Abstract
While the influence of caffeine on the regulation of brain perfusion has been the subject of multiple publications, the mechanisms involved in that regulation remain unclear. To some extent, that uncertainty is a function of a complex interplay of processes arising from multiple targets of caffeine located on a variety of different cells, many of which have influence, either directly or indirectly, on cerebral vascular smooth muscle tone. Adding to that complexity are the target-specific functional changes that may occur when comparing acute and chronic caffeine exposure. In the present review, we discuss some of the mechanisms behind caffeine influences on cerebrovascular function. The major effects of caffeine on the cerebral circulation can largely be ascribed to its inhibitory effects on adenosine receptors. Herein, we focus mostly on the A1, A2A, and A2B subtypes located in cells comprising the neurovascular unit (neurons, astrocytes, vascular smooth muscle); their roles in the coupling of increased neuronal (synaptic) activity to vasodilation; how caffeine, through blockade of these receptors, may interfere with the “neurovascular coupling” process; and receptor-linked changes that may occur in cerebrovascular regulation when comparing acute to chronic caffeine intake.
Keywords: Adenosine, arteriole, astrocyte, calcium, neurovascular coupling, synapse, vasodilation
INTRODUCTION
The widely-consumed psychostimulant, caffeine (1,3,7-trimethylxanthine), displays a broad array of actions on the brain. There are multiple targets of caffeine, giving rise to a high level of complexity that can confound experimental efforts to understand caffeine influences in the brain. Despite such “impediments”, a substantial body of literature has been accumulated in recent years that has provided some insights into the mechanisms of caffeine influence in the brain, with important clinical/translational implications. One example of the latter would be Alzheimer's disease (AD), as supported by evidence suggesting a positive influence of long-term caffeine intake on cognitive function in patients, especially memory and learning (e.g. [1]). One possible contributing factor to the onset and progression of cognitive impairment in AD, is vascular dysfunction. In particular, this relates to pathologic changes in cerebral vascular tissue linked to accumulation of amyloid-β peptide (Aβ) in and around cerebral blood vessels, resulting in a condition labeled cerebral amyloid angiopathy (CAA). Some of the characteristics of CAA that are thought to contribute to exacerbation of AD pathology include impaired neurovascular coupling, cerebral hypoperfusion and hypercontractility, loss of cholinergic innervation, blood-brain-barrier damage, and microvascular ruptures [2–5]. Of some relevance to the present discussion, Arendash and co-workers [6] recently published compelling data showing that chronic caffeine consumption was associated with reductions in cerebral Aβ levels, and cognitive improvement in AD mice. Since increased presence of Aβ may impair cerebral vascular function (see above), it is tempting to postulate that caffeine could limit the severity of CAA and, perhaps, mitigate cognitive decline, via a vasculoprotective effect. However, such a mechanism remains to be established. Thus, in the remainder of this review, no further consideration will be given to caffeine-modulated vascular-AD linkages. Instead, we will focus primarily on acute and chronic caffeine influences on hemodynamic function, with some emphasis on neurovascular coupling (functional hyperemia).
CAFFEINE TARGETS RELATED TO CEREBRAL VASCULAR CONTROL
Caffeine influence on cerebral perfusion is likely to involve its interactions with targets in vascular cells (e.g., smooth muscle; endothelium) as well as non-vascular cells (e.g., neurons; astrocytes; microglia). The literature points to at least 4 targets of caffeine in the brain – adenosine receptors, cyclic nucleotide phospodiesterases, ryanodine receptors, and GABAA receptors. With exception of the ryanodine receptors (activation), caffeine's influence on the above targets is inhibitory. Realistically, within the range of daily human caffeine consumption, among the caffeine-sensitive entities listed above, only the adenosine receptors may have any relevance, since caffeine possesses 1–2 orders of magnitude less potency toward the other targets listed [7]. Thus, the present review will focus only on caffeine effects on adenosine receptors and the implications of that interaction with respect to neurovascular coupling in particular.
NEUROVASCULAR COUPLING AND THE CONCEPT OF THE NEUROVASCULAR UNIT
The tight coupling between neuronal activity and blood flow is fundamental to brain function. When a specific brain region is activated, cerebral blood flow increases in a temporally and spatially coordinated manner, thereby improving substrate (glucose, O2) delivery to meet local metabolic demands. Multiple signaling pathways have been shown to contribute to neurovascular coupling (reviewed in [8–11]). However, there appears to be a considerable degree of overlap in the mechanisms promoting the vasodilation accompanying increased neural activity, suggesting interactions among the signaling pathways. That complexity of the neurovascular signaling process, to a large degree, arises from the participation of multiple cells, principally neurons, astrocytes, and vascular cells (smooth muscle and endothelium), collectively termed the “neurovascular unit”. Astrocytes, in fact, may represent the linchpin in transducing increased synaptic activity to local vasodilation. The important physiologic function of astrocytes in sensing neuronal activity and in turn regulating the tone of cerebral arterioles has been addressed by a number of investigators [8–11]. Astrocytic endfeet extensively ensheath cerebral microvessels, to the extent that direct neuronal contacts to cerebral arterioles are sparse. Thus, astrocytes physically link neurons and their synapses with the vasculature, and are in a strategic position to convey neuronal signals to the blood vessels. As an example, neuronal activation can evoke increases in astrocytic [Ca2+], which in turn may trigger certain enzymes or cell membrane channels to generate or release vasoactive compounds. In this review, owing to its relevance to caffeine influences, we consider only one such vasodilating pathway, one that is related to adenosine. Clearly, there are other astrocytic Ca2+-linked vasodilating mechanisms, for example, involving K+ or arachidonic acid-derived mediators [8–11]. The overall importance of astrocyte Ca 2+ regulation to the neurovascular coupling process is reflected in the impairment of that process following inhibition of certain astrocyte Ca2+-related pathways [12, 13].
Increased synaptic activity has been linked to initiation of a propagated signal between astrocytes. Thus, a signal originating at the site of increased neural (synaptic) activity can be transmitted, over multiple astrocytes, to perivascular glial endfeet and elicit changes in arteriolar tone. Calcium, as reflected in the appearance of a “Ca2+ wave” capable of traversing multiple astrocytes, appears to be a vital component in this signaling process. Other factors that may play important roles in the transmission of a neuronally-initiated vasodilating signal, via astrocytes, include excitatory neurotransmitters (e.g., glutamate), K+, and ATP released by activated neurons (see [11]). There is evidence that ATP binds to purinergic receptors on neighboring astrocytes. These receptors are termed metabotropic purinergic receptors (labeled P2Y in Fig. 1) by virtue of their linkage to phospholipase C activation, inositol trisphosphate generation, and the well-described release of Ca2+ from intracellular stores (e.g. [14]). This appears to play an important role in initiating and propagating the Ca2+ wave. The appearance of ATP in the extracellular compartment also leads to rapid formation of adenosine. This is due to the ubiquitous presence of ecto-nucleotidases on astrocytic, vascular, and neuronal surfaces in the brain [15–19], particularly ecto-nucleoside triphosphate diphosphohydrolase-1 (E-NTPDase-1) and ecto-nucleotide pyrophosphatase/phosphodiesterase (E-NPP), which mediate direct ATP to AMP conversions; and ecto-5’-nucleotidase, which catalyzes the subsequent formation of adenosine from AMP. In considering neurovascular coupling and the neurovascular unit, one cannot ignore the contributions from the pial arterioles that lie upstream from the parenchymal arterioles. Thus, although local dilation of parenchymal arterioles is important in adjusting nutrient supply to neuronal needs, that response could be ineffective in the absence of dilation in the pial arterioles. Indeed, during increased activity of cortical neurons, overlying pial arterioles dilate, despite the lack of any direct contact with activated neurons. In a recent report, we showed that astrocytes provide a key link between increased neuronal activity in the brain parenchyma and the remote arteriolar relaxation represented by pial arterioles [20].
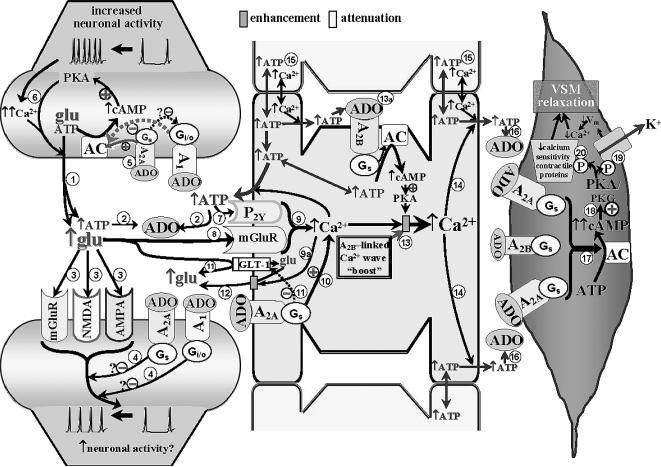
Fig. 1.
