Abstract
The head and mouthpart structures of 11 species of Eurasian scorpionflies represent three extinct and closely related families during a 62-million-year interval from the late Middle Jurassic to the late Early Cretaceous. These taxa had elongate, siphonate (tubular) proboscides and fed on ovular secretions of extinct gymnosperms. Five potential ovulate host-plant taxa co-occur with these insects: a seed fern, conifer, ginkgoopsid, pentoxylalean, and gnetalean. The presence of scorpionfly taxa suggests that siphonate proboscides fed on gymnosperm pollination drops and likely engaged in pollination mutualisms with gymnosperms during the mid-Mesozoic, long before the similar and independent coevolution of nectar-feeding flies, moths, and beetles on angiosperms. All three scorpionfly families became extinct during the later Early Cretaceous, coincident with global gymnosperm-to-angiosperm turnover.
Animal pollination, most frequently accomplished by insects (1), benefits seed plants by ensuring efficient fertilization without relying on costlier, abiotic modes such as wind and water transport (2). In return for pollination, plants provide a variety of nutritional and other rewards to pollinating animals, particularly pollen and nectar (1). Little is known about pollination mutualisms during the mid-Mesozoic (3, 4)—before the origin of flowering plants in the Early Cretaceous (5)—although evidence from the fossil record of insect mouthpart structure (6), gut contents (7), insect consumption of plant reproductive organs (8), and plant reproductive features (4, 9) indicates earlier consumption of pollen, nectar-like fluids, and other plant tissues (8). Here, we show that a major clade of extinct, siphon-bearing, fluid-feeding insects, early members of the order Mecoptera (scorpionflies), were likely feeding on gymnospermous ovulate secretions during the late Middle Jurassic to mid–Early Cretaceous. Modern scorpionflies are a relict clade of ~600 species, of which the relict Nannochoristidae have fluid-feeding mouthparts that are inferred to imbibe nectar and other plant exudates (10), perhaps representing an underappreciated legacy from distant Mesozoic ancestors. In contrast to some of their antecedents, modern Mecoptera overwhelmingly are minor predators or consumers of dead organisms (11) and rarely are implicated as floral visitors (12).
Our material consists of 21 insect specimens, six newly described (13), representing 11 species, five genera, and three families of Eurasian Mecoptera (tables S1 and S2). This mid-Mesozoic group encompasses the families Mesopsychidae (Fig. 1), Aneuretopsychidae (Fig. 2, A to E and G to I, and fig. S2), and Pseudopolycentropodidae (Fig. 2, F and J to O), for which the encompassing name Aneuretopsychina is available (14). These three lineages likely originated during the Late Permian, as judged by the co-occurrence of several closely related lineages during this epoch (15, 16). Taxa of these long-proboscid lineages are uncommon but persistent in Eurasian deposits representing an interval of 62 million years (My), from the late Middle Jurassic (Bathonian-Callovian boundary interval) to the late Early Cretaceous (late Albian Stage). We examined adpression specimens from four fine-grained deposits of late Middle Jurassic and Early Cretaceous ages, as well as one specimen from late Early Cretaceous amber (13).
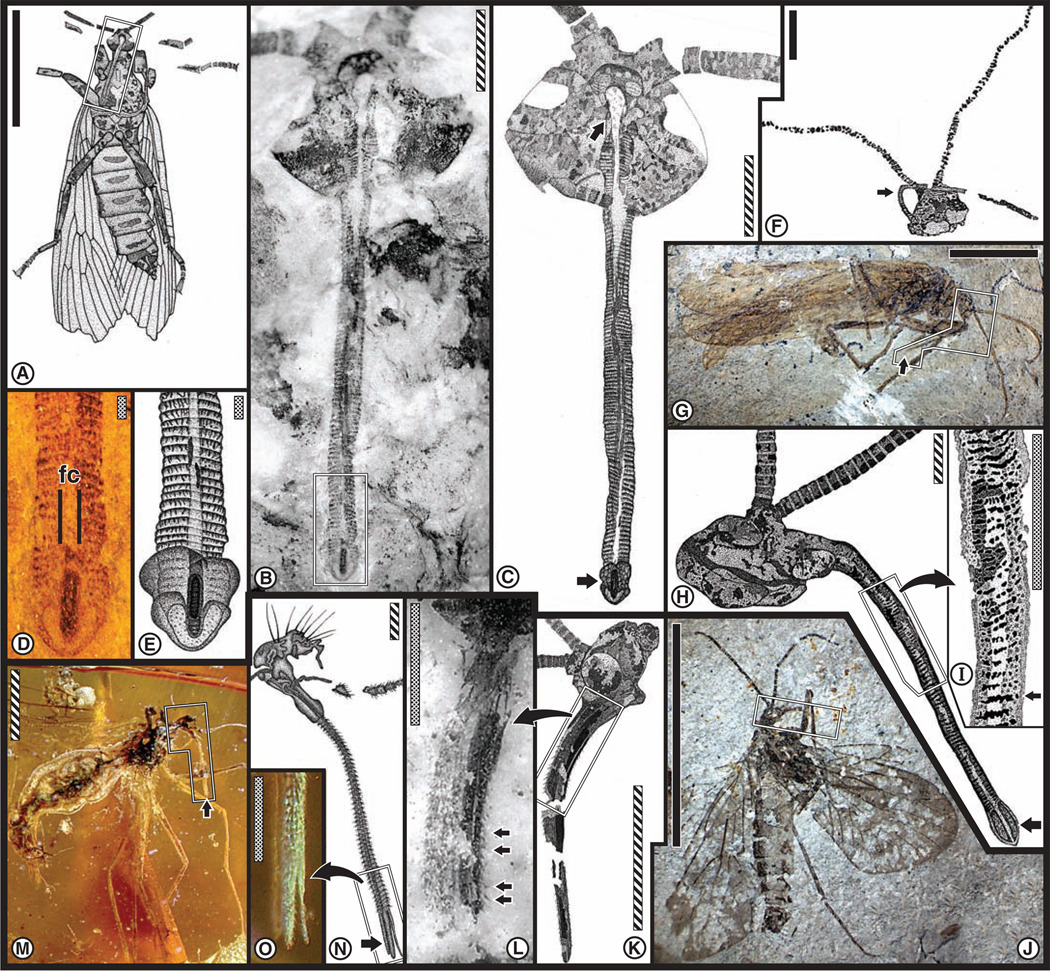
Fig. 1.
