Abstract
Death receptors of the tumor necrosis factor (TNF) receptor super family have been implicated in constitutive activation of Nuclear Factor kappa B (NF-κB) in pancreatic cancer (PaC) cells. In this study we demonstrate that fisetin, a natural flavonoid, induces apoptosis and inhibits invasion of chemoresistant PaC AsPC-1 cells through suppression of DR3 mediated NF-κB activation. Fisetin treatment resulted in dose-dependent inhibition of PaC cell growth and cell proliferation with concomitant induction of apoptosis. A cDNA array analysis revealed that fisetin modulates expression of more than 20 genes at transcription level with maximum decrease observed in DR3 expression and a parallel increase observed in the expression levels of IκBα, an NF-κB inhibitor. Down-regulation of DR3 in PaC cells was found to down regulate activated pNF-κB/p65, pIkBα/β kinases (pIKK’s), MMP9 and XIAP that mostly impart chemoresistance in PaC. Immunoblotting and EMSA analysis showed a marked decrease in pNF-κB and NF-κB DNA binding activity respectively with modest decrease in NF-κB promoter activity and significant decrease in MMP9 promoter activity with fisetin treatment. Importantly, consistent with these findings, we further found that transient down-regulation of DR3 by RNA interference significantly augmented fisetin induced changes in cell proliferation, cell invasion and apoptosis paralleled with decrease in pNF-κB, pIKKα/β, MMP9, XIAP and NF-κB DNA binding activity. Blocking of DR3 receptor with an extra cellular domain blocking antibody demonstrated similar effects. These data provide evidence that fisetin could provide a biological rationale for treatment of pancreatic cancer or as an adjuvant with conventional therapeutic regimens.
Keywords: Pancreatic cancer, Fisetin, apoptosis, Invasion, DR3
INTRODUCTION
Pancreatic cancer (PaC) represents one of the most aggressive tumor types with extremely poor prognosis.1 Constitutively activated nuclear factor kappa B (NF-κB) has been associated with a variety of aggressive tumor types, including pancreatic cancer.2-4 Constitutive activation of NF-κB and its regulated genes strongly enhance invasive properties as well as impart chemoresistance to PaC cells.5 Members of TNF superfamily including TNFR1, DR4, DR5, DR6, Fas and their ligands have been reported to play an important role in many cellular activities including proliferation, migration, differentiation, apoptosis, angiogenesis and inflammation and are highly expressed in number of pathological conditions.6 The signaling pathways induced by these receptors are similar and rely on oligomerization of the receptor by ligand binding, recruitment of death domain proteins, such as TRADD, FAD, or TRAF2, RIP1, through homophilic interaction of their death domains, and subsequent activation of the caspases apoptotic cascade or the transcription factor NF-κB.4 Members of TNF superfamily ligands have been shown to induce NF-κB activation via binding to their cognate receptors.7 NF-κB regulates the expression of genes that regulate transformation, tumor promotion, tumor invasion, angiogenesis, and metastasis.8
DR3, a member of the TNF family and a receptor for TNF, is a protein on the surface of cells that has been reported to be capable of inducing NF-κB activation when over expressed in mammalian cells.9-10 Recent studies suggest that DR3-blocking agents might be more effective at specifically treating autoimmune disease since mice engineered to lack DR3 were resistant to those diseases suggesting that blocking DR3 in mice, and possibly in humans, is a potential therapy for these diseases.11 While earlier studies mostly confined expression of DR3 to lymphoid cells, however recent studies have reported its expression in non-lymphoid tissues and pancreatic cancer cells.12-13
Fisetin (3,7,3′,4′-tetrahydroxyflavone) a naturally occurring flavonoid found in many fruits and vegetables exhibits a wide variety of functions including antioxidant, neurotropic, antiangiogenic and antiproliferative effects.14-19 Fisetin was found to suppress TNF, NF-κB, NF-κB-dependent reporter gene expression and activity.17 In this study we show that fisetin mediates its apoptotic, anti-proliferative and anti-invasive effects in chemoresistant PaC cells by modulating DR3 receptor mediated down regulation of NF-κB signaling pathway.
MATERIAL & METHODS
Reagents and Antibodies
Fisetin (3, 3′, 4′, 7-Tetrahydroxyflavone) 99% pure was purchased from Sigma (St. Louis, MO). Antibodies against XIAP, DR3, pNF-κB/p65, NF-κB/p65, IκBα, pIκBα, IKKα/β and MMP9 were obtained from Cell Signaling (Beverly, MA), anti-PARP/116 from Upstate (Lake Placid, NY) and anti-PARP/85 from Promega (Madison, WI). Horseradish peroxidase (HRP) conjugated secondary antibodies were obtained from Amersham (Arlington Height, IL). DR3-shRNA and scrambled-shRNA were purchased from Qiagen (Valencia, CA). BCA Protein assay kit was obtained from Pierce (Rockford, IL). Novex precast Tris-glycine gels were obtained from Invitrogen (Carlsbad, CA). Annexin-V-Fluos staining kit was purchased from Roche (Indianapolis, IN).
Cell culture and treatment
Human PaC cell line AsPC-1 was obtained from ATCC (Manassas, VA) and grown in appropriate media supplemented with 10% FBS and 1% Penicillin-Streptomycin under standard cell culture conditions. A stock solution of fisetin (25 mM) was prepared by dissolving in dimethyl sulfoxide (DMSO, 0.1% v/v). The cells (60-70% confluent) were treated with fisetin (0-80 μM) for 24 and 48 h in complete growth medium and cell viability was performed and lysates prepared for Western blotting or stored at −80 °C for later use.
Cell viability assay
The effect of fisetin on cell viability was determined by MTT (3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyl tetrazoliumbromide) assay as described earlier.16
Apoptosis assay
The annexin-V-Fluos staining kit was used for the detection of apoptotic cells according to vendor’s protocol and described earlier.16 The AsPC-1 cells were grown to 70% confluence and treated with fisetin (10-60 μM) for 48 h.