The neurovascular unit (represented by a synaptic, astrocytic, and vascular component) and the role of adenosine (ADO), via its receptors, in the coupling of enhanced neural activity to arteriolar vascular smooth muscle (VSM) relaxation. Synaptic Component. Increased axonal activity is characterized by enhanced Ca2+ entry and accumulation in presynaptic nerve terminals, promoting a Ca2+-dependent vesicular release of, for example, glutamate (glu) and ATP from the presynaptic terminal into the synaptic cleft . The released ATP can be rapidly converted to adenosine (ADO) via ectonucleotidases
. The released ATP can be rapidly converted to adenosine (ADO) via ectonucleotidases . The increased ADO can engage A1 and A2A receptors on pre- and post-synaptic membranes, and it can interact with A2A and A2B receptors on adjacent astrocytes (see below). Although A1 receptors may be expressed on astrocytes and blood vessels, in the Fig. 1 model, those sites are not assigned any functional significance (see Table 1). The glutamate released from the presynaptic terminal can effect post-synaptic activation via engaging metabotropic (mGluR) or ionotropic (NMDA and AMPA) receptors on post-synaptic dendrites
. The increased ADO can engage A1 and A2A receptors on pre- and post-synaptic membranes, and it can interact with A2A and A2B receptors on adjacent astrocytes (see below). Although A1 receptors may be expressed on astrocytes and blood vessels, in the Fig. 1 model, those sites are not assigned any functional significance (see Table 1). The glutamate released from the presynaptic terminal can effect post-synaptic activation via engaging metabotropic (mGluR) or ionotropic (NMDA and AMPA) receptors on post-synaptic dendrites . Post-synaptic activation of A1 and A2A receptors has been associated with repression of glutamate-linked post-synaptic function [22]. This could act as a “brake” on trans-synaptic signaling
. Post-synaptic activation of A1 and A2A receptors has been associated with repression of glutamate-linked post-synaptic function [22]. This could act as a “brake” on trans-synaptic signaling . The patterns of A1 and A2A receptor expression, as well as the neurotransmitters they modulate, vary among brain structures. Based upon information obtained from cerebrocortical synaptosomes (where evidence indicates the presence of both A1 and A2A receptor-mediated modulation of glutamate release [32,33]), the model depicted in Fig. 1 (and Fig. 2) could be taken to represent cerebral cortex. The figure depicts the presence of A1 and A2A receptors in close association with one another in the presynaptic nerve ending. This “heteromeric” arrangement represents one of several possibilities, including scenarios where the A1 or A2A receptor subtype predominates. In the heteromeric arrangement, it has been postulated that the Gs-linked A2A receptor not only will activate adenylyl cyclase (AC), but also, via a PKA-independent mechanism [32,33], prevent the Gi/o-linked A1 receptor from inhibiting AC, especially under conditions of increased neuronal activity and ADO availability [35]
. The patterns of A1 and A2A receptor expression, as well as the neurotransmitters they modulate, vary among brain structures. Based upon information obtained from cerebrocortical synaptosomes (where evidence indicates the presence of both A1 and A2A receptor-mediated modulation of glutamate release [32,33]), the model depicted in Fig. 1 (and Fig. 2) could be taken to represent cerebral cortex. The figure depicts the presence of A1 and A2A receptors in close association with one another in the presynaptic nerve ending. This “heteromeric” arrangement represents one of several possibilities, including scenarios where the A1 or A2A receptor subtype predominates. In the heteromeric arrangement, it has been postulated that the Gs-linked A2A receptor not only will activate adenylyl cyclase (AC), but also, via a PKA-independent mechanism [32,33], prevent the Gi/o-linked A1 receptor from inhibiting AC, especially under conditions of increased neuronal activity and ADO availability [35] . One consequence of this will be a PKA-driven increased Ca2+ influx at the presynaptic membrane, overcoming A1 receptor-linked depression of voltage-dependent Ca2+ entry [33], thereby potentiating Ca2+-dependent glutamate/ATP release and extracellular ADO generation
. One consequence of this will be a PKA-driven increased Ca2+ influx at the presynaptic membrane, overcoming A1 receptor-linked depression of voltage-dependent Ca2+ entry [33], thereby potentiating Ca2+-dependent glutamate/ATP release and extracellular ADO generation . Astrocytic Component. The released ATP and glutamate can interact with astrocyte metabotropic P2Y receptors
. Astrocytic Component. The released ATP and glutamate can interact with astrocyte metabotropic P2Y receptors and mGluR's
and mGluR's , respectively, leading to mobilization of Ca2+ from intracellular storage sites within astrocytes
, respectively, leading to mobilization of Ca2+ from intracellular storage sites within astrocytes . In addition, the increased presence of ADO, arising from the released ATP, activates A2A receptors on astrocytes leading to cAMP/PKA-dependent mobilization of intracellular Ca2+ from cellular stores
. In addition, the increased presence of ADO, arising from the released ATP, activates A2A receptors on astrocytes leading to cAMP/PKA-dependent mobilization of intracellular Ca2+ from cellular stores . Adenosine interaction with astrocytic A2A receptors also can contribute to blockade of the astrocytic glutamate import protein, GLT-1
. Adenosine interaction with astrocytic A2A receptors also can contribute to blockade of the astrocytic glutamate import protein, GLT-1 ; and promote Ca2+-dependent
; and promote Ca2+-dependent enhancement of glutamate efflux
enhancement of glutamate efflux . This should result in further elevations in glutamate levels in the synaptic cleft, as well as contributing to the astrocytic “Ca2+ wave”. The figure also speculates that a PKA-linked “boost” to the astrocytic Ca2+ mobilization
. This should result in further elevations in glutamate levels in the synaptic cleft, as well as contributing to the astrocytic “Ca2+ wave”. The figure also speculates that a PKA-linked “boost” to the astrocytic Ca2+ mobilization may arise from ADO binding to Gs-linked A2B receptors
may arise from ADO binding to Gs-linked A2B receptors . The “wave” of Ca2+ generated by the combined influences of glutamatergic, purinergic P2Y, and adenosinergic mechanisms will ultimately promote ATP release from astrocytes, including remote sites
. The “wave” of Ca2+ generated by the combined influences of glutamatergic, purinergic P2Y, and adenosinergic mechanisms will ultimately promote ATP release from astrocytes, including remote sites . ATP represents an important signaling molecule in astrocytes. It arises from cellular glucose and O2 metabolism and can diffuse (along with Ca2+) from astrocyte to astrocyte through gap junctions
. ATP represents an important signaling molecule in astrocytes. It arises from cellular glucose and O2 metabolism and can diffuse (along with Ca2+) from astrocyte to astrocyte through gap junctions . Additionally, ATP represents perhaps the most important molecule involved in inter-astrocytic communication. Thus, Ca2+-dependent release of ATP from one astrocyte interacts with P2Y receptors on adjacent astrocytes, contributing to the spread of the Ca2+ wave. Arteriolar Component. The release of ATP in the vicinity of arterioles is likely to result in rapid formation of ADO
. Additionally, ATP represents perhaps the most important molecule involved in inter-astrocytic communication. Thus, Ca2+-dependent release of ATP from one astrocyte interacts with P2Y receptors on adjacent astrocytes, contributing to the spread of the Ca2+ wave. Arteriolar Component. The release of ATP in the vicinity of arterioles is likely to result in rapid formation of ADO and interactions with smooth muscle A2 receptors. There is little doubt that cerebral arterioles are well-endowed with A2 receptors. Both A2 subtypes are likely to be present on cerebral resistance vessels; although the literature seems to favor the A2A receptor, especially in intraparenchymal and pial arterioles [24,50]. This is reflected in the figure. Principally, A2 activation generates cAMP
and interactions with smooth muscle A2 receptors. There is little doubt that cerebral arterioles are well-endowed with A2 receptors. Both A2 subtypes are likely to be present on cerebral resistance vessels; although the literature seems to favor the A2A receptor, especially in intraparenchymal and pial arterioles [24,50]. This is reflected in the figure. Principally, A2 activation generates cAMP , which is not only capable of activating PKA, but cGMP-dependent protein kinase (PKG) as well [46,70]
, which is not only capable of activating PKA, but cGMP-dependent protein kinase (PKG) as well [46,70] . The increased kinase function is associated with phosphorylation and opening of K+ channels
. The increased kinase function is associated with phosphorylation and opening of K+ channels , leading to smooth muscle cell hyperpolarization (↓Vm). This lowers intracellular Ca2+ levels through a reduction in Ca2+ influx via voltage-operated Ca2+ channels. Elevated PKA/PKG function also is accompanied by a reduction in the Ca2+-sensitivity of contractile proteins (e.g., myosin
, leading to smooth muscle cell hyperpolarization (↓Vm). This lowers intracellular Ca2+ levels through a reduction in Ca2+ influx via voltage-operated Ca2+ channels. Elevated PKA/PKG function also is accompanied by a reduction in the Ca2+-sensitivity of contractile proteins (e.g., myosin ). The combination of reduced VSM Ca2+ levels and diminished sensitivity to Ca2+ leads to relaxation. See text for further discussion and additional citations.
