Abstract
This study was undertaken to determine whether depression-like behavior can be observed in gonadally intact females that have experienced normal pregnancy. When tested on the forced swim test (FST) on postpartum days 1-7, previously pregnant rats spent slightly more time immobile, significantly less time swimming and diving, and defecated more than virgin controls. Subchronic treatment with nomifensine (DA reuptake inhibitor, 2.5 mg/kg) but not sertraline (serotonin reuptake inhibitor, 10 mg/kg) or desipramine (norepinephrine reuptake inhibitor, 10 mg/kg) significantly decreased immobility on postpartum day 2. In rats pre-exposed to the FST in mid-pregnancy, neither subchronic nor chronic treatment with desipramine or sertraline decreased immobility on postpartum day 2; in contrast, chronic desipramine significantly decreased immobility in virgin controls. These results indicate that postpartum female rats, compared to virgin controls, show a reduction in some “active coping behaviors” but no significant increase in immobility when tested during the early postpartum period, unlike ovariectomized females that have undergone hormone-simulated pregnancy (HSP). Additionally, immobility that is increased by FST pre-exposure is not readily prevented by treatment with standard antidepressant medications in postpartum females. Depression-like behaviors previously observed in females that have undergone HSP may result from the more dramatic changes in estradiol, prolactin or corticosterone that occur during the early “postpartum” period, compared to the more subtle changes in these hormones that occur in actual postpartum females.
Keywords: postpartum depression, females, antidepressants, forced swim test, gestational stress, estradiol, prolactin, corticosterone, pregnancy, nominfensine, sertraline, desipramine
Previous studies suggest that hormonal changes that women undergo around the time of childbirth may trigger postpartum mood disorders (Moses-Kolko et al., 2009; O’Hara, 2009; Parry et al., 2003). We and others have shown previously that ovariectomized female rats that have undergone hormone-simulated pregnancy (HSP) show depression-like behaviors during the “postpartum” period, suggesting that HSP followed by hormone withdrawal may be a useful animal model of postpartum depression (Galea et al., 2001; Green et al., 2009; Navarre et al., 2010; Stoffel and Craft, 2004). In these studies, the abrupt hormone withdrawal at the end of the HSP (modeling parturition) is considered to be the stressor; rats that have undergone HSP and are tested only once during the “postpartum” period on the forced swim test (FST) or sucrose preference test show increased immobility and decreased sucrose preference, respectively, compared to ovariectomized, vehicle-treated controls.
Although only 10-15% of women experience significant postpartum mood disturbance (Halbreich, 2005), reproductive hormones change dramatically in all women at parturition, and mild mood disturbance (“baby blues”) is estimated to occur in 75% of women within the first week following childbirth (Sit and Wisner, 2009). To determine whether this more common but milder mood disturbance can also be modeled (detected) in animals, we recently compared actual postpartum female rats with those that had undergone HSP using the sucrose preference test. Compared to HSP females tested during the “postpartum” period, actual postpartum females showed only slightly suppressed sucrose preference in the first week postpartum (Navarre et al., 2010). In the present study we employed another common measure of depression-like behavior in the rat, the FST, to determine whether actual postpartum females show any depression-like behavior during the early postpartum period. In keeping with previous studies using the HSP model, parturition itself was considered the stressor, and rats were initially tested on the FST only once during the postpartum period (i.e., there was no FST pre-exposure as commonly used by other investigators: Porsolt et al., 1978; Borsini & Meli, 1988; Cryan et al., 2005). We also subsequently tested postpartum females using a standard procedure that included FST pre-exposure, to ensure that they responded similarly to controls.
A previous study of FST behavior in pregnant/postpartum female rats reported that postpartum females showed elevated immobility (and decreased struggling) on postpartum days 3 and 7 compared to females tested during mid-pregnancy, although immobility was not elevated above that of ovariectomized controls (Molina-Hernandez and Téllez-Alcántara, 2001). Another study showed no difference in immobility between virgin and postpartum females tested on postpartum day 8-10 (Walker et al., 1995), whereas a third study showed increased immobility at 4 weeks postpartum, but only in rats that had had their pups removed on the first day postpartum (Pawluski et al., 2009). In the present study, the first goal was to compare FST behaviors between virgin control and postpartum females during the first postpartum week; in addition to the standard measures of “active coping” (swimming and struggling (climbing)) and “passive coping” (immobility), we assessed a third escape-related behavior, diving, as well as a general measure of emotionality in the rat, defecation (Broadhurst, 1957). Second, we compared virgin vs. postpartum females’ FST behaviors after administration of standard antidepressant medications, to determine whether mood-related changes in postpartum females can be prevented by antidepressant treatment. Third, we compared FST behaviors and plasma levels of several hormones in HSP vs. actual postpartum females, to determine whether increased immobility in HSP but not actual postpartum females could relate to greater hormonal changes in HSP compared to actual postpartum females during the postpartum period.
Methods
All procedures used in this study adhered to the guidelines of the NIH Guide for Care and Use of Laboratory Animals (Publication No. 85-23, revised 1985), and were approved by the Institutional Animal Care and Use Committee at Washington State University (LARC 3218).
Subjects
Gonadally intact Sprague-Dawley female rats, approximately 3-6 months old, were used (bred in-house from Taconic stock, Germantown, NY). Rats were maintained under a 12:12 hr light:dark cycle (lights on at 0600 hr), with food and water available ad libitum.
Apparatus
The FST was conducted using two cylindrical Plexiglas containers (45 cm high × 25 cm diameter) filled with tap water to 30 cm and maintained at room temperature during testing (25 ± 1.0 °C). Female rats of the age used in this study are unable to touch the bottom of a tank filled to 30 cm; deeper water, in which rats cannot touch the tank bottom, has been shown to yield more reliable antidepressant effects (Detke and Lucki, 1996). The locomotor activity test was conducted using photobeam chambers (Opto-varimex, Columbus Instruments, Columbus, OH). The 15 photobeams that cross the width of the cage were 2.5 cm apart and 8 cm above the cage floor.
Timed (and untimed) pregnancies
When we did not have to know the first day of pregnancy (i.e., in the first and third experiments), untimed pregnancies were used: each female that was intended for the “pregnant” group was simply paired with a male for a minimum of 2 weeks. Each female intended for the control group was paired with another female. All females were briefly handled daily (picked up for a few sec by the base of the tail). Beginning 2 weeks after pairing, if a female paired with a male looked visibly pregnant, the male was removed and the female was housed alone thereafter. If the female did not look pregnant, the male was left in the cage for up to one more week; at that point any females that were not clearly pregnant were paired with a different male. Control females were separated from each other at approximately the same time that pregnant females were separated from males, to ensure that the period of single-housing before testing was the same in both control and pregnant groups.
For all other experiments, because various treatments needed to occur on certain days of pregnancy, a timed pregnancy procedure was used: vaginal smears were taken daily; as soon as a female was observed to be in vaginal proestrus, a stud male was placed in her cage. Vaginal smears continued to be taken daily. As soon as sperm were observed in the smear, the male was removed from the cage and that day was designated as pregnancy day 1. A few females did not show sperm in the smear within approximately 1 week after pairing with a male; these were re-assigned to the control group. Additionally, a few females were paired with males when the females were in diestrus, and then the males were removed within 1-2 days so that females did not become pregnant. This procedure yielded pregnant and control groups that spent approximately the same number of days paired with males before being singly housed until testing.
For both untimed and timed pregnancies, females continued to be handled briefly each day until pups were born. Near the time of parturition, females were monitored 3 times/day (approximately 7-9 am, 12-2 pm and 5-10 pm) so that time of pup birth could be accurately estimated for most rats.
Determination of postpartum day
Postpartum day 1 and 2 testing occurred 24 ± 4 hr and 48 ± 4 hr, respectively, after parturition (only females whose pup births were actually observed were included in these groups). Postpartum day 4 and 7 testing occurred 72 ± 12 hr and 96 ± 12 hr, respectively, after parturition. Females continued to be handled briefly (lifted by the base of the tail) daily during the postpartum period, until testing.