[3H]-Thymidine incorporation assay
AsPC-1 cells (60% confluent) grown in 24 well culture plates were subjected to fisetin treatment for 48 h, the last 16 h of which were in the presence of [3H]thymidine (0.5 μCi/ml). Cells were then washed twice with PBS at room temperature and then with ice-cold 5% trichloroacetic acid (TCA). The cells were next incubated with TCA solution on ice for 30 min and subsequently the acid-insoluble fraction was dissolved in 1 ml of 0.5M NaOH. Incorporated [3H]thymidine was quantified by liquid scintillation counting.
Microarray analysis
Twenty four hours post incubation with fisetin, treated and untreated cells were harvested and RNA was isolated by using RNAeasy kit (Qiagen, Valencia, CA). Next, 4 μg of RNA was enzymatically converted into cDNA. The cDNA was then biotinylated with the True Labeling-AMP™ 2.0 Kit as per vendor’s protocol (Super Array, Frederick, MD). The labeled probes were hybridized on the arrays (Human Cancer Pathway Finder, Superarray, Frederick, MD) imprinted with 113 genes representative of the six biological pathways involved in transformation and tumorigenesis. Hybridization was followed by detection with the chemiluminescent reagents and x-ray film development. Data was acquired and analyzed by using GE superarray software (Superarray, Frederick, MD).
DR3-shRNA transfection
Transfections were performed by using nucleofection kit (Amaxa, Walkersville, MD). Briefly, 106 cells were transfected with 50 nM of shRNA directed against DR3. Control cells were transected with scrambled shRNA (50 nM). After overnight incubation, transfected cells were treated with fisetin (20-40 μM) and harvested after 24 h post fisetin treatment. Cell lysates were processed for immunoblot analysis. In addition, in a parallel set of experiments, annexin-V assay and cell invasion assay were performed.
Western Blot Analysis
Cell lysates were prepared in cold lysis buffer [(0.05 mmol/L Tris-HCl, 0.15 mmol/L NaCl, 1 mole/L EGTA, 1 mol/L EDTA, 20 mmol/L NaF, 100 mmol/L Na3VO4, 0.5% NP-40, 1% Triton X-100, 1 mol/L phenyl methylsulfonyl flouride (pH 7.4) with freshly added protease inhibitor cocktail (Calbiochem, La Jolla, CA). The lysate was collected and protein content measured by BCA method. For Western blotting, 40 μg protein was resolved over 12% Tris-glycine polyacrylamide gels under non-reduced conditions, transferred onto nitrocellulose membranes and subsequently incubated in blocking buffer (5% nonfat dry milk/1% Tween 20; in 20mmol/L TBS, pH 7.6) for 2 hours. The blots were incubated with appropriate primary antibody, washed and incubated with appropriate secondary HRP-conjugated antibody. The blots were detected with chemiluminescence and autoradiography, using XAR-5 film (Kodak, Rochester, NY). Equal loading of protein was confirmed by stripping the blots and reprobing with β-actin.
Wound closure assay
Cells were plated in 24 well plates. When the cells were ~90% confluent, a wound was induced on the monolayer cells by scraping a gap using a micropipette tip and then fisetin was added immediately after the wound induction. The speed of wound closure was compared between fisetin treated and untreated control group. Photographs were taken 24 h after wound incision.
Chemoinvasion assay
We used a chemoinvasion kit (Millipore, Danvers, MA) for cell invasion assay. Confluent cells were adjusted to 106 cells per 100 μl of cell suspension, resuspended in serum-free media with appropriate concentration of fisetin, and applied onto the matrigel-filled transwell chamber. After 14 h incubation, noninvasive cells were scraped off with a cotton swab and invasive cells were fixed and stained for 10 minutes with crystal violet (0.5% in 20% methanol) and washed with water.
Gelatin zymography
Cells were seeded at a density of 106 and grown in medium containing 10% FBS. After 24 h, appropriate concentrations of fisetin were added and cells were allowed to grow for additional 24 h. Supernatant was collected and cleared by centrifugation, and cells were detached from the wells with trypsin and counted. Supernatants (normalized to cell numbers) were then loaded onto zymogram gels. After electrophoresis, the gel was incubated with renaturing buffer for 30 min at room temperature and incubated overnight at 37 °C with zymogram developing buffer. The gel was stained with 0.02% Coomassie brilliant blue in 20% acetic acid. Molecular weights of the gelatinolytic bands were estimated using molecular weight markers.
Transcriptional activity of NF-κB and MMP9
The human MMP-9 promoter luciferase plasmid (pGL2-MMP-9-luc) was received as a kind gift. Empty pGL2 was procured from Upstate Laboratories (Lake Placid, NY). All plasmids were transformed in agar media and extracted by using Maxiprep kit (Qiagen, Valencia, CA). Cells plated at a density of 5 × 104 cells/well were transfected with the plasmids (200ng/well) for 24 h. Renilla luciferase (20 ng/well, pRL-TK; Promega, Madison, WI) was used as an internal control. In addition, for controls, the same amount of empty vectors, were transfected in cells. After 12 h post-transfection, cells were treated with fisetin (5-10 μM) and incubated for 24 h. The cells were then harvested and transcriptional activity was measured in terms of luciferase activity by using dual-luciferase reporter assay system (Promega, Madison, WI). Relative luciferase activity was calculated with the values from vector alone group with or without Fisetin treated group.
Nuclear extract preparation and electrophoresis mobility shift assays (EMSA)
EMSA for NF-κB was performed using lightshift™ chemiluminiscent EMSA kit (Pierce, Rockford, IL) as per manufacturer’s protocol and described earlier [20].
Effect of fisetin on cell surface expression of DR3
For analysis of cell surface expression of DR3, fisetin treated cells were harvested and suspended in Dulbecco’s PBS containing 1% FBS and 0.1% sodium azide. The cells were preincubated with 10% goat serum for 20 min and washed, and then monoclonal rabbit IgG anti-DR3 antibodies were added. Following 1 h incubation at 4 °C, cells were washed and incubated for an additional 1 h in FITC-conjugated goat anti-rabbit IgG antibody. The cells were analyzed using a FACS Calibur flow cytometer and Cell Quest acquisition and analysis programs (BD Biosciences, San Jose, CA).
Effect of blocking of DR3 extracellular domain with antibody
A DR3 specific antibody was used at a concentration of 5μg/ml to further ascertain the role of DR3 in induction of apoptosis and invasion in AsPC-1 cells. AsPC-1 cells were treated with either a DR3 antibody, 20 μM fisetin or a combination of both. Cells were analyzed for apoptosis induction, invasion and DR3 expression as detailed above.