). The combination of reduced VSM Ca2+ levels and diminished sensitivity to Ca2+ leads to relaxation. See text for further discussion and additional citations.
ADENOSINE RECEPTORS AND NEUROVASCULAR COUPLING
The role of adenosine and its receptors in the coupling of increased neuronal/synaptic activity to dilation of arterioles and arteries, and how caffeine might influence that important physiologic response, is the central theme of this review. To facilitate the following discussion of this rather complex process, we have created two cartoons representing neurovascular coupling in the absence (Fig. 1) and presence (Fig. 2) of acute caffeine exposure. In constructing these cartoons, a number of simplifying assumptions were made regarding the interplay among the major components of the neurovascular unit (synapse, astrocyte, and arteriole), adenosinergic mechanisms, and the targets of caffeine. Those assumptions, along with their rationale, supporting evidence, and caveats are summarized in Table 1.
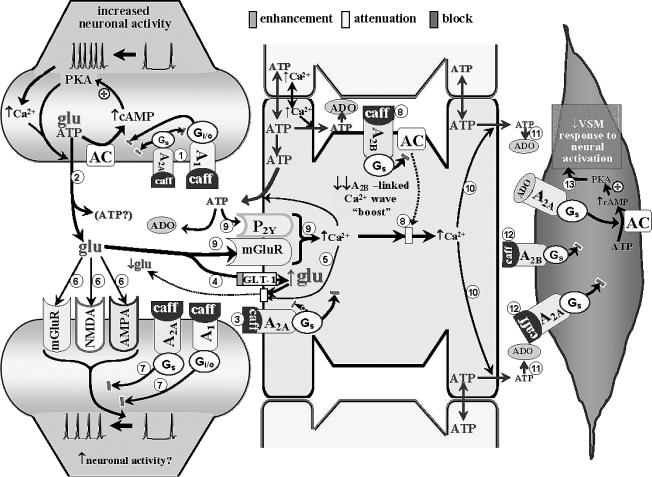
Fig. 2.
Scheme depicting diminished neurovascular coupling during acute caffeine exposure. There are several potential manifestations related to caffeine restriction of ADO interactions with A1 and A2A receptors in presynaptic nerve endings. The net effect of that “multi-receptor blockade” on glutamate (and ATP) release during increased synaptic activity depends on the A1/A2A configuration. In an A1 receptor-dominant presynaptic configuration, the presence of caffeine should remove the postulated A1 receptor-related block of voltage-dependent Ca2+ entry (e.g. [71]), thus increasing Ca2+-mediated glutamate and ATP release. In an A2A receptor-dominant presynaptic scenario (probably the least likely), caffeine blockade is likely to attenuate glutamate release during states of increased neuronal activity. In Fig. 2, the heteromeric A1/A2A receptor arrangement is represented . Although precise predictions are not possible, caffeine-related blockade of both receptors might be associated with modest or no changes in activity-evoked glutamate and ATP release
. Although precise predictions are not possible, caffeine-related blockade of both receptors might be associated with modest or no changes in activity-evoked glutamate and ATP release . Further reductions in glutamate presence in the synaptic cleft may arise from caffeine blockade of astrocytic A2A receptors
. Further reductions in glutamate presence in the synaptic cleft may arise from caffeine blockade of astrocytic A2A receptors – leading to disinhibition of GLT-1 (permitting greater uptake of glutamate from the synaptic cleft)
– leading to disinhibition of GLT-1 (permitting greater uptake of glutamate from the synaptic cleft) and diminished A2A receptor-mediated Ca2+-dependent glutamate efflux
and diminished A2A receptor-mediated Ca2+-dependent glutamate efflux . A net reduction in glutamate levels in the synaptic cleft could restrict the generation of glutamate receptor-mediated post-synaptic activation
. A net reduction in glutamate levels in the synaptic cleft could restrict the generation of glutamate receptor-mediated post-synaptic activation . Yet, the presence of caffeine will also prevent ADO-linked post-synaptic depression mediated through activation of A1 and A2A receptors
. Yet, the presence of caffeine will also prevent ADO-linked post-synaptic depression mediated through activation of A1 and A2A receptors . This may permit some post-synaptic activation to occur. Moreover, caffeine blockade of astrocytic A2A and A2B receptors
. This may permit some post-synaptic activation to occur. Moreover, caffeine blockade of astrocytic A2A and A2B receptors
 , along with the diminished contributions from metabotropic purinergic and glutamatergic receptors (arising from reduced extracellular ATP and glutamate levels
, along with the diminished contributions from metabotropic purinergic and glutamatergic receptors (arising from reduced extracellular ATP and glutamate levels ), a large reduction in the capacity to generate a Ca2+ wave might be expected
), a large reduction in the capacity to generate a Ca2+ wave might be expected , resulting in less ADO being “presented” to VSM cells
, resulting in less ADO being “presented” to VSM cells . The diminished ADO exposure, combined with caffeine blockade of VSM A receptors
. The diminished ADO exposure, combined with caffeine blockade of VSM A receptors , leaves little capacity remaining for ADO-mediated vasodilation. The potentially diminished capacity to effect “remote” increases in extracellular ADO could also interfere with heterosynaptic influences (see Fig. 1 legend); although the lack of relevant information does not permit any further discussion of this matter. The above speculation provides some (although not the only) possible explanations for the finding that acute caffeine administration profoundly attenuates the in vivo dilation of pial arterioles
, leaves little capacity remaining for ADO-mediated vasodilation. The potentially diminished capacity to effect “remote” increases in extracellular ADO could also interfere with heterosynaptic influences (see Fig. 1 legend); although the lack of relevant information does not permit any further discussion of this matter. The above speculation provides some (although not the only) possible explanations for the finding that acute caffeine administration profoundly attenuates the in vivo dilation of pial arterioles accompanying somatosensory activation in rats [54].
accompanying somatosensory activation in rats [54].
Table 1.
