Abstract
Belief in one’s ability to exert control over the environment and to produce desired results is essential for an individual’s well being. It has been repeatedly argued that the perception of control is not only desirable, but it is likely a psychological and biological necessity. In this article, we review the literature supporting this claim and present evidence for a biological basis for the need for control and for choice — that is, the means by which we exercise control over the environment. Converging evidence from animal research, clinical studies, and neuroimaging work suggest that the need for control is a biological imperative for survival, and a corticostriatal network is implicated as the neural substrate of this adaptive behavior.
The Significance of Choice
You have brains in your head,
You have feet in your shoes,
You can steer yourself in any direction you choose.
-- Dr. Seuss (Theodor Seuss Geisel—American children’s book writer and cartoonist)1
From Western philosophical and psychological theory to government constitutions and beloved children’s books, we are immersed in a vocabulary of “personal freedom” and “self-determination.” An important question is whether this societal emphasis is the cause or the consequence of the need to exercise personal autonomy. Some may argue that people feel entitled to be their own “deciders,” that is, to exercise personal autonomy, as a result of societal values that are acquired through social learning. However, converging evidence from diverse areas of research provide increasing support for a biological explanation for our need for control.
Individuals exercise control over the environment by making choices. These choices include complex and emotionally salient decisions that may occur only once in a lifetime (e.g. which university to attend), but also include basic perceptual decisions that occur hundreds of thousands of times every day (e.g. deciding where to focus your attention in the visual field). Although much of our behavior is elicited by environmental cues, and may be below the state of explicit awareness, all voluntary behavior involves choice nonetheless. Thus, to choose, is to express a preference, and to assert the self. Each choice – no matter how small – reinforces the perception of control and self-efficacy (see Figure 1), and removing choice likely undermines this adaptive belief.
Figure 1.
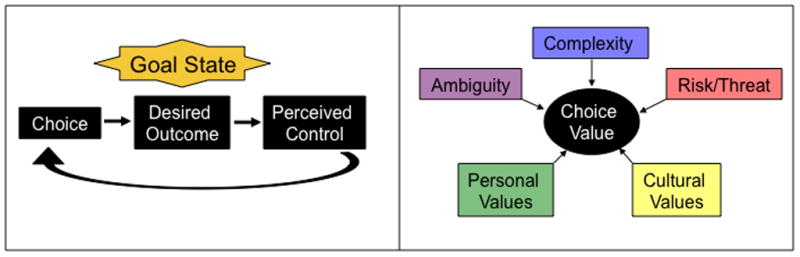
Choice is the vehicle for exercising control. (Left panel) For a given goal state, there is a desired outcome. When an individual chooses actions that lead to the desired outcome, the experienced contingency results in the perception of control. If the action results in reward (or avoids punishment), then the specific action is reinforced. However, the decision-making process itself (i.e. the opportunity for choice) is also reinforced. (Right panel) For any specific goal state, the value of the exercising control through choice may depend on certain personal and cultural values that may be learned, as well as situational factors, including the complexity of the choice, which may weigh heavily on cognitive resources (e.g. working memory), the ambiguity or uncertainty of choice outcomes, and whether or not potential risk or threat is involved.
Although there is extensive evidence that the perception of control is adaptive across diverse spheres of psychosocial functioning as well as physical health (see below), there have been no attempts to integrate these findings into a systematic review addressing why people desire choice and control. Here, we present evidence to support the claim that the need for control is biologically motivated, meaning that the biological bases for this need have been adaptively selected for evolutionary survival. First, we summarize the predominant contributions to our understanding of perceived control and its adaptive effects. We then present empirical evidence that the presence or absence of control has a profound impact on the regulation of emotion, cognition, and physiology. Finally, we examine the neural substrates of the need for control based on findings from animal studies, clinical populations, and neuroimaging research. Taken together, this evidence provides compelling support for a biological explanation of the need for choice
Choice is a Vehicle for Perceiving Control
If people did not believe they were capable of successfully producing desired results, there would be very little incentive to face even the slightest challenge. Thus, perception of control is likely adaptive for survival. The benefits of control beliefs have been reviewed extensively (Box 1), though less attention has been paid to understanding the benefits of behaviorally exercising control (but see 2). Opportunities to exercise control may be necessary to foster self-efficacy beliefs. Individuals with little experience of acting as an effective agent will likely have little belief in their ability to produce desired results, leading to feelings of helplessness and depression 3.
Text Box 1. Perceiving Control: Key Terms.
The perception that one is “in control” is a complex psychological phenomenon whose many facets have been described using various terms. Albert Bandura 73 has used terms such as “agency” and “self-efficacy” to describe the collective beliefs in one’s abilities to exert control over one’s environment and to act as an agent capable of producing desired results. A high sense of self-efficacy supports the construction of anticipation and beliefs of successful performance, and actual success on the anticipated task further strengthens beliefs of efficacy 74. In addition, research has shown that the stronger the perceived self-efficacy, the higher the goals people set for themselves 75. An extensive literature suggests that perceived self-efficacy is highly adaptive in many areas of psychosocial functioning 8, 76, 77, including work-related performance, psychosocial functioning in children and adolescents, academic achievement and persistence, and health functioning. Constructs related to self-efficacy have been proposed by others. Julian Rotter 78 coined the term “locus of control” to refer to an individual’s belief that life events are within personal control (internal locus of control), as opposed to a belief that events are uncontrollable (external locus of control). Ellen Langer 79 demonstrated the phenomenon of “illusion of control,” which is the assumption of personal control when there is no true control over the situation or event (e.g. believing you have a better chance of winning the lottery if you select the lucky numbers). More recently, Deci and Ryan have argued that “autonomy” and “self-determination”– terms describing an individual’s motivation to act as an independent and causal agent upon the environment – are fundamental psychological needs 77. While the theories behind each term have conceptual differences, they largely address the same underlying phenomenon and reach a common conclusion: the belief in one’s ability to exert control over the environment and to produce desired results is essential for an individual’s general well being.
