Abstract
Background
Continuous EEG monitoring (cEEG) of critically ill patients is frequently utilized to detect non-convulsive seizures (NCS) and status epilepticus (NCSE). The indications for cEEG, as well as when and how to treat NCS, remain unclear. We aimed to describe the current practice of cEEG in critically ill patients to define areas of uncertainty that could aid in designing future research.
Methods
We conducted an international survey of neurologists focused on cEEG utilization and NCS management.
Results
Three-hundred and thirty physicians completed the survey. 83% use cEEG at least once per month and 86% manage NCS at least five times per year. The use of cEEG in patients with altered mental status was common (69%), with higher use if the patient had a prior convulsion (89%) or abnormal eye movements (85%). Most respondents would continue cEEG for 24 h. If NCS or NCSE is identified, the most common anticonvulsants administered were phenytoin/fosphenytoin, lorazepam, or levetiracetam, with slightly more use of levetiracetam for NCS than NCSE.
Conclusions
Continuous EEG monitoring (cEEG) is commonly employed in critically ill patients to detect NCS and NCSE. However, there is substantial variability in current practice related to cEEG indications and duration and to management of NCS and NCSE. The fact that such variability exists in the management of this common clinical problem suggests that further prospective study is needed. Multiple points of uncertainty are identified that require investigation.
Keywords: Continuous EEG, Non-convulsive seizure, Non-convulsive status epilepticus, Anticonvulsant, Monitoring
Introduction
Continuous electroencephalographic monitoring (cEEG) provides a non-invasive, bedside, ongoing means of assessing brain function, and there are an expanding number of indications for cEEG in neurologic and other intensive care units [1, 2]. The most common use is for detection of non-convulsive seizures (NCS) and non-convulsive status epilepticus (NCSE) which are reported to occur in critically ill adults [3] and children [4], and information derived from cEEG is reported to impact management in the majority of patients [5]. However, little data exists to allow for evidence based cEEG implementation or management related to cEEG findings. To date, studies have not investigated whether detection and management of NCS improves outcome, but the presence of NCS has been associated with worse outcome [6-8] and NCS have been shown to affect processes that may lead to or worsen brain injury [9]. Although urgent cEEG studies in inpatients prompt changes in anticonvulsant management in 52% of adults [10], little data exist regarding optimal NCS management.
We aimed to understand current clinical practice of cEEG and management of NCS and NCSE. This information is important for two reasons. First, these findings identify points of clinical uncertainty and equipoise that require future study, are ethical to study, and will have an important impact on clinical management. Second, multicenter studies will likely be required in order to enroll a sufficient number of patients, and information related to current clinical practice will help ensure that future studies are designed in a manner that provides for feasible implementation and enrollment at a large number of centers. We describe data acquired from an international survey of adult and pediatric neurologists that describes current clinical practice.
Methods
In May 2009, a survey participation invitation was sent to all members of the American Epilepsy Society, the American Clinical Neurophysiology Society, and the Child Neurology Society using member email lists. Those members who chose to participate completed an online survey designed to take 10–15 min using the Survey-Monkey website (www.surveymonkey.com). The survey was completed anonymously, and did not ask respondents to specific the name of their University or Hospital. Both in section introductions and individual questions, the survey asked and reminded respondents to consider critically ill patients in an intensive care unit setting. Pediatric neurologists were asked and reminded to address the questions in relation to management of non-neonates.
The survey instrument had three components. The first addressed demographic and practice information. The second addressed issues related to cEEG including indications for EEG and issues related to initiation and duration of cEEG. The third addressed management of NCS and NCSE. The instrument used closed-ended questions in which answers were chosen from lists or drop-down menus, some of which were mutually exclusive and some of which allowed multiple answers. At the end of each section, respondents were asked to provide other comments related to these issues. The survey is provided in the supplemental material.
The study was approved by the Institutional Review Board at the Children’s Hospital of Philadelphia.