Behavioral procedures
In Experiment 1 (time course of FST behavior during the postpartum period), previously pregnant and control females were tested on the FST for 5 min on postpartum day 1, 2, 4 or 7 (separate rats at each time point), between 12-3 pm. Behavior was videotaped for 5 min, and scored later by an observer who was blind to treatment group assignment. A time sampling technique was used to score behavior (Detke et al., 1995): at the end of each 5-sec period, the observer recorded the predominant behavior occurring during that 5-sec period: struggling (movements of the forelimbs, usually directed at the walls of the cylinder, wherein the forepaws break the surface of the water); swimming (paddling with forelimbs and hindlimbs causing the rat to move through at least two quadrants of the cylinder within 5 sec); immobility (making only the movements necessary to keep its head above water, so that the rat stays within a single quadrant); diving (entire rat submerged under the surface of the water, paddling toward the bottom of the cylinder); headshaking. Although it is arguably an “active coping” (escape-related) behavior just like swimming and struggling, diving is not usually scored by other investigators because it occurs at a fairly low frequency and has not been shown to change with antidepressant treatment (Cryan et al., 2005). However, we noticed in a pilot study that postpartum rats could be distinguished from controls by their lesser diving behavior, so we included this measure throughout the study. Defecation (the number of fecal boli excreted) during the FST also was recorded, as a measure of emotionality (Broadhurst, 1957).
After forced swim testing, rats were towel-dried and placed under a heat lamp for 30 min. Vaginal smears were taken, and then females were returned to their pups. Spontaneous locomotor activity was assessed for 5 min on the following day (12-3 pm).
Because postpartum females in Experiment 1 were all in diestrus at the time of testing, and control females were in various stages, Experiment 2 was conducted to determine whether estrous stage affects FST behavior in virgin female rats. All rats were housed in female-female pairs. Vaginal smears were taken daily for 2 weeks. Females were then separated into individual cages, and vaginal smears were taken daily until a female was in proestrus, estrus or diestrus. A 5-min FST was then conducted between 12-3 pm. Rats were towel-dried and placed under a heat lamp for approximately 15 min. Locomotor activity was then assessed for 5 min. As much as possible, females were tested in groups of three, one female/stage on a given day, until all females were tested. A second vaginal smear was taken immediately after behavioral testing; only those females remaining in the same stage from before to after testing were included in analyses.
In Experiment 3 (subchronic antidepressant drug treatment), vehicle, nomifensine (2.5 mg/kg), desipramine (10 mg/kg), or sertraline (10 mg/kg) was administered 24, 5 and 1 hr before conducting a 5-min FST on postpartum day 2. Females were not exposed to the FST before postpartum day 2. Rats were towel-dried and placed under a heat lamp for approximately 15 min (see data analysis), and then locomotor activity was assessed for 5 min.
In Experiment 4 (FST pre-exposure), females were impregnated by the timed-pregnancy method. On pregnancy day 14 (between 12-3 pm), half of the rats were placed into the FST cylinders for 15 min; after drying, rats were returned to their home cages. The other half of rats were left undisturbed (except for brief daily handling). On postpartum day 2, all rats were tested in the FST for 5 min (and, after approximately 15 min drying, on the locomotor activity test for 5 min). This experiment was conducted to determine if pre-exposure to the FST at long vs. short intervals would increase immobility during the postpartum day 2 FST, and to determine whether increases in immobility from the first to second FST were comparable in control (virgin) vs. postpartum females.
In Experiment 5 (subchronic vs. chronic anti-depressant drug treatment in FST-pre-exposed rats), postpartum and control females were exposed to the FST for 15 min on pregnancy day 12, to increase immobility on the postpartum day 2 FST; we hypothesized that antidepressant-induced changes in postpartum FST behavior might be more readily observed if females were pre-exposed to the FST. In both chronic and sub-chronic treatment groups, drug administration began after FST pre-exposure, so that rats were drug-free during the first FST. Thus, immediately after the 15-min FST on pregnancy day 12, vehicle, desipramine (10 mg/kg) or sertraline (10 mg/kg) was administered. In the subchronic groups, vehicle was administered once daily from pregnancy day 12 through postpartum day 1; then vehicle, desipramine (10 mg/kg) or sertraline (10 or 20 mg/kg) was administered 24, 5 and 1 hr before the 5-min, postpartum day 2 FST. In the chronic groups, vehicle, desipramine (10 mg/kg) or sertraline (10 mg/kg) was administered on pregnancy day 12 and the same treatment was administered once daily thereafter, with the last injection given on postpartum day 2, 1 hr before the 5-min postpartum FST. Thus, in the chronic groups, drugs were administered once daily for 13-14 days, depending on the length of gestation (22-23 days). After approximately 15 min drying post-FST, locomotor activity was measured for 5 min.
Vaginal smears
Proestrus was identified by the predominance (approximately 75% or more of epithelial cells in sample) of nucleated epithelial cells without leukocytes; estrus was identified by the predominance of cornified epithelial cells; diestrus (metestrus and diestrus) were identified by the presence of leukocytes and scattered nucleated and/or cornified epithelial cells (Freeman, 1988).
Plasma hormone levels
To compare hormone levels between control and postpartum females, as well as ovariectomized females that had undergone HSP, trunk blood was taken from rats in each of these four groups on postpartum days 1, 2, 4 or 7 (separate rats at each time point). To determine hormone profiles in the absence of FST “stress”, no behavioral testing was conducted in these rats. They were euthanized at approximately the same time of day that behavioral testing was conducted in other rats. Blood samples were centrifuged for 20 min at 2000 rpm at 4°C; serum was removed and stored at −80°C. Hormone levels were determined via double antibody radioimmunoassay kits (estradiol, testosterone and progesterone: Diagnostic System Laboratories, Webster, TX; corticosterone: ImmuChem, MP Biomedicals, Orangeburg, NY), or by ELISA (prolactin: 12-MKVRP-1, Alpco Diagnostics, Boston, MA). Manufacturer’s instructions were followed in each case, except for estradiol, in which samples and standards were extracted twice using ether (done in duplicate); after the ether evaporated, the assay reagents were added to the extraction tubes, which were incubated overnight at 4°C.
Drugs
Nomifensine maleate and desipramine hydrochloride were purchased from Sigma-Aldrich, Inc. (St. Louis, MO); these drugs were dissolved in physiological saline and injected s.c. (nomifensine) or i.p. (desipramine). Sertraline hydrochloride was generously donated by Pfizer, Inc. (Groton, CT); it was dissolved in Tween80, which was then diluted to 5% in saline, and injected i.p. Drugs were administered in volumes of 1 ml/kg, except the 20 mg/kg dose of sertraline, which was administered in a volume of 2 ml/kg due to solubility limitations. Saline served as the control vehicle for nomifensine and desipramine, and 5% Tween80 served as the control vehicle for sertraline.
Data analysis
Frequency data for each of the 5 behaviors in the FST were converted to percent of total test behaviors: (# 5-sec periods in which behavior was predominant / total # 5-sec periods) X 100. Headshakes were not analyzed because they occur at very low frequency and have never been shown to be an escape-related behavior. Based on a random sample of 13 rats scored by both J.A.R. and K.T.T., inter-rater reliability was 0.91 (immobility), 0.86 (swimming), 0.95 (struggling), 0.94 (diving), and 0.98 (defecation). Locomotor activity was analyzed as the number of photobeam breaks in 5 min. Data from each experiment were analyzed via ANOVA, with a significance level of p ≤ 0.05. If significant main effects or interactions were observed, the Student-Newman-Keuls test was used to determine which specific groups differed from each other. In the drug experiments, Dunnett’s post hoc tests were used to compare multiple drug treatment groups to the vehicle control group, within postpartum or virgin control groups. Because no significant differences were found between rats tested with saline vs. 5% Tween80 vehicle, vehicle groups were pooled before analysis.