| Simplifying assumption | Rationale/ supporting evidence/caveats |
|---|---|
| A1, A2A, and A2B receptors possess similar sensitivities to caffeine blockade. | Only applies to conditions of enhanced synaptic activity, where endogenous adenosine rises to levels sufficient to activate low-affinity A2B receptors [7]. |
| A3 receptors are not considered. | Limited cerebral A3 expression [27]; no direct vascular actions of A3 receptor ligands [24]; order-of-magnitude less sensitive to caffeine vs A1, A2A, and A2B receptors [7]. |
| Caffeine blockade of A1 receptors primarily reflects actions toward presynaptic sites; although post-synaptic sites cannot be ignored. | Although A1 receptors exist on post-synaptic membranes, presynaptic expression may predominate [21]. A major consequence of presynaptic A1 receptor activation is inhibition of neurotransmitter release [22]. Post-synaptic A1 receptor activation may hyperpolarize post-synaptic elements, further restricting synaptic signaling. |
| Astrocytic A1 receptors are not considered. | Synaptic A1 receptor expression exceeds astrocytic expression [21]; reports are inconsistent regarding the manifestations of astrocytic A1 receptor activation (e.g., intracellular Ca2+ regulation [25,26]). |
| Cerebral arterioles are devoid of functional A1 receptor activity. | Direct applications of A1 receptor ligands are without affect on cerebral arterial/arteriolar tone [24]. |
| Caffeine influence on neurovascular coupling is likely to involve blockade of A2A receptors expressed on pre- and post-synaptic membranes, astrocytes, and vascular cells. | Presynaptic A2A receptor engagement can promote glutamate release both directly and via restricting A1 receptor effects. Adenosine A2A (and A1) receptors on post-synaptic membranes are thought to depress glutamate receptor-mediated post-synaptic activation [22]. Post-synaptic expression of A2A receptors may be of some importance in "heterosynaptic" regulation (see text), but is not assigned a major role in the synapse → astrocyte → arteriole signaling pathway. |
| Caffeine blockade of A2B receptors, during synaptic activation states, primarily reflects actions toward astrocytic and vascular sites. | Neuronal A2B receptor expression is sparse [38,39]. There is good evidence of A2B receptor expression in astrocytes [22]. Although controversial, cerebral artery/arteriolar A2B receptor expression and function may be less extensive than A2A receptors [8,24,50]. |
Adenosine receptors, also referred to as purinergic P1 receptors, consist of four known subtypes (A1, A2A, A2B, and A3). These receptors are coupled to either Gi/o (A1 and A3) or Gs (A2A and A2B) proteins, which, upon engagement with adenosine, results in inhibition or enhancement of adenylyl cyclase activity, respectively. Among the adenosine receptors, the A1 receptor is the most regionally widespread in the brain. Evidence indicates that the A1 receptor is enriched in synaptic membranes in relation to other cellular elements [21], which is consistent with its purported neuromodulatory role. A prime example of that role is the ability of A1 receptor activation to restrict presynaptic neurotransmitter release [22]. Furthermore, A1 receptor agonists have been reported to have a far greater effect toward restricting glutamate vs GABA release, indicating that presynaptic A1 activation is primarily anti-excitatory [22]. Although some cerebrovascular presence of A1 receptors has been documented [23], its functional significance is unclear, since direct applications of A1 ligands do not affect cerebral arterial/arteriolar tone [24]. The A1 receptor is expressed in astrocytes [25,26]. However, results from in vitro models (primary cultures, acutely isolated astrocytes, or brain slices) have not yielded consistent findings. That is, A1 receptor activation has been associated with PLC-linked intracellular Ca2+ elevations in some situations [25], but repression of Ca2+ influx in others (e.g. [26]). The A3 receptor also is expressed in brain tissue, but with a limited distribution [27]. Although some expression of A3 receptors has been found in brain blood vessels [23], like the A1 receptor, it does not appear to be associated with any direct hemodynamic function [24]. The A3 receptor has also been detected in astrocyte in vitro preparations [25], and may be involved in the regulation of astrocyte intracellular Ca2+ [28].
The high affinity, A2A, and low affinity, A2B, receptors are expressed in cerebral resistance vessels (see, for example [24]). Despite its lower adenosine affinity, recent findings [29] suggested that the A2B receptor plays a significant role in physiologic cerebral vasodilation. Some have speculated that the A2A and A2B receptors are differentially distributed within the cerebral arterial/arteriolar system, with A2A receptors predominating in the upstream pial arterial/arteriolar segments and A2B having greater influence in the downstream intraparenchymal segments [8]. However, others have reported a substantial A2A receptor participation in adenosine-induced dilations in rat brain parenchymal arterioles [24]. Irrespective of segmental localization, adenosine engagement of the vascular A2 receptors promotes vasodilation. Like A1 receptors, A2A receptors are found in presynaptic structures, but with a more limited regional distribution compared to A1 receptors [30]. In rodents, the A2A subtype exhibits the highest expression in the striatum, in keeping with its association with dopamine-rich structures, with comparatively low abundance (relative to A1) in the cortex and hippocampus (e.g. [31]). Nevertheless, the existence of A2A receptors in the cortex is not insignificant, in light of evidence that glutamate release in rat cortical synaptosomes is enhanced by A2A receptor activation [32,33]. In contrast to the hippocampus [30] and striatum [34], the possibility of a cortical A1/A2A colocalization has not been confirmed via immunohistochemistry. Nevertheless, the presence of both A1 and A2A receptors in cortical synaptic structures can be inferred from findings that evoked glutamate release from cortical synaptosomes exhibits a marked sensitivity to manipulations of A1 and A2A receptor function [32, 33]. One postulated manifestation of colocalization is the “heteromeric” A1/A2A arrangement [31]. Thus, the higher levels of extracellular adenosine that are likely to be associated with conditions of elevated neuronal activity are thought to favor increased A2A receptor function [35]. The activated A2A receptor can, in turn, effect an inhibition of A1 receptor function, via a mechanism involving protein kinase C, rather than PKA [36, 37]. The A2A receptor effect could also include a more conventional PKA-dependent mechanism that involves elevated presynaptic Ca2+ entry [22]. The end result of these A2A receptor-related actions is enhancement of glutamate release. Experimental evidence, obtained in synaptosomal preparations of rat cortex, hippocampus, and striatum, support an A2A receptor crosstalk repression of A1 receptor synaptic function that occurs across multiple cerebral structures [36,37].
With respect to A2B receptors, evidence indicates a rather sparse neuronal presence in the brain [38,39]. Although A2B receptors are reasonably well-expressed in astrocytes and cerebral vascular cells [8,22,24,40], in view of the low affinity of A2B receptors toward adenosine, any functional relevance may be limited to conditions of elevated extracellular adenosine presence, as may be found during higher neuronal activity states. The model depicted in Fig. 1 represents such a state. Therefore, A2B receptors on astrocytes and vascular smooth muscle are given consideration (see also Table 1).
In astrocytes, at least two functions for A2A receptors have been identified (see Fig. 1). Both of these functions are likely linked to activation of adenylyl cyclase and enhanced cAMP-dependent protein kinase (PKA)-mediated phosphorylation. The first involves restriction of glutamate uptake, via inhibition of the astrocytic GLT-1 transporter [41]. The second A2A receptor function relates to PKA-mediated release of Ca2+ from astrocytic stores [42]. This not only can contribute to trans-astrocytic signaling (i.e., the “Ca2+ wave”), but also promote Ca2+-dependent glutamate and ATP efflux (e.g. [41,43–45]). This will yield further elevations of glutamate and ATP in the synaptic cleft, potentiating neurotransmission [25,40] and providing more ATP for extracellular adenosine generation. It also has been suggested that adenosine engagement of astrocytic A2B receptors may provide a “boost” for the astrocytic Ca2+ wave during neural activation states (see review by Koehler et al. [8]). The A2 receptor-associated potentiation of astrocytic signaling, provided by a more robust Ca2+ wave, will ultimately enhance release of astrocytic ATP in the vicinity of arterioles. The ATP can then be rapidly converted to adenosine, which interacts with arteriolar smooth muscle A2 receptors. The subsequent Gs-mediated activation of smooth muscle adenylyl cyclase will increase intracellular cAMP levels and activate PKA (and, perhaps, cGMP-dependent protein kinase [PKG] [46]), which in turn, can phosphorylate targets within the smooth muscle cells. One target is K+ channels [47], leading to K+ efflux, thereby promoting smooth muscle hyperpolarization and reduced intracellular Ca2+ levels. Another key group of phosphorylatable targets are myosin light chain phosphatase, myosin light chain kinase, and RhoA, with the result being a reduced sensitivity of contractile proteins to Ca2+ [46,48]. It should be emphasized that the signaling scheme provided by Fig. 1 applies both to local dilation of parenchymal arterioles, involving a single astrocyte, or upstream pial arteriolar dilation involving Ca2+/ATP (and perhaps K+) – linked communication over multiple astrocytes (see [11,20]).
It is of some interest to note that if one replaces the vascular smooth muscle cell in Fig. 1 with another synapse, interactions of adenosine with its receptors in that synapse could lead to synaptic depression. Such a process might be found in the “heterosynaptic depression” model proposed by Cunha [31]. Thus, an astrocytic Ca2+ wave, arising as the result of synaptic activation at one locus, will ultimately result in the release of ATP and subsequent generation of adenosine at a remote astrocytic site. If that adenosine contacts an arteriole, vasodilation (neurovascular coupling) will occur. If another synapse is contacted instead, synaptic depression could occur. One might speculate that a possible contribution to that heterosynaptic depression could arise not only from the presence of A1 receptors presynaptically, but also from the presence of A1 and A2A receptors on post-synaptic membranes of these distal synapses. Post-synaptic A1 and A2A receptors are thought, if anything, to be associated with attenuation of post-synaptic excitation (e.g., glutamatergic [22,49]). Thus, adenosine-related attenuation of post-synaptic function at remote (and even proximal) synapses could act to limit the spread of electrical activity from extending much beyond the area of initial activation. This might be viewed as an efficient way to ensure sufficient perfusion and substrate delivery to active neurons while limiting the amount of electrical “noise” (see Table 1). However, it merits mention that distribution of adenosine receptors between pre-and post-synaptic membranes may exhibit regional variations. For example, for A2A receptors, evidence suggests post-synaptic localization being favored in the striatum, while a distribution favoring the presynaptic membrane appears to be the case in the cortex [49]. How such differences might affect heterosynaptic depression remains to be determined.