Head and siphonate mouthpart features associated with fluid-feeding Mesopsychidae scorpionflies from the mid-Mesozoic of northeastern China. (A to N) Six specimens of the new mesopsychid genus and species Lichnomesopsyche gloriae Ren, Labandeira & Shih gen. et sp. nov. (13), from the late Middle Jurassic (transitional Bathonian-Callovian) Jiulongshan Formation of Inner Mongolia, China. (A) Specimen CNU-M-NN2005020-1. (B) Enlargement of template in (A) as an overlay drawing of head and mouthparts. (C) Overlay drawing of specimen CNU-M-NN2005024. (D) Overlay drawing of specimen CNU-M-NN2005021-1. These images show long, tapering, filiform antennae; head and proboscis setal patterns; large, separate compound eyes; medially adpressed, three-segmented maxillary palps; and proboscis base, indicating a cibarial pump. (E) Enlargement of basal proboscis region from rectangle in (D), exhibiting, from left to right, proboscis base with setal pattern, three-segmented left maxillary palp, and right antenna. (F) Overlay drawing of a proboscis terminus, showing the central food canal, subterminal mouth, and prominent, ventrolaterally placed, type 1 pseudolabellum [CNU-M-NN2005025, counterpart of (L) below]. (G) Reconstruction of head and mouthparts based on details of all specimens. (H) Midproboscis region indicated in (G) is sectioned to illustrate the external pilosity, medial food canal reinforced by longitudinal rodlike structures, and inferred muscles for control of proboscis terminus. (I) Head and detail of basal proboscis region (CNU-M-NN2005029), exhibiting fine, transverse costae lining the food tube, probably representing a sucking pump. (J) Overlay drawing of a ventral proboscis (CNU-M-NN2005-025) from a scanning electron microscope (SEM) image of the proboscis tip in (B). (K) Enlargement of boxed area in (J), a composite of images from five tile scans using extended depth of field (EDF), illustrating the food canal (fc), reinforcing bundles (arrows), and proboscis margin, reconstructed in (H). (L) Photograph of ventral aspect of a proboscis terminus showing subterminal ventrolateral pseudolabellae, distinctive pilosity clothing the external surface, and probable food contents as a vertical, blobby line at left side of food canal [CNU-MM-2005-025, counterpart of (F) above]. (N) Lateral view of a ventrolateral terminal pad and mouth (CNU-M-NN2005-027-2), representing specimen tip in (M) outlined by a rectangle. (O to R) Specimen of a mesopsychid species, Vitimopsyche kozlovi Ren, Labandeira & Shih sp. nov. (13) (CNU-M-HP2005001), from the Early Cretaceous (Barremian) Yixian Formation of Hebei, northeastern China. (O) Camera lucida drawing. (P) Head and siphonate mouthpart region from rectangle in (O), enlarged as a SEM image integrating five EDF tile scans. (Q) Drawing of image in (P), showing formerly conjoined, subsurface, labial premental-palpal halves with possible tongue-and-groove interlocking. (R) Drawing of an associated pectinate antennal fragment occurring adjacent to the head in (O). Scale bars: solid, 10 mm; striped, 1 mm; dotted, 0.1 mm.
Fig. 2.
Head and mouthpart features associated with fluid-feeding in two specimens of siphonate Aneuretopsychidae (A to E and G to I) and in three specimens of siphonate (F and J to L) and stylate (M to O) Pseudopolycentropodidae, scorpionflies from the Middle Jurassic to late Early Cretaceous, from northeastern China and northern Myanmar (Burma). Shown in (A) to (E) is the head and mouthpart structure of Jeholopsyche liaoningensis Ren, Shih & Labandeira gen et sp. nov. (13), illustrating ventral body aspect (A) and dorsal view of head and mouthparts in photo (B) and drawing (C) (CNU-M-LB2005002). Note the filiform antennae, palp absence, compound eyes surrounded by prominent ocular sclerite (ring), and bracketing of a prominent clypeal region housing a cibarial pump. The long siphon has thickened striate walls basally [(B), upper arrow in (C)] and is annulated by small transverse ridges, bearing a food canal (fc) and terminus with a V-shaped pseudolabellum (type 2), elongate-ellipsoidal mouth with opaque contents, and lateral fleshy lobes [lower arrow in (C)] in the photo (D) and drawing (E). A specimen (CNU-M-NN2005003) of Pseudopolycentropus janeannae Ren, Shih & Labandeira sp. nov. (13) in (F), exhibiting a siphon curving 160° without fracture (arrow). (G) Another specimen (CNU-M-LB2005001) of an undetermined palpless species of Jeholopsyche shown in lateral view bearing a proboscis (arrow), probably in life position. (H) Enlargement of head and mouthparts boxed in (G), illustrating a robust proboscis base housing a cibarial pump and a terminus expanded to form a type 3 pseudolabellum (at arrow), homologous to that of (D) and (E). (I) Siphonal area boxed in (H) is enlarged to show segmented transverse ridges and food canal. Shown in (J) to (L) is the small species Pseudopolycentropus janeannae Ren, Shih & Labandeira sp. nov. (13) (CNU-M-NN2005004), illustrated in right lateral view in (J) and left head profile of counterpart in (K). In (K) are broad circular eyes with diminutive ocular sclerite, absence of palps, well defined labrum (base of arrow), an externally smooth, finely pilose, external siphonal surface, and absence of any lobe-like modification of the rounded terminus. Dense, fine setae on the proboscis are enlarged in (L) (arrows). Shown in (M) to (O) is a specimen of Parapolycentropus burmiticus (AMNH-Bu-1444) from Burmese amber. Entire insect (M) bears head and mouthparts, emphasizing stylate mouthparts within a labial tube. (N) Drawing [enlargement of boxed area in (M)] shows robust setae emerging from proboscis annulae and a distinct stylet, the terminus housing separated type 4 pseudolabellae [magnified in (O)]. Scale bars: solid, 10 mm; striped, 1 mm; dotted, 0.1 mm.
