flowmeter (Periflux5010; Perimed) and calibrated using a motility standard. Skin blood flow was also assessed using a laser-Doppler imaging system (Periscan PIM3; Perimed) at a site adjacent to the laser-Doppler flow probe sites. An outline of the imaged site was marked to confirm that scans were obtained from the same region during each of the perturbations. The scanned area was 33 mm × 50 mm and required ∼60 s per scan.
Forearm blood flow (FBF).
Brachial artery diameter and blood velocity were obtained from the same arm used to index skin blood flow, using commercially available Doppler ultrasound equipment (iE33, Philips Ultrasound, Bothell, WA). These values were used for subsequent calculation of FBF. Ultrasound imaging of brachial artery diameter was performed from the perpendicular image along the central axis of the scanned area. A linear array transducer was positioned at a site medial to the biceps brachii muscle and approximately 4–5 cm proximal to the elbow joint where the best spatial resolution was achieved. The blood velocity profiles were obtained using the same transducer with a sample volume depth of 2.0–3.5 mm and size adjusted to cover the entire width of the artery. During all blood velocity measurements the probe was appropriately positioned in a fixed manner to maintain an insonation angle of ≤60°. Brachial artery diameter images were stored onto the hard drive of the Doppler ultrasound machine and were later measured, using on-screen calipers. Angle-corrected, time- and space-averaged, and intensity-weighted mean brachial artery blood velocities were calculated using on-screen calipers immediately following each data collection period. FBF was also assessed from the opposite arm using venous occlusion plethymography (Hokanson, Bellevue, WA) as has been previously described (26). Before Doppler ultrasound and plethysmography data collection periods, a cuff on each wrist was inflated to 220 mmHg to arrest circulation to the hand.
Experimental Protocol
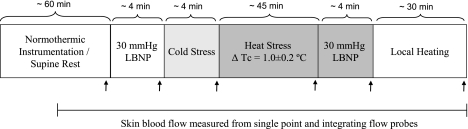
An outline of the experimental protocol is provided in Fig. 1.
Fig. 1.
Protocol schematic. Measures of skin blood flow using the single-point laser-Doppler flow probe and the integrating laser-Doppler flow probe were continuously obtained throughout the protocol. The arrows indicate the timing of laser-Doppler scanning of cutaneous tissue which was followed by Doppler ultrasound and plethysmographic measures of forearm blood flow. The exception is during local heating when measures of forearm blood flow were not obtained. LBNP, lower-body negative pressure; Tc, core temperature.
Laser-Doppler evaluation of skin blood flow.
Following instrumentation, which lasted ∼30 min, subjects rested on a patient table in the supine position while thermoneutral water (34 °C) circulated through the suit. After an ∼30-min stabilization period, scan imaging of the forearm site was performed, while continuous measures of skin blood flow from the single-point and the integrating laser-Doppler flow probes were obtained at this time and throughout all subsequent perturbations. Approximately 5 min after these baseline measurements, subjects were exposed to a 4-min, 30-mmHg LBNP challenge with the aforementioned scan being obtained in the final minute of LBNP. Approximately 5 min after LBNP exposure, the subjects were exposed to an acute 4-min cold stress, which was accomplished by perfusing ∼8°C water through the suit with the aforementioned scan image of skin blood flow being obtained during the final minute of cold stress. If shivering occurred, the water temperature was increased slightly to offset this response. Following completion of cold stress data collection, whole body heating began by circulating 49°C water through the suit until internal temperature increased ∼1.0°C above baseline temperature. Once this increase in internal temperature was attained, the temperature of the water circulating the suit was slightly decreased in an effort to attenuate the rate of rise in internal temperature during data collection. At this point the scan image of skin blood flow was repeated, which was followed ∼5 min later by the onset of a 4-min, 30-mmHg LBNP challenge. Another scan image was obtained during the final minute of LBNP. Immediately following completion of the whole body heat stress + LBNP data collection, two local heating elements (3-cm diameter, Peritemp4005; Perimed) were attached to the site where the scanned images were obtained and the temperature of all four local heating elements (one around each of the single and integrated laser-Doppler sites, and two on the scan site) were increased to 42°C for at least 30 min to achieve maximal skin blood flow (28). After this period, the local heating elements were removed from the scanned area site and an image of that site was immediately obtained. Throughout the duration of data collection, internal temperature, mean skin temperature, and skin blood flow from the single and integrated laser-Doppler flow probes were continuously measured.