Statistical analyses
Student’s t test for independent analysis was applied to evaluate differences between the treated and untreated groups with respect to the expression of various proteins. A p-value of <0.05 was considered to be statistically significant.
RESULTS
Effect of fisetin on cell growth and viability
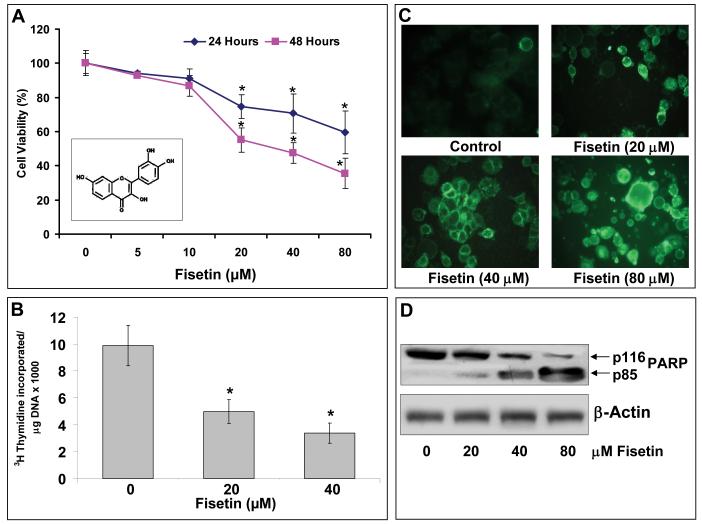
Recently, it has been shown that fisetin caused significant growth-inhibitory effects on different cancer cells in a time and dose-dependent manner [14-19]. To evaluate the effect of fisetin on the growth of human PaC cells we selected AsPC-1 cells. The choice of these cells was based on the fact that these cells demonstrate resistance to conventional chemotherapeutic regimens. Treatment of AsPC-1 cells with fisetin resulted in a dose-dependent growth inhibition with an IC50 of 38 μM at 48 h (Figure 1A). These results suggested that the cell line AsPC-1 that is highly resistant to currently available chemotherapeutic drugs remarkably showed sensitivity to fisetin treatment.
Figure 1. Effect of fisetin of AsPC-1 cells on cell growth & viability, cell proliferation and induction of apoptosis.
(A) Cell growth and viability of human PaC AsPC-1cells treated with fisetin (0-80 μM for 24 and 48 h) as measured by MTT assay. Each concentration of fisetin was repeated in ten wells. The values are represented as percent viable cells where vehicle (DMSO)-treated cells were regarded as 100% viable. Data is represented as mean ± SE of three independent experiments. Inset: Structure of fisetin. (B) Histogram showing rate of 3[H]thymidine uptake in AsPC-1 cells treated with fisetin. Cells were subjected to fisetin treatment (0-40 μM) for 48 h, the last 16 h of which was in the presence of [3H]thymidine (0.5 μCi/ml). Each bar represents mean ± SE of three independent experiments. *indicates p<0.05. (C) Effect of fisetin (0-80 μM) treatment on apoptosis induction in AsPC-1 cells as demonstrated by annexin-V staining (green fluorescence). (D) Effect of fisetin (0-80 μM) treatment on the expression and cleavage of PARP as determined by immunoblot analysis. Blots were stripped and reporbed with β-actin for equal protein loading. The immunoblots shown here are representative of three independent experiments with similar results. *indicates p<0.05
Effect of fisetin on AsPC-1 cell proliferation
It is well known that proliferating cells exhibit increased [3H]-thymidine incorporation into DNA which arises from increased growth factor expression and activity in cancer cells. Next, we determined the effect of fisetin treatment (20-40 μM) on the rate of proliferation of AsPC-1 cells by measuring the rate of uptake of thymidine by the dividing cells. We observed that treatment of cells with fisetin significantly decreased the thymidine incorporation in a dose-dependent manner, further validating the anti-proliferative efficacy of fisetin against human PaC AsPC-1 cells (Figure 1B).
Effect of fisetin on apoptosis induction in AsPC-1 cells
To investigate whether fisetin induced apoptosis in AsPC-1 cells we used the annexin-V staining and PARP cleavage as a measure of apoptosis. Annexin-V specially binds to phosphatidylserine and has been employed for determination of apoptotic cells. AsPC-1 cells treated with fisetin (10-60 μM; 48 h) showed dose-dependent induction of apoptosis (Figure 1C). Additionally, PARP cleavage as detected by immunoblot analysis showed that the full size PARP (116 KD) protein was cleaved to yield an 85 KD fragment after treatment with fisetin (Figure 1D).
Gene expression profile of fisetin treated AsPC-1 cells
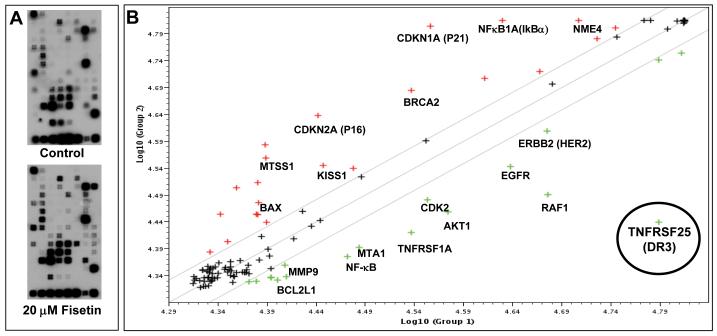
Next, we analyzed the effect of fisetin treatment of AsPC-1 cells on a subset of 113 genes with known relevance to cancer cell transformation and tumorigenesis (Figure 2). Compared to vehicle treated cells, fisetin treatment caused significant modulation in the expression of many genes that were either unregulated or downregulated ranging between 1.5- and 7-fold (supplementary table 1). The highly upregulated genes were mostly related to cell cycle control, invasion, and metastasis (p21, p16, IκBα, NME4, KISS1) and downregulated genes reported to be antiapoptotic, such as BCL2L1 and genes having their role in cell invasion, proliferation and metastasis like NF-κB, MMP9, EGFR, HER-2. Interestingly, death domain containing TNFR superfamily members TNFR1 and TNFRSF25 (DR3) receptors were highly down regulated on fisetin treatment, with DR3 showing the maximum modulation among all the selected genes. These data support the hypothesis that fisetin has a broader effect on cell survival, proliferation, invasion and metastasis of PaC cells.