Results
Survey Respondents
The survey was completed by 330 physicians and 82% completed all questions. Although the survey was sent to all organization members, only 22 non-physicians completed the survey (including 13 EEG technologists). These were excluded from analysis since there were too few responses in any group to be analyzed. Characteristics of the respondents and the respondents’ institutions are shown in Table 1. The mean number of years in practice was 15 ± 11 years.
Table 1.
Characteristics of respondents and respondents’ institutions, n = 330
| Level of training | |
| Attending | 90% |
| Resident/fellow | 10% |
| Practice type | |
| Pediatrics | 57% |
| Adult | 32% |
| Both | 11% |
| Practice specialty (more than 1 permitted) | |
| Epilepsy | 81% |
| Neurocritical care | 29% |
| General neurology | 64% |
| Other | 17% |
| Practice location | |
| United States | 84% |
| Europe | 6% |
| Canada | 5% |
| Other | 5% |
| Respondent institution | |
| Academic/tertiary care | 85% |
| Community hospital | 12% |
| Office only | 3% |
| Does respondent interpret EEGs? | |
| Yes—with formal qualifications | 60% |
| Yes—without formal qualifications | 26% |
| No | 14% |
| Institution EEG availability | |
| All times (24/7) | 63% |
| Limited additional hours | 25% |
| Only standard weekday hours | 12% |
| Institution cEEG availability | |
| All times (24/7) | 80% |
| Limited additional hours | 11% |
| Only standard weekday hours | 9% |
| Institution remote EEG reading availability | |
| Possible for all records | 35% |
| Possible for some records | 43% |
| Not possible for any records. | 22% |
| Non-convulsive seizure management | |
| ≥5 patients per year | 86% |
| <5 patients per year | 14% |
| Patients undergoing cEEG Per Month | |
| <1 | 17% |
| 1–5 | 41% |
| 6–20 | 29% |
| >20 | 13% |
Pediatric Versus Adult Practice
When results regarding cEEG monitoring indications and management of NCS and NCSE were stratified by practice type (pediatric or adult), only one question related to Propofol use for NCSE was answered differently adult and pediatric respondents. Propofol was selected as the first line coma inducing medication for 30% of adult and 3% of pediatric respondents.
EEG Monitoring Indications and Duration
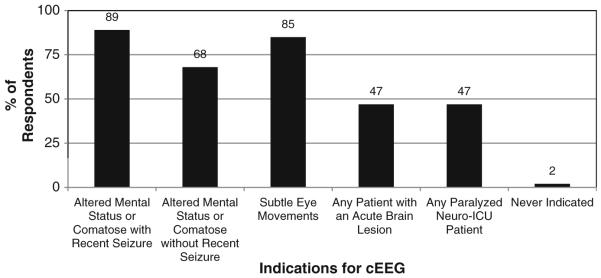
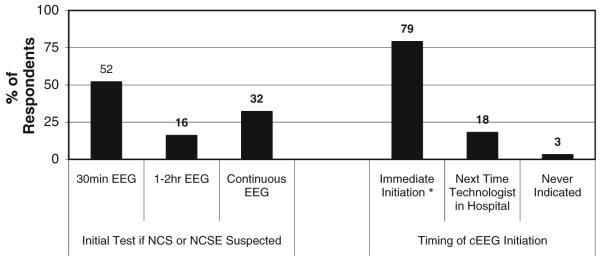
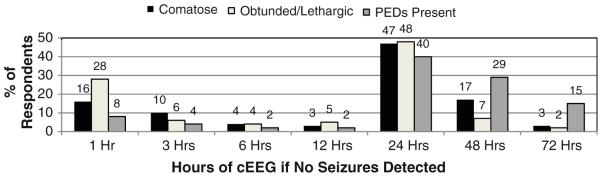
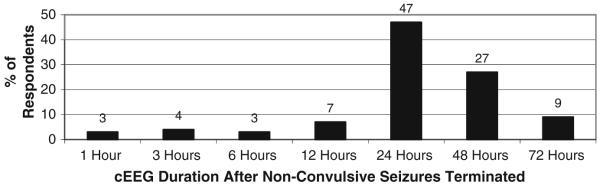
Figure 1 depicts the proportion of respondents that used EEG to identify NCS and NCSE for each of several indications. Figure 2 shows the type of EEG performed and urgency of initiation when cEEG is considered indicated for a patient. Figure 3 shows how long respondents would continue EEG monitoring if no seizures were detected in a patient who was comatose, a patient who is obtunded/lethargic, and if periodic epileptiform discharges (PEDs) are present. If seizures are detected, treated with an anticonvulsant, and terminate, the duration of cEEG after seizure termination to ensure seizures do not recur is shown in Fig. 4.