Comparisons between Doppler ultrasound and venous occlusion plethysmography measures of FBF.
Measures of FBF using both devices were obtained during the same perturbations as those outlined above for assessment of skin blood flow. However, ∼1 min before each measure a cuff on each wrist was inflated to 220 mmHg to arrest circulation to the hand. Although unconventional, a cuff was inflated on the wrist of the arm from which Doppler ultrasound measures of FBF were obtained to allow more precise comparisons to plethysmographic measures of FBF.
Data Analysis
Thermal and hemodynamic data were sampled at 50 Hz via a data-acquisition system (Biopac System, Santa Barbara, CA). All indexes of skin blood flow are represented as a percentage of each respective maximal skin blood flow achieved during local heating. Because of the differing units for plethysmography (ml·100 ml tissue−1·min−1) and Doppler ultrasound (ml/min), the FBF responses for these methods are represented as a percent change relative to their respective baseline normothermic values (or relative to pre-LBNP for the heat stress + LBNP component). Five to six plethysmographic measures that were obtained under each condition were averaged, whereas the average of three measurements of brachial artery diameter and an average of 20–30 s of intensity-weighted time-averaged mean brachial blood velocities (Vmean) were used for Doppler ultrasound FBF determination. For Doppler ultrasound, FBF was calculated as FBF (ml/min) = Vmean·π·(brachial artery diameter/2)2·60 (4).
Two-way repeated-measures analyses of variance (RM-ANOVA) were used to evaluate the effects of the thermal and baroreceptor provocations on the skin blood flow responses between the three measurement devices (single-point laser-Doppler flow probe, integrated laser-Doppler Flow probe, and scanner).
For each perturbation, FBF responses relative to control normothermic conditions between the two measurement devices (Doppler ultrasound and plethysmography) within a given perturbation (normothermia + LBNP, skin-surface cooling, whole body heating, and whole body heating + LBNP) were evaluated using separate paired t-tests. Likewise, the effect of LBNP during whole body heating was evaluated using a paired t-test. All statistical analyses were performed using a commercially available statistical software package (SigmaStat 3.11, Chicago, IL). All values are reported as means ± SD. P values < 0.05 were considered statistically significant.