Results
FST behaviors in postpartum vs. control females on postpartum days 1-7
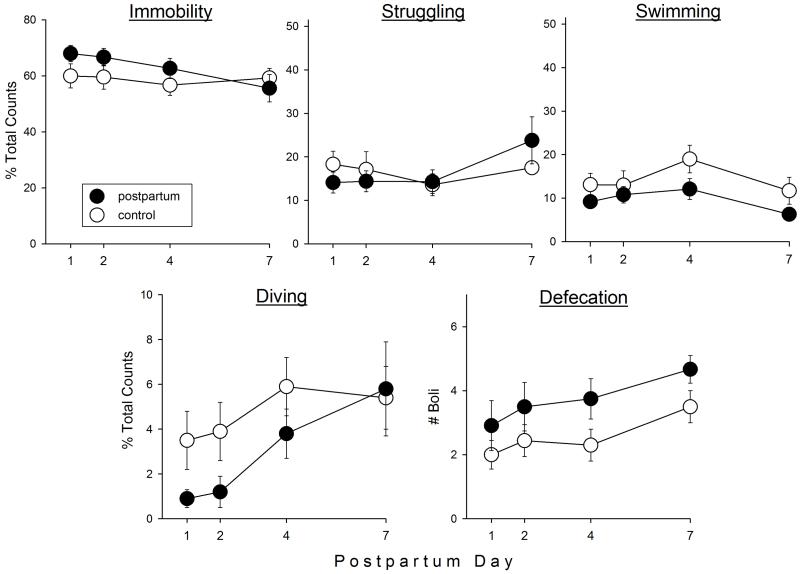
Figure 1 shows FST behaviors and defecation in postpartum vs. control females tested on postpartum days 1, 2, 4 or 7. Postpartum females spent slightly but not significantly more time immobile than control females (F(1,69)=2.79, p=0.10). Struggling did not differ between the two groups; however, postpartum females spent significantly less time swimming (F(1,69)=7.43, p=0.008) and diving (F(1,69)=4.08, p=0.047) than controls, and also defecated significantly more during the FST than control females (F(1,69)=8.36, p=0.005). There were no differences between postpartum and control females on the locomotor activity test (data not shown).
Figure 1.
Forced swim test (FST) behaviors in postpartum vs. virgin control female rats on postpartum days 1, 2, 4 and 7. Rats were not pre-exposed to the FST; parturition served as the “inescapable stressor.” Each point is the mean ± 1 S.E.M., N=8-12 rats. *significantly greater than control group, p≤0.05.
Estrous stage and FST behaviors
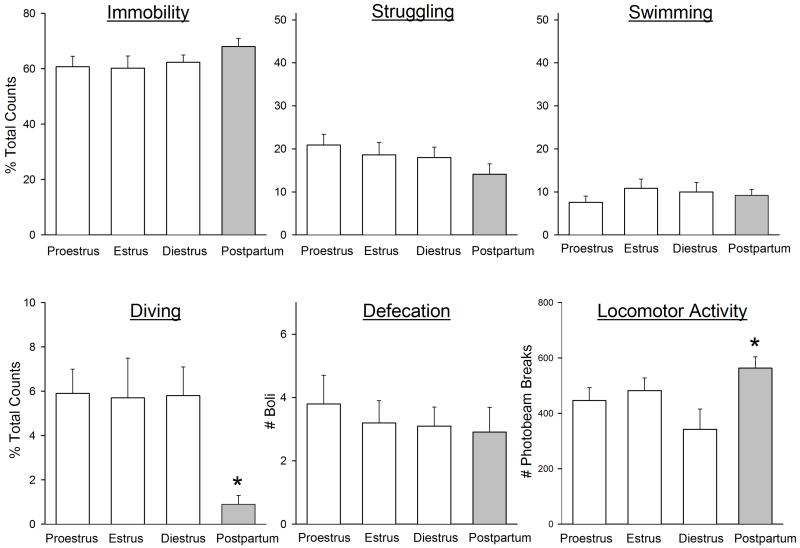
One possible confound in the first experiment was the difference in estrous stages between groups. Whereas all postpartum females were in diestrus at the time of testing, the control females were in various estrous stages. To determine whether this may have affected the results of the first experiment, a separate experiment was conducted to compare FST behaviors in control (virgin) females that were in proestrus, estrus or diestrus, so that similar numbers of females could be selected in each stage. Figure 2 shows that no significant differences were observed between rats in proestrus, estrus or diestrus in any FST behavior or locomotor activity. When data from previously pregnant females tested on postpartum day 1 (from Experiment 1) were compared to cycling females in various stages, postpartum females showed significantly less diving than all three groups of cycling females (Figure 2: F(3,35)=5.10, p=0.005), and significantly more locomotor activity than diestrous females (Figure 2: F(3,35)=4.11, p=0.013).
Figure 2.
FST behaviors and locomotor activity in virgin control female rats tested during proestrus, estrus or diestrus (open bars) vs. postpartum females (gray bars) tested on postpartum day 1 (data from postpartum females are re-plotted from Fig. 1). Rats were not pre-exposed to the FST; parturition served as the “inescapable stressor.” Each bar is the mean ± 1 S.E.M., N=9-12 rats. *significantly different from all cycling females (diving), or diestrous females (locomotor activity), p≤0.05.
Subchronic treatment with antidepressant drugs in postpartum vs. control females on postpartum day 2
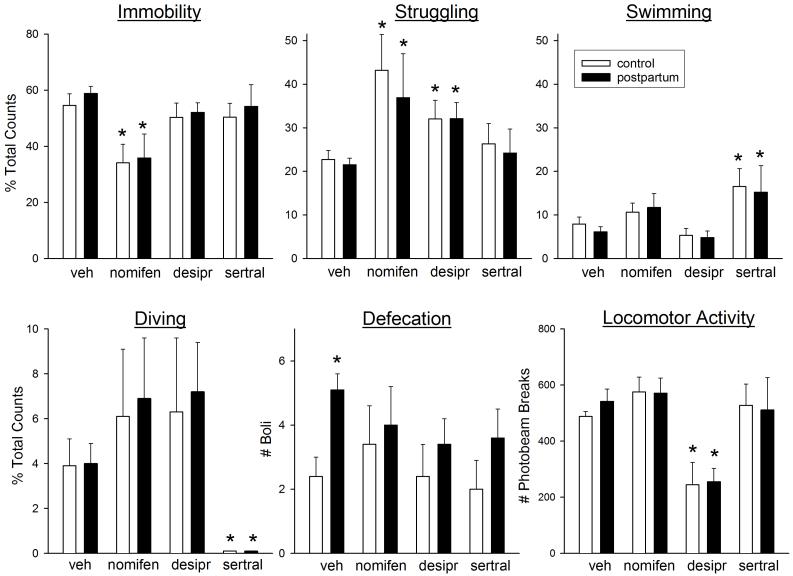
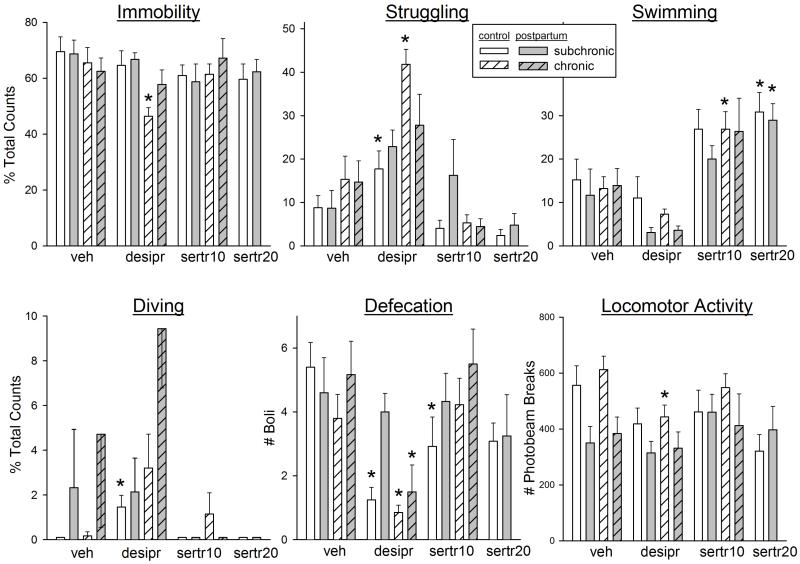
Figure 3 shows the effects of subchronic treatment with nomifensine (2.5 mg/kg), desipramine (10 mg/kg) and sertraline (10 mg/kg) on FST behaviors and locomotor activity in postpartum and control females tested on postpartum day 2. Immobility was significantly decreased only by nomifensine (F(3,72)=7.67, p=0.001). This decreased immobility was primarily accounted for by increased struggling (F(3,72)=6.28, p=0.001), with both nomifensine and desipramine significantly increasing struggling compared to the vehicle group. In contrast, sertraline significantly increased swimming (F(3,72)=6.02, p=0.001). Nomifensine and desipramine also tended to increase diving, whereas sertraline significantly decreased diving (F(3,72)=5.56, p=0.002). Similar to Experiment 1, defecation was significantly greater in postpartum compared to control females (F(1,60)=5.93, p=0.018); drug pretreatment did not significantly alter defecation in either group. Locomotor activity was decreased only by desipramine (F(3,63)=12.55, p<0.001). There were no significant differences between postpartum and control rats in the effects of any of the three antidepressants on any behavior.