ACUTE CAFFEINE EFFECTS ON NEUROVASCULAR COUPLING
A scheme summarizing acute caffeine effects on neural activation-evoked arteriolar dilation is presented in Fig. 2. A number of the simplifying assumptions listed in Table 1 relate specifically to caffeine influences. First, we assumed a roughly equivalent caffeine potency toward blocking A1, A2A, and A2B receptors [7]. However, despite that similarity, the A2B receptors, owing to their low adenosine affinity, may be affected by caffeine only under conditions of enhanced neuronal (synaptic) activity, where endogenous adenosine levels are likely to be elevated. Since Fig. 2, like Fig. 1, depicts an increased synaptic activity state, caffeine influences on A2B receptors are included. Second, possible contributions from A3 receptors are not considered. This is due to limited A3 receptor distribution in the brain [27] and an apparent caffeine inhibitory potency toward the A3 receptor that is lower by approximately one order of magnitude, in relation to the other 3 receptors [7]. Third, we postulated that caffeine effects toward A1 receptors primarily involve preventing presynaptic manifestations of A1 receptor activation (i.e., the capacity to restrict neurotransmitter release); although post-synaptic A1 receptor blockade is given some consideration [49]. Fourth, when examining the effects of caffeine-mediated blockade of A2A receptors on arteriolar responses during neuronal activation states, we took into account pre- and post-synaptic, astrocytic, and vascular smooth muscle A2A receptor sites (see earlier). For A2B receptor-related influences of caffeine on arteriolar tone, under conditions of increased synaptic activity, it seems reasonable to limit the focus to blockade of astrocytic and smooth muscle A2B receptors.
Nevertheless, in the context of neurovascular coupling, the assumption of an A1 receptor effect of caffeine being largely confined to presynaptic elements is bolstered by findings that topical application of the A1 receptor antagonist, DPCPX, actually potentiates pial arteriolar dilations evoked by sciatic nerve stimulation in rats [50]. This might be explained on the basis of an A1 receptor-linked repression of neurotransmitter (e.g., glutamate) release, during increased axonal activity, acting as a “brake” on synaptic function. The upshot of this is that A1 receptor blockade will be accompanied by enhanced synaptic activity. In fact, there appears to be a significant A1 receptor-related tonic inhibition of synaptic transmission [51]. This could account for the well-known arousal effects associated with caffeine consumption. However, caffeine-induced arousal was reported to be absent in A2A receptor knockout mice [52]. Although this seems to imply an important role for A2A receptor inhibition in the arousal response to caffeine, those findings have been questioned in a recent report [53]. On the other hand, acute caffeine administration in rats constricts pial arterioles [54] and lowers CBF in rats [55] and human subjects [56,57]. This could be explained by the caffeine-induced loss of a basal A2 receptor-associated vasodilating “tone”. The importance of a vascular (presumably A2 receptor) site for the vasoconstrictive actions of caffeine is underscored by findings showing that direct application of caffeine was accompanied by a ~70% reduction in adenosine-induced dilation of isolated rat brain arterioles [54]. The acute effects of caffeine on vascular tone and CBF not only may be related to the presence of A2A and A2B receptors on vascular smooth muscle, but also on astrocytes. Thus, especially under conditions of elevated synaptic activity, blockade of astrocytic A2 receptors could disrupt Ca2+ signaling mechanisms, diminishing ATP release, and reducing the amount of adenosine to which arterioles are exposed. Furthermore, one also cannot ignore effects arising from caffeine blockade of A2A (and A1) receptors on synaptic membranes. During enhanced axonal activity, A2A receptor-mediated facilitation may override A1 receptor-mediated repression of glutamate release from nerve endings [31]. In the presence of caffeine, which blocks both receptors, it is difficult to predict what the net effect on glutamate release will be. Caffeine-associated blockade of astrocytic A2A receptors, as depicted in Fig. 2, may favor net glutamate uptake into astrocytes, reducing glutamate levels in the synaptic cleft. Thus, there is a measure of uncertainty regarding the net effect of caffeine on activation of post-synaptic glutamate receptors. Adding to the uncertainty, caffeine will also block post-synaptic A1 and A2A receptors [22]. Since this could remove adenosine-associated inhibitory influences on glutamate-related post-synaptic function, the overall net effect could be enhanced post-synaptic activity. It is tempting to speculate that caffeine blockade of post-synaptic adenosine receptors might enhance the post-synaptic electrical response to glutamate during states of elevated neural activity. This might lead to a greater spread of electrical “noise” beyond the initial site of increased axonal firing, perhaps contributing to increased heterosynaptic activity and caffeine-related arousal. Thus, the ability to maintain or increase neural activity, in the face of direct vasoconstrictive actions (caffeine blockade of VSM A2 receptors) is consistent with findings showing neurovascular “uncoupling” in rats and humans given acute caffeine treatment [54,56,64].
CHRONIC CAFFEINE EXPOSURE AND CEREBRAL PERFUSION
Although there is agreement among studies, with respect to the vasoconstrictive, CBF-lowering actions of acute caffeine treatments, the effects of chronic caffeine exposure on cerebral perfusion cannot be easily generalized. There are a number of factors one might consider regarding cerebrovascular regulation in conjunction with chronic caffeine use. Certainly, adenosine-linked factors merit prime consideration. This includes (but is not limited to), changes in adenosine receptor expression and agonist sensitivity associated with caffeine tolerance. One example is the upregulation of platelet A2A receptor expression observed in human subjects following chronic caffeine exposure [58]. One could also consider how variations in adenosine receptor distribution, within the cerebral arterial system and the other components of the neurovascular unit, may impact on caffeine effects. A number of publications appearing over the past two decades point to cerebral A1 (but not A2A) receptor upregulation and sensitization in rodents in association with chronic caffeine intake (reviewed in [59]). Consistent with a lack of influence of chronic caffeine intake on A2A receptor function, one study in rats revealed that the tolerance that develops toward the striatal motor stimulant effects of caffeine, with chronic caffeine intake, could not be recapitulated with prolonged administration of a selective antagonist of A2A receptors [60]. However, the results of other investigations showed seemingly opposite effects of chronic caffeine treatment. Thus, an enhanced A2A receptor ligand binding in the striatum was reported in one study [61], while a downregulation of striatal A2A mRNA and protein expression was observed in another [62]. Adding to the uncertainty, Arendash and co-workers [63] reported no changes in A2A and A1 expression in hippocampus and frontal cortex of mice following chronic caffeine intake. While this might reflect regional selectivity in adenosine receptor responses to chronic caffeine exposure, it should be noted that the results in the latter study were obtained in AβPP transgenic mice, and, thus, should be viewed with some caution.
Another potentially interesting aspect of the role of adenosine receptors in caffeine tolerance development relates to the postulated presence of presynaptic A1-A2A receptor heterodimers in the brain [31], as touched upon earlier. In one postulated scenario [35], at least in the striatum, receptor tolerance to chronic caffeine exposure affects A2A more than A1 receptors. This relative enhancement of A2A receptor function could affect further inhibition of caffeine-repressed A1 receptors. In this way, chronic caffeine exposure would favor a greater A2A over A1 receptor function and enhanced presynaptic activity, as opposed to caffeine-naïve rats. It might be speculated that such a scheme could be associated with a partial recovery of the repressed neurovascular coupling accompanying acute caffeine exposure. However, this co-localization of adenosine receptors is not uniformly distributed among brain regions and may simply function as a limited local “fine-tuning” mechanism [30,31,34]. Furthermore, the potential impact of A1-A2A receptor heterodimerization as a facilitator of neurovascular coupling may only be manifested in higher brain activation states [31]. In summary, there remains a high level of uncertainty surrounding this issue, and further study is clearly warranted.