RESULTS
Thermal and Hemodynamic Data in Response to Vasoconstrictor Stimuli While Normothermic
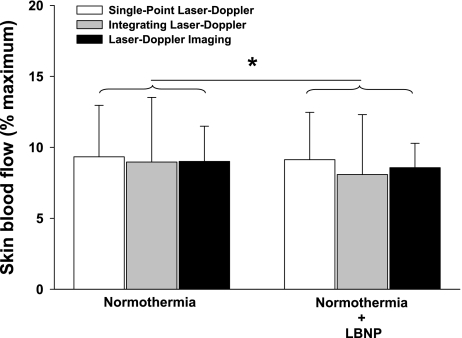
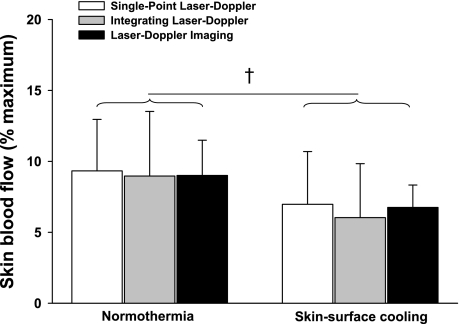
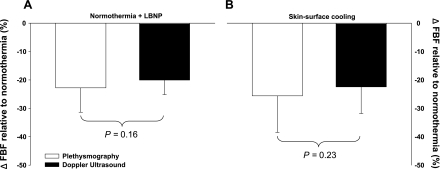
Before either vasoconstrictor stimuli, internal and mean skin temperatures were 37.0 ± 0.2°C and 34.4 ± 0.4°C, respectively. LBNP had no effect on mean skin or internal temperatures (P > 0.05 for both variables). Skin-surface cooling had no effect on internal temperature (P > 0.05), but mean skin temperature was reduced by 4.2 ± 0.5°C (P < 0.001). Statistical analyses revealed a small yet significant condition effect of LBNP (Fig. 2; P = 0.03) and skin-surface cooling (Fig. 3; P < 0.01) on skin blood flow responses; however, the magnitude of vasoconstriction for both perturbations were similar between laser-Doppler devices for both LBNP (Fig. 2; P = 0.89) and skin-surface cooling (Fig. 3; P = 0.89). The reductions in FBF relative to baseline normothermic values during LBNP (see Fig. 6A; P = 0.16) and during skin-surface cooling (see Fig. 6B; P = 0.23) were likewise similar between measurement devices (i.e., Doppler ultrasound and plethysmography).
Fig. 2.
Normalized skin blood flow during normothermic baseline and 30-mmHg LBNP. Skin blood flow during normothermic baseline was similar between the 3 measurement devices (P = 0.89). Baroreceptor unloading via 30-mmHg LBNP induced a very small but significant decrease in skin blood flow (main effect of LBNP; P = 0.03); however, the response to LBNP was similar between measurement devices (P = 0.53 for the interaction). Data are represented as a % of maximal skin perfusion assessed during 42°C local heating. *Significant condition effect of normothermic LBNP relative to normothermic baseline (P < 0.05).
Fig. 3.
Normalized skin blood flow response to skin-surface cooling relative to normothermic baseline. Skin-surface cooling induced significant decreases in skin blood flow (main effect of skin-surface cooling; P < 0.01); however, the response to skin-surface cooling was similar between measurement devices (P = 0.60 for the interaction). †Significant condition effect of skin-surface cooling relative to normothermic baseline (P < 0.01).
Fig. 6.
Reductions in forearm blood flow to LBNP and skin-surface cooling. There were no differences in the reduction in forearm blood flow responses to LBNP (A: P = 0.16) or skin-surface cooling (B: P = 0.23) between devices. Data are represented as a % change relative to normothermic baseline values.
Thermal and Hemodynamic Responses to Heat Stress With and Without LBNP
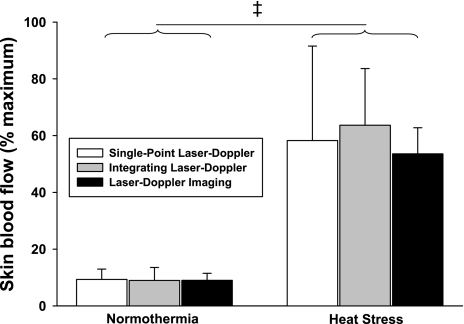
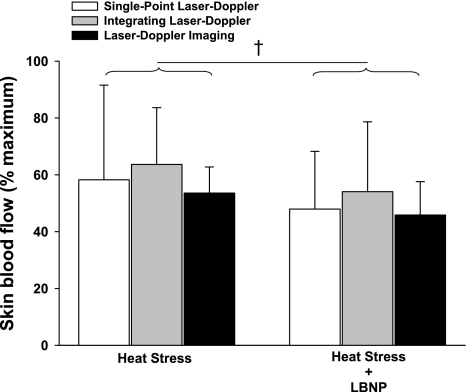
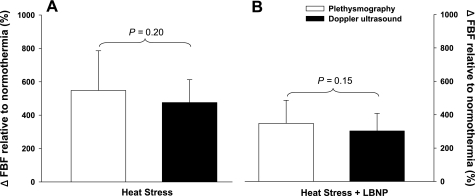
Whole body heating increased mean skin temperature by 4.4 ± 0.6°C and internal temperature by 1.0 ± 0.2°C (P < 0.001 for both variables). As expected, skin blood flow was significantly greater following whole body heating relative to normothermic conditions (Fig. 4; P < 0.001); however, the magnitude of this increase was similar between laser-Doppler devices (Fig. 4; P = 0.71). Subsequent LBNP resulted in small yet significant reductions skin blood flow (Fig. 5; P < 0.01), with the magnitude of these reductions also being similar between laser-Doppler measurement devices (Fig. 5; P = 0.61). Similar to the normothermia plus LBNP (Fig. 6A) and the skin-surface cooling (Fig. 6B) condition, the increases in FBF in response to the heat stress (Fig. 7A; P = 0.20), as well as the subsequent reduction in FBF to LBNP were not different between plethysmography and Doppler ultrasound devices (Fig. 7B; P = 0.15).