We conducted a cladistic analysis using 53 morphological characters, 19 extinct taxa, and 12 extant taxa (tables S3 to S5). In the best-supported cladogram (Fig. 3 and fig. S1), the three siphonate lineages—Mesopsychidae, Aneuretopsychidae, and Pseudopolycentropodidae—strongly support monophyly of the Aneuretopsychina (16), with unique head and mouthpart apomorphies, but retain generalized symplesiomorphies for most other characters, such as wing venation, leg characters, and genitalic structure. The inclusion of basal Permochoristidae (as Pseudonannochoristinae) and a terminal Permotipulidae in the Aneuretopsychina, currently based on wing features, suggests siphonate mouthparts in these taxa. We consider the Aneuretopsychina the sister taxon of the remaining, dominantly modern Mecoptera, rather than a stem group or paraphyletic lineage as previously proposed (16).
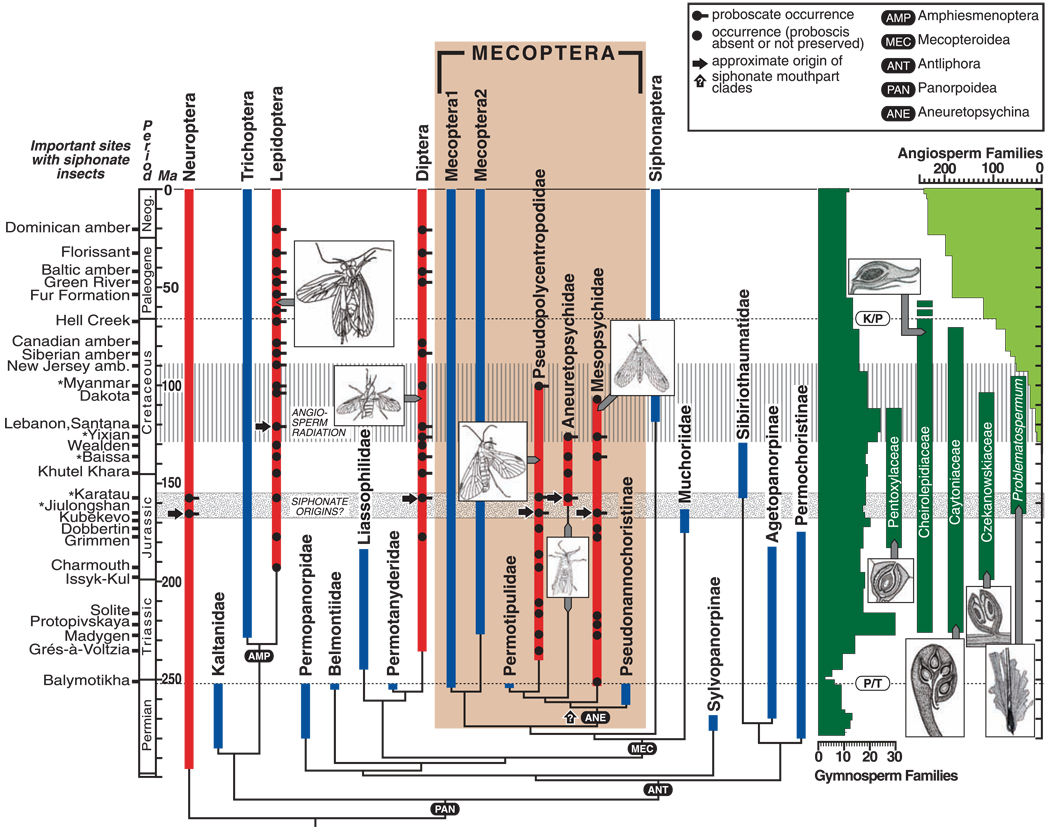
Fig. 3.
Major clades of panorpoid insects with siphonate mouthparts and their phylogenetic relationships (center) and likely associations with gymnospermous seed plants (right), presented in geochronologic context (left). At left are geologic periods calibrated to a time scale in millions of years (43); major Mesozoic localities are at the far left; asterisks denote fossil deposits in this report. Major extinction events are the Permian-Triassic (P/T) and Cretaceous-Paleogene (K/P) boundaries, indicated by the plant-insect associational record. At the center are broad, phylogenetic relationships derived from an analysis (13) of a morphology-based character matrix (tables S3 to S5) using a heuristic search in PAUP resulting in one of two best trees, shown here (length = 121, consistency index = 0.521, retention index = 0.725, rescaled consistency index = 0.378, homoplasy index = 0.479, and Goloboff fit criterion = −14.900) (fig. S1). Note the Mesozoic clade of long-proboscid Mecoptera (ANE) basal to remaining Mecoptera. All extant taxa and almost all geochronologically recent, nonsiphonate extinct taxa cluster into Mecoptera 1 ([Robinjohniidae + Bittacidae] + [Nannochoristidae + Boreidae]) and Mecoptera 2 (Panorpidae sensu stricto + [Dinopanorpidae + {Parachoristidae + |Eomeropidae + (Meropeidae + Thaumatomeropidae)|}]) clades. Alternative hypotheses for the origin of long-proboscid Mecoptera are presented as (i) basally within the clade during the Late Permian (bottom arrow with “?”), or (ii) convergently in three constituent lineages during a ~13-My window in the mid-Jurassic, indicated by a stippled horizontal bar. The latter hypothesis is complemented by penecontemporaneous appearances of siphonate proboscides in a neuropteran and multiple brachyceran fly lineages (24), minimally representing five independent origins, indicated by vertical red bars and five accompanying arrows. By contrast, monophyletic siphonate Lepidoptera are delayed, paralleling the ecologic rise of angiosperms. At right are gymnosperm (dark green) and angiosperm (light green) seed-plant diversity patterns (not to the same scale), compiled at conventional family levels (5, 44–50). Dark green vertical bars delineate known ranges of five gymnospermous clades indicated by cross sections of ovulate organs that could represent pollination mutualisms contemporaneous with long-proboscid scorpionflies (table S6). Illustrated are Caytonia sewardi Harris of Caytoniaceae (33, 51, 52), Alvinia bohemica Kvaček of Cheirolepidiaceae (8, 27), Leptostrobus cancer Harris of Czekanowskiaceae (53), Carnoconites compactus Srivastava of Pentoxylaceae (36, 54), and gnetalean Problematospermum ovale Turutanova-Ketova (28, 55). The transverse hatched bar within the Cretaceous (40 My deep) is the global transition from gymnosperm- to angiosperm-dominated floras; the topmost margin indicates the earliest angiosperm’s tubular flowers with nectar (5).