Opportunities for choice have been shown to create the illusion of control (see Box 1). For example, healthy individuals tend to overestimate their personal control and ability to achieve success in chance situations involving choice 4, whereas depressed individuals are more accurate in judging the degree of personal control 5. When attempts to control events are unsuccessful, healthy individuals tend to rationalize outcomes rather than admit any compromise of personal control 6, 7. The findings of such research suggest that the illusion of control may protect individuals against the development of maladaptive cognitive and affective responses.
Additionally, the default assumption of control by healthy individuals when provided with opportunity for choice4 suggests that the assumption of control is adaptive. Violation of this assumption may result in abnormal behavior and compromised functioning. Individuals who do not perceive control over their environments may seek to gain control in any way possible, potentially engaging in maladaptive behaviors8. Furthermore, while the illusion of control seems to be adaptive for psychosocial functioning, extreme overestimates of control may contribute to dangerous risk-taking 9, 10. Struggles to augment or diminish control are believed to be at the core of anxiety and mood disorders, eating disorders, and substance abuse8. Therapeutic treatments from diverse theoretical perspectives focus on issues of control to promote patients’ well being11. Ultimately, the automatic perception of control seems to be essential for healthy functioning, whereas disruptions to perceived control can result in various manifestations of psychopathology.
Choice is Desirable
Choice allows organisms to exert control over the environment by selecting behaviors that are conducive to achieving desirable outcomes and avoiding undesirable outcomes. As a result, one would expect that opportunities to exercise choice would be desirable, in and of themselves (see Figure 1). In fact, animals and humans alike demonstrate a preference for choice over non-choice, even when that choice affords no improvement in outcome reward. For example, it has been found that when deciding between two options, animals 12, 13 and humans 14, 15 prefer the option that leads to a second choice over one that does not, even though the expected value of both options is the same, and making a second choice requires greater expenditure of energy. In economic terms, the preference for choice and control in such conditions may be considered irrational. Yet, if we consider that exercising control could be rewarding in and of itself, then such behavior could be considered rational for maximizing utility.
People report that tasks involving choice, however inconsequential, are more enjoyable than tasks without choice, and the provision of choice often leads to improved performance on a task 16. In a classic study by Langer & Rodin 17, even having a single opportunity to exercise choice was shown to influence subsequent mood, quality of life, and longevity in nursing-home residents. It seems that choice can induce greater feelings of confidence and success 18, 19, which likely further reinforces choice behavior and efficacy beliefs. Collectively, these findings imply that choice is rewarding per se.
In fact, simply expressing a preference, through choice, may reinforce an individual’s perception of control if choices are perceived as optimal for producing desired results. In an early variant of the now classic “free-choice” paradigm (Figure 2), subjects rated their liking for eight appliances and then decided which of two similarly rated appliances they would like to own 20. Subsequent ratings of all appliances showed that subjects tended to rate the chosen appliance higher and the rejected appliance lower than they had initially. This and many similar results established that choice can motivate attitude change, and spawned theories of post-choice rationalization 21 and self-perception 22 as processes that enacted the changes. More recent work suggests, however, that the subjective optimization of choice outcomes is a natural, and relatively automatic, byproduct of choice. This conclusion is supported by findings that that choice-induced attitude change a) occurs in preschool aged children and in monkeys 23 b) happens for amnesic patients who can’t explicitly remember that they made a choice 24 (which precludes post-choice rationalization), c) when choices are made under conditions of divided attention 24 (which precludes the use of controlled processes to change attitudes during the act of choosing), d) is predicted by choice-related activity in the striatum 25 (a region involved in reward processing and implicit learning), and e) is greater for happy as compared to unhappy individuals26 (who presumably are happy in part because they effectively make the best of their choices).
Figure 2.
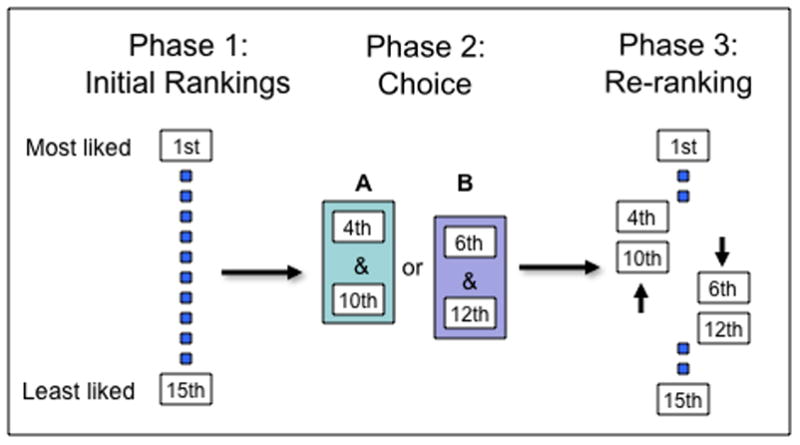
Experimental paradigm for examining post-choice attitude change. In this variant of the paradigm, used by Lieberman and colleagues [23], subjects rank order 15 art prints from most liked to least liked in Phase 1. Next, subjects are asked to choose between two pairs of prints in Phase 2. Choice A includes those prints ranked 4th and 10th, and Choice B includes those prints ranked 6th and 12th by the participant. In Phase 3 of the experiment, subjects are asked to re-rank all 15 art prints. Subjects typically rank the chosen options higher and the rejected options lower in Phase 3 than they did in Phase 1, demonstrating a post-choice change in attitude.