Fig. 1.
Which indications lead you to order cEEG to detect non-convulsive seizures or non-convulsive status epilepticus? (296 respondents)
Fig. 2.
If NCS (non-convulsive seizures) or NCSE (non-convulsive status epilepticus) is suspected then what type of EEG do you obtain and how urgently do you obtain the EEG? (294 respondents). * Including initiation by a 24/7 in-hospital EEG technologist or calling in an on-call technologist
Fig. 3.
How long do you continue cEEG if no seizures are detected in a patient who is comatose (292 respondents), obtunded/lethargic (291 respondents), or if PEDs (periodic epileptiform discharges) were detected (289 respondents)?
Fig. 4.
How long do you continue cEEG after non-convulsive seizures terminated? (288 respondents)
EEG Monitoring Review and Reporting
If EEG is being used to screen for NCS, it is reviewed once per day (21%), twice per day (29%), three or four times per day (17%), or almost continuously (18%) (293 respondents). Reports were provided daily (72%), twice per day (11%), and rarely only on weekdays (7%) (290 respondents). Daily reports are provided through an electronic medical record (48%), hand written in the hospital chart (25%), or verbally relayed to the primary physicians (27%) (293 respondents). The use of quantitative EEG trending software is relatively uncommon: compressed spectral array is used by 18% and amplitude integrated EEG is used by 13% of physicians, whereas 66% used neither of these tools (286 respondents).
Non-Convulsive Seizure and Non-Convulsive Status Epilepticus Management
Physicians would initiate treatment if any NCS are identified (79%), if multiple NCS are identified (12%), only if NCSE is identified (5%), or never if all seizures are non-convulsive (0.5%) (277 respondents). The management aim is to terminate all NCS (63%) or to terminate frequent NCS but tolerate up to 5 (26%) or 10 (6%) NCS per day. Rarely the aim was to induce burst suppression (3%), induce electrocerebral silence (1%), or tolerate all NCS as long as none had a clinical correlate (1%) (277 respondents).
The most common medications used to treat NCS and NCSE are shown in Fig. 5. If NCS were detected, physicians would induce coma if NCS persisted after 3rd line treatment (56%), 2nd line treatment (19%), or first line treatment (4%), while 21% would never induce coma if all seizures were non-convulsive (277 respondents). If NCSE was detected, physicians would induce coma if NCSE persisted after 3rd line medication (60%), 2nd line medication (29%), or 1st line medication (5%), while 6% would never induce coma for NCSE (268 respondents). The most common medications used for coma induction if needed are shown in Fig. 6. Willingness to intubate for NCS and NCSE is shown in Fig. 7. If seizures are detected and treated with a continuous coma inducing medication, physicians would continue cEEG while on the coma inducing medication (12%), and while on the coma inducing medication plus an additional 24 h (51%), an additional 48 h (16%), or an additional 12 h (8%) (273 respondents).
Fig. 5.