Fig. 4.
Normalized skin blood flow response to whole body heating-induced elevations in skin and internal temperatures relative to normothermic baseline. Whole body heating induced significant increases in skin blood flow (main effect of heating; P < 0.001); however, the response to whole body heating was similar between measurement devices (P = 0.54 for the interaction). ‡Significant condition effect of whole body heating relative to normothermic baseline (P < 0.001).
Fig. 5.
Normalized skin blood flow response to 30-mmHg LBNP during whole body heating relative to whole body heating without LBNP: 30-mmHg LBNP induced small but significant decreases in skin blood flow (main effect of LBNP; P < 0.01); however, the response to LBNP was similar between measurement devices (P = 0.78 for the interaction). †Significant condition effect of LBNP relative to control whole body heating (P < 0.01).
Fig. 7.
Forearm blood flow responses during whole body heating and subsequent 30-mmHg LBNP. Forearm blood flow responses to whole body heating compared with normothermic baseline were similar between venous occlusion plethysmography and Doppler ultrasound devices (A: P = 0.20). Likewise, the forearm blood flow response to LBNP relative to control whole body heating was similar between venous occlusion plethysmography and Doppler ultrasound devices (B: P = 0.15).
DISCUSSION
The primary findings of this study are that changes in skin blood flow to two different thermal stimuli (skin-surface cooling and whole body heat stress) and to LBNP were similar between single-point laser-Doppler flowmetry, integrated laser-Doppler flowmetry, and laser-Doppler imaging. Likewise, these results demonstrate that the change in FBF during the aforementioned perturbations were similar when assessed using venous occlusion plethysmography and Doppler ultrasound. These findings suggest that while there may be some heterogeneity in measures of absolute blood flow from site to site in the human skin vasculature (9, 13, 21), cutaneous and limb responses to the performed perturbations are similarly tracked regardless of measurement technique.
Previous reports indicate large variations in skin blood flow recordings obtained from multiple sites separated by only a few millimeters within the same subject (9, 13, 21). It is likely that this heterogeneous response is related to the spacing of ascending arterioles, which have been reported to be separated by an average of 1.7 mm (1), resulting in a high probability of neighboring laser-Doppler flow probes reporting different flow values (13, 21). This is particularly true when considering the single-point laser-Doppler flow probes sample from a relatively small area, estimated to be ∼1 mm3 of skin. In this regard, the nonnormalized numeric values obtained from each device for a given perturbation were always largest with the laser-Doppler imaging system, followed by the integrating laser-Doppler flow probe, and least with the single-point laser-Doppler flow probe (Table 1). A common approach used to take into account regional vascular heterogeneity in the assessment of skin blood flow is to normalize the measurements to a maximal value obtained via local administration of the vasodilator sodium nitroprusside or via locally heating the site to ∼42–43°C for at least 30 min. Despite the aforementioned differences in absolute skin blood flow readings, when the data were normalized to each respective maximal flow value, the normothermic baseline values as well as responses to each of the perturbations were strikingly similar among the three measurement techniques (Figs. 2–5). These findings reinforce the importance of normalizing measurements of skin blood flow to a maximal value when making comparisons between different sites, especially if differing measurement technologies are used between sites. Furthermore, the findings suggest that when normalized for differences in maximal skin blood flow, interpretations regarding vascular control are similar regardless of measurement technology.