However, none of the studies cited above provided evidence regarding expression or functional changes in vascular-specific adenosine receptor populations. Based upon the earlier discussion, it is difficult to envisage chronic caffeine effects that do not involve vascular smooth muscle A2 receptors (see [54]). Indeed, it has been suggested that the increased CBF that occurs in human subjects within 24 h of caffeine withdrawal results from adenosine binding to a more sensitized population of receptors or a greater abundance of cerebral arterial receptors (see [65,66] for references). Due to the lack of evidence supporting any direct adenosine A1 receptor effects on cerebral arteriolar tone [54], one might ascribe the apparent “upregulation” of adenosine receptor function to vascular A2 receptors. Furthermore, in human subjects, a partial (or incomplete) tolerance to acute caffeine-related CBF reductions was found in association with chronic caffeine intake [66, 67]. While this could be a reflection of A2A receptor up-regulation (as postulated by Addicott et al. [65]), in the absence of any data regarding A2 receptor expression or ligand affinity in cerebral arteries and arterioles, one cannot eliminate the possibility that these chronic caffeine effects may be related to adenosine receptors residing in astrocytes or neurons. One also cannot ignore the role of altered abundance of non-adenosinergic receptor populations. For example, increased expression of brain tissue acetylcholine, serotonin, and GABAA receptors has been documented in the mouse in conjunction with chronic caffeine ingestion [59,68]. As such, there is the possibility that chronic caffeine effects on neurovascular coupling (or vascular function in general) may arise from altered expression of nonadenosinergic receptors, or even ion channels [59,68], residing in one or more of the cellular elements of the neurovascular unit. These and related issues will make interesting subjects for future experimentation.
CONCLUDING REMARKS
In conclusion, caffeine can have multiple effects on cerebral arteries and arterioles, resulting in a complex influence on brain perfusion both under resting (basal) conditions and during states of increased synaptic activity (neural activation-induced hyperemia). At the levels of caffeine achieved in the circulation and brain during normal human consumption, caffeine is known to exert measurable effects on CBF. Those effects can be largely attributed to actions toward adenosine receptors and not other potential targets of caffeine, such as phosphodiesterases and ryanodine receptors. Both animal and human studies have indicated that acute caffeine exposure leads to constriction of cerebral vessels and reduced CBF, as well as diminished neurovascular coupling. It can be postulated that those effects of caffeine likely arise from inhibitory actions on A1, A2A, and A2B receptors distributed among the cellular components of the neurovascular unit – namely, neurons, astrocytes and vascular smooth muscle. The principal site of acute caffeine influence on A1 receptors is likely presynaptic nerve endings. Yet, if this were the only target of caffeine to affect neurovascular coupling, one should observe an enhanced cerebral vasodilating response to neural/synaptic activation, as was reported by Meno et al. [50] in the presence of an A1 receptor antagonist. Since caffeine suppresses neurovascular coupling (e.g., in the cortex [54]), it is probable that this reflects caffeine actions toward A2A receptors, and, perhaps to a lesser extent, A2B receptors. The A2 receptors contributing to the caffeine effect are likely to be found in multiple cellular sites. Indeed, A2A receptors have been identified in presynaptic, post-synaptic, astrocytic, and vascular smooth muscle sites; whereas A2B receptors are, at the least, expressed in astrocytes and vascular smooth muscle cells.
Attempts to understand the effects of chronic caffeine intake on cerebral hemodynamics have been confronted with more uncertainty. A principal problem here arises from a lack of consensus with respect to changes in the expression and/or adenosine sensitivity of A1 vs A2 receptors in association with prolonged exposure to an antagonist, like caffeine. While we cannot ignore A1 receptor changes, there is some indirect evidence to suggest that vascular A2A receptors might selectively upregulate during chronic caffeine exposure. This could explain the repeated observation that acute caffeine withdrawal from chronic caffeine ingestion is associated with an increase in CBF – one that is directly proportional to the daily caffeine intake immediately preceding the onset of abstinence (e.g. [69]). However, further experimentation is clearly warranted. Such experimentation could include, in animal models, a simple verification of whether there is at least a trend toward normalization of an acutely depressed neurovascular coupling in the presence of continuous caffeine intake. In association with such studies, one might consider the use of conditional knockouts to characterize the participation of adenosine receptors associated with specific cellular elements within the neurovascular unit.
ACKNOWLEDGMENTS
This work was supported by grant number HL088259 from the NIH (DAP); the American Heart Association (HLX); and the Juvenile Diabetes Research Foundation (FV).
Authors’ disclosures available online (http://www.jalz.com/disclosures/view.php?id=247).
REFERENCES
- 1.van Gelder BM, Buijsse B, Tijhuis M, Kalmijn S, Giampaoli S, Nissinen A, Kromhout D. Coffee consumption is inversely associated with cognitive decline in elderly European men: the FINE Study. Eur J Clin Nutr. 2007;61:226–232. doi: 10.1038/sj.ejcn.1602495. [DOI] [PubMed] [Google Scholar]
- 2.Bell RD, Zlokovic BV. Neurovascular mechanisms and blood-brain barrier disorder in Alzheimer's disease. Acta Neuropathol. 2009;118:103–113. doi: 10.1007/s00401-009-0522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen X, Gawryluk JW, Wagener JF, Ghribi O, Geiger JD. Caffeine blocks disruption of blood brain barrier in a rabbit model of Alzheimer's disease. J Neuroinflammation. 2008;5:12. doi: 10.1186/1742-2094-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iadecola C, Park L, Capone C. Threats to the mind: aging, amyloid, and hypertension. Stroke. 2009;40:S40–S44. doi: 10.1161/STROKEAHA.108.533638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benarroch EE. Neurovascular unit dysfunction: a vascular component of Alzheimer disease? Neurology. 2007;68:1730–1732. doi: 10.1212/01.wnl.0000264502.92649.ab. [DOI] [PubMed] [Google Scholar]
- 6.Arendash GW, Mori T, Cao C, Mamcarz M, Runfeldt M, Dickson A, Rezai-Zadeh K, Tan J, Citron BA, Lin X, Echeverria V, Potter H. Caffeine reverses cognitive impairment and decreases brain amyloid-beta levels in aged Alzheimer's disease mice. J Alzheimers Dis. 2009;17:661–680. doi: 10.3233/JAD-2009-1087. [DOI] [PubMed] [Google Scholar]
- 7.Fredholm BB, Battig K, Holmen J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev. 1999;51:83–133. [PubMed] [Google Scholar]
- 8.Koehler RC, Gebremedhin D, Harder DR. Role of astrocytes in cerebrovascular regulation. J Appl Physiol. 2006;100:307–317. doi: 10.1152/japplphysiol.00938.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koehler RC, Roman RJ, Harder DR. Astrocytes and the regulation of cerebral blood flow. Trends Neurosci. 2009;32:160–169. doi: 10.1016/j.tins.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10:1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- 11.Paulson OB, Hasselbalch SG, Rostrup E, Knudsen GM, Pelligrino D. Cerebral blood flow response to functional activation. J Cereb Blood Flow Metab. 2010;30:2–14. doi: 10.1038/jcbfm.2009.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alkayed NJ, Birks EK, Narayanan J, Petrie KA, Kohlercabot AE, Harder DR. Role of p-450 arachidonic acid epoxygenase in the response of cerebral blood flow to glutamate in rats. Stroke. 1997;28:1066–1072. doi: 10.1161/01.str.28.5.1066. [DOI] [PubMed] [Google Scholar]
