Abstract
The lipids of Treponema innocens, type strain B256, formerly considered a nonpathogenic isolate of T. hyodysenteriae, have been analyzed and compared with the lipids of T. hyodysenteriae. The lipids of T. innocens comprised 16% of the cell dry weight. Polar lipids amounted to about two-thirds of the total lipids and consisted of 61.9% phospholipids and 38.1% glycolipid. Neutral lipids consisted mainly of sterols. The phospholipids were principally phosphatidylglycerol, phosphatidylcholine, and cardiolipin. Minor amounts of lysophosphatidylcholine, sphingomoyelin, and a relatively nonpolar, unidentified phospholipid were present. The latter lipid has not been detected in T. hyodysenteriae. The glycolipid fraction of T. innocens contained a single component, monoglucosyldiglyceride, in contrast to the occurrence in T. hyodysenteriae of two components: monogallactosyldiglyceride and a less-polar glycolipid tentatively identified as acylmonogalactosyldiglyceride (the additional acyl moieties being 86.6% acetyl, 11.6% propionyl, and 1.6% n-butyryl groups). Alk-1-enyl ether analogs comprised 24.6% of the total phospholipids and glycolipid of T. innocens, or about one-third of the amount in T. hyodysenteriae. The acyl and alk-1-enyl moieties of T. innocens consisted of greater than or equal to 92% of 14:0, iso-15:0, and 16:0 chains. In contrast to T. hyodysenteriae, anteiso-15:0 moieties were not detected, and a reversed distribution of 14:0 and iso-15:0 alk-1-enyl moieties occurred in the two species.
Full text
PDF
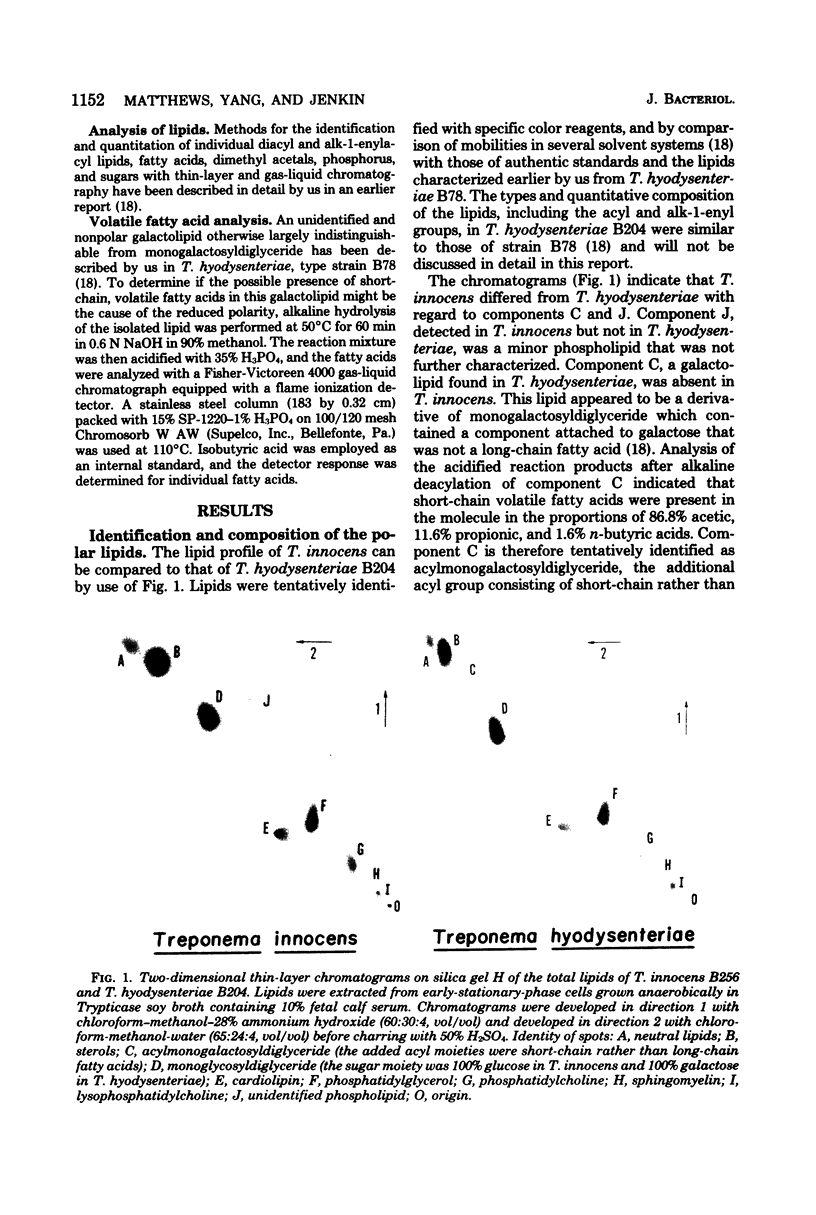

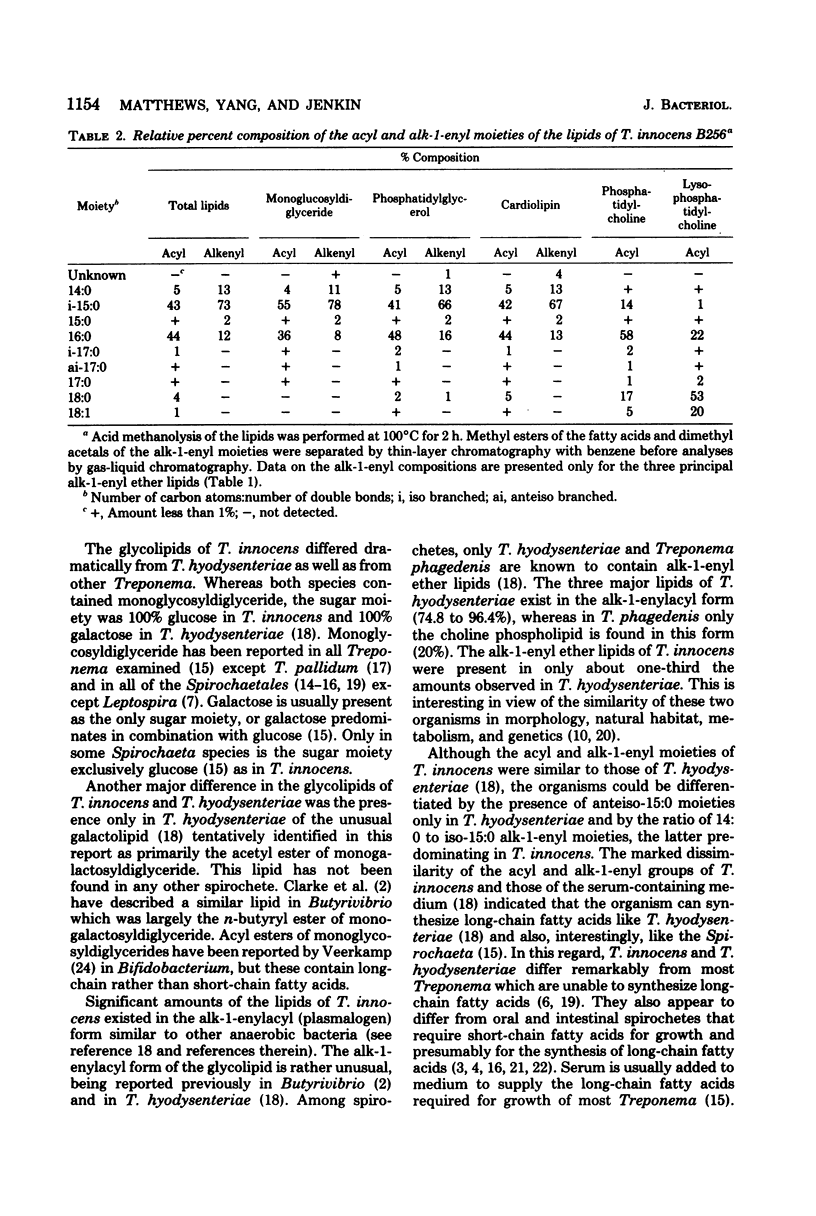

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baum D. H., Joens L. A. Partial purification of a specific antigen of Treponema hyodysenteriae. Infect Immun. 1979 Dec;26(3):1211–1213. doi: 10.1128/iai.26.3.1211-1213.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke N. G., Hazlewood G. P., Dawson R. M. Novel lipids of Butyrivibrio spp. Chem Phys Lipids. 1976 Oct;17(2-3):222–232. doi: 10.1016/0009-3084(76)90067-0. [DOI] [PubMed] [Google Scholar]
- Cwyk W. M., Canale-Parola E. Treponema succinifaciens sp. nov., an anaerobic spirochete from the swine intestine. Arch Microbiol. 1979 Sep;122(3):231–239. doi: 10.1007/BF00411285. [DOI] [PubMed] [Google Scholar]