Restriction of Choice is Aversive
The argument for an inherent need for control is strengthened by the findings of physiological and behavioral detriments when personal control is absent. In animals and humans, the perception of control over a stressor has been shown to inhibit autonomic arousal, stress hormone release, immune system suppression, and maladaptive behaviors (e.g. learned helplessness) observed when stressors are uncontrollable 3, 27, 28. Behavioral control has been implicated in decreasing arousal during anticipation of aversive photographs, and in increasing tolerance to pain and aversive noise 29. The benefits of perceived control can exist even in the absence of true control over aversive events, or if the individual has the opportunity to exert control but never actually exercises that option 29. Thus, the perception of control seems to be important for regulation of emotional responses to stressful situations.
In the absence of other stressors, however, the removal of choice, in and of itself, can be very stressful. It has been found that restriction of behaviors, particularly behaviors that are highly valued by a species, contributes to behavioral and physiological manifestations of stress 30, 31. In fact, physical restraint is one of the most popular methods for experimentally inducing stress in rodents. While this procedure does not physically harm the animals in any way, the simple restriction of motion nonetheless results in robust behavioral and physiological indices of stress, such as increased heart rate, increased norepinephrine and cortisol release, and the production of gastric ulcers 32.
On a similar note, animals raised in captivity are markedly disadvantaged compared to their free-ranging conspecifics 33. Lack of control over the environment is believed to be a major cause of the abnormal stereotypic behaviors, failure to thrive and impaired reproduction commonly observed in animals raised in captivity 31. It seems that the aversive effects of captivity may depend on the extent to which behavioral choices have been reduced relative to what could be performed in the natural environment. When animals are provided access to an additional region of their habitat, they show a significant reduction in the stress-related stereotypic behaviors commonly observed in captive animals (e.g. pacing), increased social play, and decreased levels of the stress-hormone cortisol 34, 35. Furthermore, species with larger natural home-range size tend to have higher frequencies of stereotypic behaviors and higher rates of infant mortality 31, but this relationship applies only to animals in captivity, and not for their free-ranging conspecifics.
Humans demonstrate similar patterns of negative affect in response to the removal or restriction of choices. Once control over an aversive stimulus has been established, the removal of this control produces greater fear, more negative perceptions of the stimulus, narrowing of attention, and greater effort placed on regaining control 36. Negative responses to losing control have been observed even in very young human infants. In four-month old infants, disrupting a learned contingency between behavior and rewards, results in negative emotional reactions, even if rewards are still delivered, but are not dependent on the infants’ actions 37. Additionally, once children master a skill (e.g. feeding themselves), they become resistant to adult attempts to influence or control this ability 38. The preference for control, and aversion to its removal, observed in very young infants, suggests that these preferences may be present at birth, or at least very early in development, and thus likely reflect a fundamental need that is biologically motivated.
Neural Bases of Choice and Control: Implications for Emotion Regulation
To make our case for a biological basis for the need for control, we have provided evidence that the perception of control is adaptive, that choice is desirable, and that the removal of choice is aversive. While the behavioral evidence is compelling on its own, it is critical that we identify potential biological substrates of choice and control. The evidence described above suggests that perceived control influences cognition and behavior by modulating affective and motivational processing. Thus, we would expect that the experience of control, as it is exercised through choice, would engage primarily brain regions that have been associated with emotion processing and regulation. Though the affective experience of choice itself has not been examined directly, there is converging evidence that implicates a corticostriatal network as the neural substrate for perceiving control (see Figure 3).
Figure 3. Hypothesized Neural Circuitry for Choice.
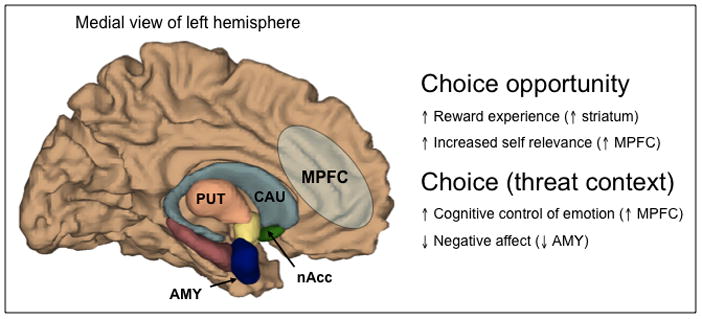
If the opportunity to exercise control, via choice, is intrinsically rewarding, one would expect choice to recruit brain regions commonly involved in reward processing, such as the striatum, which is comprised of the caudate (CAU), putamen (PUT), and nucleus accumbens (nAcc). Other reward regions in the ventral medial prefrontal cortex (MPFC) may also be preferentially activated to reflect increased reward value under increased self relevance. In the context of potential threats, the opportunity to exercise control may recruit regions of the brain that are critical for adaptive emotion regulation, such as the MPFC, and consequently result in decreases in brain regions involved in negative emotional responses, such as the amygdala (AMY).