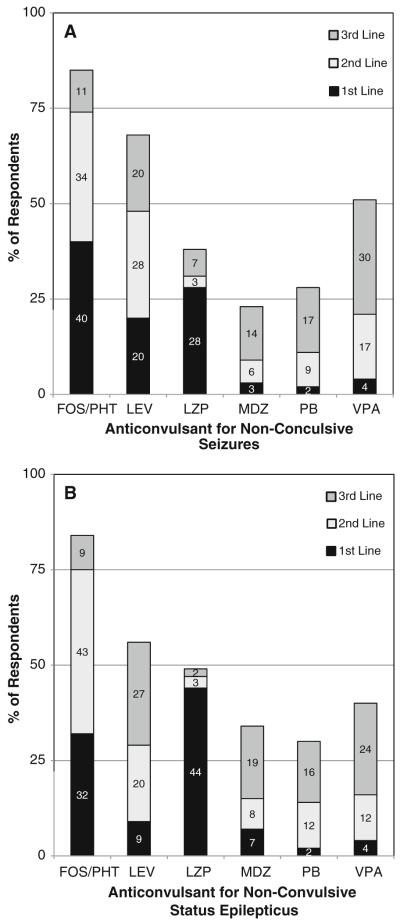
What anticonvulsant do you administer as a first, second, and third line medication of (a) non-convulsive seizures (271 respondents) or (b) non-convulsive status epilepticus? (268 respondents). FOS fosphenytoin, LEV levetiracetam, LZP lorazepam, MDZ midazolam, PB phenobarbital, PHT phenytoin, VPA valproic acid
Fig. 6.
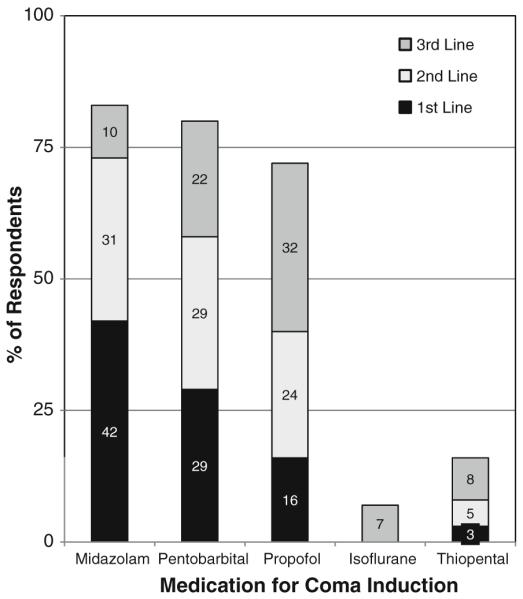
If non-convulsive seizures or status epilepticus persist despite initial anticonvulsants and you want to initiate coma, which medications do you use as first, second, and third line choices? (267 respondents)
Fig. 7.
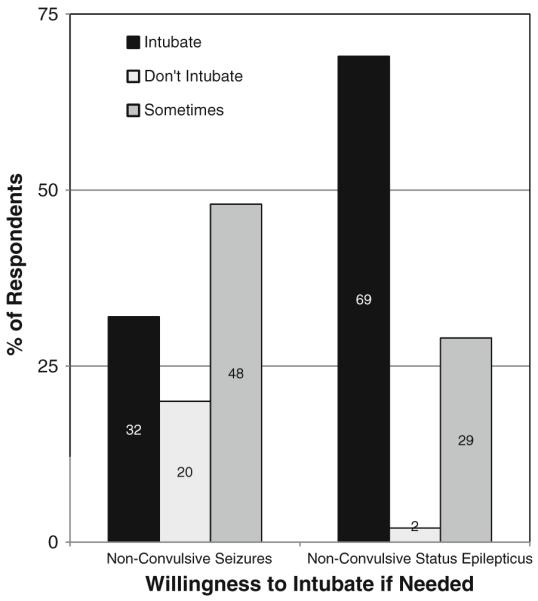
If non-convulsive seizures or non-convulsive status epilepticus is present, are you willing to intubate the patient to escalate treatment? (273 respondents)
If PEDs are present and occur at a frequency faster than 1.5 Hz but never evolve into a seizure, then anticonvulsants would be administered by 63% and not administered by 37% (276 respondents). If an anticonvulsant is administered, the treatment goal is to provide prophylaxis against seizures but not specifically target the PEDs for treatment (66%), aim to reduce PEDs to less than 1.5 Hz (14%), or aim to terminate PEDs (20%) (273 respondents).