Figure 2. Effect of fisetin treatment of AsPC-1 cells on the expression of genes involved in transformation and tumorigenesis.
(A) Autoradiographic image of cDNA array from control (upper panel) and fisetin treated (20 μM, bottom panel) cells. (B) Scatter plot analysis of gene expression of AsPC-1 cells treated with fisetin (20 μM). Genes whose expression remained unchanged fall along the diagonal line, while upregulated genes appear above the line and down regulated genes appear below the line. DR3 gene encircled in the picture was significantly down regulated by fisetin treatment.
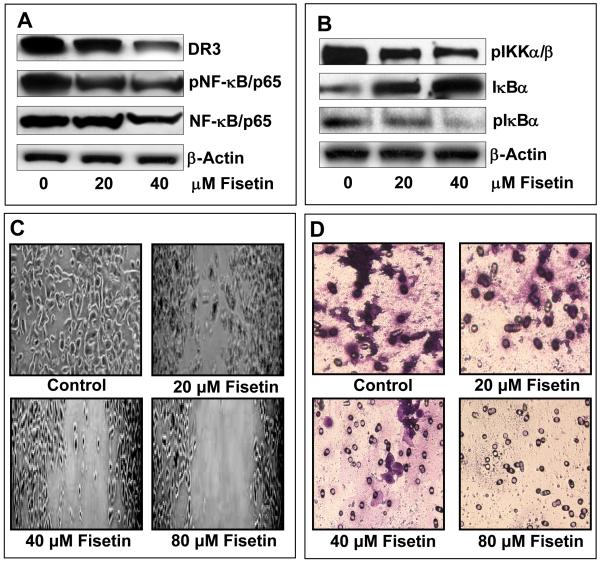
Effect of fisetin treatment of AsPC-1 cells on invasion and on protein expression of DR3 and MMP9 and on NF-κB signaling
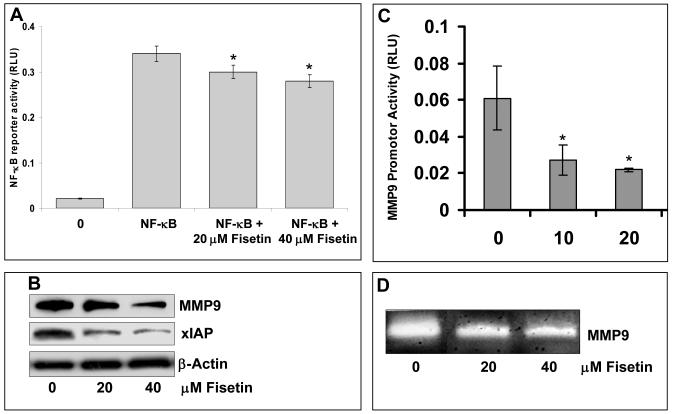
To further validate the results of the gene array, we conducted immunoblot analysis and observed dose-dependent decrease in the protein expression of DR3, phosphorylation of NF-κB, IKKα/β and IκBα (Figure 3A–B). A crucial step in the activation of NF-κB is the phosphorylation of IκB by IκB kinase complex. These results indicate that fisetin treatment of AsPC-1 cells results in inhibition of IKKα/β, phosphorylation and degradation of IκBα and subsequent activation of NF-κB. Fisetin treatment of AsPC-1 cells also resulted in inhibition of NF-κB luciferase reporter activity (Figure 4A). Since we observed inhibition of genes related to metastasis and invasion, we next performed wound closure and chemo-invasion assays. Fisetin treatment resulted in dose-dependent inhibition of migration of AsPC-1 cells in the wound closure assay and across the matrigel in the invasion assay (Figure 3C-D). Since NF-κB has been recognized as a major regulator of invasion and metastasis, we performed further studies on MMP9 whose expression was found to be inhibited by fisetin as revealed by gene array analysis. Fisetin inhibited in a dose-dependent manner the luciferase reporter activity of MMP9 (Figure 4B), decreased the protein expression of MMP9 (Figure 4C) and also inhibited its activity as demonstrated by gelatin zymography (Figure 4D).
Figure 3. Effect of fisetin treatment of AsPC-1 cells on protein expression of DR3, phosphorylation of NF-κB, IKKα/β and IκBα and on migration and invasion.
(A) Representative immunoblots showing expression of DR3, pNF-κB/p65 and NF-κB/p65 in cells treated with fisetin (0-40 μM). (B) Representative immunoblots showing expression of pIKKα/β, IκBα and pIκBα in cells treated with fisetin (0-40 μM). Equal loading was confirmed by stripping the blots and reprobing for β-actin. (C) Representative images to show the effect of fisetin (0-80 μM) treatment on migration of AsPC-1 cells as determined by wound closure assay. (D) Representative images to show the effect of fisetin (0-80 μM) treatment on the invasion of AsPC-1 cells across matrigel as determined by chemo-invasion assay. The details are described under Materials and Methods.
Figure 4. Effect of fisetin treatment of AsPC-1 cells on the protein expression of MMP9, XIAP, reporter activity of MMP9 and NF-κB and on the activity of MMP9.
(A). Histogram shows the effect of fisetin (0-40 μM) treatment of AsPC-1 cells on NF-κB reporter activity. Data is represented as mean ± SE of three independent experiments. (B) Representative immunoblots showing effect of fisetin (0-40 μM) treatment on protein expression of MMP9 and XIAP. Equal loading was confirmed by stripping immunoblots and reprobing them for β-actin. (C) Histogram shows the effect of fisetin (0-40 μM) treatment of AsPC-1 cells on promoter activity of MMP9. Fisetin treated cells were transfected with 1 μg of MMP9 luciferase reporter plasmid or 50 ng of renilla luciferase reporter plasmid as an internal control as described in Materials and Methods. Data is represented as mean ± SE of three independent experiments. (D) A representative zymogram showing MMP9 activity in AsPC-1 cells treated with fisetin (0-40 μM). *indicates p<0.05.