Excluding appendages, the 11 species range in body length from 3 mm in Parapolycentropus burmiticus to 28 mm in Lichnomesopsyche gloriae Ren, Labandeira & Shih gen. et sp. nov. (13, 17) (Fig. 1A). Wing venation indicates that these insects were modest flyers and could not hover, similar to modern scorpionflies but unlike some coeval nematocerous flies (8, 18). The heads are rather small and triangular or sub-circular in frontal aspect, with large but nonconverging spheroidal, compound eyes; filiform, pectinate, or moniliform antennae; and a distinctive clypeal sclerite indicating an underlying cibarial sucking pump. The external mouthparts in all but one species bear structures typical of extant, long-proboscid, pollinating insects (19) and prominent, long, siphonate mouthparts with or without accompanying maxillary palps. The prolonged tubular siphons are labial in origin and anatomically constitute the two sutured halves of the labial palps that are anchored at the premental base (19, 20). Many of the labial siphons bear diverse external cuticular ornamentation and vestiture, various terminal processes associated with feeding, and a subterminal circular to ellipsoidal mouth. Conspicuously absent are the downward extension of the head capsule and a terminally located, complete series of mandibulate mouthparts typical of modern Mecoptera (11, 20).
Representing the Mesopsychidae is L. gloriae (13) (Fig. 1, A to N, and table S2), our most fully documented species. Its head is triangular, posteriorly clothed with stiff setae between moderately bulging eyes; the head lacks ocelli and has a prominent, trapezoidal, clypeal sclerite situated immediately below the insertion of compact, filiform, tapering antennae (Fig. 1, B to D). The base of the siphon internally is characterized by distinctive transverse costae lining the outer food-tube surface, suggesting a second dilatory pump (Fig. 1I). Three-segmented maxillary palps are present on either side of a narrow labrum and proboscis base, all of which support local fields of moderately coarse setae and bear a falcately shaped third segment (Fig. 1, D and E). The forwardly directed, smooth surfaced siphon is 9.3 mm long (all dimensions herein reported are arithmetic averages; σ = 0.033, N = 6), 0.28 mm in unreconstructed diameter (σ = 0.33, N = 7), and houses a food tube 0.128 mm in unreconstructed diameter (σ = 7.33 × 10−3, N = 7). The siphon is covered by a vestiture of setae arranged in a distinctive chaetotaxic pattern along its lateral margins (Fig. 1, E, F, L, and N), deployed as nearly parallel longitudinal rows of similarly oriented setae. These setae occur from the proboscis base to the terminus, but they are absent from the immediate region of the subterminal circular mouth and at the ventrolaterally positioned, ovoidal labial pads (Fig. 1, F, J, L, and N), here termed pseudolabellae (type 1), which consist of fleshy tissue for likely capillary uptake of fluids. We interpret the siphon as exhibiting moderate flexibility and supported by ~10 flexible internal strands surrounding the food canal (Fig. 1, J and K). We infer that musculature, originating from the head capsule and attached along the proboscis, was responsible for siphonal flexion and directional control.
Congeneric with L. gloriae is L. daohugouensis Ren, Labandeira & Shih sp. nov. (13) (fig. S3 and table S2), a smaller, rarer species of half the body length (14 mm) and bearing a shorter siphon covered with a vestiture of dense, fine setae. The two studied specimens have a siphon that is 8.8 mm long, with an unreconstructed mean diameter of 0.325 mm and a food tube diameter of 0.105 mm. A larger, incompletely preserved, yet different mesopsychid species with a more ellipsoidal head is Vitimopsyche kozlovi Ren, Labandeira & Shih gen. et sp. nov. (13) (Fig. 1, O to R, and table S2). This single specimen bears distinctive pectinate antennae (Fig. 1R), broad, laterally placed eyes, and a rectangularly shaped clypeus (Fig. 1, P and Q). The distinctive, forwardly deployed siphon, 9.0 mm long, has a restored width of 0.58 mm, contains a food canal 0.14 mm in diameter, and lacks pseudolabellae (table S2). The siphon is preserved as two separated labial halves, each part of which has a dark, longitudinal ridge that is matched by an analogous but offset ridge on the counterpart, indicating an interlocking tongue-and-groove attachment mechanism. These longitudinal structures are unlikely to be stylets (which would be considerably more robust, sclerotized, and continuous), would exhibit a separate and partly nonparallel position within the opened labial siphon, and would display modifications for tissue penetration (such as retorse or serrated lateral edges or an acuminate tip). The absence of external cuticular ornamentation or pilosity on the siphon may be attributable to iron mineral replacement, disallowing preservation of fine detail; alternatively, the proboscis of V. kozlovi may have been unornamented in life.