Table 1.
Absolute skin blood flow values measured from the three measurement devices during the various thermal and baroreceptor perturbations
| Single-Point Flow Probe | Integrating Flow Probe | Scan Imager | |
|---|---|---|---|
| Normothermia | 9 ± 3 | 21 ± 8* | 35 ± 14† |
| Normothermia + LBNP | 9 ± 3 | 19 ± 7* | 33 ± 11† |
| Cold stress | 7 ± 2 | 13 ± 5* | 26 ± 8† |
| Heat stress | 58 ± 29 | 148 ± 28* | 206 ± 47† |
| Heat stress + LBNP | 49 ± 27 | 125 ± 43* | 177 ± 54† |
| Local heating | 107 ± 46 | 244 ± 52* | 385 ± 49† |
Values are means ± SD in ml. LBNP, lower-body negative pressure. Measures of skin blood flow obtained by the 3 measurement devices within each perturbation were evaluated using 1-way repeated-measures ANOVAs (main factor of device) followed by Tukey post hoc analysis to identify group differences.
Greater relative to the single-point flow probe (P < 0.05).
Greater relative to the single-point and integrating flow probes (P < 0.05).
Our findings of significant changes in normalized skin blood flow to both skin-surface cooling (Fig. 3: P < 0.01) and elevated core body temperature (Fig. 4: P < 0.001), regardless of measurement device, are consistent with previously published reports using laser-Doppler imaging (13) and laser-Doppler flow probes (2, 5, 10, 15). In contrast to the expected and consistent results obtained during steady-state thermal conditions, cutaneous vascular conductance has been reported to be either unaffected (13, 15, 25) or reduced (2, 10, 23) during baroreceptor unloading by LBNP. In the present study we showed a significant condition effect of LBNP, indicating reductions in skin blood flow in both normothermic (Fig. 2) and heat stress conditions (Fig. 4). The reason for the aforementioned inconsistencies in cutaneous vascular responses to baroreceptor unloading is unknown. Peters et al. reported a heterogeneous response of the cutaneous vasculature to baroreceptor unloading between individuals (15), which, in combination with anatomic studies indicating heterogeneity of the cutaneous vasculature in the human forearm (1), could result in these varied responses. Mack (13) also reported profound heterogeneity in skin blood flow (measured via single-point laser-Doppler flowmetry at 6 neighboring sites) and cutaneous vascular conductance (CVC) (measured by laser-Doppler imaging) responses to baroreceptor unloading induced by 40-mmHg LBNP during heat stress conditions. They found that only ∼50% of the evaluated pixels within the scanned area decreased CVC during LBNP. In contrast, our results indicate a significant condition effect of LBNP in causing reductions in skin blood flow regardless of the measurement device and thus size of the area being sampled by each device.
Venous occlusion plethysmography and Doppler ultrasound are two methodologies commonly used for the assessment of whole limb blood flow. Numerous studies have assessed limb blood flow in normothermic individuals using both of these technologies and have reported similar responses during perturbations such as reactive hyperemia (6), forearm handgrip exercise (24), and LBNP (12). The present findings of similar responses between the two methodologies during normothermia + LBNP are in agreement with these aforementioned reports and extend those findings by demonstrating that responses to thermal stimuli (skin-surface cooling and whole body heating), as well as to baroreceptor unloading during heat stress, are similar between these measurement devices.