- Hardy P. H., Jr, Munro C. O. Nutritional requirements of anaerobic spirochetes. I. Demonstration of isobutyrate and bicarbonate as growth factors for a strain of Treponema microdentium. J Bacteriol. 1966 Jan;91(1):27–32. doi: 10.1128/jb.91.1.27-32.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris D. L., Glock R. D., Christensen C. R., Kinyon J. M. Inoculation of pigs with Treponema hyodysenteriae (new species) and reproduction f the disease. Vet Med Small Anim Clin. 1972 Jan;67(1):61–64. [PubMed] [Google Scholar]
- Johnson R. C., Livermore B. P., Jenkin H. M., Eggebraten L. Lipids of Treponema pallidum Kazan 5. Infect Immun. 1970 Nov;2(5):606–609. doi: 10.1128/iai.2.5.606-609.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Livermore B. P., Walby J. K., Jenkin H. M. Lipids of parasitic and saprophytic leptospires. Infect Immun. 1970 Sep;2(3):286–291. doi: 10.1128/iai.2.3.286-291.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kean E. L. Separation of gluco- and galactocerebrosides by means of borate thin-layer chromatography. J Lipid Res. 1966 May;7(3):449–452. [PubMed] [Google Scholar]
- Kinyon J. M., Harris D. L., Glock R. D. Enteropathogenicity of various isolates of Treponema hyodysenteriae. Infect Immun. 1977 Feb;15(2):638–646. doi: 10.1128/iai.15.2.638-646.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinyon J. M., Harris D. L. Growth in Treponema hyodysenteriae in liquid medium. Vet Rec. 1974 Sep 7;95(10):219–220. doi: 10.1136/vr.95.10.219. [DOI] [PubMed] [Google Scholar]
- Lemcke R. M., Burrows M. R. A disc growth-inhibition test for differentiating Treponema hyodysenteriae from other intestinal spirochaetes. Vet Rec. 1979 Jun 16;104(24):548–551. doi: 10.1136/vr.104.24.548. [DOI] [PubMed] [Google Scholar]
- Lemcke R. M., Burrows M. R. Sterol requirement for the growth of Treponema hyodysenteriae. J Gen Microbiol. 1980 Feb;116(2):539–543. doi: 10.1099/00221287-116-2-539. [DOI] [PubMed] [Google Scholar]
- Livermore B. P., Bey R. F., Johnson R. C. Lipid metabolism of Borrelia hermsi. Infect Immun. 1978 Apr;20(1):215–220. doi: 10.1128/iai.20.1.215-220.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livermore B. P., Johnson R. C. Lipids of the Spirochaetales: comparison of the lipids of several members of the genera Spirochaeta, Treponema, and Leptospira. J Bacteriol. 1974 Dec;120(3):1268–1273. doi: 10.1128/jb.120.3.1268-1273.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livermore B. P., Johnson R. C. The lipids of four unusual non-pathogenic host-associated spirochetes. Can J Microbiol. 1975 Nov;21(11):1877–1880. doi: 10.1139/m75-271. [DOI] [PubMed] [Google Scholar]