Figure 3.
Effects of the antidepressant drugs nomifensine (nomifen, a DA reuptake inhibitor, 2.5 mg/kg), desipramine (desipr, a NE reuptake inhibitor, 10 mg/kg), and sertraline (sertral, an SSRI, 10 mg/kg) on FST behaviors in postpartum vs. virgin control female rats tested on postpartum day 2. Rats were not pre-exposed to the FST; parturition served as the “inescapable stressor.” Vehicle or drug was administered subchronically (24, 5 and 1 hr before the FST). Locomotor activity was assessed for 5 min, approximately 15 min after the FST. Each bar is the mean + 1 S.E.M., N=5-13 control rats or 8-19 postpartum rats. *significantly different from vehicle-treated group, p≤0.05.
Effect of FST pre-exposure on postpartum day 2 FST behaviors
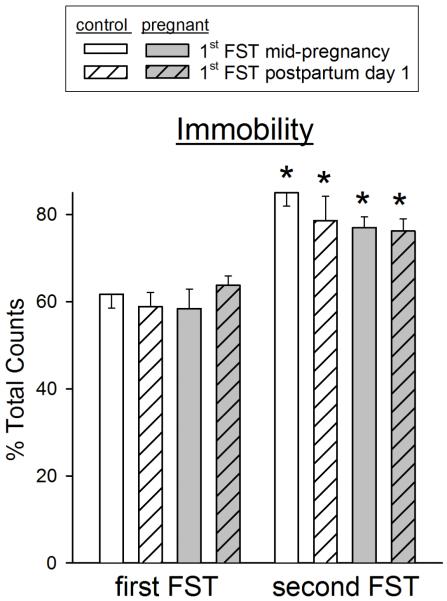
Figure 4 shows that both control and postpartum rats pre-exposed to the FST – either at mid-pregnancy or on postpartum day 1 – showed significantly and similarly elevated immobility scores on the second FST that was conducted on postpartum day 2 (F(1, 22)=70.88, p<0.001). The increase in immobility was primarily accounted for by decreases in struggling behavior, with small decreases in diving behavior as well (data not shown). There were no significant differences in any FST behaviors between control vs. postpartum females on either the first or second FST.
Figure 4.
Effect of pre-exposure to the FST on subsequent FST behaviors on postpartum day 2, in postpartum vs. virgin control female rats. Rats were exposed to the FST on either day 14 of pregnancy or day 1 postpartum (“first FST”), and then re-tested on postpartum day 2 (“second FST”). Each bar is the mean + 1 S.E.M., N=4-6 control rats or 7-9 postpartum rats. *significantly different from first FST, p≤0.05.
Subchronic vs. chronic treatment with antidepressant drugs in FST-pre-exposed postpartum vs. control females on postpartum day 2
Figure 5 shows the effects of subchronic vs. chronic treatment with desipramine (10 mg/kg) or sertraline (10 or 20 mg/kg) on FST behaviors and locomotor activity in control vs. postpartum females that were pre-exposed to the FST in mid-pregnancy. The only treatment that significantly decreased immobility was chronic desipramine (chronicity x drug: F(2,108)=3.47, p=0.035), and this effect was significant only in control females. Struggling behavior was also significantly increased by desipramine, given subchronically or chronically, in the control group only (chronicity x drug: F(2,108)=7.36, p<0.001; group x chronicity: F(1,108)=6.35, p=0.013). In contrast, swimming behavior was increased by the low dose of sertraline given either subchronically or chronically, to approximately the same extent in control and postpartum groups (drug: F(3,108)=21.63, p<0.001). Diving behavior was increased by desipramine (drug: F(3,108)=6.78, p<0.001), significantly so in control females. Defecation was significantly decreased by desipramine in most groups, and subchronic sertraline decreased defecation in the control females (drug: F(3,108)=9.43, p<0.001). Finally, locomotion was significantly decreased only by chronic desipramine in the control group. In general, locomotor activity was lower in postpartum females than in controls in this experiment, even after vehicle treatment (group: F(1,108)=7.60, p=0.007).
Figure 5.
Effects of subchronic (24-hr) vs. chronic (2-week) administration of the antidepressant drugs desipramine (10 mg/kg) or sertraline (10 or 20 mg/kg) on FST behaviors in postpartum vs. virgin control female rats tested on postpartum day 2. The 10 mg/kg dose of sertraline administered chronically (during pregnancy) caused labor complications and pup death, so the 20 mg/kg dose of sertraline was only tested subchronically. Rats were pre-exposed the FST on pregnancy day 12, to elevate postpartum immobility scores. Each bar is the mean + 1 S.E.M., N=8-13 control rats or 5-7 postpartum rats. *significantly different from vehicle-treated rats within same treatment regimen, p≤0.05.
Females in the chronic drug-treated groups had prolonged labor times, and in approximately half of their litters, one or more pups disappeared from the time of parturition to the time of testing on postpartum day 2. No pups were lost from litters of subchronic or chronic vehicle-treated dams, and only one pup was lost from one litter each in the subchronic drug-treated groups. Thus, chronic drug treatment significantly decreased litter size: at the time of testing on postpartum day 2, the vehicle-treated group had a mean litter size of 10.8 ± 1.8 pups, whereas the desipramine and sertraline-treated groups had litter sizes of 5.7 ± 1.9 and 5.8 ± 2.0 pups, respectively; in contrast, litter sizes in the subchronic treatment groups were not significantly different from each other, with means of 10.4-12.4 pups/litter (chronicity: F(1,30)=10.96, p=0.002; drug: F(2,30)=3.36, p=0.048).
Plasma hormone levels on postpartum days 1-7 in previously pregnant vs. hormone-simulated pregnant females
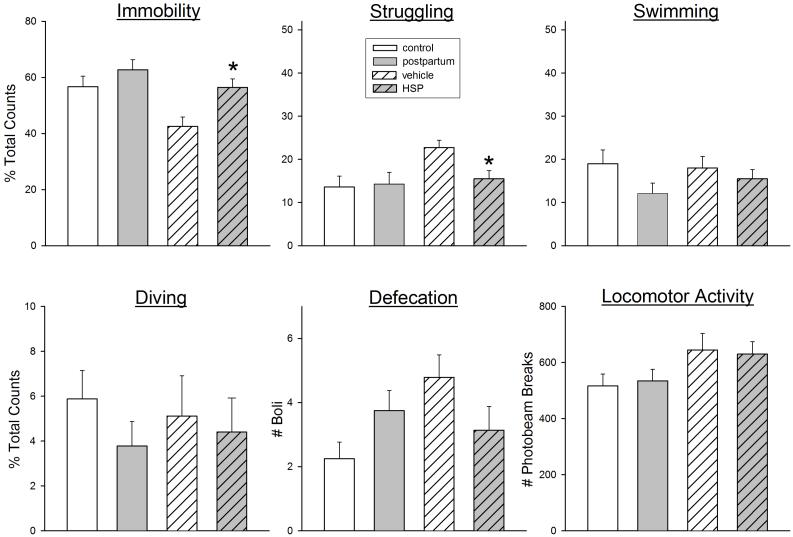
The difference in FST behaviors between postpartum vs. control females was slight compared to the differences in FST behaviors between HSP vs. control (ovariectomized) females we have previously tested (Stoffel & Craft, 2004). Figure 6 shows a comparison of FST behaviors in actual postpartum females tested on postpartum day 4 (re-plotted from Figure 1) vs. HSP females also tested on “postpartum” day 4. Whereas immobility was only slightly elevated in actual postpartum females compared to virgin controls, immobility was significantly elevated in HSP females compared to their ovariectomized, vehicle-treated controls (F(1,28)=10.10, p=0.004). This increased immobility was primarily due to significantly decreased struggling behavior in HSP compared to vehicle control females (F(1,28)=8.59, p=0.007).
Figure 6.