Discussion
Respondents indicated frequently using cEEG in critically ill patients and managing NCS and NCSE. The majority of respondents used cEEG at least once per month and managed at least several patients with NCS per year. However, there was substantial variability related to cEEG indications, cEEG initiation urgency, cEEG duration, anticonvulsant choices, and overall management approaches. The fact that cEEG is frequently used and NCS and NCSE are commonly managed yet such substantial variability exists in clinical practice reflects the paucity of prospective research in this area and establishes the need for additional study.
Most respondents utilized EEG when critically ill patients had altered mental status, whether preceded by clinical convulsions or not. This is consistent with studies reporting that NCS and NCSE occur in 8–48% of comatose adults [3, 5, 7, 9, 11-17] and 16–47% of children with altered mental status [4, 18-24], with variability based on underlying etiology, duration of cEEG, definition of seizures, and inclusion or exclusion of patients with preceding convulsions or subtle clinical signs of seizure activity.
Most physicians called in a technologist if needed. The clinical practice of initiating cEEG on an urgent basis may relate to existing data that delay to NCS diagnosis and longer NCS duration are associated with increased mortality [6]. However, this practice is likely costly and resource intensive, and the fact that it is so common suggests that further study is needed to determine whether this practice positively impacts outcome.
If cEEG was utilized and no seizures were detected, most physicians monitored for 24 h, although a similar number of respondents monitored for 1 or 48 h. Monitoring was slightly longer if the patient was comatose (as compared to obtunded/lethargic) and monitoring was slightly longer if PEDs were present. A 30-min EEG may detect less than half of seizures eventually identified by longer cEEG [15]. Studies in both adults [3] and children [4, 21] have reported that 80–95% of seizures are detected within 24 h, with slightly longer durations needed if patients are not comatose or have PEDs [3].
The frequency of cEEG review is extremely variable, and is only very rarely continuous. The fact that most respondents review the cEEG only intermittently suggests that although often referred to as “continuous EEG monitoring,” current practice really consists of continuous EEG recording with periodic review. If NCS were detected and terminated, most monitored for 24 h after seizure termination, although a substantial number monitored for 48 h after seizure termination. The frequency of cEEG review and cEEG duration may have a large impact on cEEG implementation feasibility and cost and requires further study.
If NCS or NCSE are detected, most respondents would initiate treatment, although the management approach is extremely variable. The aim of treatment is usually to terminate all NCS, but a substantial number aim to induce burst suppression or electrocerebral silence. There is also variability in the medications utilized. Respondents tended to treat NCSE slightly more aggressively than NCS, with a trend toward less use of newer anticonvulsants like levetiracetam and more willingness to induce coma and intubate if required. Little data exist related to treatment of NCS and NCSE for adult or pediatric patients, forcing physicians to use whatever limited data is available, and this may explain why management is similar across age groups. Both animal models and clinical studies are needed to develop evidence-based management algorithms [25].
Many respondents initiate an anticonvulsant if PEDs were present, although there is variability in whether the aim is to provide prophylaxis against seizures or impact the PEDs themselves. PEDs refer to a category of discharges, of which there are many types, including periodic lateralized, bilateral independent, generalized, and stimulus induced varieties [26]. These have been associated with an increased risk of seizures [20] and worse outcome [7, 16, 27, 28], but it remains unclear whether these should be targeted for treatment in most patients. Our survey did not ask respondents to differentiate between management of different types of PEDs which may have led to response variability.
The substantial variability in practice has implications for the design of any future multi-center studies investigating NCS and NCSE. First, the extensive variability in clinical practice among experienced specialists suggests that there is great clinical uncertainty and that clinical equipoise exists at many diagnostic and therapeutic decision points. This suggests that prospective study of these points is both needed and ethical. Second, if such studies are to achieve high enrollment, they will need to be planned with extensive input from centers that might potentially enroll subjects to ensure that enrollment criteria and management approaches are feasible and considered appropriate. Third, given the variability in cEEG duration, care must be taken to determine the proportion of EEG or cEEG that is clinically indicated, with any additional recording presented to families and funded as clinical research. Fourth, there are considerable differences in cEEG review frequency and since time to detection of NCS (duration of NCS) may be an important component of outcome, standardization of review parameters will be important.