Previously Johnson et al. (9) compared cutaneous to limb vasodilator responses during whole body heating via laser-Doppler flowmetry (presumably from a single-point system) and limb plethysmography, respectively. They found that although the relationship between these two devices correlated very well (R value equal to or greater than 0.94), there was a fair degree of heterogeneity in the slope of these responses between subjects (range: 0.04 to 0.12 V·ml−1·100 ml·min). In that study multiple FBF measures (i.e., upward to 100+ measure per subject) were obtained throughout the heating perturbation. In contrast, in the present protocol during heat stress plethysmographic and Doppler ultrasound measures were only obtained just before the onset of heating and at the end of heating just before LBNP. Thus we are unable to make similar comparisons, relative to that performed by Johnson et al. (9), between integrated probe/laser-scanner responses and plethysmography and Doppler ultrasound responses.
In summary, when normalized to maximum, the skin blood flow responses during a variety of thermal and/or vasoconstrictor stimuli are similar regardless of the size of the sample area. Additionally, FBF responses, when normalized to preperturbation baselines, to these perturbations are similar between venous occlusion plethysmography and Doppler ultrasound.
GRANTS
This research was supported by National Institutes of Health Grants HL-61388, HL-84072, and GM-68865.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We express appreciation to the subjects for their willing participation in this project.
REFERENCES
- 1. Braverman IM, Keh A, Goldminz D. Correlation of laser Doppler wave patterns with underlying microvascular anatomy. J Invest Dermatol 95: 283–286, 1990 [DOI] [PubMed] [Google Scholar]
- 2. Crandall CG, Johnson JM, Kosiba WA, Kellogg DL., Jr Baroreceptor control of the cutaneous active vasodilator system. J Appl Physiol 81: 2192–2198, 1996 [DOI] [PubMed] [Google Scholar]
- 3. Cui J, Arbab-Zadeh A, Prasad S, Durand S, Levine BD, Crandall CG. Effects of heat stress on thermoregulatory responses in congestive heart failure patients. Circulation 112: 2286–2292, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Dinenno FA, Joyner MJ, Halliwill JR. Failure of systemic hypoxia to blunt alpha-adrenergic vasoconstriction in the human forearm. J Physiol 549: 985–994, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Durand S, Cui J, Williams K, Crandall CG. Skin surface cooling improves orthostatic tolerance in normothermic individuals. Am J Physiol Regul Integr Comp Physiol 286: R199–R205, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Green D, Cheetham C, Reed C, Dembo L, O'Driscoll G. Assessment of brachial artery blood flow across the cardiac cycle: retrograde flows during cycle ergometry. J Appl Physiol 93: 361–368, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Holowatz LA, Houghton BL, Wong BJ, Wilkins BW, Harding AW, Kenney WL, Minson CT. Nitric oxide and attenuated reflex cutaneous vasodilation in aged skin. Am J Physiol Heart Circ Physiol 284: H1662–H1667, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Johnson JM, Proppe DW. Cardiovascular adjustments to heat stress. In: Handbook of Physiology. Environmental Physiology. Bethesda, MD: Am. Physiol. Soc., 1996, sect. 4, vol. I, pt. 2, chapt. 11, p. 215–243 [Google Scholar]
- 9. Johnson JM, Taylor WF, Shepherd AP, Park MK. Laser-Doppler measurement of skin blood flow: comparison with plethysmography. J Appl Physiol 56: 798–803, 1984 [DOI] [PubMed] [Google Scholar]
- 10. Kellogg DL, Jr, Johnson JM, Kosiba WA. Baroreflex control of the cutaneous active vasodilator system in humans. Circ Res 66: 1420–1426, 1990 [DOI] [PubMed] [Google Scholar]