FST behaviors and locomotor activity in postpartum vs. virgin control rats (re-plotted from Fig. 1, open bars) compared to ovariectomized females that had undergone hormone-simulated pregnancy (HSP) vs. vehicle injections (vehicle) (hatched bars); all rats were tested on postpartum day 4. Rats were not pre-exposed to the FST; parturition (or hormone withdrawal in HSP rats) served as the “inescapable stressor.” Each bar is the mean ± 1 S.E.M., N=8-14 rats. *HSP group significantly different from vehicle control group, p≤0.05.
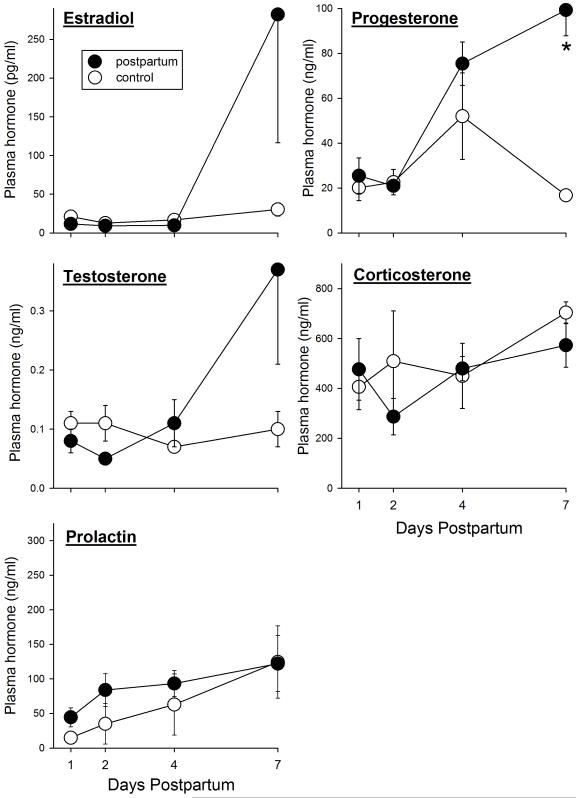
Plasma hormone levels were assessed in separate groups of rats – those that had not been tested on the FST – to determine whether hormone differences were smaller between postpartum vs. control (gonadally intact, virgin) females than between HSP vs. control (ovariectomized) females. All postpartum females were in diestrus at the time blood was taken, and control females were in various estrous stages (12% early proestrus, 29% late proestrus, 17% estrus, 42% diestrus). In contrast, ovariectomized females that had undergone HSP were mostly in proestrus to estrus at the time blood was taken, whereas their vehicle controls were all in diestrus. Figure 7 shows that there were very few significant differences in hormone levels between postpartum and virgin control rats on postpartum days 1-7. Both estradiol and testosterone were slightly suppressed in postpartum compared to control rats on postpartum days 1-2, and both of these hormones as well as progesterone were elevated compared to controls on day 7. The group differences in progesterone levels were statistically significant (Progesterone, group x day: F(3,39)=7.45, p<0.001; Testosterone, group x day: F(3,39)=2.32, p=0.09; Estradiol, group x day: F(3,39)=2.11, p=0.12). Although prolactin levels were somewhat higher in postpartum females than controls on postpartum days 1-4, neither prolactin nor corticosterone levels differed significantly between postpartum and control females on any postpartum day. A subsequent examination of hormone levels by estrous stage indicated that prolactin levels were significantly higher in late proestrous to estrous females than in diestrous females (as shown previously: Adler, 1981), and thus control females in late proestrus to estrus probably accounted for the lack of significant difference in prolactin levels between postpartum and control females, particularly at postpartum day 7, because that control group contained no diestrous females (all were late proestrous to estrous) whereas the postpartum days 1, 2 and 4 control groups each contained 50-67% diestrous females. Estrous stage differences in plasma hormone levels did not appear to affect postpartum vs. control comparisons for any other hormone except prolactin.
Figure 7.
Plasma hormone levels in postpartum vs. virgin control female rats on postpartum days 1, 2, 4 and 7. Each point is the mean + or − 1 S.E.M., N=4-6 samples. *significantly greater than control group, p≤0.05.
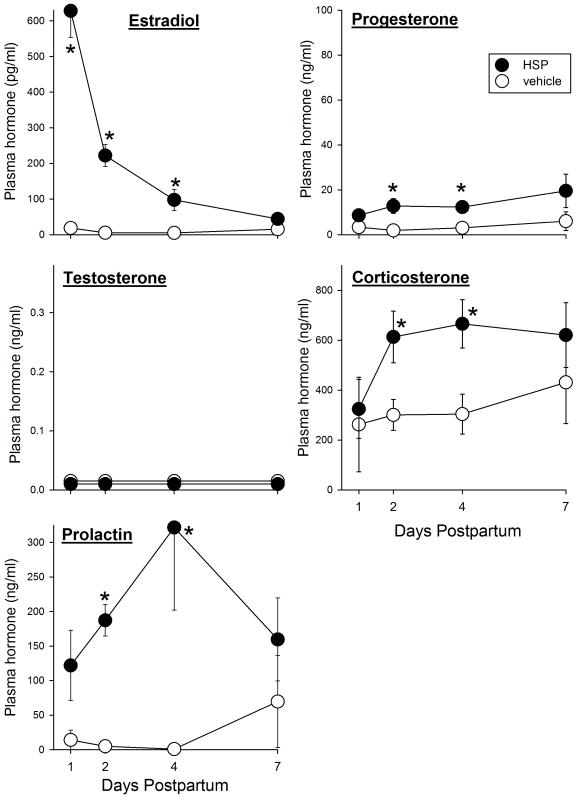
In contrast to the modest differences in hormone levels in actual postpartum females vs. controls, “postpartum” hormone levels differed substantially between ovariectomized rats that had undergone HSP compared to ovariectomized controls. Figure 8 shows that in rats that had undergone HSP, plasma estradiol was very high on “postpartum” day 1 and gradually declined such that it was no different than that in controls by “postpartum” day 7 (group x day: F(3,27)=17.24, p<0.001). Progesterone was also higher in HSP than in control rats (group: F(1,27)=7.34, p=0.012), significantly so on “postpartum” days 2 and 4. Corticosterone and prolactin levels were also significantly higher in HSP than in ovariectomized control females (Corticosterone, group: F(1,27)=9.35, p=0.005; Prolactin, group: F(1,26)=9.96, p=0.004), significantly so on “postpartum” days 2-4.
Figure 8.
Plasma hormone levels on “postpartum” days 1, 2, 4 and 7 in ovariectomized rats that had undergone hormone-simulated pregnancy (HSP) or vehicle treatment. Rats that have undergone HSP show significantly elevated immobility in the FST on “postpartum” days 2 and 4 compared to vehicle controls (Stoffel and Craft, 2004; and see Fig. 6). Each point is the mean + or − 1 S.E.M., N=3-6 samples. Note that the y-axis scales are identical in Fig. 7 and 8 except for the estradiol scale, which is compressed in Fig. 8. *significantly greater than vehicle control group, p≤0.05.
Discussion
Using the FST, a standard rodent model of depression-like behavior (Borsini and Meli, 1988; Cryan et al., 2005), the present study shows that postpartum female rats do not spend significantly more time immobile than virgin controls during the first postpartum week. However, three behavioral measures differed significantly between postpartum and virgin controls: postpartum females spent less time swimming and diving, and defecated more than control females. To the extent that the first two behaviors reflect active escape attempts that are primarily observed within the first 5 min in the tank (Porsolt et al., 1978), and the latter reflects “emotionality” to a novel situation (Broadhurst, 1957), postpartum females appear to be more stressed than control females by the FST. It is unlikely that decreased swimming and diving simply reflect a compromised ability to move, as struggling (climbing) frequency and locomotor activity did not differ between postpartum and control females. Furthermore, group differences (or lack thereof) found in Experiment 1 cannot be attributed to estrous stage differences between postpartum and control rats, as there were no estrous stage-related differences in FST behaviors in virgin females (Experiment 2). FST pre-exposure – whether it occurred 1 day or approximately 2 weeks before the 5-min FST – produced very similar increases in immobility in postpartum and control females (Experiment 4), demonstrating that the effect of FST pre-exposure on behavioral response to a subsequent stressor is maintained in pregnant/postpartum females, despite their dramatically different hormonal state compared to controls.