Effect of fisetin and DR3-silencing alone and in combination on apoptosis, cell-proliferation, and invasion in AsPC-1 cells
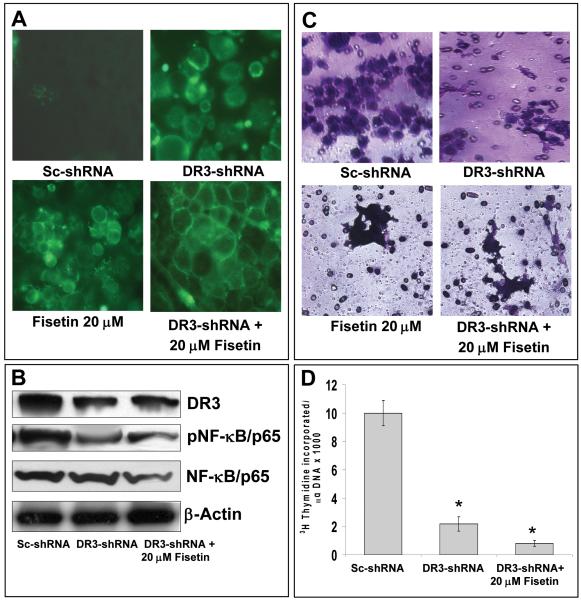
Since, fisetin was observed to decrease the viability of AsPC-1 cells and significantly decrease the expression levels of DR3 mRNA we asked whether such effects of fisetin are mediated through DR3. For this purpose, knockdown of DR3 was achieved by transfecting cells with a DR3 specific shRNA. The expression level of DR3 was significantly suppressed (50-90%) at the doses of 50-100 nM shRNA at 24 h post transfection, however at higher dose (100 nM) cell viability was highly compromised (data not shown). Therefore, a dose of 70 nM shRNA and a time point of 24 h for fisetin treatment were selected in subsequent experiments. We first determined the effect of fisetin (20 μM) treatment on apoptosis in shRNA-transfected cells. Fisetin alone treated cells as well as DR3 suppressed cells exhibited apoptosis, however, DR3-suppressed cells showed increased apoptosis when treated further with 20 μM fisetin (Figure 5A). The scrambled shRNA-transfected cells served as control. Protein expression of DR3 and pNF-κB were significantly decreased in cells treated with DR3 specific shRNA and fisetin compared to shRNA alone treated cells (Figure 5B). We next evaluated the effect of shRNA mediated suppression of DR3 on cell invasion. As evident from the Figure 5C, fisetin treatment of AsPC-1 cells led to decrease in cell migration which was further potentiated in DR3-knocked down cells. These observations paralleled with a significant decrease in the expression level of DR3 (Figure 5B). Next, we evaluated effect of DR3 knockdown on cell proliferation in fisetin treated cells by 3H-thymidine incorporation assay. DR3 knockdown led to a significant decrease in cell proliferation, however the decrease in cell proliferation was significantly more pronounced in DR3 silenced cells that were treated with fisetin (Figure 5D).
Figure 5. Effect of fisetin treatment and DR3-silencing alone and in combination on apoptosis, protein expression of DR3, pNF-κB, NF-κB, invasion and cell-proliferation in AsPC-1 cells.
(A) Representative images showing effect of fisetin (20 μM) treatment on apoptosis induction in AsPC-1 cells treated with DR3 silencing sh-RNA as demonstrated by annexin-V staining (green fluorescence). (B) Representative immunoblots showing expression of DR3, pNF-κB/p65 and NF-κB/p65 in cells treated with fisetin (20 μM) and DR3-sh-RNA. Equal loading was confirmed by stripping the blots and reprobing for β-actin. (C) Representative images to show the effect of fisetin (0-80 μM) treatment and DR3-silencing of AsPC-1 cells on invasion across matrigel as determined by chemo-invasion assay. The details are described under Materials and Methods. (D) Histogram showing rate of 3[H]thymidine uptake a measure of cell proliferation in AsPC-1 cells treated with 20 μM fisetin and DR3-sh-RNA. Cells were subjected to DR3-shRNA and fisetin treatment for 48 h, the last 16 h of which was in the presence of [3H]thymidine (0.5 μCi/ml). Each bar represents mean ± SE of three independent experiments. *indicates p<0.05
Effect of fisetin treatment and DR3-silencing alone and in combination on NF-κB signaling, XIAP, MMP9 expression in PaC AsPC-1 cells
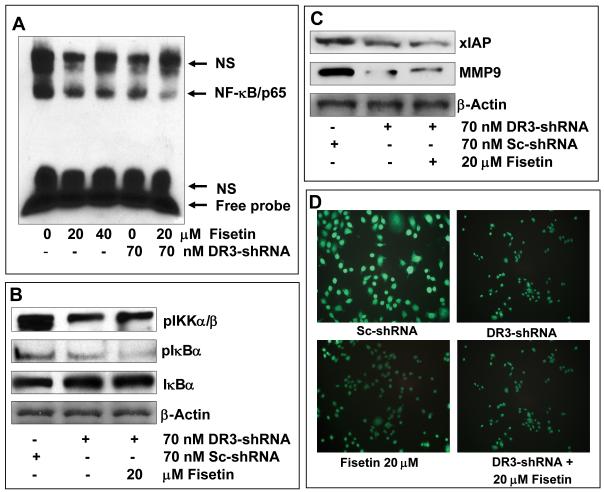
Since fisetin treatment of AsPC-1 cells inhibited NF-κB signaling we asked whether such effects of fisetin would be abrogated with silencing of DR3. Interestingly, combination of fisetin with DR3 silencing augmented the inhibition of NF-κB signaling. We observed almost complete inhibition of NF-κB DNA binding activity (Figure 6A) in DR3 silenced cells treated with fisetin. DR3 silencing also increased the inhibition of phosphorylation of IκBα (Figure 6B) and NF-κB reporter activity (Supplementary Figure 1). In parallel studies we observed augmentation of inhibition of XIAP and MMP9 (Figure 6C). Fisetin alone suppressed DR3 expression, however, DR3-suppressed cells showed increased inhibition of DR3 expression (Figure 6D). These observations suggested that DR3 silencing augments fisetin induced alterations in NF-κB signaling.