This study aimed to describe clinical practice in adults and children, but not neonates. Pediatric neurologists were asked to focus on management in the pediatric and not neonatal intensive care units since there is already an extensive literature related to neonatal seizures that has demonstrated variability in practice [29]. In contrast, there has been little study of cEEG or seizure management in critically ill children.
There are several important limitations to this study. First, this study did not actually track cEEG use or management decisions and merely asked respondents for their opinion, and there may be differences in reported use of cEEG and NCS management and actual practice. Second, although this survey demonstrates frequent use of cEEG and NCS management, it is possible that physicians involved in this type of work were more likely to complete the survey than physicians with other specialties or interests. The fact that all respondents had cEEG available strongly suggests that this is the case, and that the sample is biased towards those with more experience and interest in cEEG. Further, the fact that most respondents practice in academic or tertiary care setting suggests this group may not be representative of practice as a whole and that it may be inappropriate to generalize this data to all settings. Third, this study utilized primarily closed ended questions. While this allowed tabulation of results, many situations relating to the management of critically ill patients are complex and cannot be easily fit into a rigid management algorithm, so actual clinical practice may be more variable and intricate. Management of seizures might be affected by the underlying etiology, potential risk of further seizures, seizure frequency, and seizure duration, and this survey did not address these more complex but important clinical issues. Fourth, the survey was distributed to the members of several organizations and was not distributed to individual hospitals or epilepsy centers. Thus, multiple respondents may have been from the same center. This approach chosen since our aim was to assess individual practice and not to assess practice at only the larger epilepsy centers. However, had we instead asked one individual from each of certain epilepsy centers to complete the survey, the results may have been different and may have been less variable, since larger programs may have more clinical algorithms in place to standardize care among practitioners. Fifth, while organizations sent out our survey using their email lists, to protect members’ privacy we could not access the actual email address list and could not determine the total number of physicians who received the survey to calculate a survey response rate. Finally, compared to the proportion of adult and pediatric neurologists in practice, a large number of pediatric neurologists completed the survey. In an attempt to ensure we had sufficient pediatric respondents, we distributed the survey using the Child Neurology listhost, and this likely led to the high number of pediatric responses. However, respondents focused on adult and pediatric care had identical response profiles for all questions except for Propofol use. The fact that care is similar in adult and pediatric settings likely reflects that little data is available and so practitioners are making decisions based on both adult and pediatric data.
Our results indicate that while many neurologists often make decisions related to cEEG use and management of NCS and NCSE, substantial variability exists. Clarifying the optimal use of cEEG and management of NCS and NCSE is important and future study is needed, likely in the context of large prospective multicenter studies. While the variability in practice may make such a study complex to design, it may also portend high interest and participation.
Supplementary Material
Acknowledgments
The Child Neurology Society, American Epilepsy Society, American Clinical Neurophysiology Society, and the Critical Care EEG Consortium (funded by the American Epilepsy Society) all generously supported this work by sending group email messages to their member lists.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s12028-010-9337-2) contains supplementary material, which is available to authorized users.