The Aneuretopsychidae bore differently shaped heads, downwardly deployed proboscides, and distinctively ornamented siphonal surfaces that lacked setae. Jeholopsyche liaoningensis Ren, Shih & Labandeira gen. et sp. nov. (13) (Fig. 2, A to E, fig. S2, and table S2) is a superbly preserved specimen of medium body size, 24 mm long, with a head and proboscis whose dorsal aspect is preserved on the ventral side of the insect. The head is transversely oriented and stoutly ellipsoidal, has compact filiform antennae, prominent ocular sclerite encompassing large circular eyes positioned from the ventral to dorsal portion of the head capsule for a wide visual field, and lacks palps (Fig. 2, B and C). The distinctively shaped clypeus housed an inner cibarial pump, as determined by positional homology in a wide variety of modern scorpionfly taxa. The distinctively annulated siphon lacks setae, is 6.8 mm long and 0.34 mm wide, and has walls that expand at its base, below the clypeus, indicating a possible second dilatory suction pump, but positioned differently than in L. gloriae. Along the length of each transverse annulus is a series of perpendicularly arranged, short ridges. The siphonal terminus (Fig. 2, D and E, and fig. S2) bears a subterminal ellipsoidal mouth containing an opaque substance and is partly surrounded terminally by a V-shaped pad and by lateral fleshy pseudolabellae (type 2) that extend beyond the siphonal margin. These and other pseudolabellae probably are homologous with the functionally similar, distal palpal patch of modern Mecoptera and perhaps the labellum of Diptera (true flies). A conspicuous food tube, 0.10 mm in diameter, contains occasional blobs of opaque material identical to a nonparticulate substance lodged in the mouth (fig. S2). A second aneuretopsychid specimen, Jeholopsyche sp. (Fig. 2, G to I), similarly has a downturned proboscis as in other Aneuretopsychidae and Pseudopolycentropodidae, rather than a siphon directed anteriorly, as in the Mesopsychidae. Jeholopsyche sp. has medium-sized eyes, but its head shape, antennae, and absence of palps are similar to those of J. liaoningensis. The siphon is 5.8 mm long, 0.28 mm wide, and houses a food tube 0.085 mm in diameter (table S2), but in contrast to its congener, it lacks annuli and bears transverse, interrupted cuticular ridges that have regular spacing throughout the organ, and terminates in a unique, expanded pseudolabellum (type 3). The siphonal ridges are reminiscent of similar structures in some butterflies, such as Pieris (20) or Dryas (21), suggesting a similar function for probing into confined structures of host plants.
The much smaller Pseudopolycentropodidae is represented by two specimens of Pseudopolycentropus janeannae Ren, Shih & Labandeira sp. nov. (13) (Fig. 2, J to L, and table S2). This species has a triangularly shaped head, moderately-sized, circular compound eyes positioned laterally, a prominent labrum, compact filiform antennae, and a siphon in an anatomically downturned position lacking external cuticular ornamentation but presenting very fine setae, pushing the limit of preservational resolution. The siphon is ~1.6 mm in length and ~0.13 mm in diameter and lacks terminal structures related to feeding. The food canal, ~0.038 mm in diameter, is narrow for the proboscis width. A related larger specimen of P. janeannae, for which only one head and mouthpart ensemble have been examined, possesses an observable proboscis at least 1.9 mm long, displaying 160° of flexion without brittle fracture (Fig. 2F). The second pseudopolycentropodid species, amber Parapolycentropus burmiticus (Fig. 2, M to O), has a miniscule body length of 3.0 mm, a triangular head with prominent occipital setae, modest, laterally placed eyes, setate moniliform antennae, and a distinctive, robust labrum (17). The downwardly deflected proboscis is stylate rather than siphonate, annulated, has prominent setae arising from interannular sulci, and bears at least one protruding, robust stylet above two, elongate labial lobes that form pseudolabellae (type 4). The siphon is small, 1.33 mm long, 0.12 mm in width, and houses a barely detectable food canal 0.014 mm in width. P. burmiticus is the only species examined to bear stylate rather than siphonate mouthparts, consistent with at least some blood feeding (17). The thickened, buttressing labrum of P. burmiticus (Fig. 2, M and N) is interpreted as a major, force-absorbing brace analogous to the similarly sized, highly musculated, and anatomically homologous labrum in extant, blood-feeding mosquitoes (22). Extensive labral development in P. burmiticus is absent in the other examined fossil taxa; conversely, head and mouthpart features of the aneuretopsychine mouthparts are consistent with passive, nonpenetrative uptake of surface-accessible plant fluids.
Modern, long-proboscate, fluid-feeding dipteran lineages include nonstylate, nectar-feeding individuals that often are pollinators, as well as stylate, blood-feeding individuals. Blood-feeding taxa occur either as different species of the same higher-level taxon or as male (nectar-feeding) versus female (blood-feeding or both) sexes of the same species (23). This dual feeding strategy in several modern fly lineages may be analogous to some Early Cretaceous pseudopolycentropodids. By contrast, the presence of siphonate proboscides in the 10 other mecopteran taxa examined is likely homologous with the newly documented head and mouthpart structures of the most ancient, extant lineage of scorpionflies: the poorly known, relict, Gondwanan Nannochoristidae (10), which has a fossil record extending to the Early Jurassic (16), including thousands of specimens from the Middle Jurassic of China. Modern nannochoristids provide an anatomical context and valuable morphological link for assessing mouthpart differences between the Mesozoic Aneuretopsychina (Fig. 3) and modern Mecoptera.
Extant Nannochoristidae have been assigned variously to a basal lineage within modern Mecoptera, a separate order of insects, a sister group to Diptera, or to a clade including the Siphonaptera (fleas), among other options (10, 24). The feeding apparatus of extant Nannochoristidae is unique among extant scorpionflies. Fluid feeding apparently consists of initial uptake by capillary forces at the labial palp tip (the contact organ) followed by suction through modification of elongate, conjoined, mostly fleshy labial palps and labrum into a tubular structure. Each two-segmented palpus forms a half-tube that seals, together with the labrum, a short, projecting siphon powered by an enlarged pharyngeal pump centered under a frontal, quadrangular clypeus. In addition to tube-like elongation of the labium, there are other fluid-feeding, siphonate modifications shared between modern nannochoristid and extinct aneuretopsychine scorpionflies. These structures include a pilose and desclerotized proboscis outer surface, modification of the labial tip into a region of scales perhaps homologous with the aneuretopsychine pseudolabellum, absence of glossal and paraglossal medial labial lobes, reduction or loss of mandibles, expansion of the pharyngeal chamber into a significant suction pump, probable strengthening of intrinsic palpal musculature, and external muscles originating from the internal head capsule. Unlike the more simplified aneuretopsychine proboscis, the nannochoristid condition also includes maxillary elements, principally an egesting salivary tube within an ingesting food tube. When compared to the nannochoristid condition (10), there is no evidence that the Aneuretopsychina ever included maxillary salivary structures within their proboscis, indicating considerable mouthpart reduction and simplification in early mecopteran evolution (24).