It should be noted that several previous studies demonstrate estrous stage-dependent FST behavior, contrary to our finding of no estrous stage effect. For example, Contreras and colleagues (2000) found that the latency to first immobility was significantly longer in proestrous rats compared to those in diestrus, with a corresponding lesser (though non-significant) total time spent immobile. Similarly, proestrous mice showed reduced immobility compared to diestrous mice (Walf et al., 2009), and estrous rats showed reduced immobility compared to diestrous rats (Marvan et al., 1996). In contrast, two studies reported that female rats in proestrus-estrus spent significantly more time immobile than those in diestrus (Paré and Redei, 1993; Consoli et al., 2005). Unfortunately, it is impossible to reconcile these disparate findings given the many procedural differences among these studies (and the present one), such as the number of FST pre-exposures, the depth of the tank, the frequency of handling, and the procedure for categorizing estrous stage.
A few previous studies have examined FST behavior in female rats during the postpartum period. For example, one study reported no difference in immobility between virgin and postpartum females tested on postpartum day 8-10, either on the first FST or on a second FST conducted 24 hr later; no other FST behaviors were reported (Walker et al., 1995). In two other studies only data from the second FST were reported. In the first study, immobility in postpartum female rats did not differ from that in controls (which were ovariectomized) on postpartum days 3 and 7, although it was elevated compared to females tested during mid-pregnancy (Molina-Hernandez and Téllez-Alcántara, 2001). In another study, females tested 4 weeks after parturition showed elevated immobility if their pups had been removed on postpartum day 1 (compared to females that had suckled pups, or virgin females that had been exposed to pups); however, immobility in postpartum females was not different from virgin females that had had no pup exposure (Pawluski et al., 2009). Thus, in terms of the immobility measure, the present study agrees with these previous studies in that immobility did not differ significantly between postpartum and virgin control females during the postpartum period. Thus, based on the immobility measure alone, it cannot be concluded that postpartum females show depression-like behavior. Group differences in the three other behaviors during the FST, however, suggest that postpartum females are more sensitive than virgin females to this inescapable stressor.
The predictive validity of the FST, as determined by the ability of antidepressant medications (but not other psychotherapeutic medications) to decrease immobility, has been tested extensively and found to be quite robust (Borsini and Meli, 1988). However, nearly all testing to date has been conducted in male rodents. To test the predictive validity of the FST in postpartum females, we compared the effects of several antidepressant medications in postpartum vs. virgin females. First, we employed the subchronic (sometimes called “acute” or “subacute”) treatment regimen, in which three doses of a drug are administered over 24 hr before the (second) FST. This treatment regimen has been shown to prevent increased immobility in the rat FST using a wide variety of antidepressants (Borsini and Meli, 1988; Porsolt et al., 1977). In our first experiment, in which rats were not pre-exposed to the FST, only nomifensine significantly decreased immobility, an effect that did not differ between postpartum and control females. Nomifensine, a DA reuptake inhibitor, has been shown previously at the dose and treatment regimen we used to prevent increased immobility in the FST in male rats (Borsini et al., 1981; Porsolt et al., 1978), so it appears to be equally effective in postpartum and virgin females. In contrast, despite the fact that the noradrenergic drug desipramine (10 mg/kg) and the SSRI sertraline (10 mg/kg) significantly increased struggling and swimming, respectively, as previously shown in males (Detke et al., 1995), these antidepressants did not significantly decrease immobility in postpartum or control females. Furthermore, sertraline completely eliminated diving, another escape-related behavior, in both groups. It should be noted that although desipramine and sertraline have been shown to be effective in males at the doses we used, given subchronically, higher doses or chronic administration have been more consistently effective (Borsini et al., 1981; Detke et al., 1997; Harkin et al., 1999; Porsolt et al., 1977). Rats were also treated before the first FST in our first experiment, rather than in between a first and second FST as more commonly done by other investigators.
Therefore, in our second drug experiment we compared the effects of desipramine and sertraline given subchronically vs. chronically in females that were pre-exposed to the FST, to determine whether antidepressant effects could be detected under these conditions. Subchronic desipramine was still ineffective, whereas chronic desipramine significantly decreased immobility – but only in control females. The only behavior that was significantly altered by desipramine in postpartum females was defecation. However, whereas both subchronic and chronic desipramine decreased defecation in control females, only chronic desipramine did so in postpartum females. Taken together, these results suggest that desipramine is a less effective antidepressant in pregnant/postpartum females than in virgin females, up to a dose that, when given chronically, decreased pup births.
In contrast to desipramine, sertraline was ineffective in both control and postpartum females, even when it was administered at a higher dose (20 mg/kg) subchronically, or when administered chronically at the 10 mg/kg dose. Similar to the first drug experiment, sertraline increased swimming but eliminated diving in most groups. It is possible that a higher dose of sertraline would have been effective; previous studies in male rats demonstrate effects in the FST in the range of 5-35 mg/kg given subchronically (Cervo et al., 1991; Kelly and Leonard, 1994). Given the adverse effects of chronic administration of 10 mg/kg sertraline on pup births, we chose not to test higher doses; however, more complete acute dose-effect functions in each group would clearly provide a more robust comparison of drug potency/efficacy between control and postpartum females.
Taken together, our results indicate that postpartum females are less sensitive than virgin females to the antidepressant effects of noradrenergic and serotonergic agonists, as measured by the FST. We are not aware of any previous comparisons of antidepressant drug effects between postpartum and virgin females; however, serotonergic agonists such as 8-OH-DPAT (though not buspirone) have been reported to lack anxiolytic activity in postpartum rats (Fernández-Guasti et al., 1998; 2001). Additionally, studies in women suggest that antidepressant doses need to be increased to maintain euthymia during the second half of pregnancy (Hostetter et al., 2000; Sit et al., 2008), due to increased drug metabolism (Sit et al., 2008). Similar to the present findings, there are also previous reports of peri- and postnatal complications from antidepressant drug treatment during late pregnancy in rodents (Swerts et al., 2009; Van den Hove et al., 2008). However, in humans the primary adverse outcome of SSRI exposure during pregnancy appears to be preterm birth, which may also result from untreated depression (Bigos et al., 2009; Wisner et al., 2009).
It is possible that ovarian hormone withdrawal during the early postpartum period also contributed to postpartum females’ insensitivity to antidepressants. The present study shows that ovarian hormones such as estradiol and testosterone were below levels in control rats at postpartum days 1-2. A growing number of studies reports that antidepressant efficacy is diminished in both women and female rodents under conditions of low ovarian hormones, and conversely enhanced by hormone replacement or supplementation. For example, estradiol has been shown to enhance ovariectomized rats’ sensitivity to serotonergic and noradrenergic antidepressants (Estrada-Camarena et al., 2004; 2008; Sell et al., 2008), Moreover, testosterone replacement in gonadectomized male rats has been shown to restore sensitivity to desipramine (but not to serotonergic antidepressants) on the FST (Martinez-Mota and Fernández-Guasti, 2004). Women’s response to serotonergic antidepressants has also been shown to be improved when combined with hormone replacement therapy (Jacobs and Hyland, 2003; Rasgon et al., 2006; Thase et al., 2005; Zanardi et al., 2007). Thus, both increased drug metabolism during pregnancy and low ovarian hormone levels during the early postpartum period may contribute to postpartum females’ insensitivity to antidepressants. It would be useful to test the latter hypothesis by supplementing postpartum females with ovarian hormones, to determine whether they enhance antidepressant efficacy in this population as they have been shown to do in ovariectomized females.