- 13.Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]
- 14.Franke H, Krugel U, Illes P. P2 receptors and neuronal injury. Pflugers Arch. 2006;452:622–644. doi: 10.1007/s00424-006-0071-8. [DOI] [PubMed] [Google Scholar]
- 15.Volonté C, D'Ambrosi N. Membrane compartments and purinergic signalling: the purinome, a complex interplay among ligands, degrading enzymes, receptors and transporters. FEBS J. 2009;276:318–329. doi: 10.1111/j.1742-4658.2008.06793.x. [DOI] [PubMed] [Google Scholar]
- 16.Braun N, Sevigny J, Robson SC, Enjyoji K, Guckelberger O, Hammer K, Di Virgilio F, Zimmermann H. Assignment of ecto-nucleoside triphosphate diphosphohydrolase-1/cd39 expression to microglia and vasculature of the brain. Eur J Neurosci. 2000;12:4357–4366. [PubMed] [Google Scholar]
- 17.Grobben B, Anciaux K, Roymans D, Stefan C, Bollen M, Esmans EL, Slegers H. An ecto-nucleotide pyrophosphatase is one of the main enzymes involved in the extracellular metabolism of ATP in rat C6 glioma. J Neurochem. 1999;72:826–834. doi: 10.1046/j.1471-4159.1999.0720826.x. [DOI] [PubMed] [Google Scholar]
- 18.Langer D, Hammer K, Koszalka P, Schrader J, Robson S, Zimmermann H. Distribution of ectonucleotidases in the rodent brain revisited. Cell Tissue Res. 2008;334:199–217. doi: 10.1007/s00441-008-0681-x. [DOI] [PubMed] [Google Scholar]
- 19.Xu HL, Pelligrino DA. ATP release and hydrolysis contribute to rat pial arteriolar dilatation elicited by neuronal activation. Exp Physiol. 2007;92:647–651. doi: 10.1113/expphysiol.2006.036863. [DOI] [PubMed] [Google Scholar]
- 20.Xu HL, Mao L, Ye S, Paisansathan C, Vetri F, Pelligrino DA. Astrocytes are a key conduit for upstream signaling of vasodilation during cerebral cortical neuronal activation in vivo. Am J Physiol Heart Circ Physiol. 2008;294:H622–H632. doi: 10.1152/ajpheart.00530.2007. [DOI] [PubMed] [Google Scholar]
- 21.Rebola N, Pinheiro PC, Oliveira CR, Malva JO, Cunha RA. Subcellular localization of adenosine A1 receptors in nerve terminals and synapses of the rat hippocampus. Brain Res. 2003;987:49–58. doi: 10.1016/s0006-8993(03)03247-5. [DOI] [PubMed] [Google Scholar]
- 22.Stone TW, Ceruti S, Abbracchio MP. Adenosine receptors and neurological disease: neuroprotection and neurode-generation. Handb Exp Pharmacol. 2009;193:535–587. doi: 10.1007/978-3-540-89615-9_17. [DOI] [PubMed] [Google Scholar]
- 23.Di Tullio MA, Tayebati SK, Amenta F. Identification of adenosine A1 and A3 receptor subtypes in rat pial and intracerebral arteries. Neurosci Lett. 2004;366:48–52. doi: 10.1016/j.neulet.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Ngai AC, Coyne EF, Meno JR, West GA, Winn HR. Receptor subtypes mediating adenosine-induced dilation of cerebral arterioles. Am J Physiol Heart Circ Physiol. 2001;280:H2329–H2335. doi: 10.1152/ajpheart.2001.280.5.H2329. [DOI] [PubMed] [Google Scholar]
- 25.Verkhratsky A, Krishtal OA, Burnstock G. Purinoceptors on neuroglia. Mol Neurobiol. 2009;39:190–208. doi: 10.1007/s12035-009-8063-2. [DOI] [PubMed] [Google Scholar]
- 26.Alloisio S, Cugnoli C, Ferroni S, Nobile M. Differential modulation of ATP-induced calcium signalling by A1 and A2 adenosine receptors in cultured cortical astrocytes. Br J Pharmacol. 2004;141:935–942. doi: 10.1038/sj.bjp.0705707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borea PA, Gessi S, Bar-Yehuda S, Fishman P. A3 adenosine receptor: pharmacology and role in disease. Handb Exp Pharmacol. 2009;193:297–327. doi: 10.1007/978-3-540-89615-9_10. [DOI] [PubMed] [Google Scholar]
- 28.Chen Y, Rathbone MP, Hertz L. Guanosine-induced increase in free cytosolic calcium concentration in mouse astrocytes in primary cultures: does it act on an A3 adenosine receptor? J Neurosci Res. 2001;65:184–189. doi: 10.1002/jnr.1141. [DOI] [PubMed] [Google Scholar]
- 29.Kusano Y, Echeverry G, Miekisiak G, Kulik TB, Aronhime SN, Chen JF, Winn HR. Role of adenosine A2 receptors in regulation of cerebral blood flow during induced hypotension. J Cereb Blood Flow Metab. 2009 doi: 10.1038/jcbfm.2009.244. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rebola N, Rodrigues RJ, Lopes LV, Richardson PJ, Oliveira CR, Cunha RA. Adenosine A1 and A2A receptors are co-expressed in pyramidal neurons and co-localized in glutamatergic nerve terminals of the rat hippocampus. Neuroscience. 2005;133:79–83. doi: 10.1016/j.neuroscience.2005.01.054. [DOI] [PubMed] [Google Scholar]
- 31.Cunha RA. Different cellular sources and different roles of adenosine: A1 receptor-mediated inhibition through astrocytic-driven volume transmission and synapse-restricted A2A receptor-mediated facilitation of plasticity. Neurochem Int. 2008;52:65–72. doi: 10.1016/j.neuint.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 32.Marchi M, Raiteri L, Risso F, Vallarino A, Bonfanti A, Monopoli A, Ongini E, Raiteri M. Effects of adenosine A1 and A2A receptor activation on the evoked release of glutamate from rat cerebrocortical synaptosomes. Br J Pharmacol. 2002;136:434–440. doi: 10.1038/sj.bjp.0704712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang SJ. Caffeine facilitation of glutamate release from rat cerebral cortex nerve terminals (synaptosomes) through activation protein kinase C pathway: an interaction with presynaptic adenosine A1 receptors. Synapse. 2007;61:401–411. doi: 10.1002/syn.20384. [DOI] [PubMed] [Google Scholar]
- 34.Ciruela F, Casado V, Rodrigues RJ, Lujan R, Burgueno J, Canals M, Borycz J, Rebola N, Goldberg SR, Mallol J, Cortes A, Canela EI, Lopez-Gimenez JF, Milligan G, Lluis C, Cunha RA, Ferré S, Franco R. Presynaptic control of striatal glutamatergic neurotransmission by adenosine A1–A2A receptor heteromers. J Neurosci. 2006;26:2080–2087. doi: 10.1523/JNEUROSCI.3574-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferré S, Ciruela F, Borycz J, Solinas M, Quarta D, Antoniou K, Quiroz C, Justinova Z, Lluis C, Franco R, Goldberg SR. Adenosine A1 –A2A receptor heteromers: new targets for caffeine in the brain. Front Biosci. 2008;13:2391–2399. doi: 10.2741/2852. [DOI] [PubMed] [Google Scholar]
- 36.Lopes LV, Cunha RA, Ribeiro JA. Cross talk between A1 and A2A adenosine receptors in the hippocampus and cortex of young adult and old rats. J Neurophysiol. 1999;82:3196–3203. doi: 10.1152/jn.1999.82.6.3196. [DOI] [PubMed] [Google Scholar]
- 37.Dixon AK, Widdowson L, Richardson PJ. Desensitisation of the adenosine A1 receptor by the A2A receptor in the rat striatum. J Neurochem. 1997;69:315–321. doi: 10.1046/j.1471-4159.1997.69010315.x. [DOI] [PubMed] [Google Scholar]
- 38.Kessey K, Mogul DJ. Adenosine A2 receptors modulate hippocampal synaptic transmission via a cyclic-AMP-dependent pathway. Neuroscience. 1998;84:59–69. doi: 10.1016/s0306-4522(97)00504-6. [DOI] [PubMed] [Google Scholar]
- 39.Dixon AK, Gubitz AK, Sirinathsinghji DJ, Richardson PJ, Freeman TC. Tissue distribution of adenosine receptor mRNAs in the rat. Br J Pharmacol. 1996;118:1461–1468. doi: 10.1111/j.1476-5381.1996.tb15561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dare E, Schulte G, Karovic O, Hammarberg C, Fredholm BB. Modulation of glial cell functions by adenosine receptors. Physiol Behav. 2007;92:15–20. doi: 10.1016/j.physbeh.2007.05.031. [DOI] [PubMed] [Google Scholar]