We scanned all examined insects under epifluorescence microscopy, particularly heads and mouthparts for surface pollen and food canals for ingested, lodged grains (13). Previous studies indicate that pollen occurs as insect gut contents (7) or rarely as clumps on the head and proboscis base, such as a brachyceran fly from another Eurasian site of mid–Early Cretaceous age (25). Our results were negative for both surface and food-canal pollen in the compression and amber material. The apparent absence of pollen may be due to taphonomic factors (e.g., relatively low carbon content in some specimens, relatively high oxidation level in the matrix of many of the specimens, loss of pollen during diagenesis); alternatively, the absence of pollen may be genuine. The head and mouthparts of the amber specimen were well preserved and readily imaged, so the lack of pollen likely reflects true absence rather than taphonomic artifact.
We also examined the chemical contents of the proboscis food canal from the best-preserved specimen, J. liaoningensis (Fig. 2, A to E), by energy-dispersive spectroscopy (EDS). We produced elemental results from EDS for four regions: the matrix surrounding the fossil, the femur of the right metathoracic leg, a targeted plug of food canal material approximately midway between the proboscis base and tip, and another plug of dark material in the mouth at the proboscis tip (fig. S2); the first two of these regions were controls. As expected, the matrix was highly depleted in carbon relative to the scorpionfly, but the relative abundance of carbon in the legs and proboscis plugs was indistinguishable. One major result was the absence of iron enrichment in the food canal and mouth plugs relative to the leg or matrix. Sulfur exhibited a slight elevation in the insect relative to the matrix, and was uninformative regarding the presence of S-bearing biomolecules or their degradative by-products in the food tube or mouth. EDS data support a diet lacking in blood, suggesting that the food consisted of pollination droplets (1, 8, 26), and are noncommittal regarding the presence of any S-bearing amino acids in the food canal (13).
Several gymnospermous plant hosts are present in the same or contemporaneous Eurasian deposits and could have been food sources for long-proboscid insects (27, 28). Coexisting seed-fern and conifer taxa secreted pollination droplets with surface-exposed fluid connected to deeper-seated pollination chambers by long tubules, enclosed channels, or analogous features (table S6). Species of Caytoniaceae, Cheirolepidiaceae, Czekanowskiaceae, Pentoxylaceae, and Gnetales (Fig. 3 and table S6) bore ovulate organs that were either unusual for wind pollination or possessed structures that would be expected for long-proboscid fluid feeding. Another possibility is that the immediate ancestors of angiosperms had incomplete carpel closure, with access to probing insects of deeper-seated pollination fluids. However, angiosperm taxa with floral features amenable to pollination by long-proboscid scorpionflies, such as tubular flowers or nectarial spurs, appear later in the fossil record (5, 8).
Insect proboscis dimensions from examined siphonate taxa could have been accommodated by micropylar or other connecting ovular tubule diameters. The data reveal three size patterns specific to each of the three insect clades (tables S2 and S6). Most scorpionfly taxa examined had food canal dimensions that could not accommodate pollen diameters of the inferred host-plant taxa (tables S2 and S6); this finding suggests that the mechanism for pollen transfer was bodily surface transport on mouthpart and head surfaces, analogous to most bee flies and hover flies (1). The first group were mesopsychids bearing elongate proboscides ~8.8 to ~10.1 mm long, whose relatively modest widths could accommodate the pollen catchment funnel (~7.0 mm long) of the ovulate organ in the cheirolepidiaceous conifer Alvinia bohemica. The pollen catchment funnel of A. bohemica, lined by uniseriate trichomes distally and short, thickened glands proximally, was connected to a tube that traversed ovulate tissue to terminate immediately below the micropylar terminus adjacent to the cone axis (8, 27). The gnetalean Problematospermum ovale (28) potentially allowed access to pollination fluids through a long, tubular, exserted micropyle up to 12.0 mm long that was surrounded by a bracteate envelope of finely veined, petaloid structures. This feeding mode apparently was duplicated by coexisting, long-proboscate brachyceran flies (8, 29), analogous to extant nemognathine blister beetles that have proboscis lengths of ~10.0 mm (30), and by bee flies (31) and mydid flies such as Rhaphiomidas (32) having approximately the same proboscis lengths, pollinating deep-throated flowers. In the second group, Aneuretopsychidae, various species collectively possessed shorter siphons, 4.7 to 7.3 mm long, consistent with consumption of fluids produced by taxa such as Caytonia in tubules 4.0 to 6.5 mm long and 0.35 to 0.5 mm in diameter (33). Species of Leptostrobus (Czekanowskiaceae) also may have been a target, through which 2.5 to 5.0 mm of similar distance would have been traversed for access to micropylar fluid opposite the entry area. Modern taxa bearing tubular proboscides of this length range include the tabanid fly Mesopangonius (proboscis length ~4.5 mm) (34) and the long-tongued vespid wasp Ceramius hispanicus (~5.6 mm) (35). The third group, diminutive species of Pseudopolycentropodidae, supported proboscides ranging from 0.9 to 1.8 mm in length, with diameters sufficiently small to enable them to target plants with equally short micropylar lengths. Plant host candidates include Carnoconites compactus (Pentoxylaceae) with extended micropylar-integumental lengths of 1.0 to 2.0 mm (36), overlapping with Leptostrobus, and conceivably surface accessible ovules of some bennettitalean lineages. Such short proboscides occur in several related species of nectar-feeding tabanids in the tribe Pangoniini (37), ranging in length from 0.6 to 1.7 mm, which lack stouter mouthparts that would characterize blood feeding.