Because the differences in FST behavior between postpartum vs. virgin females were more subtle than those between the HSP vs. ovariectomized controls that we and others have studied previously (Galea et al., 2001; Stoffel and Craft, 2004), we examined plasma levels of five hormones that change from late pregnancy to the early postpartum period. Group differences in hormone levels were substantially greater in the HSP vs. control groups than in the postpartum vs. control groups. During the first “postpartum” week, estradiol, progesterone, prolactin and corticosterone were all significantly higher in rats that had undergone HSP compared to ovariectomized controls. In contrast, only progesterone was significantly higher in postpartum compared to virgin control rats, and this difference was only significant at the end of the first week postpartum. The relatively greater differences in hormone levels in the HSP-vs.-control compared to the postpartum-vs.-control groups are partly due to the fact that the control group in the HSP experiment is ovariectomized females, which would be expected to have very low levels of ovarian hormones and prolactin compared to the gonadally intact, virgin control group. However, some differences between HSP and control females would have been significant even if the HSP females had been compared to gonadally intact controls – most notably estradiol and prolactin, which were very high in HSP females. Estradiol levels in HSP rats are very high compared to those previously pregnant rats on postpartum day 1 because HSP rats are injected with high estradiol doses (50 μg) for the last 6 days of “pregnancy”, which is necessary for eliciting maternal behaviors rapidly in virgin, ovariectomized females (Bridges, 1984). High plasma prolactin levels are known to be induced by high-dose estradiol treatment (Carrillo et al., 1991). It has been hypothesized that the rapid decline in estradiol beginning at parturition serves as a trigger for postpartum mood changes, both in rats (Galea et al., 2001; Green et al., 2009; Navarre et al., 2010; Stoffel and Craft, 2004) and in women (Bloch et al., 2003; Parry et al., 2003). The present hormone profiles suggest that differences in progesterone, prolactin and corticosterone may also contribute to behavioral differences between HSP and control rats. Changes in each of these three hormones have been associated with changes in mood-related behaviors in female rats and/or women (e.g., progesterone: Andreen et al., 2009; Mostallino et al., 2009; prolactin: Sobrinho, 2003; Torner and Neumann, 2002; corticosterone/cortisol: Taylor et al., 2009; Walf and Frye, 2005), although it is not yet known how they might function (or dysfunction) in concert to result in postpartum mood disorder.
In conclusion, the present study demonstrates that postpartum female rats do not show elevated immobility in the FST compared to virgin controls in the first postpartum week; however, postpartum females show significant changes in other behaviors that may reflect heightened sensitivity to this inescapable stressor – perhaps modeling the commonly experienced, relatively mild “baby blues” that occur in a majority of women within the first week postpartum (Sit and Wisner, 2009). Furthermore, postpartum female rats respond similarly to controls to FST pre-exposure and to a dopaminergic antidepressant, but are less sensitive than controls to noradrenergic and serotonergic antidepressants. The depression-like behaviors previously observed in ovariectomized females that have undergone HSP and hormone withdrawal may result from the more dramatic changes in estradiol, prolactin and corticosterone that occur during the early “postpartum” period, compared to actual postpartum females. Further testing that includes manipulation of these other hormones which are known to affect mood will further determine whether the HSP-hormone withdrawal procedure in the female rat is a good model for postpartum depression in women.
Acknowledgments
The authors thank Jenny Browning, Brittany Navarre and Katt Kelley for excellent technical assistance. This research was funded by a grant from the National Institute of Mental Health (MH070381) to R.M.C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler NJ, editor. Neuroendocrinology of Reproduction. Plenum Press; New York: 1981. p. 282. [Google Scholar]
- Andreen L, Nyberg S, Turkmen S, van Wingen G, Fernandez G, Backstrom T. Sex steroid induced negative mood may be explained by the paradoxical effects mediated by GABAA modulators. Psychoneuroendocrinology. 2009;34:1121–32. doi: 10.1016/j.psyneuen.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Bigos KL, Pollock BG, Stankevich BA, Bies RR. Sex differences in the pharmacokinetics and pharmacodynamics of antidepressants: An updated review. Gender Med. 2009;6:522–43. doi: 10.1016/j.genm.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Bloch M, Daly R, Rubinow D. Endocrine factors in the etiology of postpartum depression. Comp Psychiatry. 2003;44:234–46. doi: 10.1016/S0010-440X(03)00034-8. [DOI] [PubMed] [Google Scholar]
- Borsini F, Bendotti C, Velkov V, Rech R, Samanin R. Immobility test: effects of 5-hydroxytryptaminergic drugs and role of catecholamines in the activity of some antidepressants. J Pharm Pharmacol. 1981;33:33–7. doi: 10.1111/j.2042-7158.1981.tb13697.x. [DOI] [PubMed] [Google Scholar]
- Borsini F, Meli A. Is the forced swimming test a suitable model for revealing antidepressant activity? Psychopharmacology. 1988;94:147–60. doi: 10.1007/BF00176837. [DOI] [PubMed] [Google Scholar]
- Bridges RS. A quantitative analysis of the roles of dosage, sequence, and duration of estradiol and progesterone exposure in the regulation of maternal behavior in the rat. Endocrinology. 1984;114:930–40. doi: 10.1210/endo-114-3-930. [DOI] [PubMed] [Google Scholar]
- Broadhurst PL. Determinants of emotionality in the rat. I. Situational factors. Gen Psych. 1957;48:1–12. doi: 10.1111/j.2044-8295.1957.tb00594.x. [DOI] [PubMed] [Google Scholar]
- Carrillo AJ, Doherty PC, Guan X, Sturtevant JR, Walro DG. Preferential increase in pituitary prolactin versus vasoactive intestinal peptide as a function of estradiol benzoate dose in the ovariectomized rat. Endocrinology. 1991;128:131–8. doi: 10.1210/endo-128-1-131. [DOI] [PubMed] [Google Scholar]
- Cervo L, Grignaschi G, Rossi C, Samanin R. Role of central serotonergic neurons in the effect of sertraline in rats in the forced swimming test. Eur J Pharmacol. 1991;196:217–22. doi: 10.1016/0014-2999(91)90433-q. [DOI] [PubMed] [Google Scholar]
- Consoli D, Fedotova J, Micale V, Sapronov NS, Drago F. Stressors affect the response of male and female rats to clomipramine in a model of behavioral despair (forced swim test) Eur J Pharmacol. 2005;520:100–7. doi: 10.1016/j.ejphar.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Contreras CM, Molina M, Saavedra M, Martinez-Mota L. Lateral septal firing rate increases during proestrus-estrus in the rat. Physiol Behav. 2000;68:279–84. doi: 10.1016/s0031-9384(99)00169-9. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev. 2005;29:547–69. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology. 1995;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Johnson J, Lucki I. Acute and chronic antidepressant drug treatment in the rat forced swimming test model of depression. Exp Clin Psychopharmacol. 1997;5:107–12. doi: 10.1037//1064-1297.5.2.107. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Lucki I. Detection of serotonergic and noradrenergic antidepressants in the rat forced swim test: the effects of water depth. Behav Brain Res. 1996;73:43–6. doi: 10.1016/0166-4328(96)00067-8. [DOI] [PubMed] [Google Scholar]
- Estrada-Camarena E, Fernández-Guasti A, Lopez-Rubalcava C. Interaction between estrogens and antidepressants in the forced swimming test in rats. Psychopharmacology. 2004;173:139–45. doi: 10.1007/s00213-003-1707-4. [DOI] [PubMed] [Google Scholar]
- Estrada-Camarena E, Vega Rivera NM, Berlanga C, Fernández-Guasti A. Reduction in the latency of action of antidepressants by 17 β-estradiol in the forced swimming test. Psychopharmacology. 2008;201:351–60. doi: 10.1007/s00213-008-1291-8. [DOI] [PubMed] [Google Scholar]
- Fernández-Guasti A, Picazo O, Ferreira A. Blockade of the anxiolytic action of 8-OH-DPAT in lactating rats. Pharmacol Biochem Behav. 1998;59:45–50. doi: 10.1016/s0091-3057(97)00392-4. [DOI] [PubMed] [Google Scholar]