The prefrontal cortex (PFC) and the striatum are known to be highly interconnected 39 and have been implicated consistently in affective and motivational processes 40, 41. Certain regions within this corticostriatal network explicitly code for actions that are most adaptive in a given context. For example, a subset of neurons in the monkey striatum respond to the expected value of a specific action (e.g. choose left vs. choose right), but do not predict the magnitude of potential rewards, or the probability of selecting of an action, independent of its reward outcome 42. This suggests that there is a biological basis for organisms to be causal agents, rather than passive observers, in their interactions with the environment. The exercise of choice allows organisms to select behaviors that will optimize rewards and minimize punishments, or at least to perceive such effectiveness. Below, we describe evidence from the extant literature supporting the striatum and PFC as key neural substrates of this adaptive behavior.
Recent neuroimaging studies have demonstrated that choice recruits neural circuitry involved in reward and motivation processing. For example, rewards that are instrumentally delivered activate the striatum to a greater extent than rewards that are passively received 43, 44. In an emotional oddball task, Tricomi and colleagues 45 found that rewards following choices activated the striatum to a greater extent than the same rewards following responses where no choice was available. Participants in that study reported feeling greater control over the monetary outcomes when choice was available. Increased activity in the striatum as a function of choice observed by Tricomi 45 and others 46 may reflect increased motivational incentive under choice conditions. Increased activity in the dorsal striatum is also observed when there is greater motivational incentive to perform a task 47 or in response to highly salient stimuli 48. If free choice preferentially activates the striatum, a region involved in reward processing and goal-directed behavior, we might hypothesize that choice, itself, may be inherently rewarding.
In stressful situations, perceived control may modulate emotion by reducing negative affect. Though it is adaptive to automatically generate emotional responses to threats in the environment, it is necessary for an individual to appropriately modulate those responses for the given context. The PFC likely plays an important role in the exertion of top-down regulation of emotional responses 49, 50. Work by Maier and colleagues has been critical for understanding the neural bases of controllability effects on stress regulation 51. In their work, Maier et al found that rodents typically demonstrate stress-related behavior and physiological response when faced with uncontrollable stress (inescapable shock) but not when faced with controllable stress (escapable shock). They found that the relationship between stressor controllability and reduced stress response is mediated by increased activity in the medial PFC (MPFC). Subsequent experiments demonstrated that if the MPFC is lesioned or deactivated, rats respond to escapable shock as if it is inescapable 52, 53. Additionally, they found that if the MPFC is stimulated during inescapable shock, rats demonstrate a reduced stress response. Thus, the protective effects of controllability depend on the integrity and activity of the MPFC.
The rodent MPFC functionally overlaps with both the medial and lateral portions of the primate PFC 54, regions that have been implicated in perception of control in recent neuroimaging studies. The perception of control in the absence of any true control has been associated with increased activity in the MPFC 55. Additionally, there is evidence that recruitment of the lateral and medial PFC when exercising control may mitigate negative emotional responses to aversive situations, such as pain 56, 57 and choices involving increased risk 58. These findings are consistent with results demonstrating an inverse relationship between PFC activity and limbic activity (i.e. amygdala) during both the downregulation of negative affect and fear extinction 50, 59. Thus, in threatening situations, the opportunity to exercise control may alleviate stress by engaging MPFC mechanisms of emotion modulation. In fact, individuals with major depression fail to normally recruit ventral portions of the MPFC when attempting to downregulate negative affect 60.
Control-related activity in the MPFC may also reflect behaviors associated with increased self-relevance, since the MPFC is implicated in perception of the “self” 61–65. Several studies have found the ventral MPFC to be preferentially active when making choices that are self-relevant (e.g. preferences) as compared to choices involving only a perceptual discrimination 66, 67. Nearby regions of the ventrolateral PFC have been found to respond more to choices that have greater personal consequence 68. Thus, opportunity for choice may be more desirable because it engages self-processing networks that increase the subjective value of the actions and their associated rewards.
Above, we present evidence that regions in the PFC and striatum play important roles in perceiving control. It is likely that these regions form a corticostriatal network that interacts to produce the motivational states associated with control and choice. The PFC and striatum are highly interconnected 39 and are commonly coactivated in studies of affective and motivational processes. Furthermore, disruption to motivation, or even complete apathy, can occur as a result of damage or disease of either the striatum or the PFC 39, 69, suggesting the communication between these regions is critical for motivational processes.
Disruptions to the perception of, and desire for control are also associated with alterations in functioning of the MPFC. Profound apathy accompanying Alzheimer’s disease is associated with reduced metabolic activity in the MPFC 70. Schizophrenic individuals with delusions of control and persecution show reduced activity in the ventral MPFC when assessing whether a threat is self-relevant 71. These findings support the hypothesis that corticostriatal regions are involved in perceiving control. Moreover, they demonstrate that the desire for control is an essential component of what it means to be human, since the extreme compromise in this desire and in an individual’s sense of self is observed only in what are arguably some of the most devastating and debilitating psychiatric disorders.