Our case for insect pollination is based on a variety of evidence for these three lineages of scorpionflies and their inferred pollinated plants. First, scorpionfly proboscis morphology and surface features, such as hairy and obliquely ridged surfaces (Figs. 1 and 2), are highly convergent with extant dipteran, blister beetle (19, 30), and glossate lepidopteran mouthparts engaged in active pollination (1, 18, 19, 21, 31, 35). Second, co-occurring with these scorpionflies are seed-fern and conifer ovulate organs (table S6), which present pollen-receptive areas that are hidden or poorly exposed to wind-dispersed grains and lead into long integumental channels, prolonged catchment funnels, or extended tubular micropyles, ending in deeper-seated pollen chambers. Third, the levels of amino acids, sugars, inorganic ions, and other constituents found in modern insect-pollinated gymnosperms (26) suggest that the pollination droplets that filled these elongate tubular structures would be nutritionally similar to angiosperm nectar, thereby sustaining high levels of insect activity (8). Fourth, unisexual pollen-bearing organs typically are separated some distance from the conspecific ovulate organs, either on the same plant or different individual plants, thus favoring outcrossing for more efficient modes of pollination, such as remote insect transport. This contrasts with the bisexual system of known bennettitaleans and magnoliid angiosperms, in which female and male organs are adjacent to each other (38). Fifth, pollen extracted from affiliated pollen organs range in length from 17 to 65 µm for the diverse ovulate taxa that we implicate for pollination (table S6), approximating the modern 25- to 50-µm range for entomophilous cycads (39) and within the broader range of 10 to 80 µm for similarly pollinated gnetaleans (40). The pollen of Caytonia sewardi possesses bisaccate pollen typically not associated with entomophily [but see (41)]; however, its compact pollen was within the entomophilous size range. Last, cheirolepidiaceous and gnetalean pollen occur as gut contents in several Mesozoic insect lineages (7, 8), serving as a nutritional source and/or as a reward for pollination.
Two hypotheses may explain the timing for the origin of the mecopteran siphonate proboscis from proposed nannochoristid-like ancestors: (i) The siphon was established during the Late Permian, for which there is phylogenetic but no fossil evidence (15, 16). (ii) Alternatively, if we consider only fossil data, three separate originations occurred in a ~13-My interval during the mid-Jurassic that also parallel the origin of dipteran and neuropteran siphons (Fig. 3). The latter hypothesis would indicate minimally a fivefold convergence among coeval aneuretopsychine scorpionfly and two other unrelated siphon-bearing lineages. The ~62-My subsequent phase of fluid-feeding on gymnospermous seed plants, and their probable pollination, eventually was replaced by a newly evolved, lepidopteran, siphon-bearing clade, Glossata, during the Early Cretaceous, probably as specialists on early angiosperm hosts (3, 42). Contemporaneously, this origin was accompanied by host-switching of siphonate, brachyceran fly lineages onto angiosperms and extinction of three siphonate lineages of scorpionflies.
Supplementary Material
Footnotes
Supporting Online Material
www.sciencemag.org/cgi/content/full/326/5954/840/DC1
Materials and Methods
SOM Text
Figs. S1 to S4
Tables S1 to S6
References
References and Notes
- 1.Proctor M, Yeo P, Lack A. The Natural History of Pollination. Portland, OR: Timber; 1996. [Google Scholar]
- 2.Midgley JJ, Bond WJ. Philos. Trans. R. Soc. London Ser. B. 1991;333:209. [Google Scholar]
- 3.Grimaldi DA. Ann. Mo. Bot. Gard. 1999;86:373. [Google Scholar]
- 4.Gorelick R. Biol. J. Linn. Soc. 2001;74:407. [Google Scholar]
- 5.Crepet WL. Ann. Mo. Bot. Gard. 2008;95:3. [Google Scholar]
- 6.Labandeira CC. Annu. Rev. Ecol. Syst. 1997;28:153. [Google Scholar]
- 7.Krassilov VA, Rasnitsyn AP, Afonin SA. Afr. Invertebr. 2007;48:3. [Google Scholar]
- 8.Labandeira CC, Kvaček J, Mostovski MB. Taxon. 2007;56:663. [Google Scholar]
- 9.Axsmith BJ, Krings M, Waselkov K. J. Paleontol. 2004;78:402. [Google Scholar]
- 10.Beutel RG, Baum E. J. Zool. Syst. Evol. Res. 2008;46:346. [Google Scholar]
- 11.Byers GW, Thornhill R. Annu. Rev. Entomol. 1983;28:203. [Google Scholar]
- 12.Porsch O. Plant Syst. Evol. 1957;104:115. [Google Scholar]
- 13.See supporting material on Science Online.