- Matthews H. M., Yang T. K., Jenkin H. M. Alk-1-enyl ether phospholipids (plasmalogens) and glycolipids of Treponema hyodysenteriae. Analysis of acyl and alk-1-enyl moieties. Biochim Biophys Acta. 1980 May 28;618(2):273–281. doi: 10.1016/0005-2760(80)90033-8. [DOI] [PubMed] [Google Scholar]
- Matthews H. M., Yang T. K., Jenkin H. M. Unique lipid composition of Treponema pallidum (Nichols virulent strain). Infect Immun. 1979 Jun;24(3):713–719. doi: 10.1128/iai.24.3.713-719.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer H., Meyer F. Lipid metabolism in the parasitic and free-living spirochetes Treponema pallidum (Reiter) and Treponema zuelzerae. Biochim Biophys Acta. 1971 Feb 2;231(1):93–106. doi: 10.1016/0005-2760(71)90257-8. [DOI] [PubMed] [Google Scholar]
- Miao R. M., Fieldsteel A. H., Harris D. L. Genetics of Treponema: characterization of Treponema hyodysenteriae and its relationship to Treponema pallidum. Infect Immun. 1978 Dec;22(3):736–739. doi: 10.1128/iai.22.3.736-739.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOCRANSKY S. S., LOESCHE W. J., HUBERSAK C., MACDONALD J. B. DEPENDENCY OF TREPONEMA MICRODENTIUM ON OTHER ORAL ORGANISMS FOR ISOBUTYRATE, POLYAMINES, AND A CONTROLLED OXIDATION-REDUCTION POTENTIAL. J Bacteriol. 1964 Jul;88:200–209. doi: 10.1128/jb.88.1.200-209.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smibert R. M., Claterbaugh R. L., Jr A chemically defined medium for Treponema strain PR-7 isolated from the intestine of a pig with swine dysentery. Can J Microbiol. 1972 Jul;18(7):1073–1078. doi: 10.1139/m72-166. [DOI] [PubMed] [Google Scholar]
- Taylor D. J., Alexander T. J. The production of dysentery in swine by feeding cultures containing a spirochaete. Br Vet J. 1971 Nov;127(11):58–61. doi: 10.1016/s0007-1935(17)37282-2. [DOI] [PubMed] [Google Scholar]
- Veerkamp J. H. Biochemical changes in Bifidobacterium bifidum var. pennsylvanicus after cell wall inhibition. V. Structure of the galactosyldiglycerides. Biochim Biophys Acta. 1972 Jul 19;273(2):359–367. doi: 10.1016/0304-4165(72)90227-9. [DOI] [PubMed] [Google Scholar]
- Whipp S. C., Robinson I. M., Harris D. L., Glock R. D., Matthews P. J., Alexander T. J. Pathogenic synergism between Treponema hyodysenteriae and other selected anaerobes in gnotobiotic pigs. Infect Immun. 1979 Dec;26(3):1042–1047. doi: 10.1128/iai.26.3.1042-1047.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]