Contributor Information
Nicholas S. Abend, Division of Neurology, The Children’s Hospital of Philadelphia, 34th Street and Civic Center Blvd, Philadelphia, PA 19104, USA
Dennis J. Dlugos, Division of Neurology, The Children’s Hospital of Philadelphia, 34th Street and Civic Center Blvd, Philadelphia, PA 19104, USA
Cecil D. Hahn, Division of Neurology, The Hospital For Sick Children, Toronto, Canada
Lawrence J. Hirsch, Comprehensive Epilepsy Center, Neurological Institute, Columbia University College of Physicians and Surgeons, New York, USA
Susan T. Herman, Department of Neurology, Beth Israel Deaconess Medical Center, Boston, USA
References
- 1.Friedman D, Claassen J, Hirsch LJ. Continuous electroencephalogram monitoring in the intensive care unit. Anesth Analg. 2009;109:506–23. doi: 10.1213/ane.0b013e3181a9d8b5. [DOI] [PubMed] [Google Scholar]
- 2.Kurtz P, Hanafy KA, Claassen J. Continuous EEG monitoring: is it ready for prime time? Curr Opin Crit Care. 2009;15:99–109. doi: 10.1097/MCC.0b013e3283294947. [DOI] [PubMed] [Google Scholar]
- 3.Claassen J, Mayer SA, Kowalski RG, Emerson RG, Hirsch LJ. Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology. 2004;62:1743–8. doi: 10.1212/01.wnl.0000125184.88797.62. [DOI] [PubMed] [Google Scholar]
- 4.Jette N, Claassen J, Emerson RG, Hirsch LJ. Frequency and predictors of nonconvulsive seizures during continuous electroencephalographic monitoring in critically ill children. Arch Neurol. 2006;63:1750–5. doi: 10.1001/archneur.63.12.1750. [DOI] [PubMed] [Google Scholar]
- 5.Vespa PM, Nenov V, Nuwer MR. Continuous EEG monitoring in the intensive care unit: early findings and clinical efficacy. J Clin Neurophysiol. 1999;16:1–13. doi: 10.1097/00004691-199901000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Young GB, Jordan KG, Doig GS. An assessment of nonconvulsive seizures in the intensive care unit using continuous EEG monitoring: an investigation of variables associated with mortality. Neurology. 1996;47:83–9. doi: 10.1212/wnl.47.1.83. [DOI] [PubMed] [Google Scholar]
- 7.Oddo M, Carrera E, Claassen J, Mayer SA, Hirsch LJ. Continuous electroencephalography in the medical intensive care unit. Crit Care Med. 2009;37:2051–6. doi: 10.1097/CCM.0b013e3181a00604. [DOI] [PubMed] [Google Scholar]
- 8.Carrera E, Claassen J, Oddo M, Emerson RG, Mayer SA, Hirsch LJ. Continuous electroencephalographic monitoring in critically ill patients with central nervous system infections. Arch Neurol. 2008;65:1612–8. doi: 10.1001/archneur.65.12.1612. [DOI] [PubMed] [Google Scholar]
- 9.Vespa PM, Miller C, McArthur D, et al. Nonconvulsive electrographic seizures after traumatic brain injury result in a delayed, prolonged increase in intracranial pressure and metabolic crisis. Crit Care Med. 2007;35:2830–6. [PMC free article] [PubMed] [Google Scholar]
- 10.Kilbride RD, Costello DJ, Chiappa KH. How seizure detection by continuous electroencephalographic monitoring affects the prescribing of antiepileptic medications. Arch Neurol. 2009;66:723–8. doi: 10.1001/archneurol.2009.100. [DOI] [PubMed] [Google Scholar]
- 11.Privitera M, Hoffman M, Moore JL, Jester D. EEG detection of nontonic-clonic status epilepticus in patients with altered consciousness. Epilepsy Res. 1994;18:155–66. doi: 10.1016/0920-1211(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 12.Jordan KG. Neurophysiologic monitoring in the neuroscience intensive care unit. Neurol Clin. 1995;13:579–626. [PubMed] [Google Scholar]
- 13.DeLorenzo RJ, Waterhouse EJ, Towne AR, et al. Persistent nonconvulsive status epilepticus after the control of convulsive status epilepticus. Epilepsia. 1998;39:833–40. doi: 10.1111/j.1528-1157.1998.tb01177.x. [DOI] [PubMed] [Google Scholar]