Caveats and Conclusions
In this review, we present evidence that suggests the desire for control is not something we acquire through learning, but rather, is innate, and thus likely biologically motivated. We are born to choose. The existence of the desire for control is present in animals and even very young infants before any societal or cultural values of autonomy can be learned. It is possible that organisms have adapted to find control rewarding – and its absence aversive – since the perception of control seems to play an important role in buffering an individual’s response to environmental stress. We propose that brain regions in a corticostriatal network are integral for the perception of control. If the desire for control is imperative for survival, it makes sense that the neural bases of these adaptive behaviors would be in phylogenetically older regions of the brain that are involved in affective and motivational processes.
Although we present evidence supporting a biological basis for the need for control, we do not make assumptions about the boundaries of the preference for control and choice (see Box 2), nor do we claim that this preference is unchangeable. The desire for control is distinguishable from an individual’s experienced perception of control. While the basic need for control may be biologically motivated, it is possible, as well as probable, that the perception of control, and the preference to exert control, can be altered as a result of personal experience with control 3, as well as learning what is most rewarding in a social context, which may explain cultural differences in how choice is valued (see Box 3). Nonetheless, personal autonomy seems to be highly valued in very young children from diverse cultures 72. Exactly what content is perceived to be included in the personal domain may vary across cultures, but what is important cross-culturally is that the exercise of choice acts to energize and reinforce an individual’s sense of agency18, 19. Anything that undermines this perception of control may be harmful to an individual’s well-being.
Text Box 2. When is choice undesirable?
Choice may not be desirable in all situations, particularly in the context of complex or emotionally difficult decisions. However, there may be a difference between the desire to have choices and the desire to make choices. For example, a study by Iyengar and Lepper 80 found that found that although greater choices seem to be more attractive at first, a larger assortment ultimately may result in deferral of choice. In this study, individuals who were given the opportunity to select from an array of six gourmet jams were more likely to purchase a jam than were participants who chose from an array of 24 or 30 choices. Moreover, individuals were more satisfied with their selections when they were chosen from the limited array of options. In a similar vein, a recent study investigating choice preferences in the context of healthcare 81 found that of the 823 participants included in the study, 95.6% indicated that having choices was extremely important, whereas only 30.3% indicated that making choices was extremely important. If we are motivated to choose the best option, then choosing a non-optimal option means that we were unsuccessful, and so avoiding decision may reflect anticipatory regret or fear of failure or blame for poor decisions. This may explain why people tend to defer to default options (i.e. status-quo) when choice difficulty is increased, a phenomenon which has recently been linked to connectivity between PFC and the basal ganglia 82. In the absence of sufficient knowledge or resources to make an optimal decision, choice by a proxy agent, such as a trusted friend, family member, or physician, may be more desirable than personal choice. In any case, individuals are still exercising control by choosing to engage in or to abstain from decisions to promote their best interest.
Box 3. Questions for future research.
Is the mere act of exercising choice rewarding, independent of its consequences? Does choice opportunity activate neural circuitry involved in affective processing and emotion regulation? How does choice opportunity regulate affective responses to aversive events? How does prior experience with control affect future impact of choice opportunity on emotion regulation?
Under what conditions is choice undesirable? (see Box 2) How do cognitive load, ambiguity and risk influence the desirability of choice? How do these variables modulate corticostriatal brain activity to choice?
How is the value of choice and exercising control impacted by individual differences in development, personality, learning history, and cultural experiences more broadly?
Collectively, the evidence suggests the desire to exercise control, and thus, the desire to make choices, is paramount for survival. The opportunity for choice enhances an individual’s perception of control, and thus, exercising choice may serve as the primary means by which humans and animals foster this psychologically adaptive belief. Just as we respond to physiological needs (e.g. hunger) with specific behaviors (i.e. food consumption), we may fill a fundamental psychological need by exercising choice. While eating is undoubtedly necessary for survival, we argue that exercising control may be critical for an individual to thrive. Thus, we propose that exercising choice and the need for control – much like eating and hunger – are biologically motivated. We argue that while people may be biologically programmed to desire the opportunity for choice, the value of exercising specific choices likely depends on the available cognitive resources of the decision-maker in the given context, as well as the subjective value of the choice contents, influenced by personal experience and social and cultural learning.