- 11. Kimmerly DS, Shoemaker JK. Hypovolemia and neurovascular control during orthostatic stress. Am J Physiol Heart Circ Physiol 282: H645–H655, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Kitano A, Shoemaker JK, Ichinose M, Wada H, Nishiyasu T. Comparison of cardiovascular responses between lower body negative pressure and head-up tilt. J Appl Physiol 98: 2081–2086, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Mack GW. Assessment of cutaneous blood flow by using topographical perfusion mapping techniques. J Appl Physiol 85: 353–359, 1998 [DOI] [PubMed] [Google Scholar]
- 14. Oberg PA, Tenland T, Nilsson GE. Laser-Doppler flowmetry—a non-invasive and continuous method for blood flow evaluation in microvascular studies. Acta Med Scand Suppl 687: 17–24, 1984 [DOI] [PubMed] [Google Scholar]
- 15. Peters JK, Nishiyasu T, Mack GW. Reflex control of the cutaneous circulation during passive body core heating in humans. J Appl Physiol 88: 1756–1764, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Rowell LB. Thermal stress. In: Human Circulation Regulation During Physical Stress. New York: Oxford Univ. Press, 1986, p. 174–212 [Google Scholar]
- 17. Rowell LB, Brengelmann GL, Murray JA. Cardiovascular responses to sustained high skin temperature in resting man. J Appl Physiol 27: 673–680, 1969 [DOI] [PubMed] [Google Scholar]
- 18. Sokolnicki LA, Roberts SK, Wilkins BW, Basu A, Charkoudian N. Contribution of nitric oxide to cutaneous microvascular dilation in individuals with type 2 diabetes mellitus. Am J Physiol Endocrinol Metab 292: E314–E318, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Sokolnicki LA, Strom NA, Roberts SK, Kingsley-Berg SA, Basu A, Charkoudian N. Skin blood flow and nitric oxide during body heating in type 2 diabetes mellitus. J Appl Physiol 106: 566–570, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Taylor WF, Johnson JM, Kosiba WA, Kwan CM. Cutaneous vascular responses to isometric handgrip exercise. J Appl Physiol 66: 1586–1592, 1989 [DOI] [PubMed] [Google Scholar]
- 21. Tenland T, Salerud EG, Nilsson EG, Oberg PA. Spatial and temporal variations in human skin blood flow. Int J Microcirc Clin Exp 2: 81–90, 1983 [PubMed] [Google Scholar]
- 22. Thompson CS, Holowatz LA, Kenney WL. Cutaneous vasoconstrictor responses to norepinephrine are attenuated in older humans. Am J Physiol Regul Integr Comp Physiol 288: R1108–R1113, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Tripathi A, Nadel ER. Forearm skin and muscle vasoconstriction during lower body negative pressure. J Appl Physiol 60: 1535–1541, 1986 [DOI] [PubMed] [Google Scholar]
- 24. Tschakovsky ME, Shoemaker JK, Hughson RL. Beat-by-beat forearm blood flow with Doppler ultrasound and strain-gauge plethysmography. J Appl Physiol 79: 713–719, 1995 [DOI] [PubMed] [Google Scholar]
- 25. Vissing SF, Secher NH, Victor RG. Mechanisms of cutaneous vasoconstriction during upright posture. Acta Physiol Scand 159: 131–138, 1997 [DOI] [PubMed] [Google Scholar]
- 26. Whitney RJ. The measurement of volume changes in human limbs. J Physiol 121: 1–27, 1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wick DE, Roberts SK, Basu A, Sandroni P, Fealey RD, Sletten D, Charkoudian N. Delayed threshold for active cutaneous vasodilation in patients with Type 2 diabetes mellitus. J Appl Physiol 100: 637–641, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Wilson TE, Carter R, 3rd, Cutler MJ, Cui J, Smith ML, Crandall CG. Active recovery attenuates the fall in sweat rate but not cutaneous vascular conductance after supine exercise. J Appl Physiol 96: 668–673, 2004 [DOI] [PubMed] [Google Scholar]