Figure 6. Effect of fisetin treatment and DR3-silencing alone and in combination on NF-κB signaling, XIAP, MMP9 and DR3 expression in AsPC-1 cells.
(A) NF-κB DNA binding activity in AsPC-1 cells treated with fisetin and a DR3 silencing sh-RNA alone and in combination. NS indicates non-specific binding. (B) Representative immunoblots showing expression of pIKKα/β, IκBα and pIκBα in cells treated with fisetin and a DR3 silencing sh-RNA. (C) Representative immunoblots showing expression of XIAP and MMP9 in cells treated with fisetin and a DR3 silencing sh-RNA alone and in combination. Equal loading was confirmed by stripping the blots and reprobing for β-actin. (D) Representative images to show the effect of fisetin and a DR3 silencing sh-RNA alone and in combination fisetin the cell surface expression of DR3. Treated cells were prepared for fluorescent staining as detailed in the Materials and Methods by using a primary anti-DR3 antibody and a secondary FITC-conjugated secondary antibody. Intensity of staining indicates the level of DR3 expression.
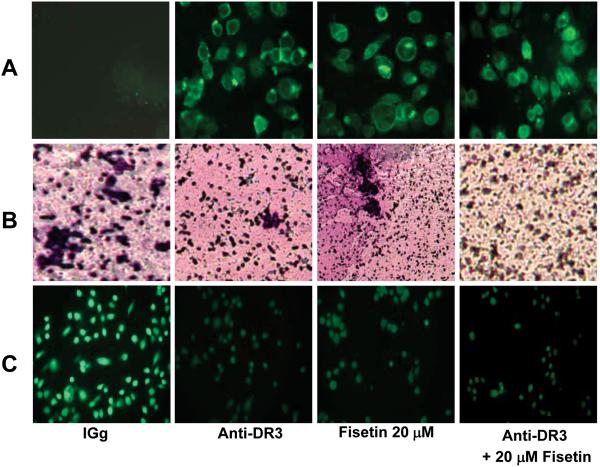
Effect of fisetin treatment in combination with a DR3-antagonist antibody on apoptosis, invasion and DR3 expression in AsPC-1 cells
To further substantiate our findings, we treated cells with an antagonistic antibody against the DR3 receptor in combination with fisetin. Combination with a DR3 antagonistic antibody augmented the effects of fisetin as seen by an enhanced induction of apoptosis (Figure 7A), enhanced inhibition of invasion (Figure 7B) and an enhanced inhibition of the expression of DR3 (Figure 7C). These observations further suggest that fisetin induced effects on PaC AsPC-1 cells are mediated through inhibition of the DR3 receptor.
Figure 7. Effect of fisetin treatment and DR3 antagonistic antibody, alone and in combination on apoptosis, invasion and DR3 expression.
(A) Effect of fisetin (20 μM) treatment alone and in combination with a DR3 antagonistic antibody on apoptosis induction in AsPC-1 cells as demonstrated by annexin-V staining (green fluorescence). (B) Representative images to show the effect of fisetin (20 μM) treatment alone and in combination with a DR3 antagonistic antibody on invasion across matrigel as determined by chemo-invasion assay. The details are described under Materials and Methods. (C) Representative images to show the effect of fisetin alone and in combination with a DR3 antagonistic antibody on the cell surface expression of DR3. Treated cells were prepared for fluorescent staining as detailed in the Materials and Methods by using a primary anti-DR3 antibody and a secondary FITC-conjugated secondary antibody. Intensity of staining indicates the level of DR3 expression.
DISCUSSION
Pancreatic cancer is the most aggressive of all cancers with the lowest survival rate. According to recent estimates, for all stages of pancreatic cancer combined, the one-year relative survival rate is 20%, and the five-year rate is 4%.21 These low survival rates are attributable to resistance of pancreatic cancer to current therapeutic treatment protocols. Thus, progress in therapeutic approaches is required to improve clinical outcome.
In this study we demonstrate that fisetin, a flavonoid inhibits cell proliferation of human pancreatic cancer AsPC-1 cells via DR3 mediated inhibition of NF-κB. Fisetin induced apoptosis and inhibited cell growth as well as metastasis in human PaC AsPC-1 cells. This observation in itself was significant since this cell line is resistant to several chemotherapeutic drugs. A cDNA array, containing genes related to transformation and tumorigenesis, suggested modulation of several genes related to cell proliferation, apoptosis and metastasis by fisetin. Most notably we observed significant inhibition of DR3 and induction of the inhibitor of NF-κB gene suggesting involvement of a DR3 mediated NF-κB pathway in the anti-proliferative and anti-apoptotic effects of fisetin. The protein encoded by DR3 gene is a member of the TNF-receptor super-family.9 This receptor is expressed preferentially in the tissues enriched in lymphocytes, and it is suggested that it may play a role in regulating lymphocyte homeostasis.11,22 The signal transduction of this receptor is mediated by various death domain containing adaptor proteins and shown to stimulate NF-κB activity and regulate cell apoptosis.23
To further corroborate the findings of the cDNA array we observed that fisetin treatment resulted in significant inhibition of the NF-κB signaling pathway. It is well known that NF-κB exerts its effects through induction of anti-apoptotic factors. While we observed increased expression of the XIAP protein in AsPC-1 cells, fisetin treatment was found to significantly decrease its expression. Activation of DR3 induces the formation of a signaling complex containing TRADD, TRAF2, and RIP and activates the NF-κB pathway.22 Induction of DR3 by an agonistic antibody has been shown to induce NF-κB activation which was observed to be responsible for resistance to apoptosis in human erythroleukemic TF-1 cells that express DR3 endogenously.22 Furthermore, inhibition of DR3 by RNA interference significantly sensitized PaC cells to fisetin-induced apoptosis. XIAP is suggested to contribute to drug resistance in PaC because XIAP blocks apoptosis at a key node of the apoptotic machinery through inhibition of caspase-3 and caspase-9.24 Downregulation of XIAP could restore sensitivity to TRAIL-induced apoptosis in pancreatic cancer.25
There are consensus recognition sequences with 85 to 95% homology for NF-κB binding both upstream and downstream of the genomic human DR3 sequence, consistent with the possibility that the DR3 promoter is regulated by NF-κB.26 This could suggest induction of NF-κB-dependent genes through DR3 that may include both pro-inflammatory genes and anti-apoptotic genes.26 DR3 also recruits TRAF2 via TRADD and thus activates the transcription factor, NF-κB that induces the transcription of a number of genes.27-28 Importantly, NF-κB has been reported to promote both angiogenesis and metastasis in certain tumor models, potentially through the regulation of vascular endothelial growth factor (VEGF) and MMPs.29 Our observed inhibition of invasion and metastasis by fisetin could be attributed to inhibition of the NF-κB signaling.