Glossary
- Expected value
For a given variable (e.g. monetary reward), the expected value is equal to the product of the actual value and its probability of occurring
- Learned helplessness
When animals or humans have experienced uncontrollable stress they may display helpless behavior when presented with controllable stress in the future
- Post-choice rationalization
Cognitive dissonance arises when one’s choices (or actions more generally) conflict with one’s prior attitudes about choice options. This dissonant state is unpleasant and motivates a change in attitudes about what was chosen and/or not chosen (or done or not done, more generally), which serves to both justify the choice and reduce dissonance.21
- Theory of self-perception
Theory presented by Daryl Bem22, which argues that people draw inferences about their attitudes or beliefs based on observations of their own behavior, rather than direct access to mental states
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Lauren A. Leotti, Email: laurenleotti@psychology.rutgers.edu, Department of Psychology, Rutgers University Newark, Smith Hall, Room 301, 101 Warren Street, Newark, NJ 07102
Sheena S. Iyengar, Email: ss957@columbia.edu, Department of Management, Columbia University, Uris Hall, Room 714, 3022 Broadway, New York, NY 10027
Kevin N. Ochsner, Email: kochsner@psych.columbia.edu, Department of Psychology, Columbia University, 406 Schermerhorn Hall, 1190 Amsterdam Ave, New York, NY 10027
References
- 1.Seuss D. Oh, the Places You'll Go! Random House; 1990. [Google Scholar]
- 2.Patall EA, et al. The effects of choice on intrinsic motivation and related outcomes: a meta-analysis of research findings. Psychol Bull. 2008;134:270–300. doi: 10.1037/0033-2909.134.2.270. [DOI] [PubMed] [Google Scholar]
- 3.Mineka S, Hendersen RW. Controllability and predictability in acquired motivation. Annu Rev Psychol. 1985;36:495–529. doi: 10.1146/annurev.ps.36.020185.002431. [DOI] [PubMed] [Google Scholar]
- 4.Lewinsohn PM, et al. Social competence and depression: the role of illusory self-perceptions. Journal of abnormal psychology. 1980;89:203–212. doi: 10.1037//0021-843x.89.2.203. [DOI] [PubMed] [Google Scholar]
- 5.Alloy LB, Abramson LY. Judgment of contingency in depressed and non-depressed students: Sadder but wiser? Journal of Experimental Psychology: General. 1979;108:441–485. doi: 10.1037//0096-3445.108.4.441. [DOI] [PubMed] [Google Scholar]
- 6.Peterson C, Seligman ME. Explanatory style and illness. Journal of personality. 1987;55:237–265. doi: 10.1111/j.1467-6494.1987.tb00436.x. [DOI] [PubMed] [Google Scholar]
- 7.Cannon DR. Cause or control? The temporal dimension in failure sense-making. The Journal of Applied Behavioral Science. 1999;35:416. [Google Scholar]
- 8.Shapiro DH, Jr, et al. Controlling ourselves, controlling our world. Psychology’s role in understanding positive and negative consequences of seeking and gaining control. The American psychologist. 1996;51:1213–1230. doi: 10.1037//0003-066x.51.12.1213. [DOI] [PubMed] [Google Scholar]
- 9.Goodie AS. The role of perceived control and overconfidence in pathological gambling. Journal of Gambling Studies. 2005;21:481–502. doi: 10.1007/s10899-005-5559-1. [DOI] [PubMed] [Google Scholar]
- 10.Fenton-O’Creevy M, et al. Trading on illusions: Unrealistic perceptions of control and trading performance. Journal of Occupational and Organizational Psychology. 2003;76:53–68. [Google Scholar]
- 11.Strupp HH. Specific versus non-specific factors in psychotherapy and the problem of control. Archives of General Psychiatry. 1970;23:393–401. doi: 10.1001/archpsyc.1970.01750050009002. [DOI] [PubMed] [Google Scholar]
- 12.Catania AC, Sagvolden T. Preference for free choice over forced choice in pigeons. Journal of the experimental analysis of behavior. 1980;34:77–86. doi: 10.1901/jeab.1980.34-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki S. Selection of force- and free-choice by monkeys. Perceptual and motor skills. 1999;88:242–250. [Google Scholar]
- 14.Bown NJ, et al. The lure of choice. Journal of Behavioral Decision Making. 2003;16:297–308. [Google Scholar]
- 15.Suzuki S. Effects of number of alternatives on choice in humans. Behavioural Processes. 1997;39:205–214. doi: 10.1016/s0376-6357(96)00049-6. [DOI] [PubMed] [Google Scholar]
- 16.Cordova DI, Lepper MR. Intrinsic Motivation and the Process of Learning: Beneficial Effects of Contextualization, Personalization, and Choice. Journal of Educational Psychology. 1996;88:715–730. [Google Scholar]
- 17.Langer EJ, Rodin J. The effects of choice and enhanced personal responsibility for the aged: a field experiment in an institutional setting. J Pers Soc Psychol. 1976;34:191–198. doi: 10.1037//0022-3514.34.2.191. [DOI] [PubMed] [Google Scholar]
- 18.Henry RA, Sniezek JA. Situational factors affecting judgments of future performance. Organizational Behavior and Human Decision Processes. 1993;54:104–132. [Google Scholar]
- 19.Tafarodi RW, et al. The confidence of choice: Evidence for an augmentation effect on self-perceived performance. Personality and Social Psychology Bulletin. 1999;25:1405–1416. [Google Scholar]
- 20.Brehm JW. Post-decision changes in the desirability of alternatives. Journal of Abnormal and Social Psychology. 1956;52:384–389. doi: 10.1037/h0041006. [DOI] [PubMed] [Google Scholar]
- 21.Festinger L. A theory of cognitive dissonance. Row, Peterson; 1957. [Google Scholar]
- 22.Bem DJ. An experimental analysis of self-persuasion. Journal of Experimental Social Psychology. 1965;1:199–218. [Google Scholar]