- Fernández-Guasti A, Ferreira A, Picazo O. Diazepam, but not buspirone, induced similar anxiolytic-like actions in lactating and ovariectomized Wistar rats. Pharmacol Biochem Behav. 2001;70:85–93. doi: 10.1016/s0091-3057(01)00586-x. [DOI] [PubMed] [Google Scholar]
- Freeman ME. The neuroendocrine control of the ovarian cycle of the rat. In: Knobil E, Neill J, editors. The physiology of reproduction. Raven Press; New York: 1988. pp. 1893–1928. [Google Scholar]
- Galea LA, Wide JD, Barr AM. Estradiol alleviates depressive-like symptoms in a novel animal model of post-partum depression. Behav Brain Res. 2001;122:1–9. doi: 10.1016/s0166-4328(01)00170-x. [DOI] [PubMed] [Google Scholar]
- Green AD, Barr AM, Galea LAM. Role of estradiol withdrawal in ‘anhedonic’ sucrose consumption: a model of postpartum depression. Physiol Behav. 2009;97:259–65. doi: 10.1016/j.physbeh.2009.02.020. [DOI] [PubMed] [Google Scholar]
- Halbreich U. Postpartum disorders: Multiple interacting underlying mechanisms and risk factors. J Affect Dis. 2005;88:1–7. doi: 10.1016/j.jad.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Harkin A, Kelly JP, McNamara M, Connor TJ, Dredge K, Redmond A, et al. Activity and onset of action of reboxetine and effect of combination with sertraline in an animal model of depression. Eur J Pharmacol. 1999;364:123–32. doi: 10.1016/s0014-2999(98)00838-3. [DOI] [PubMed] [Google Scholar]
- Hostetter A, Stowe ZN, Strader JR, Jr, McLaughlin E, Llewellyn A. Dose of selective serotonin uptake inhibitors across pregnancy: clinical implications. Depress Anxiety. 2000;11:51–7. [PubMed] [Google Scholar]
- Jacobs PA, Hyland ME. An evaluation of the benefits of taking hormone replacement therapy with other prescription drugs. Maturitas. 2003;46:273–281. doi: 10.1016/s0378-5122(03)00198-1. [DOI] [PubMed] [Google Scholar]
- Kelly JP, Leonard BE. The effect of tianeptine and sertraline in three animal models of depression. Neuropharmacology. 1994;33:1011–6. doi: 10.1016/0028-3908(94)90160-0. [DOI] [PubMed] [Google Scholar]
- Martinez-Mota L, Fernández-Guasti A. Testosterone-dependent antidepressant-like effects of noradrenergic but of serotonergic drugs. Pharmacol Biochem Behav. 2004;78:711–18. doi: 10.1016/j.pbb.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Marvan ML, Chavez-Chavez L, Santana S. Clomipramine modifies fluctuations of forced swimming immobility in different phases of the rat estrous cycle. Arch Med Res. 1996;27:83–6. [PubMed] [Google Scholar]
- Molina-Hernandez M, Téllez-Alcántara NP. Antidepressant-like actions of pregnancy, and progesterone in Wistar rats forced to swim. Psychoneuroendocrinology. 2001;26:479–91. doi: 10.1016/s0306-4530(01)00007-5. [DOI] [PubMed] [Google Scholar]
- Moses-Kolko EL, Berga SL, Kalro B, Sit DKY, Wisner KL. Transdermal estradiol for postpartum depression: A promising treatment option. Clin Obstet Gyn. 2009;52:516–29. doi: 10.1097/GRF.0b013e3181b5a395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostallino MC, Sanna E, Concas A, Biggio G, Follesa P. Plasticity and function of extrasynaptic GABAA receptors during pregnancy and after delivery. Psychoneuroendocrinology. 2009;34S:S74–S83. doi: 10.1016/j.psyneuen.2009.06.013. [DOI] [PubMed] [Google Scholar]
- Navarre BM, Laggart JD, Craft RM. Anhedonia in postpartum rats. Physiol Behav. 2010;99:59–66. doi: 10.1016/j.physbeh.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hara MW. Postpartum depression: what we know. J Clin Psychol. 2009;65:1258–69. doi: 10.1002/jclp.20644. [DOI] [PubMed] [Google Scholar]
- Paré WP, Redei E. Sex differences and stress response of WKY rats. Physiol Behav. 1993;54:1179–85. doi: 10.1016/0031-9384(93)90345-g. [DOI] [PubMed] [Google Scholar]
- Parry BL, Sorenson DL, Meliska CJ, Basavaraj N, Zirpoli GG, Gamst A, et al. Hormonal basis of mood and postpartum disorders. Curr Women’s Health Rep. 2003;3:230–5. [PubMed] [Google Scholar]
- Pawluski JL, Lieblich SE, Galea LAM. Offspring-exposure reduces depressive-like behaviour in the parturient female rat. Behav Brain Res. 2009;197:55–61. doi: 10.1016/j.bbr.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol. 1978;47:379–91. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–2. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Rasgon NL, Dunkin J, Fairbanks L, Altshuler LL, Troung C, Elman S, Wroolie TE, Brunhuber MV, Rapkin A. Estrogen and response to sertraline in postmenopausal women with major depressive disorder: A pilot study. J Psychiatric Res. 2006;41:338–43. doi: 10.1016/j.jpsychires.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Sell SL, Craft RM, Seitz PK, Stutz SJ, Cunningham KA, Thomas ML. Estradiol-sertraline synergy in ovariectomized rats. Psychoneuroendocrinology. 2008;33:1051–60. doi: 10.1016/j.psyneuen.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Sit DKY, Wisner KL. Identification of postpartum depression. Clin Obstet Gyn. 2009;3:456–68. doi: 10.1097/GRF.0b013e3181b5a57c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sit DKY, Perel JM, Helsel JC, Wisner KL. Changes in antidepressant metabolism and dosing across pregnancy and early postpartum. J Clin Psychiatry. 2008;69:652–8. doi: 10.4088/jcp.v69n0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobrinho LG. Prolactin, psychological stress and environment in humans: adaptation and maladaptation. Pituitary. 2003;6:35–9. doi: 10.1023/a:1026229810876. [DOI] [PubMed] [Google Scholar]
- Stoffel EC, Craft RM. Ovarian hormone withdrawal-induced “depression” in female rats. Physiol Behav. 2004;83:505–13. doi: 10.1016/j.physbeh.2004.08.033. [DOI] [PubMed] [Google Scholar]
- Swerts CAS, Dias Costa AMD, Esteves A, Borato CES, Swerts MSO. Effects of fluoxetine and imipramine in rat fetuses treated during a critical gestational period: a macro and microscopic study. Rev Bras Psiquiatr. 2009 Dec 18; doi: 10.1590/s1516-44462009005000015. pii: S1516-44462009005000015. [Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Taylor A, Glover V, Marks M, Kammerer M. Diurnal pattern of cortisol output in postnatal depression. Psychoneuroendocrinology. 2009;34:1184–8. doi: 10.1016/j.psyneuen.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Thase ME, Entsuah R, Cantillon M, Kornstein SG. Relative antidepressant efficacy of venlafaxine and SSRIs: sex-age interactions. J Womens Health (Larchmt) 2005;14:609–16. doi: 10.1089/jwh.2005.14.609. [DOI] [PubMed] [Google Scholar]
- Torner L, Neumann ID. The brain prolactin system: involvement in stress response adaptations in lactation. Stress. 2002;5:249–57. doi: 10.1080/1025389021000048638. [DOI] [PubMed] [Google Scholar]
- Van den Hove DL, Blanco CE, Scheepens A, Desbonnet L, Myint AM, Leonard BE, Prickaerts J, Steinbusch HW. Prenatal maternal paroxetine treatment and neonatal mortality in the rat: a preliminary study. Neonatology. 2008;93:52–5. doi: 10.1159/000106433. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. Antianxiety and antidepressive behavior produced by physiological estradiol regimen may be modulated by hypothalamic-pituitary-adrenal axis activity. Neuropsychopharmacology. 2005;30:1288–1301. doi: 10.1038/sj.npp.1300708. [DOI] [PubMed] [Google Scholar]
- Walf AA, Koonce CJ, Frye CA. Adult female wildtype, but not oestrogen receptor β knockout, mice have decreased depression-like behavior during pro-estrus and following administration of oestradiol or diarylpropionitrile. J Psychopharm. 2009;23:442–50. doi: 10.1177/0269881108089598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker C-D, Trottier G, Rochford J, Lavallee D. Dissociation between behavioral and hormonal responses to the forced swim stress in lactating rats. J Neuroendocrinology. 1995;7:615–22. doi: 10.1111/j.1365-2826.1995.tb00799.x. [DOI] [PubMed] [Google Scholar]
- Wisner KL, Sit DKY, Hanusa BH, Moses-Kolko EL, Bogen DL, Hunker DF, et al. Major depression and antidepressant treatment: impact on pregnancy and neonatal outcomes. Am J Psychiatry. 2009;166:557–66. doi: 10.1176/appi.ajp.2008.08081170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanardi R, Rossini D, Magri L, Malaguti A, Colombo C, Smeraldi E. Response to SSRIs and role of the hormonal therapy in post-menopausal depression. Eur Neuropsychopharmacology. 2007;17:400–5. doi: 10.1016/j.euroneuro.2006.11.001. [DOI] [PubMed] [Google Scholar]