In view of these facts, DR3 seems to be capable of inducing both apoptosis and expression of survival/activation genes and is likely to have multiple functions depending on the context of its expression. This fact seems to be the case in place for DR3 signaling in chemoresistant human PaC AsPC-1 cells. DR3 upregulation in PaC cells could result in induction of NF-κB signaling which in turn activates a host of anti-apoptotic genes including XIAP and metastatic genes such as MMP9. Although DR3 activation is related to activation of pro-apoptotic mechanisms, its induction of NF-κB in PaC could have opposing effects. In this regard, it is important to identify the molecular mechanisms that dictate these outcomes. Our earlier studies and studies from other laboratories also suggest that fisetin targets the NF-κB pathway suggesting that these effects may not be cell or cancer type specific.14,17 It is important to distinguish the situations where NF-κB functions prooncogenic from those where it may function a proapoptotic factor and identify inhibitors of NF-κB or its upstream regulatory pathways to clinical cancer therapy in a manner consistent with an antiapoptotic/proproliferation role, either as stand-alone therapies or as adjuvants with existing or new therapies. It is essential that additional preclinical studies in relevant animal models of human pancreatic cancer be undertaken to establish the anticancer efficacy of fisetin and evaluate if it acts via similar mechanisms. Our studies suggest that fisetin could be developed for treatment of PaC or as an adjuvant with conventional therapeutic regimens.
Supplementary Material
Supplementary figure 1: Effect of fisetin treatment and DR3-silencing alone and in combination on NF-κB reporter in AsPC-1 cells. Histogram showing the effect of fisetin in combination with a DR3 silencing sh-RNA on NF-κB reporter activity. Data is represented as mean ± SE of three independent experiments. *indicates p<0.05
Acknowledgement
This work was supported by US PHS Grants RO1CA78809, RO1CA101039 and RO1CA120451. Author, Imtiyaz Murtaza was a recipient of Overseas Associateship Award (BT/IN/BTON/Nich/09/2007) from the Department of Biotechnology, Ministry of Science and Technology, Government of India. We thankfully acknowledge Dr. Douglas D. Boyd, Department of Cancer Biology, MD Anderson Cancer Center, Houston, Texas for providing pGL3-MMP9-luc reporter plasmid.
Footnotes
Current Address: Sher-e-Kashmir University of Agricultural Sciences & Technology, Shalimar Campus, Srinagar, 191121, J&K India
REFERENCES
- 1.Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2006;20:1218–49. doi: 10.1101/gad.1415606. [DOI] [PubMed] [Google Scholar]
- 2.Sebens S, Arlt A, Schäfer H. NF-kappaB as a molecular target in the therapy of pancreatic carcinoma. Recent Results Cancer Res. 2008;177:151–64. doi: 10.1007/978-3-540-71279-4_17. [DOI] [PubMed] [Google Scholar]
- 3.Lee CH, Jeon YT, Kim SH, Song YS. NF-kappaB as a potential molecular target for cancer therapy. Biofactors. 2007;29:19–35. doi: 10.1002/biof.5520290103. [DOI] [PubMed] [Google Scholar]
- 4.Holcomb B, Yip-Schneider M, Schmidt CM. The role of nuclear factor kappaB in pancreatic cancer and the clinical applications of targeted therapy. Pancreas. 2008;36:225–35. doi: 10.1097/MPA.0b013e31815b3207. [DOI] [PubMed] [Google Scholar]
- 5.Wang W, Abbruzzese JL, Evans DB, Larry L, Cleary KR, Chiao PJ. The nuclear factor-kappa B RelA transcription factor is constitutively activated in human pancreatic adenocarcinoma cells. Clin Cancer Res. 1999;5:119–27. [PubMed] [Google Scholar]
- 6.Ashkenazi A. Targeting death and decoy receptors of the tumor-necrosis factor superfamily. Nat Rev Cancer. 2002;2:420–30. doi: 10.1038/nrc821. [DOI] [PubMed] [Google Scholar]
- 7.Jackson-Bernitsas DG, Ichikawa H, Takada Y, Myers JN, Lin XL, Darnay BG, Chaturvedi MM, Aggarwal BB. Evidence that TNF-TNFR1-TRADD-TRAF2-RIP-TAK1-IKK pathway mediates constitutive NF-kappaB activation and proliferation in human head and neck squamous cell carcinoma. Oncogene. 2007;26:1385–97. doi: 10.1038/sj.onc.1209945. [DOI] [PubMed] [Google Scholar]
- 8.Van Waes C. Nuclear factor-kappa B in development, prevention, and therapy of cancer. Clin Cancer Res. 2007;13:1076–82. doi: 10.1158/1078-0432.CCR-06-2221. [DOI] [PubMed] [Google Scholar]
- 9.Chinnaiyan AM, O’Rourke K, Yu GL, Lyons RH, Garg M, Duan DR, Xing L, Gentz R, Ni J, Dixit VM. Signal transduction by DR3, a death domain-containing receptor related to TNFR-1 and CD95. Science. 1996;274:990–2. doi: 10.1126/science.274.5289.990. [DOI] [PubMed] [Google Scholar]
- 10.Grenet J, Valentine V, Kitson J, Li H, Farrow SN, Kidd VJ. Duplication of the DR3 gene on human chromosome 1p36 and its deletion in human neuroblastoma. Genomics. 1998;49:385–93. doi: 10.1006/geno.1998.5300. [DOI] [PubMed] [Google Scholar]