- 23.Egan LC, et al. The origins of cognitive dissonance: evidence from children and monkeys. Psychol Sci. 2007;18:978–983. doi: 10.1111/j.1467-9280.2007.02012.x. [DOI] [PubMed] [Google Scholar]
- 24.Lieberman MD, et al. Do amnesics exhibit cognitive dissonance reduction? The role of explicit memory and attention in attitude change. Psychol Sci. 2001;12:135–140. doi: 10.1111/1467-9280.00323. [DOI] [PubMed] [Google Scholar]
- 25.Sharot T, et al. How choice reveals and shapes expected hedonic outcome. J Neurosci. 2009;29:3760–3765. doi: 10.1523/JNEUROSCI.4972-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lyubomirsky S, Ross L. Changes in attractiveness of elected, rejected, and precluded alternatives: a comparison of happy and unhappy individuals. Journal of personality and social psychology. 1999;76:988–1007. doi: 10.1037//0022-3514.76.6.988. [DOI] [PubMed] [Google Scholar]
- 27.Bandura A, et al. Catecholamine secretion as a function of perceived coping self-efficacy. Journal of Consulting and Clinical Psychology. 1985;53:406–414. doi: 10.1037//0022-006x.53.3.406. [DOI] [PubMed] [Google Scholar]
- 28.Maier SF, et al. Stressor controllability, immune function, and endogenous opiates. In: Brush FR, Overmier JB, editors. Affect, conditioning, and cognition: Essays on the determinants of behavior. Erlbaum; 1985. pp. 183–201. [Google Scholar]
- 29.Thompson SC. Will it hurt less if I can control it? A complex answer to a simple question. Psychological Bulletin. 1981;90:89–101. [PubMed] [Google Scholar]
- 30.Friend TH. Recognizing behavioral needs. Applied Animal Behaviour Science. 1989;22:151–158. [Google Scholar]
- 31.Clubb R, Mason G. Animal welfare: captivity effects on wide-ranging carnivores. Nature. 2003;425:473–474. doi: 10.1038/425473a. [DOI] [PubMed] [Google Scholar]
- 32.Glavin GB, et al. Restraint stress in biomedical research: an update. Neuroscience and biobehavioral reviews. 1994;18:223–249. doi: 10.1016/0149-7634(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 33.Morgan KN, Tromberg CT. Sources of stress in captivity. Applied Animal Behaviour Science. 2007;102:262–302. [Google Scholar]
- 34.Owen MA, et al. Enclosure choice and well-being in giant pandas: Is it all about control? Zoo Biology. 2005;24:475–481. [Google Scholar]
- 35.Ross SR. Issues of choice and control in the behaviour of a pair of captive polar bears (Ursus maritimus) Behavioural Processes. 2006;73:117–120. doi: 10.1016/j.beproc.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 36.Crombez G, et al. Is it better to have controlled and lost than never to have controlled at all? An experimental investigation of control over pain. Pain. 2008;137:631–639. doi: 10.1016/j.pain.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 37.Sullivan MW, Lewis M. Contextual determinants of anger and other negative expressions in young infants. Dev Psychol. 2003;39:693–705. doi: 10.1037/0012-1649.39.4.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kochanska G, Aksan N. Development of mutual responsiveness between parents and their young children. Child development. 2004;75:1657–1676. doi: 10.1111/j.1467-8624.2004.00808.x. [DOI] [PubMed] [Google Scholar]
- 39.Levy R, Dubois B. Apathy and the functional anatomy of the prefrontal cortex-basal ganglia circuits. Cereb Cortex. 2006;16:916–928. doi: 10.1093/cercor/bhj043. [DOI] [PubMed] [Google Scholar]
- 40.Berridge KC, Kringelbach ML. Affective neuroscience of pleasure: reward in humans and animals. Psychopharmacology. 2008;199:457–480. doi: 10.1007/s00213-008-1099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Delgado MR. Reward-related responses in the human striatum. Annals of the New York Academy of Sciences. 2007;1104:70–88. doi: 10.1196/annals.1390.002. [DOI] [PubMed] [Google Scholar]
- 42.Samejima K, et al. Representation of action-specific reward values in the striatum. Science (New York, N Y) 2005;310:1337–1340. doi: 10.1126/science.1115270. [DOI] [PubMed] [Google Scholar]
- 43.Bjork JM, Hommer DW. Anticipating instrumentally obtained and passively-received rewards: a factorial fMRI investigation. Behavioural brain research. 2007;177:165–170. doi: 10.1016/j.bbr.2006.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Doherty J, et al. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science (New York, N Y) 2004;304:452–454. doi: 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- 45.Tricomi EM, et al. Modulation of caudate activity by action contingency. Neuron. 2004;41:281–292. doi: 10.1016/s0896-6273(03)00848-1. [DOI] [PubMed] [Google Scholar]
- 46.Coricelli G, et al. Regret and its avoidance: a neuroimaging study of choice behavior. Nat Neurosci. 2005;8:1255–1262. doi: 10.1038/nn1514. [DOI] [PubMed] [Google Scholar]
- 47.Delgado MR, et al. Motivation-dependent responses in the human caudate nucleus. Cereb Cortex. 2004;14:1022–1030. doi: 10.1093/cercor/bhh062. [DOI] [PubMed] [Google Scholar]