- 14.Rasnitsyn AP, Kozlov MV. Trans. U.S.S.R. Acad. Sci. Earth Sci. Sect. 1991;310:233. [Google Scholar]
- 15.Novokshonov VG. Early Evolution of Scorpionflies (Insecta: Panorpida) Moscow: Nauka; 1997. [in Russian] [Google Scholar]
- 16.Grimaldi DA, Engel MS. Evolution of the Insects. New York: Cambridge Univ. Press; 2005. [Google Scholar]
- 17.Grimaldi DA, Zhang J-F, Fraser NC, Rasnitsyn AP. Insect Syst. Evol. 2005;36:443. [Google Scholar]
- 18.Stuckenberg BR. Ann. Natal Mus. 2000;41:181. [Google Scholar]
- 19.Borrell BJ, Krenn HW. In: Ecology and Biomechanics: A Mechanical Approach to the Ecology of Animals and Plants. Herrel H, Speck T, Rowe NP, editors. Boca Raton, FL: Taylor & Francis; 2006. pp. 185–212. [Google Scholar]
- 20.Chaudonneret J. Les Pièces Buccales des Insectes: Thème et Variations. France: Berthier, Dijon; 1990. [Google Scholar]
- 21.Krenn HW, Plant JD, Szucsich NU. Arthropod Struct. Dev. 2005;34:1. [Google Scholar]
- 22.Snodgrass RE. Smithson. Misc. Coll. 1943;104:1. [Google Scholar]
- 23.Kniepert FW. Oecologia. 1980;46:125. doi: 10.1007/BF00346976. [DOI] [PubMed] [Google Scholar]
- 24.Beutel RG, Kristensen NP, Pohl H. Arthropod Struct. Dev. 2009;38:427. doi: 10.1016/j.asd.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Labandeira CC. In: The Evolutionary Biology of Flies. Yeates DK, Wiegmann BM, editors. New York: Columbia Univ. Press; 2005. pp. 217–273. [Google Scholar]
- 26.Nepi M, et al. Ann. Bot. 2009;104:205. doi: 10.1093/aob/mcp124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kvaček J. Rev. Palaeobot. Palynol. 2000;112:51. doi: 10.1016/s0034-6667(00)00035-x. [DOI] [PubMed] [Google Scholar]
- 28.Sun G, et al. Early Angiosperms and Their Associated Plants from Western Liaoning, China. Shanghai: Shanghai Scientific and Technical Education Publishing House; 2001. [Google Scholar]
- 29.Ren D. Science. 1998;280:85. doi: 10.1126/science.280.5360.85. [DOI] [PubMed] [Google Scholar]
- 30.Grinfel’d EK. Entomol. Rev. (Engl. Transl.) 1975;54:18. [Google Scholar]
- 31.Szucsich NU, Krenn HW. Zoomorphology. 2000;120:79. [Google Scholar]
- 32.Cazier MA. Bull. Am. Mus. Nat. Hist. 1985;182:181. [Google Scholar]
- 33.Harris TM. New Phytol. 1933;32:97. [Google Scholar]
- 34.Burger JF. Proc. Entomol. Soc. Wash. 1988;90:12. [Google Scholar]
- 35.Krenn HW, Mauss V, Plant J. Arthropod Struct. Dev. 2002;31:103. doi: 10.1016/s1467-8039(02)00025-7. [DOI] [PubMed] [Google Scholar]
- 36.Bose MN, Pal PK, Harris TM. Philos. Trans. R. Soc. London Ser. B. 1985;310:77. [Google Scholar]
- 37.González CR, Flores P. Zootaxa. 2004;579:1. [Google Scholar]
- 38.Crepet W. Am. J. Bot. 1972;59:1048. [Google Scholar]
- 39.Dehgan B, Dehgan NB. Am. J. Bot. 1988;75:1501. [Google Scholar]
- 40.Tekleva MV, Krassilov VA. Rev. Palaeobot. Palynol. 2009;156:130. doi: 10.1016/s0034-6667(00)00075-0. [DOI] [PubMed] [Google Scholar]
- 41.Stelleman P, Meeuse ADJ. Tijdschr. Entomol. 1976;119:15. [Google Scholar]
- 42.Labandeira CC, Dilcher DL, Davis DR, Wagner DL. Proc. Natl. Acad. Sci. U.S.A. 1994;91:12278. doi: 10.1073/pnas.91.25.12278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ogg JG, Ogg G, Gradstein FM. The Concise Geologic Time Scale. Cambridge: Cambridge Univ. Press; 2008. [Google Scholar]
- 44.Collinson ME, Boulter MC, Holmes PL. In: The Fossil Record 2. Benton MJ, editor. London: Chapman & Hall; 1993. pp. 809–849. [Google Scholar]
- 45.Friis EM, Pedersen KR, Crane PR. Ann. Mo. Bot. Gard. 1999;86:259. [Google Scholar]
- 46.Magallón S, Sanderson MJ. Evolution. 2001;55:1762. doi: 10.1111/j.0014-3820.2001.tb00826.x. [DOI] [PubMed] [Google Scholar]
- 47.Soltis PS, Soltis DE. Am. J. Bot. 2004;91:1614. doi: 10.3732/ajb.91.10.1614. [DOI] [PubMed] [Google Scholar]
- 48.Crepet WL, Nixon KC, Gandolfo MA. Am. J. Bot. 2004;91:1666. doi: 10.3732/ajb.91.10.1666. [DOI] [PubMed] [Google Scholar]
- 49.Anderson CL, Bremer K, Friis EM. Am. J. Bot. 2005;92:1737. doi: 10.3732/ajb.92.10.1737. [DOI] [PubMed] [Google Scholar]
- 50.Anderson JM, Anderson HM, Cleal C. Strelitzia 20: Brief History of the Gymnosperms: Classification, Biodiversity, Phytogeography and Ecology. Pretoria: South African National Biodiversity Institute; 2008. [Google Scholar]
- 51.Harris TM. Ann. Bot. 1940;4:713. [Google Scholar]
- 52.Harris TM. Phytomorphology. 1951;1:29. [Google Scholar]
- 53.Harris TM. Philos. Trans. R. Soc. London Ser. B. 1951;235:483. [Google Scholar]
- 54.Sahni B. Bot. Gaz. 1948;110:47. [Google Scholar]
- 55.Wang X. personal communication. 2009 July [Google Scholar]
- 56.This is contribution 152 of the Evolution of Terrestrial Ecosystems consortium at the National Museum of Natural History and contribution 614 of the Florida Museum of Natural History. Supported by the National Science Foundation of China (grants 30430100 and 40872022), the Natural Science Foundation of Beijing (grant 5082002), and the Key and PHR Program of the Beijing Municipal Commission of Education (D.R.); the Origin and Evolution of the Biosphere program of the Presidium of the Russian Academy of Sciences (A.B.); and the Intramural Research Program of the National Library of Medicine. We thank F. Marsh for drafting the figures, T. Lott for a presubmission review, P. Crane for comments, T. Rose for assistance on the geochemical analyses, J. Corman for collection of data, B. Wagner and R. Johnson for translations, P. Peltier for proofreading, and W. Wu for general assistance. X. Wang provided data and images for Problematospermum ovale, and D. Grimaldi of the American Museum of Natural History (New York) made an important specimen available for study.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.