- 41.Nishizaki T, Nagai K, Nomura T, Tada H, Kanno T, Tozaki H, Li XX, Kondoh T, Kodama N, Takahashi E, Sakai N, Tanaka K, Saito N. A new neuromodulatory pathway with a glial contribution mediated via A2a adenosine receptors. GLIA. 2002;39:133–147. doi: 10.1002/glia.10100. [DOI] [PubMed] [Google Scholar]
- 42.Nishizaki T. ATP- and adenosine-mediated signaling in the central nervous system: adenosine stimulates glutamate release from astrocytes via A2A adenosine receptors. J Pharmacol Sci. 2004;94:100–102. doi: 10.1254/jphs.94.100. [DOI] [PubMed] [Google Scholar]
- 43.Pryazhnikov E, Khiroug L. Sub-micromolar increase in [Ca2+]i triggers delayed exocytosis of ATP in cultured astrocytes. GLIA. 2008;56:38–49. doi: 10.1002/glia.20590. [DOI] [PubMed] [Google Scholar]
- 44.Blum AE, Joseph SM, Przybylski RJ, Dubyak GR. Rho-family GTPases modulate Ca2+ -dependent ATP release from astrocytes. Am J Physiol Cell Physiol. 2008;295:C231–C241. doi: 10.1152/ajpcell.00175.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Montana V, Malarkey EB, Verderio C, Matteoli M, Parpura V. Vesicular transmitter release from astrocytes. GLIA. 2006;54:700–715. doi: 10.1002/glia.20367. [DOI] [PubMed] [Google Scholar]
- 46.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- 47.Ko EA, Han J, Jung ID, Park WS. Physiological roles of K+ channels in vascular smooth muscle cells. J Smooth Muscle Res. 2008;44:65–81. doi: 10.1540/jsmr.44.65. [DOI] [PubMed] [Google Scholar]
- 48.Porter M, Evans MC, Miner AS, Berg KM, Ward KR, Ratz PH. Convergence of Ca2+-desensitizing mechanisms activated by forskolin and phenylephrine pretreatment, but not 8-bromo-cGMP. Am J Physiol Cell Physiol. 2006;290:C1552–C1559. doi: 10.1152/ajpcell.00534.2005. [DOI] [PubMed] [Google Scholar]
- 49.Fredholm BB, Chen JF, Cunha RA, Svenningsson P, Vaugeois JM. Adenosine and brain function. Int Rev Neurobiol. 2005;63:191–270. doi: 10.1016/S0074-7742(05)63007-3. [DOI] [PubMed] [Google Scholar]
- 50.Meno JR, Crum AV, Winn HR. Effect of adenosine receptor blockade on pial arteriolar dilation during sciatic nerve stimulation. Am J Physiol Heart Circ Physiol. 2001;281:H2018–H2027. doi: 10.1152/ajpheart.2001.281.5.H2018. [DOI] [PubMed] [Google Scholar]
- 51.Halassa MM, Fellin T, Haydon PG. Tripartite synapses: roles for astrocytic purines in the control of synaptic physiology and behavior. Neuropharmacology. 2009;57:343–346. doi: 10.1016/j.neuropharm.2009.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang ZL, Qu WM, Eguchi N, Chen JF, Schwarzschild MA, Fredholm BB, Urade Y, Hayaishi O. Adenosine A2A, but not A1, receptors mediate the arousal effect of caffeine. Nat Neurosci. 2005;8:858–859. doi: 10.1038/nn1491. [DOI] [PubMed] [Google Scholar]
- 53.Van Dort CJ, Baghdoyan HA, Lydic R. Adenosine A(1) and A(2A) receptors in mouse prefrontal cortex modulate acetylcholine release and behavioral arousal. J Neurosci. 2009;29:871–881. doi: 10.1523/JNEUROSCI.4111-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meno JR, Nguyen TS, Jensen EM, Alexander WG, Groysman L, Kung DK, Ngai AC, Britz GW, Winn HR. Effect of caffeine on cerebral blood flow response to somatosensory stimulation. J Cereb Blood Flow Metab. 2005;25:775–784. doi: 10.1038/sj.jcbfm.9600075. [DOI] [PubMed] [Google Scholar]
- 55.Gotoh J, Kuang TY, Nakao Y, Cohen DM, Melzer P, Itoh Y, Pak H, Pettigrew K, Sokoloff L. Regional differences in mechanisms of cerebral circulatory response to neuronal activation. Am J Physiol Heart Circ Physiol. 2001;280:H821–H829. doi: 10.1152/ajpheart.2001.280.2.H821. [DOI] [PubMed] [Google Scholar]
- 56.Chen Y, Parrish TB. Caffeine's effects on cerebrovascular reactivity and coupling between cerebral blood flow and oxygen metabolism. Neuroimage. 2009;44:647–652. doi: 10.1016/j.neuroimage.2008.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mathew RJ, Barr DL, Weinman ML. Caffeine and cerebral blood flow. Br J Psychiatry. 1983;143:604–608. doi: 10.1192/bjp.143.6.604. [DOI] [PubMed] [Google Scholar]
- 58.Varani K, Portaluppi F, Merighi S, Ongini E, Belardinelli L, Borea PA. Caffeine alters A2A adenosine receptors and their function in human platelets. Circulation. 1999;99:2499–2502. doi: 10.1161/01.cir.99.19.2499. [DOI] [PubMed] [Google Scholar]
- 59.Jacobson KA, von Lubitz DK, Daly JW, Fredholm BB. Adenosine receptor ligands: differences with acute versus chronic treatment. Trends Pharmacol Sci. 1996;17:108–113. doi: 10.1016/0165-6147(96)10002-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Popoli P, Reggio R, Pezzola A. Effects of SCH 58261, an adenosine A2A receptor antagonist, on quinpirole-induced turning in 6-hydroxydopamine-lesioned rats. Lack of tolerance after chronic caffeine intake. Neuropsychopharmacology. 2000;22:522–529. doi: 10.1016/S0893-133X(99)00144-X. [DOI] [PubMed] [Google Scholar]
- 61.Johansson B, Georgiev V, Lindstrom K, Fredholm BB. A1 and A2A adenosine receptors and A1 mRNA in mouse brain: effect of long-term caffeine treatment. Brain Res. 1997;762:153–164. doi: 10.1016/s0006-8993(97)00378-8. [DOI] [PubMed] [Google Scholar]
- 62.Singh S, Singh K, Gupta SP, Patel DK, Singh VK, Singh RK, Singh MP. Effect of caffeine on the expression of cytochrome P450 1A2, adenosine A2A receptor and dopamine transporter in control and 1-methyl 4-phenyl 1, 2, 3, 6-tetrahydropyridine treated mouse striatum. Brain Res. 2009;1283:115–126. doi: 10.1016/j.brainres.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 63.Arendash GW, Schleif W, Rezai-Zadeh K, Jackson EK, Zacharia LC, Cracchiolo JR, Shippy D, Tan J. Caffeine protects Alzheimer's mice against cognitive impairment and reduces brain beta-amyloid production. Neuroscience. 2006;142:941–952. doi: 10.1016/j.neuroscience.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 64.Perthen JE, Lansing AE, Liau J, Liu TT, Buxton RB. Caffeine-induced uncoupling of cerebral blood flow and oxygen metabolism: a calibrated BOLD fMRI study. Neuroimage. 2008;40:237–247. doi: 10.1016/j.neuroimage.2007.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Addicott MA, Yang LL, Peiffer AM, Burnett LR, Burdette JH, Chen MY, Hayasaka S, Kraft RA, Maldjian JA, Laurienti PJ. The effect of daily caffeine use on cerebral blood flow: How much caffeine can we tolerate? Hum Brain Mapp. 2009;30:3102–3114. doi: 10.1002/hbm.20732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sigmon SC, Herning RI, Better W, Cadet JL, Griffiths RR. Caffeine withdrawal, acute effects, tolerance, and absence of net beneficial effects of chronic administration: cerebral blood flow velocity, quantitative EEG, and subjective effects. Psychopharmacology (Berl) 2009;204:573–585. doi: 10.1007/s00213-009-1489-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Watson J, Deary I, Kerr D. Central and peripheral effects of sustained caffeine use: tolerance is incomplete. Br J Clin Pharmacol. 2002;54:400–406. doi: 10.1046/j.1365-2125.2002.01681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shi D, Daly JW. Chronic effects of xanthines on levels of central receptors in mice. Cell Mol Neurobiol. 1999;19:719–732. doi: 10.1023/A:1006901005925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Field AS, Laurienti PJ, Yen YF, Burdette JH, Moody DM. Dietary caffeine consumption and withdrawal: confounding variables in quantitative cerebral perfusion studies? Radiology. 2003;227:129–135. doi: 10.1148/radiol.2271012173. [DOI] [PubMed] [Google Scholar]
- 70.Pelligrino DA, Wang Q. Cyclic nucleotide crosstalk and the regulation of cerebral vasodilation. Prog Neurobiol. 1998;56:1–18. doi: 10.1016/s0301-0082(98)00009-4. [DOI] [PubMed] [Google Scholar]
- 71.Wu LG, Saggau P. Adenosine inhibits evoked synaptic transmission primarily by reducing presynaptic calcium influx in area CA1 of hippocampus. Neuron. 1994;12:1139–1148. doi: 10.1016/0896-6273(94)90321-2. [DOI] [PubMed] [Google Scholar]