- 48.Zink CF, et al. Human striatal activation reflects degree of stimulus saliency. Neuroimage. 2006;29:977–983. doi: 10.1016/j.neuroimage.2005.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kober H, et al. Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. Neuroimage. 2008;42:998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 51.Maier SF, et al. Behavioral control, the medial prefrontal cortex, and resilience. Dialogues in clinical neuroscience. 2006;8:397–406. doi: 10.31887/DCNS.2006.8.4/smaier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Amat J, et al. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- 53.Amat J, et al. Previous experience with behavioral control over stress blocks the behavioral and dorsal raphe nucleus activating effects of later uncontrollable stress: role of the ventral medial prefrontal cortex. J Neurosci. 2006;26:13264–13272. doi: 10.1523/JNEUROSCI.3630-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Uylings HB, et al. Do rats have a prefrontal cortex? Behavioural brain research. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 55.Tanaka SC, et al. Calculating consequences: brain systems that encode the causal effects of actions. J Neurosci. 2008;28:6750–6755. doi: 10.1523/JNEUROSCI.1808-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Salomons TV, et al. Individual differences in the effects of perceived controllability on pain perception: critical role of the prefrontal cortex. J Cogn Neurosci. 2007;19:993–1003. doi: 10.1162/jocn.2007.19.6.993. [DOI] [PubMed] [Google Scholar]
- 57.Wiech K, et al. Neurocognitive aspects of pain perception. Trends Cogn Sci. 2008;12:306–313. doi: 10.1016/j.tics.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 58.Rao H, et al. Neural correlates of voluntary and involuntary risk taking in the human brain: an fMRI Study of the Balloon Analog Risk Task (BART) Neuroimage. 2008;42:902–910. doi: 10.1016/j.neuroimage.2008.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Delgado MR, et al. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron. 2008;59:829–838. doi: 10.1016/j.neuron.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Johnstone T, et al. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci. 2007;27:8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kelley WM, et al. Finding the self? An event-related fMRI study. Journal of cognitive neuroscience. 2002;14:785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- 62.Gusnard DA, et al. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Johnson SC, et al. Neural correlates of self-reflection. Brain. 2002;125:1808–1814. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- 64.Platek SM, et al. Where am I? The neurological correlates of self and other. Brain research. 2004;19:114–122. doi: 10.1016/j.cogbrainres.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 65.Heatherton TF, et al. Medial prefrontal activity differentiates self from close others. SCAN. 2006;1:18–25. doi: 10.1093/scan/nsl001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Johnson SC, et al. The cerebral response during subjective choice with and without self-reference. Journal of cognitive neuroscience. 2005;17:1897–1906. doi: 10.1162/089892905775008607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Paulus MP, Frank LR. Ventromedial prefrontal cortex activation is critical for preference judgments. Neuroreport. 2003;14:1311–1315. doi: 10.1097/01.wnr.0000078543.07662.02. [DOI] [PubMed] [Google Scholar]
- 68.Turk DJ, et al. From facial cue to dinner for two: the neural substrates of personal choice. Neuroimage. 2004;22:1281–1290. doi: 10.1016/j.neuroimage.2004.02.037. [DOI] [PubMed] [Google Scholar]
- 69.Fuster V. Human lesion studies. Annals of the New York Academy of Sciences. 1997;811:207–224. doi: 10.1111/j.1749-6632.1997.tb52003.x. discussion 224–205. [DOI] [PubMed] [Google Scholar]
- 70.Marshall GA, et al. Positron emission tomography metabolic correlates of apathy in Alzheimer disease. Archives of neurology. 2007;64:1015–1020. doi: 10.1001/archneur.64.7.1015. [DOI] [PubMed] [Google Scholar]
- 71.Blackwood NJ, et al. Persecutory delusions and the determination of self-relevance: an fMRI investigation. Psychological medicine. 2004;34:591–596. doi: 10.1017/S0033291703008997. [DOI] [PubMed] [Google Scholar]
- 72.Helwig CC. The development of personal autonomy throughout cultures. Cognitive Development. 2006;21:458–473. [Google Scholar]
- 73.Bandura A. Self-efficacy: the exercise of control. Freeman; 1997. [Google Scholar]
- 74.Kazdin AE. Imagery elaboration and self-efficacy in the covert modeling treatment of unassertive behavior. Journal of Consulting and Clinical Psychology. 1979;47:725–233. doi: 10.1037//0022-006x.47.4.725. [DOI] [PubMed] [Google Scholar]
- 75.Bandura A, Wood RE. Effect of perceived controllability and performance standards on self-regulation of complex decision-making. Journal of Personality and Social Psychology. 1989;56:805–814. doi: 10.1037//0022-3514.56.5.805. [DOI] [PubMed] [Google Scholar]
- 76.Bandura A, et al. Role of Affective Self-Regulatory Efficacy in Diverse Spheres of Psychosocial Functioning. Child development. 2003;74:769–782. doi: 10.1111/1467-8624.00567. [DOI] [PubMed] [Google Scholar]
- 77.Ryan RM, Deci EL. Self-regulation and the problem of human autonomy: does psychology need choice, self-determination, and will? Journal of personality. 2006;74:1557–1585. doi: 10.1111/j.1467-6494.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 78.Rotter JB. Generalized expectancies of internal versus external control of reinforcements. Psychological Monographs. 1966:80. [PubMed] [Google Scholar]
- 79.Langer EJ. The Illusion of Control. Journal of Personality and Social Psychology. 1975;32:311–328. [Google Scholar]
- 80.Iyengar SS, Lepper M. When Choice is Demotivating: Can One Desire Too Much of a Good Thing? Journal of Personality and Social Psychology. 2000;79:995–1006. doi: 10.1037//0022-3514.79.6.995. [DOI] [PubMed] [Google Scholar]
- 81.Ogden J, et al. The value of choice: development of a new measurement tool. Br J Gen Pract. 2008;58:614–618. doi: 10.3399/bjgp08X330735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fleming SM, et al. Overcoming status quo bias in the human brain. Proc Natl Acad Sci U S A. 2010;107:6005–6009. doi: 10.1073/pnas.0910380107. [DOI] [PMC free article] [PubMed] [Google Scholar]