- 11.Meylan F, Davidson TS, Kahle E, Kinder M, Acharya K, Jankovic D, Bundoc V, Hodges M, Shevach EM, Keane-Myers A, Wang EC, Siegel RM. The TNF-family receptor DR3 is essential for diverse T cell-mediated inflammatory diseases. Immunity. 2008;29:1–11. doi: 10.1016/j.immuni.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Lamki RS, Wang J, Thiru S, Pritchard NR, Bradley JA, Pober JS, Bradley JR. Expression of silencer of death domains and death-receptor-3 in normal human kidney and in rejecting renal transplants. Am J Pathol. 2003;163:401–11. doi: 10.1016/S0002-9440(10)63670-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ringel B, Ibrahim SM, Köhler H, Ringel J, Koczan D, Liebe S, Löhr M, Thiesen HJ. Apoptotic molecules in pancreatic carcinoma cell lines. Ann N Y Acad Sci. 1999;880:175–8. doi: 10.1111/j.1749-6632.1999.tb09521.x. [DOI] [PubMed] [Google Scholar]
- 14.Suh Y, Afaq F, Johnson JJ, Mukhtar H. A plant flavonoid fisetin induces apoptosis in colon cancer cells by inhibition of COX2 and Wnt/EGFR/NF-kappaB signaling pathways. Carcinogenesis. 2008 doi: 10.1093/carcin/bgn269. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan N, Asim M, Afaq F, Abu Zaid M, Mukhtar H. A novel dietary flavonoid fisetin inhibits androgen receptor signaling and tumor growth in athymic nude mice. Cancer Res. 2008;68:8555–63. doi: 10.1158/0008-5472.CAN-08-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan N, Afaq F, Syed DN, Mukhtar H. Fisetin, a novel dietary flavonoid, causes apoptosis and cell cycle arrest in human prostate cancer LNCaP cells. Carcinogenesis. 2008;29:1049–56. doi: 10.1093/carcin/bgn078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sung B, Pandey MK, Aggarwal BB. Fisetin, an inhibitor of cyclin-dependent kinase 6, down-regulates nuclear factor-kappaB-regulated cell proliferation, antiapoptotic and metastatic gene products through the suppression of TAK-1 and receptor-interacting protein-regulated IkappaBalpha kinase activation. Mol Pharmacol. 2007;71:1703–14. doi: 10.1124/mol.107.034512. [DOI] [PubMed] [Google Scholar]
- 18.Lu X, Jung J, Cho HJ, Lim DY, Lee HS, Chun HS, Kwon DY, Park JH. Fisetin inhibits the activities of cyclin-dependent kinases leading to cell cycle arrest in HT-29 human colon cancer cells. J Nutr. 2005;135:2884–90. doi: 10.1093/jn/135.12.2884. [DOI] [PubMed] [Google Scholar]
- 19.Hou DX, Fukuda M, Johnson JA, Miyamori K, Ushikai M, Fujii M. Fisetin induces transcription of NADPH:quinone oxidoreductase gene through an antioxidant responsive element-involved activation. Int J Oncol. 2001;18:1175–9. doi: 10.3892/ijo.18.6.1175. [DOI] [PubMed] [Google Scholar]
- 20.Afaq F, Adhami VM, Ahmad N, Mukhtar H. Inhibition of ultraviolet B-mediated activation of nuclear factor kappaB in normal human epidermal keratinocytes by green tea Constituent (−)-epigallocatechin-3-gallate. Oncogene. 2003;22:1035–44. doi: 10.1038/sj.onc.1206206. [DOI] [PubMed] [Google Scholar]
- 21.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 22.Bodmer J, Burns K, Schneider P, Hofmann K, Steiner V, Thome M, Bornand T, Hahne M, Schröter M, Becker K, Wilson A, French LE, et al. TRAMP, a novel apoptosis-mediating receptor with sequence homology to tumor necrosis factor receptor 1 and Fas(Apo-1/CD95) Immunity. 1997;6:79–88. doi: 10.1016/s1074-7613(00)80244-7. [DOI] [PubMed] [Google Scholar]
- 23.Wen L, Zhuang L, Luo X, Wei P. TL1A-induced NF-kappaB activation and c-IAP2 production prevent DR3-mediated apoptosis in TF-1 cells. J Biol Chem. 2003;278:39251–8. doi: 10.1074/jbc.M305833200. [DOI] [PubMed] [Google Scholar]
- 24.Salvesen GS, Duckett CS. IAP proteins: blocking the road to death’s door. Nat Rev Mol Cell Biol. 2002;3:401–10. doi: 10.1038/nrm830. [DOI] [PubMed] [Google Scholar]
- 25.Vogler M, Walczak H, Stadel D, Haas TL, Genze F, Jovanovic M, Gschwend JE, Simmet T, Debatin KM, Fulda S. Targeting XIAP bypasses Bcl-2-mediated resistance to TRAIL and cooperates with TRAIL to suppress pancreatic cancer growth in vitro and in vivo. Cancer Res. 2008;68:7956–65. doi: 10.1158/0008-5472.CAN-08-1296. [DOI] [PubMed] [Google Scholar]
- 26.Marsters SA, Sheridan JP, Donahue CJ, Pitti RM, Gray CL, Goddard AD, Bauer KD, Ashkenazi A. Apo-3, a new member of the tumor necrosis factor receptor family, contains a death domain and activates apoptosis and NF-κB. Curr Biol. 1996;6:1669–76. doi: 10.1016/s0960-9822(02)70791-4. [DOI] [PubMed] [Google Scholar]
- 27.Hsu H, Shu SB, Pan MG, Goeddel DV. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell. 1996;84:299–308. doi: 10.1016/s0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- 28.Hunter T, Karin M. The regulation of transcription by phosphorylation. Cell. 1992;70:375–387. doi: 10.1016/0092-8674(92)90162-6. [DOI] [PubMed] [Google Scholar]
- 29.Bassères DS, Baldwin AS. Nuclear factor-kappaB and inhibitor of kappaB kinase pathways in oncogenic initiation and progression. Oncogene. 2006;25:6817–30. doi: 10.1038/sj.onc.1209942. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure 1: Effect of fisetin treatment and DR3-silencing alone and in combination on NF-κB reporter in AsPC-1 cells. Histogram showing the effect of fisetin in combination with a DR3 silencing sh-RNA on NF-κB reporter activity. Data is represented as mean ± SE of three independent experiments. *indicates p<0.05