- 14.Towne AR, Waterhouse EJ, Boggs JG, et al. Prevalence of nonconvulsive status epilepticus in comatose patients. Neurology. 2000;54:340–5. doi: 10.1212/wnl.54.2.340. [DOI] [PubMed] [Google Scholar]
- 15.Pandian JD, Cascino GD, So EL, Manno E, Fulgham JR. Digital video-electroencephalographic monitoring in the neurological-neurosurgical intensive care unit: clinical features and outcome. Arch Neurol. 2004;61:1090–4. doi: 10.1001/archneur.61.7.1090. [DOI] [PubMed] [Google Scholar]
- 16.Claassen J, Jette N, Chum F, et al. Electrographic seizures and periodic discharges after intracerebral hemorrhage. Neurology. 2007;69:1356–65. doi: 10.1212/01.wnl.0000281664.02615.6c. [DOI] [PubMed] [Google Scholar]
- 17.Dennis LJ, Claassen J, Hirsch LJ, Emerson RG, Connolly ES, Mayer SA. Nonconvulsive status epilepticus after subarachnoid hemorrhage. Neurosurgery. 2002;51:1136–43. doi: 10.1097/00006123-200211000-00006. discussion 44. [DOI] [PubMed] [Google Scholar]
- 18.Hyllienmark L, Amark P. Continuous EEG monitoring in a paediatric intensive care unit. Eur J Paediatr Neurol. 2007;11:70–5. doi: 10.1016/j.ejpn.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Hosain SA, Solomon GE, Kobylarz EJ. Electroencephalographic patterns in unresponsive pediatric patients. Pediatr Neurol. 2005;32:162–5. doi: 10.1016/j.pediatrneurol.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 20.Abend NS, Topjian A, Herman ST, et al. Electroencephalographic monitoring during hypothermia after pediatric cardiac arrest. Neurology. 2009;72:1931–40. doi: 10.1212/WNL.0b013e3181a82687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abend NS, Dlugos DJ. Nonconvulsive status epilepticus in a pediatric intensive care unit. Pediatr Neurol. 2007;37:165–70. doi: 10.1016/j.pediatrneurol.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 22.Saengpattrachai M, Sharma R, Hunjan A, et al. Nonconvulsive seizures in the pediatric intensive care unit: etiology, EEG, and brain imaging findings. Epilepsia. 2006;47:1510–8. doi: 10.1111/j.1528-1167.2006.00624.x. [DOI] [PubMed] [Google Scholar]
- 23.Alehan FK, Morton LD, Pellock JM. Utility of electroencephalography in the pediatric emergency department. J Child Neurol. 2001;16:484–7. doi: 10.1177/088307380101600704. [DOI] [PubMed] [Google Scholar]
- 24.Tay SK, Hirsch LJ, Leary L, Jette N, Wittman J, Akman CI. Nonconvulsive status epilepticus in children: clinical and EEG characteristics. Epilepsia. 2006;47:1504–9. doi: 10.1111/j.1528-1167.2006.00623.x. [DOI] [PubMed] [Google Scholar]
- 25.Walker MC. Treatment of nonconvulsive status epilepticus. Int Rev Neurobiol. 2007;81:287–97. doi: 10.1016/S0074-7742(06)81019-6. [DOI] [PubMed] [Google Scholar]
- 26.Chong DJ, Hirsch LJ. Which EEG patterns warrant treatment in the critically ill? Reviewing the evidence for treatment of periodic epileptiform discharges and related patterns. J Clin Neurophysiol. 2005;22:79–91. doi: 10.1097/01.wnp.0000158699.78529.af. [DOI] [PubMed] [Google Scholar]
- 27.San-Juan OD, Chiappa KH, Costello DJ, Cole AJ. Periodic epileptiform discharges in hypoxic encephalopathy: BiPLEDs and GPEDs as a poor prognosis for survival. Seizure. 2009;18(5):365–8. doi: 10.1016/j.seizure.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Claassen J, Hirsch LJ, Frontera JA, et al. Prognostic significance of continuous EEG monitoring in patients with poor-grade subarachnoid hemorrhage. Neurocrit Care. 2006;4:103–12. doi: 10.1385/NCC:4:2:103. [DOI] [PubMed] [Google Scholar]
- 29.Bartha AI, Shen J, Katz KH, et al. Neonatal seizures: multicenter variability in current treatment practices. Pediatr Neurol. 2007;37:85–90. doi: 10.1016/j.pediatrneurol.2007.04.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.